Abstract
Two dissociable learning processes underlie instrumental behaviour. Whereas goal-directed behaviour is controlled by knowledge of the consequences, habitual behaviour is elicited directly by antecedent Pavlovian stimuli without knowledge of the consequences. Predominance of habitual control is thought to underlie psychopathological conditions associated with corticostriatal abnormalities, such as impulsivity and drug dependence. To explore this claim, smokers were assessed for nicotine dependence, impulsivity, and capacity for goal-directed control over instrumental performance in an outcome devaluation procedure. Reduced goal-directed control was selectively associated with the Motor Impulsivity factor of Barrett's Impulsivity Scale (BIS), which reflects propensity for action without thought. These data support the claim that human impulsivity is marked by impaired use of causal knowledge to make adaptive decisions. The predominance of habit learning may play a role in psychopathological conditions that are associated with trait impulsivity.
Keywords: Outcome specific devaluation, Goal-directed learning, Habit, Drug dependence, Nicotine, Impulsivity
Animal behavioural neuroscience has identified two dissociable learning processes underlying instrumental behaviour (Dickinson & Balleine, 2010). Goal-directed, or intentional, instrumental behaviour is mediated by explicit knowledge of the instrumental contingency between response (R) and receipt of the outcome (O; intention), combined with knowledge of the incentive value of the O (desire), which provides evaluative feedback to determine the propensity to perform the R. By contrast, habitual instrumental behaviour comes under the direct control of stimuli (S) in which the response (R) has been previously reinforced, through classic S-R/reinforcement learning. Thus, whereas goal-directed behaviour is mediated by knowledge of the outcome, such knowledge plays no role in habitual behaviour because the S, when reencountered, elicits the R directly.
The revaluation procedure is the accepted method for determining the goal-directed or habitual status of an instrumental response. In a commonly employed animal revaluation design, rats first learn two instrumental responses for distinct outcomes (such as pellets and sucrose), before one outcome is devalued by specific satiety or taste aversion conditioning in a separate context. In the test phase that follows, rats again have the opportunity to perform the two instrumental responses, but this time in extinction—that is, where the two responses are no longer effective in producing their respective outcomes. A selective reduction of responding for the devalued outcome is interpreted as evidence that the response is goal directed in the sense of being controlled by knowledge of the R-O contingencies established in training combined with knowledge of the current low incentive value of the devalued outcome. By contrast, equivalent responding for the devalued and nondevalued outcome indicates that these responses have become autonomous of the current incentive value of the outcomes—that is, they have become S-R habits elicited directly by the instrumental context without knowledge of their consequences.
This dual-process theory of instrumental learning is supported by behavioural dissociation studies. These studies have found that whereas habit learning is favoured by conditions in which a single response-outcome contingency is overtrained (Dickinson, Balleine, Watt, Gonzalez, & Boakes, 1995; Holland, 2004; Kosaki & Dickinson, 2010), where the response is proximal to consumption in a seeking-taking chain (Corbit & Balleine, 2003) and where the causal contingency between the response and the outcome is degraded (Dickinson, Nicholas, & Adams, 1983), the opposite conditions favour goal-directed learning. Furthermore, neural dissociation studies have established that whereas goal-directed learning is mediated by a circuit including the prelimbic cortex and dorsomedial striatum, habit learning is primarily mediated by the somatosensory cortices and dorsolateral striatum (Balleine & O'Doherty, 2010). Finally, although the S -R controller may predominate in invariant conditions, agents readily switch back to the R-O controller when a prediction error is encountered (Daw, Niv, & Dayan, 2005).
The dominance of the habit learning system has been argued to underlie individual differences in impulsivity and associated conditions such as drug dependence (Everitt et al., 2008). Evidence for this claim comes from the finding that impulsivity confers a risk factor for dependence in both humans and animals (Belin, Mar, Dalley, Robbins, & Everitt, 2008; Verdejo-García, Lawrence, & Clark, 2008), and both conditions are associated with abnormalities in the corticostriatal pathways (Biederman et al., 2008; Volkow, Fowler, Wang, Baler, & Telang, 2009) underlying goal-directed and habit learning (Balleine & O'Doherty, 2010; Jedynak, Uslaner, Esteban, & Robinson, 2007). Finally, drug preexposure enhances impulsivity (Perry & Carrol, 2008) and habit learning (Nelson & Killcross, 2006; Nordquist et al., 2007; Schoenbaum & Setlow, 2005). The implication is that a preexisting habitual/impulsive trait increases the risk of uptake and maintenance of drug use because the individual fails to use knowledge of adverse consequences to regulate consumption. In turn, drug exposure exacerbates the individuals’ habitual/impulsive trait by impacting on the neural substrates of instrumental learning, thus rendering them more prone to develop habitual drug-seeking behaviours. The vulnerable individuals thus enter a vicious circle that longitudinally spirals into clinical dependence (Everitt et al., 2008).
To explore this claim, the current study used a human revaluation procedure to assess the relationship between individual differences in goal-directed control, impulsivity, and nicotine dependence in a group of student smokers. Revaluation procedures have been developed for humans to explore the neural substrates (de Wit, Corlett, Aitken, Dickinson, & Fletcher, 2009; Tricomi, Balleine, & O'Doherty, 2009; Valentin, Dickinson, & O'Doherty, 2007), developmental maturation (Kenward, Folke, Holmberg, Johansson, & Gredeback, 2009; Klossek, Russell, & Dickinson, 2008), and effects of conflict (de Wit, Niry, Wariyar, Aitken, & Dickinson, 2007) and stress (Schwabe & Wolf, 2009) on goal-directed and habit learning. However, as far as we are aware, this study is the first to examine the link between goal-directed control and impulsivity in humans.
