Abstract
Background
Analysis of occupational mortality in England and Wales during 1991-2000 showed no decline in work-attributable deaths from asbestosis.
Aims
To explore why there was no decline in mortality from asbestosis despite stricter controls on asbestos exposure over recent decades.
Methods
Using data from registers of all deaths in Great Britain with mention of mesothelioma or asbestosis on the death certificate, we plotted death rates by five-year age group within five-year birth cohorts for a) mesothelioma and b) asbestosis without mention of mesothelioma.
Results
Analysis was based on a total of 33,751 deaths from mesothelioma and 5,396 deaths from asbestosis. For both diseases, mortality showed a clear cohort effect; and within birth cohorts, death rates increased progressively with age through to 85 years and older. However, highest mortality from mesothelioma was in men born during 1939-43, whereas mortality from asbestosis peaked in men born during 1924-38.
Conclusion
Our findings suggest that in Britain, mortality from asbestosis has been determined mainly by cumulative exposure to asbestos before 45 years of age, and that the effect of such exposure continues through to old age. That mortality from asbestosis peaked in earlier birth cohorts than mortality from mesothelioma may reflect a difference in exposure-response relationships for the two diseases. The discrepancy could be explained if risk of asbestosis increased more steeply than that of mesothelioma at higher levels of exposure to asbestos, and if the highest prevalence of heavy exposure occurred in earlier birth cohorts than the highest prevalence of less intense exposures.
Keywords: Asbestos, asbestosis, mesothelioma, trends, cohort effect
Introduction
The most recent national analysis of mortality by occupation in England and Wales, covering the period 1991-2000, found no decline in work-attributable deaths from asbestosis or mesothelioma, despite increasingly strict controls on asbestos exposure over recent decades.(1) While the finding for mesothelioma was predictable, given the long induction period for development of such tumours, the absence of any reduction in deaths from asbestosis was unexpected. To explore possible explanations, we carried out a detailed analysis of national trends in mortality from asbestos-related disease by age and birth cohort.
Methods
Since 1968, the Health and Safety Executive (HSE) has maintained registers of all deaths in Great Britain with mention of mesothelioma or asbestosis on the death certificate.(2) We abstracted data from these registers for mesothelioma deaths in men aged 20-89 years during 1968 to 2008 and for asbestosis deaths in men aged 20-89 years during 1978-2008, the shorter period of observation for asbestosis being determined by the availability of data in electronic format. Deaths were ascribed to mesothelioma where the disease was certified as either the underlying or a contributory cause. Deaths were assigned to asbestosis if the certificate mentioned asbestosis but not mesothelioma.
We summarised death rates in five-year birth cohort and age at death categories. Deaths were assigned to these categories using the dates of birth and death recorded on each death certificate. We used national population data from the Office for National Statistics (ONS) and the National Records of Scotland (NRS) by single calendar year and age to derive the person-years denominators for calculation of corresponding death rates. Results were summarised graphically by plotting death rates by age within each birth cohort. In these graphs, each age category was represented by the average age at death in that category, thereby accounting for any restrictions in person-time as a consequence of the limited periods of observation.
Analysis was based entirely on anonymised data, and ethical approval was not required.
Results
Analysis was based on a total of 33,751 deaths from mesothelioma and 5,396 deaths from asbestosis. The majority of birth cohorts (15/20 for mesothelioma; 10/14 for asbestosis) had at least three age-categories with five or more deaths.
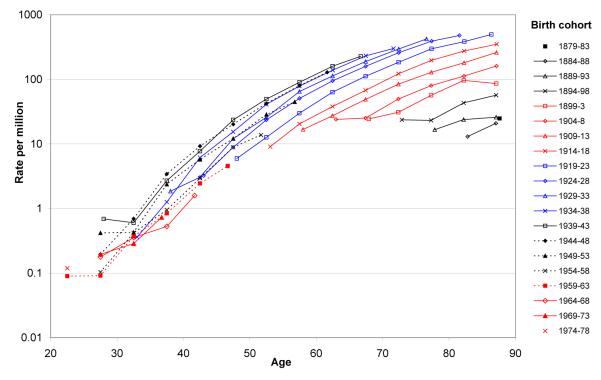
Figure 1 shows mortality from mesothelioma in men by birth cohort and age during 1968-2008. In each birth cohort, death rates increased steeply with age through to the oldest age group analysed (85-89 years). Moreover, a clear trend was apparent across birth cohorts. Age-specific mortality increased progressively in successive birth cohorts up to that born in 1939-43, and thereafter declined progressively.
Figure 1.
Death rates for mesothelioma in Great Britain by age group for twenty birth cohorts