Method
Participants
Student smokers (n = 64) were recruited and were dichotomized into those who smoked every day (n = 32) and those who smoked less than every day of the week (n = 32). This behavioural criterion was employed because the early uptake of smoking measured in this way has been prospectively associated with the longitudinal perseveration of smoking and development of clinical dependence and thus provides a proxy for individual difference in vulnerability to dependence amongst individuals who have tried the drug (Hiroi & Scott, 2009). Gender was balanced within each group. All participants accurately reported knowledge of the response-outcome contingencies at the end of training. A further 8 participants were excluded and replaced who failed to accurately report this instrumental knowledge.
Apparatus and materials
Impulsivity was measured with Version 11 of Barratt's Impulsivity Scale (BIS) because this scale has been well validated as an marker for dependence and neuropsychiatric conditions (Stanford et al., 2009). This questionnaire contains three subscales: (a) Motor Impulsivity (e.g., “I do things without thinking”) assesses propensity for action without thought; (b) Nonplanning Impulsivity (e.g., “I plan tasks carefully”) assesses future orientation; and (c) Attentional Impulsivity (e.g., “I don't pay attention”) assesses capacity for sustained attention. The median of each subscale was used to dichotomize the sample to establish three impulsivity criteria. Programming was conducted in E-prime software (Psychology Software Tools, Inc.) with standard PC and monitor.
It should be noted that impulsivity is a multidimensional construct, and there are many methods for measuring this trait (Cyders, Flory, Rainer, & Smith, 2009; Perry & Carrol, 2008; Verdejo-García et al., 2008; Whiteside & Lynam, 2001). We employed the BIS because this scale has been associated with drug dependence in many studies (Stanford et al., 2009), and we wanted to avoid increasing the likelihood of obtaining a false positive due to obtaining multiple indices of impulsivity. It will be important for future work to assess the relationship between goal-directed control and other measures of impulsivity.
Procedure
Participants provided informed consent and their breath carbon monoxide level (through Bedfont Smokerlyzer). Questionnaires then established age, gender, smoking days per week, cigarettes smoked on smoking days, age of smoking onset, smoking years, time of their last cigarette, DSM-IV (Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition; American Psychiatric Association, 1994) nicotine dependence (Donny & Dierker, 2007), cigarette dependence score (Etter, Le Houezec, & Perneger, 2003), smoking urges (Brief Questionnaire of Smoking Urges, QSU-brief; Cappelleri et al., 2007), and substance misuse (Willner, 2000). The study was approved by the University of Nottingham Psychology Ethics Committee.
Concurrent choice training
The purpose of concurrent choice training was to establish two instrumental responses, each of which earned a distinct reward. The training session was initiated with the following onscreen instructions: “This is a game in which you can win chocolate bars and bottles of water. In each trial, press the D or H key to try and win these rewards. You will only win on some trials. Press the space bar to begin.” Participants were informed verbally at this stage that they would not get to keep the rewards they earned during the task. Each trial began with the centrally presented text, “Select a key,” which remained until either the D or the H key was pressed. A single response on one key immediately replaced this text with the outcome, “You win 1/4 of chocolate bar,” whereas the other key produced the outcome, “You win 1/4 of a bottle of water,” accompanied by a picture of that reward. The key-reward assignment was counterbalanced between participants. Each key had only a 50% chance of yielding its respective outcome. On nonrewarded trials, the text “You win nothing” was presented. The outcomes (chocolate, water, nothing) were presented for 1,500 ms, followed by a random intertrial interval between 1,000 and 2,500 ms prior to the next trial.
Training comprised five 16 trial blocks, and each block contained two cycles of 8 trials. Each cycle scheduled 4 chocolate and 4 water outcomes in random order. At the end of each block a “totalizer” screen reported the quantity of each reward type earned and instructed participants to move whole chocolate bars or water bottles into “their” boxes present on the table (remainders were carried over to the next block). There were 10 each of Cadbury Dairy Milk Treatsize chocolate bars (15 g) and Acqua Panna S. Pellegrino still water bottles (250 ml) on the table, which participants cached in two adjacent boxes upon these totalizer instructions.
Specific satiety
Following training, participants in each smoker group were randomly assigned to a chocolate and water satiety group (balancing gender). At the start of the satiety treatment, participants were asked to consume either eight chocolate bars or two bottles of water, respectively, to rate the pleasantness of each unit (chunk/sip) consumed on a visual analogue scale, to record the number of units consumed and the decline in palatability across units. Beyond these instructions, participants were not compelled to consume more than they wished, for ethical reasons, and whether they consumed the total amount or not was not recorded because the decline in hedonic evaluation of the devalued commodity was deemed to be the critical factor rather than the absolute amount consumed. When participants had finished consuming as much as possible of the reward, they reported their desire to consume chocolate and water on a 7-point Likert scale, to estimate the effectiveness of the satiety treatment at reducing desire for the devalued compared to the nondevalued outcome. The data of interest were the number of units consumed (consumption), the decline in the pleasantness ratings from the first to the last unit consumed (reduction in pleasure), and the reduction in desire for the devalued compared to the nondevalued outcome (reduction in desire).
Extinction test
The purpose of the extinction test was to assess the effect of the satiety treatment on choice between the two keys in the absence of feedback from the outcomes. Immediately following the satiety treatment, participants were presented with the on-screen instructions: “In this phase, you can earn chocolate bars and bottles of water as before but you will only be told how many you have earned at the end of the experiment. Press the space bar to begin“. By telling subjects that rewards were earned despite the absence of outcome feedback, we ensured that responding was maintained rather than rapidly extinguished (Schwabe & Wolf, 2009; Tricomi et al., 2009; Valentin et al., 2007).
The extinction test was identical to training except that outcomes were omitted from trials (thus each key selection simply launched the intertrial interval, ITI), the totalizer screen was omitted (each block ran seamlessly into the next), and there were 72 trials. Immediately following the test session, participants were asked which key had produced chocolate and which had produced water in the first part of the experiment. Participants were included only if they accurately reported these instrumental contingencies. Finally, participants were paid £5 and were debriefed.
Results
Devaluation effect
Percentage choice of the chocolate key increased linearly across the five blocks of training—50.2, 51.8, 52.3, 54.9, 55.8, respectively, F(1, 63) = 5.83, p < .05—but stabilized by the final two blocks, F < 1, so the mean of the final two blocks was treated as the baseline choice. This baseline differed reliably from the economic indifference value of 50%, t(63) = 3.42, p < .005, indicating that there was a 5.3% preference for chocolate over water at baseline. However, there was no reliable difference in baseline chocolate choice between groups dichotomized by the three BIS impulsivity factors, smoking status, and satiety treatment, Fs(1, 62) < 1.51, ps > .22. Thus, we could examine the percentage reduction in choice of the devalued outcome in the extinction test compared to baseline in the knowledge that baseline choice was matched between groups.
Percentage chocolate choice showed no linear change across six 12-trial blocks of the extinction test in either satiety group, Fs(1, 31) < 2.21, ps > .05, so the overall mean was treated as the test session. Devaluation magnitude scores were calculated by subtracting the choice of the devalued outcome in the test phase from that at baseline, such that positive values reflect the percentage decrease in choice of the devalued outcome following the satiety treatment.
Figure 1A shows that the groups dichotomized by the median BIS motor impulsivity score (25) differed with respect to devaluation magnitude scores, F(1, 62) = 7.96, p < .007, although the devaluation effect of both the low, t(32) = 7.03, p < .001, and the high, t(32) = 2.69, p < .05, motor impulsive group was significantly greater than zero, indicating that the difference was in degree of goal-directed control. Importantly, the group effect was significant relative to the Bonferroni-corrected alpha level of p < .01 employed to control for the inflated Type 1 error rate due to conducting five group contrasts (tobacco dependence status, three BIS scores, and satiety treatment). The group effect did not interact with satiety treatment, F < 1, indicating that the group effect was equivalent in the two satiety treatments. Finally, when devaluation magnitude scores were segmented into six blocks of testing, the group effect did not interact with block, nor was there a main effect of block, Fs < 1, indicating that the group effect was stable across test blocks and cannot be attributed to a differential return of appetitive for the devalued outcome.
Figure 1.
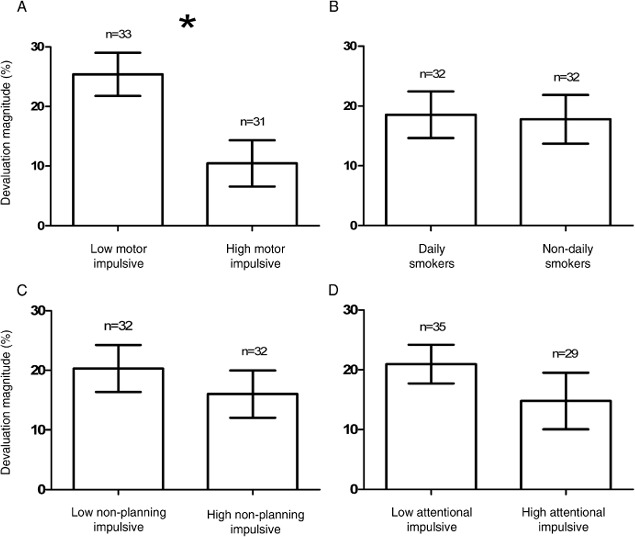
Mean percentage reduction in choice of the devalued outcome in the extinction test relative to baseline, in groups split by daily smoking and the median of the three BIS impulsivity scores.
Additionally, as shown in Table 1, the high and low BIS motor groups were matched for consumption and reduction in subjective pleasure and desire for the devalued outcome in the satiety treatment, indicating equal effectiveness of the satiety treatment at reducing the hedonic appraisal of the devalued reward. Moreover, the group effect in Figure 1A remained significant when all measures listed in Table 1 were entered separately as covariates, Fs(1, 61) > 6.23, ps ≤ .01. Likewise, correlations revealed an association between BIS motor impulsivity scores and the devaluation effect, r = — .26, p < .05 (see Table 2), and this association remained significant when partial correlations controlled all variables listed in Table 1, rs > — .25, p ≤ .052. This analysis shows that differential goal-directed control across levels of motor impulsivity could not be attributed to confounding variables reflected in questionnaire data, including the other impulsivity subscales, or differential experience of the satiety treatment.
Table 1.
Mean questionnaire and satiety data from the sample split by daily smoking, median BIS motor, nonplanning, and impulsivity scores, and satiety treatment
| Smoker groups | BIS motor groups | BIS nonplanning | BIS attention groups | Satiety groups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Daily (n = 32) |
Nondaily (n = 32) |
p ≤ |
Low (n = 33) |
High (n = 31) |
p ≤ |
Low (n = 32) |
High (n = 32) |
p ≤ |
Low (n = 35) |
High (n = 29) |
p ≤ |
Chocolate (n = 32) |
Water (n = 32) |
p ≤ | |
| Number of males | 16 | 16 | 1 | 17 | 15 | .80 | 17 | 15 | .62 | 21 | 11 | .08 | 16 | 16 | 1 |
| Age (years) | 21.2 | 21.1 | .82 | 21.2 | 21.2 | .97 | 21.0 | 21.3 | .69 | 21.5 | 20.8 | .28 | 21.4 | 21.0 | .57 |
| (3.6) | (1.6) | (1.8) | (3.6) | (1.7) | (3.6) | (3.6) | (1.2) | (3.6) | (1.6) | ||||||
| Smoking days per week | 7.0 | 3.2 | .00 | 4.5 | 5.8 | .01 | 5.0 | 5.2 | .69 | 5.0 | 5.2 | .66 | 4.7 | 5.5 | .15 |
| (0.0) | (1.5) | (2.3) | (1.8) | (2.3) | (2.1) | (2.2) | (2.1) | (2.4) | (1.8) | ||||||
| Cigarettes on smoking days | 9.2 | 4.0 | .00 | 5.8 | 7.4 | .17 | 7.0 | 6.2 | .49 | 6.8 | 6.3 | .66 | 7.2 | 6.0 | .29 |
| (5.1) | (2.2) | (3.3) | (5.7) | (5.7) | (3.4) | (4.5) | (5.0) | (5.0) | (4.3) | ||||||
| Hours since a cigarette | 4.7 | 76.1 | .00 | 34.2 | 46.9 | .32 | 23.3 | 57.5 | .08 | 28.3 | 55.1 | .37 | 41.2 | 39.6 | .75 |
| (5.5) | (124) | (39.1) | (131) | (31) | (129) | (38) | (134) | (67) | (117) | ||||||
| Breath CO | 9.7 | 2.4 | .00 | 5.5 | 6.6 | .48 | 7.2 | 4.8 | .14 | 6.5 | 5.4 | .51 | 7.0 | 5.0 | .22 |
| (7.6) | (1.4) | (6.1) | (7.0) | (8.1) | (4.4) | (6.6) | (6.6) | (7.3) | (5.6) | ||||||
| Years spent smoking | 4.8 | 4.1 | .42 | 4.3 | 4.6 | .77 | 4.5 | 4.5 | .96 | 5.2 | 3.6 | .09 | 4.7 | 4.3 | .67 |
| (4.6) | (2.4) | (2.3) | (4.7) | (2.4) | (4.6) | (4.6) | (1.8) | (4.5) | (2.6) | ||||||
| Age of smoking onset (years) | 16.4 | 17.1 | .16 | 16.8 | 16.7 | .81 | 16.7 | 16.8 | .84 | 16.3 | 17.2 | .07 | 16.7 | 16.8 | .85 |
| (2.2) | (1.5) | (1.4) | (2.3) | (1.5) | (2.3) | (2.0) | (1.7) | (1.5) | (2.2) | ||||||
| DSM nicotine dependence | 5.2 | 4.2 | .00 | 4.6 | 4.7 | .67 | 4.8 | 4.5 | .43 | 4.7 | 4.6 | .87 | 4.5 | 4.8 | .34 |
| (1.4) | (1.6) | (1.4) | (1.7) | (1.4) | (1.7) | (1.3) | (1.8) | (1.5) | (1.6) | ||||||
| Cigarette dependence score | 13.9 | 7.5 | .00 | 9.9 | 11.5 | .15 | 10.9 | 10.5 | .73 | 10.7 | 10.7 | .99 | 10.4 | 11.0 | .61 |
| (3.6) | (2.1) | (3.7) | (4.9) | (4.7) | (4.1) | (4.3) | (4.5) | (4.5) | (4.3) | ||||||
| QSU Factor 1 | 3.7 | 2.4 | .00 | 3.0 | 3.2 | .49 | 3.1 | 3.1 | .94 | 2.8 | 3.5 | .08 | 2.9 | 3.3 | .27 |
| (1.7) | (1.2) | (1.6) | (1.6) | (1.7) | (1.6) | (1.5) | (1.6) | (1.3) | (1.9) | ||||||
| QSU Factor 2 | 1.8 | 1.7 | .78 | 1.7 | 1.7 | .97 | 1.9 | 1.6 | .19 | 1.7 | 1.8 | .48 | 1.5 | 2.0 | .06 |
| (1.1) | (0.8) | (1.1) | (0.8) | (1.1) | (0.8) | (1.0) | (0.9) | (0.8) | (1.1) | ||||||
| Substance misuse score | 2.7 | 1.7 | .06 | 1.8 | 2.6 | .15 | 1.9 | 2.4 | .38 | 2.3 | 2.1 | .79 | 1.7 | 2.7 | .10 |
| (2.2) | (2.3) | (2.1) | (2.4) | (2.2) | (2.4) | (2.3) | (2.3) | (2.2) | (2.3) | ||||||
| BIS motor | 26.6 | 24.4 | .08 | 21.6 | 29.6 | .00 | 23.2 | 27.8 | .00 | 24.4 | 26.8 | .07 | 24.3 | 26.7 | .06 |
| (4.7) | (5.3) | (3.2) | (3.1) | (5.1) | (4.0) | (4.8) | (5.2) | (3.6) | (6.1) | ||||||
| BIS nonplanning | 25.2 | 25.4 | .86 | 23.2 | 27.5 | .00 | 21.3 | 29.3 | .00 | 24.1 | 26.8 | .03 | 24.8 | 25.8 | .44 |
| (4.9) | (5.1) | (4.7) | (4.4) | (3.3) | (2.7) | (4.0) | (5.7) | (4.1) | (5.8) | ||||||
| BIS attentional | 17.7 | 17.2 | .64 | 16.5 | 18.4 | .07 | 16.0 | 18.8 | .00 | 14.2 | 21.3 | .00 | 16.7 | 18.1 | .21 |
| (4.4) | (4.2) | (3.8) | (4.6) | (3.7) | (4.3) | (2.0) | (2.7) | (3.4) | (4.9) | ||||||
| Chunks/sips consumed | 21.0 | 22.1 | .60 | 20.4 | 22.7 | .29 | 21.0 | 22.1 | .62 | 21.8 | 21.2 | .78 | 18.3 | 24.8 | .00 |
| (8.7) | (8.9) | (8.9) | (8.4) | (9.3) | (8.2) | (8.9) | (8.6) | (8.3) | (8.0) | ||||||
| Reduction in pleasure | 57.6 | 69.8 | .06 | 62.9 | 64.5 | .79 | 66.7 | 60.7 | .34 | 63.5 | 63.8 | .97 | 68.2 | 59.2 | .15 |
| (27.1) | (21.6) | (27.1) | (23.2) | (23.8) | (26.3) | (26.0) | (24.4) | (25.0) | (24.7) | ||||||
| Reduction in desire | 4.2 | 4.3 | .94 | 4.2 | 4.2 | .97 | 4.3 | 4.2 | .85 | 4.5 | 4.0 | .31 | 5.3 | 3.2 | .00 |
| (1.9) | (1.9) | (1.9) | (2.0) | (1.7) | (2.1) | (1.9) | (2.0) | (1.1) | (2.0) | ||||||
| % Rewarded trials | 52.5 | 52.7 | .89 | 53.6 | 51.5 | .14 | 54.0 | 51.1 | .05 | 54.2 | 50.6 | .02 | 53.4 | 51.8 | .28 |
| (5.7) | (6.1) | (6.8) | (4.5) | (5.9) | (5.5) | (5.6) | (5.6) | (6.1) | (5.6) | ||||||
Note: The columns headed p ≤ shows where each group pair differed reliably or not. Standard deviations in parentheses. BIS = Barrett's Impulsivity Scale. CO = carbon monoxide. DSM = Diagnostic and Statistical Manual of Mental Disorders. QSU = Questionnaire of Smoking Urges.
Table 2.
Correlations between the devaluation effect and key individual differences: Smoking days per week and the three subscales of the BIS Impulsivity Questionnaire
|
Devaluation magnitude |
||
|---|---|---|
| r | p | |
| Smoking days per week | −.03 | .80 |
| BIS Motor | −.26 | <.05 |
| Nonplanning | −.14 | .26 |
| Attention | −.07 | .56 |
Note: N = 64. BIS = Barrett's Impulsivity Scale. The significant correlation between BIS motor impulsivity and devaluation remained significant when the other three individual differences were controlled in partial correlations (see text).
By contrast, the two smoker groups did not differ with respect to the devaluation effect, F < 1 (Figure 1B) despite their difference in multiple proxies of nicotine dependence shown in Table 1, and smoking days per week did not correlate with the devaluation effect, r = −.03, p = .80 (see Table 2). Moreover, Table 1 shows a positive association between smoking days per week and motor impulsivity, which was borne out by a correlation between these scores, r = .27, p < .04. By contrast, smoking days per week did not correlate with BIS nonplanning or attentional impulsivity scores, rs < .09, ps > .52. Finally, the correlation between smoking days per week and BIS motor scores remained significant or marginal when all variables listed in Table 1 were partialled out in partial correlations, rs > .21, ps < .09. Thus, despite smoking days per week being selectively associated with motor impulsivity in this student sample, smoking days was not itself significantly associated with the devaluation effect.
Finally, there was no difference in devaluation effect between groups split by the median BIS nonplanning score (25; Figure 1C), attentional impulsivity score (17; Figure 1D) or satiety treatment, Fs(1, 62) < 1.60, ps > .21, and these two impulsivity subscales did not correlate with the devaluation effect (see Table 2). We conclude, therefore, that impaired goal-directed control in this human outcome devaluation procedure was selectively associated with BIS motor impulsivity.
Satiety treatment
The satiety treatment reduced the desire for the devalued compared to the nondevalued outcome by an average of 4.2 points on the 7-point Likert scale. This reduction in desire was greater for the chocolate (mean = 5.3, SEM = 0.2) than for the water (mean = 3.2, SEM = 0.4) treatment, F(1, 62) = 26.62, p < .001, although both treatments were reliably greater than zero, ts(31) > 8.90, ps < .001, demonstrating the effectiveness of the treatments in reducing desire for the devalued outcome selectively. Similarly, the satiety treatments reduced the pleasure rating of the first sip/chunk consumed relative to the last by an average of 63.7% on a visual analogue scale. Moreover, this decline in palatability was equal for the chocolate (mean = 68.2%, SEM = 4.4) and water (mean = 59.2%, SEM = 4.4) treatments, F(1, 62) = 2.11, p = .15, and the overall mean was significantly greater than zero, t(63) = 20.30, p < .001, indicating that both treatments reduced palatability of the consumed reward. Finally, fewer chunks/sips were consumed in the chocolate (mean = 18.3, SEM = 1.5) than the water (mean = 24.8, SEM = 1.4) satiety treatment, F(1, 62) = 10.09, p < .001.
Discussion
The sample as a whole selectively reduced responding for the devalued outcome in the extinction test, indicating that their choice was goal directed in the sense of being controlled by an integration of knowledge about the R-O contingencies established in training with knowledge of the current incentive value of the Os. This outcome-specific devaluation effect accords with previous demonstrations of goal-directed control over instrumental behaviour in humans (de Wit et al., 2009; de Wit et al., 2007; Kenward et al., 2009; Klossek et al., 2008; Schwabe & Wolf, 2009; Tricomi et al., 2009; Valentin et al., 2007).
The unique contribution of the current study was the finding that the outcome-specific devaluation effect was associated with BIS motor impulsivity; specifically, the devaluation effect was reduced in participants who scored high in motor impulsivity. This subscale required participants to endorse questions about their propensity for action without thought—for example, “I make up my mind quickly” and “I act on the spur of the moment”. These questions fit the intuitive sense of the phenomenology of habit, that behaviour is prompted by the situation rather than chosen following an evaluation of alternatives. The claim that impulsive individuals are more strongly cue driven accords with a finding by Kirkeby and Robinson (2005) that impulsive individuals showed reduced variation in reaction times in the Stroop task, suggesting they were more reliably prompted by the colour cue and reflected less about the meaning of the word than did low impulsive individuals.
The differential devaluation effect of the high and low motor impulsive groups could not be attributed to potential confounding variables. With regard to the satiety treatment, the motor impulsive groups showed the same consumption and reduction in the palatability and desire for the devalued outcome, plus these scores were ineffective as covariates. Thus, the differential devaluation effect could not be explained by differential sensitivity to the satiety treatment. Second, the questionnaire data were ineffective as covariates, suggesting that the differential devaluation effect could not be attributed to group differences in questionnaire measures including the other impulsivity subscales. Third, all participants included in the study accurately reported knowledge of the R-O contingencies established in training, at the end of the test session, so the differential devaluation effect could not be attributed to differences in knowledge of these contingencies during the test period. Finally, the differential devaluation effect remained stable over test blocks, so could not be attributed to a differential return of appetite for the devalued outcome during the test period. We conclude, therefore, that the impaired devaluation performance in the high motor impulsive group was mediated by a relative decoupling of instrumental choice from the explicit hedonic appraisal of the outcomes. This decoupling may have arisen from high impulsive participants’ regarding their instrumental choices as being irrelevant to their current motivational state, or from a predominance of habitual S-R control over choice. Either way, this decoupling is reminiscent of the wanting-liking distinction (Berridge & Kringelbach, 2008; Robinson & Berridge, 1993) inasmuch as action was selected in contradiction to reduced hedonic evaluation of the outcome.
Total BIS impulsivity scores have been associated with a variety of neuropsychiatric conditions including attention-deficit/hyperactivity disorder (ADHD), adolescent risk taking, reduced error processing, gambling, violent offending, neurodegeneration, and brain injury (Stanford et al., 2009). In addition, the Motor Impulsivity subscale in particular (compared to the other subscales) has shown associations with cocaine use, craving, and treatment retention (Moeller et al., 2001; see also Leblond, Ladouceur, & Blaszczynski, 2003), binge drinking (Carlson, Johnson, & Jacobs, 2010), major depression (Hur & Kim, 2009),
duration of bipolar depression (Swann, Lijffijt, Lane, Steinberg, & Moeller, 2009), mania (Swann, Steinberg, Lijffijt, & Moeller, 2008), suicide attempts (Dougherty et al., 2004), binge eating disorder (Nasser, Gluck, & Geliebter, 2004), as well as alterations in Wisconsin card sorting performance (Cheung, Mitsis, & Halperin, 2004), working memory performance (Whitney, Jameson, & Hinson, 2004), executive function (Spinella, 2005), and stop signal task performance (Gorlyn, Keilp, Tryon, & Mann, 2005), underactivation of the dorsolateral prefrontal cortex (DLPFC; Asahi, Okamoto, Okada, Yamawaki, & Yokota, 2004) and overactivation of the ventrolateral PFC (Goya-Maldonado et al., 2010) in no-go trials, and overactivation of the DLPFC in a rapid cue-matching task (Valdes et al., 2006). Our finding that motor impulsivity is associated with impaired goal-directed control raises the possibility that underperformance of the R-O controller and/or predominance of the S -R controller may be present in all of these neuropsychological conditions, but this remains to be tested.
By contrast, there was no difference in the magnitude of the devaluation effect between the daily and nondaily smoker groups recruited for this study, indicating that our proxy of tobacco dependence vulnerability (smoking days per week) was not associated with differential goal-directed control. In explaining this null result, it is noteworthy that adult drug dependence is typically associated with higher BIS scores on all three subscales (Stanford et al., 2009), yet in our student sample, only one proxy for dependence level, smoking days per week, was associated with only the Motor Impulsivity subscale. The restrictedness of this association compared to older/more dependent drug users fits the argument that although impulsivity is associated with the early acquisition of drug taking, this association becomes stronger longitudinally with the transition to clinical dependence (Belin et al., 2008; Biederman et al., 1997; Flory & Manuck, 2009). The implication is that impaired goal-directed control should have a larger effect size in older addicts, especially those who have experienced drug-related neuroadaptations or neurotoxicity. This proposal could explain why psychostimulant preexposed rats show accelerated habit learning (Nelson & Killcross, 2006; Nordquist et al., 2007; Schoenbaum & Setlow, 2005) whereas our more dependent student smoker did not. To conclude, adaptive decision making requires that the cognitively demanding R-O controller overrides established S-R habits when circumstances change. Our data suggest that human motor impulsivity is associated with impairment in this capacity. It seems reasonable, therefore, that dual-process theory of instrumental learning could make an important contribution to the understanding of neuropsychiatric conditions of which impulsivity is a core feature.
Acknowledgments
This work was supported by an MRC grant to Lee Hogarth (G0701456) at the University of Nottingham.
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Asahi S. Okamoto Y. Okada G. Yamawaki S., Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 2004;254(4):245–251. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- Balleine B. W., O'Doherty J. P. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D. Mar A. C. Dalley J. W. Robbins T. W., Everitt B. J. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320(5881):1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K. C., Kringelbach M. L. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology. 2008;199(3):457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J. Makris N. Valera E. M. Monuteaux M. C. Goldstein J. M. Buka S., et al. Towards further understanding of the co-morbidity between attention deficit hyperactivity disorder and bipolar disorder: A MRI study of brain volumes. Psychological Medicine. 2008;38(7):1045–1056. doi: 10.1017/S0033291707001791. [DOI] [PubMed] [Google Scholar]
- Biederman J. Wilens T. Mick E. Faraone S. V. Weber W. Curtis S., et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(1):21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Cappelleri J. C. Bushmakin A. G. Baker C. L. Merikle E. Olufade A. O., Gilbert D. G. Multivariate framework of the brief questionnaire of smoking urges. Drug and Alcohol Dependence. 2007;90(2-3):234–242. doi: 10.1016/j.drugalcdep.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Carlson S. R. Johnson S. C., Jacobs P. C. Disinhibited characteristics and binge drinking among university student drinkers. Addictive Behaviors. 2010;35(3):242–251. doi: 10.1016/j.addbeh.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Cheung A. M. Mitsis E. M., Halperin J. M. The relationship of behavioral inhibition to executive functions in young adults. Journal of Clinical and Experimental Neuropsychology. 2004;26(3):393–404. doi: 10.1080/13803390490510103. [DOI] [PubMed] [Google Scholar]
- Corbit L. H., Balleine B. W. Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29(2):99–106. doi: 10.1037/0097-7403.29.2.99. [DOI] [PubMed] [Google Scholar]
- Cyders M. A. Flory K. Rainer S., Smith G. T. The role of personality dispositions to risky behavior in predicting first-year college drinking. Addiction. 2009;104(2):193–202. doi: 10.1111/j.1360-0443.2008.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. D. Niv Y., Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience. 2005;8(12):1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- de Wit S. Corlett P. R. Aitken M. R. Dickinson A., Fletcher P. C. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. Journal of Neuroscience. 2009;29(36):11330–11338. doi: 10.1523/JNEUROSCI.1639-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit S. Niry D. Wariyar R. Aitken M. R. F., Dickinson A. Stimulus-outcome interactions during instrumental discrimination learning by rats and humans. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33(1):1–11. doi: 10.1037/0097-7403.33.1.1. [DOI] [PubMed] [Google Scholar]
- Dickinson A., Balleine B. The cognitive/ motivational interface. In: Kringelbach M. L., Berridge K. C., editors. Pleasures of the brain. The neural basis of taste, smell and other rewards. Oxford, UK: Oxford University Press; 2010. pp. 74–84. [Google Scholar]
- Dickinson A. Balleine B. Watt A. Gonzalez F., Boakes R. A. Motivational control after extended instrumental training. Animal Learning & Behavior. 1995;23(2):197–206. [Google Scholar]
- Dickinson A. Nicholas D. J., Adams C. D. The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. Quarterly Journal of Experimental Psychology, Section B: Comparative and Physiological Psychology. 1983;35:35–51. [Google Scholar]
- Donny E. C., Dierker L. C. The absence of DSM-IV nicotine dependence in moderate-to-heavy daily smokers. Drug and Alcohol Dependence. 2007;89(1):93–96. doi: 10.1016/j.drugalcdep.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty D. M. Mathias C. W. Marsh D. M. Papageorgiou T. D. Swann A. C., Moeller F. G. Laboratory measured behavioral impulsivity relates to suicide attempt history. Suicide and Life-Threatening Behavior. 2004;34(4):374–385. doi: 10.1521/suli.34.4.374.53738. [DOI] [PubMed] [Google Scholar]
- Etter J. F. Le Houezec J., Perneger T. V. A self-administered questionnaire to measure dependence on cigarettes: The Cigarette Dependence Scale. Neuropsychopharmacology. 2003;28(2):359–370. doi: 10.1038/sj.npp.1300030. [DOI] [PubMed] [Google Scholar]
- Everitt B. J. Belin D. Economidou D. Pelloux Y. Dalley J. W., Robbins T. W. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society B. 2008. pp. 3125–3135. [DOI] [PMC free article] [PubMed]
- Flory J. D., Manuck S. B. Impulsiveness and cigarette smoking. Psychosomatic Medicine. 2009;71(4):431–437. doi: 10.1097/PSY.0b013e3181988c2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlyn M. Keilp J. G. Tryon W. W., Mann J. J. Performance test correlates of component factors of impulsiveness. Personality and Individual Differences. 2005;38(7):1549–1559. [Google Scholar]
- Goya-Maldonado R. Walther S. Simon J. Stippich C. Weisbrod M., Kaiser S. Motor impulsivity and the ventrolateral prefrontal cortex. Psychiatry Research: Neuroimaging. 2010;183(1):89–91. doi: 10.1016/j.pscychresns.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Hiroi N., Scott D. Constitutional mechanisms of vulnerability and resilience to nicotine dependence. Molecular Psychiatry. 2009;14:653–667. doi: 10.1038/mp.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. C. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30(4):258–258. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- Hur J. W., Kim Y. K. Comparison of clinical features and personality dimensions between patients with major depressive disorder and normal control. Psychiatry Investigation. 2009;6(3):150–155. doi: 10.4306/pi.2009.6.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak J. P. Uslaner J. M. Esteban J. A., Robinson T. E. Methamphetamine-induced structural plasticity in the dorsal striatum. European Journal of Neuroscience. 2007;25(3):847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Kenward B. Folke S. Holmberg J. Johansson A., Gredeback G. Goal directedness and decision making in infants. Developmental Psychology. 2009;45(3):809–819. doi: 10.1037/a0014076. [DOI] [PubMed] [Google Scholar]
- Kirkeby B. S., Robinson M. D. Impulsive behavior and stimulus-response variability in choice reaction time. Journal of Research in Personality. 2005;39(2):263–277. [Google Scholar]
- Klossek U. M. H. Russell J., Dickinson A. The control of instrumental action following outcome devaluation in young children aged between 1 and 4 years. Journal of Experimental Psychology: General. 2008;137(1):39–51. doi: 10.1037/0096-3445.137.1.39. [DOI] [PubMed] [Google Scholar]
- Kosaki Y., Dickinson A. Choice and contingency in the development of behavioral autonomy during instrumental conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36(3):334–342. doi: 10.1037/a0016887. [DOI] [PubMed] [Google Scholar]
- Leblond J. Ladouceur R., Blaszczynski A. Which pathological gamblers will complete treatment? British Journal of Clinical Psychology. 2003;42:205–209. doi: 10.1348/014466503321903607. [DOI] [PubMed] [Google Scholar]
- Moeller F. G. Dougherty D. M. Barratt E. S. Schmitz J. M. Swann A. C., Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Abuse Treatment. 2001;21(4):193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Nasser J. A. Gluck M. E., Geliebter A. Impulsivity and test meal intake in obese binge eating women. Appetite. 2004;43(3):303–307. doi: 10.1016/j.appet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Nelson A., Killcross S. Amphetamine exposure enhances habit formation. Journal of Neuroscience. 2006;26(14):3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist R. E. Voorn P. Malsen J. Joosten R. Pennartz C. M. A., Vanderschuren L. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. European Neuropsychopharmacology. 2007;17(8):532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Perry J. L., Carrol M. E. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200(1):1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Berridge K. C. The neural basis of drug craving: An incentive-sensitization theory of drug addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G., Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: Implications for altered orbitofrontal-amygdalar function. Cerebral Cortex. 2005;15(8):1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Wolf O. T. Stress prompts habit behavior in humans. The Journal of Neuroscience. 2009;29(22):7191–7198. doi: 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella M. Self-rated executive function: Development of the executive function index. International Journal of Neuroscience. 2005;115(5):649–667. doi: 10.1080/00207450590524304. [DOI] [PubMed] [Google Scholar]
- Stanford M. S. Mathias C. W. Dougherty D. M. Lake S. L. Anderson N. E., Patton J. H. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences. 2009;47(5):385–395. [Google Scholar]
- Swann A. C. Lijffijt M. Lane S. D. Steinberg J. L., Moeller F. G. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disorders. 2009;11(3):280–288. doi: 10.1111/j.1399-5618.2009.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann A. C. Steinberg J. L. Lijffijt M., Moeller F. G. Impulsivity: Differential relationship to depression and mania in bipolar disorder. Journal of Affective Disorders. 2008;106(3):241–248. doi: 10.1016/j.jad.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E. Balleine B. W., O'Doherty J. P. A specific role for posterior dorsolateral striatum in human habit learning. European Journal of Neuroscience. 2009;29(11):2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes I. H. Steinberg J. L. Narayana P. A. Kramer L. A. Dougherty D. M. Swann A. C., et al. Impulsivity and BOLD fMRI activation in MDMA users and healthy control subjects. Psychiatry Research-Neuroimaging. 2006;147(2-3):239–242. doi: 10.1016/j.pscychresns.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Valentin V. Dickinson A., O'Doherty J. P. Determining the neural substrates of goal-directed learning in the human brain. The Journal of Neuroscience. 2007;27(15):4019–4026. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A. Lawrence A. J., Clark L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow N. D. Fowler J. S. Wang G. J. Baler R., Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside S. P., Lynam D. R. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. [Google Scholar]
- Whitney P. Jameson T., Hinson J. M. Impulsiveness and executive control of working memory. Personality and Individual Differences. 2004;37(2):417–428. [Google Scholar]
- Willner P. Further validation and development of a screening instrument for the assessment of substance misuse in adolescents. Addiction. 2000;95(11):1691–1698. doi: 10.1046/j.1360-0443.2000.951116919.x. [DOI] [PubMed] [Google Scholar]


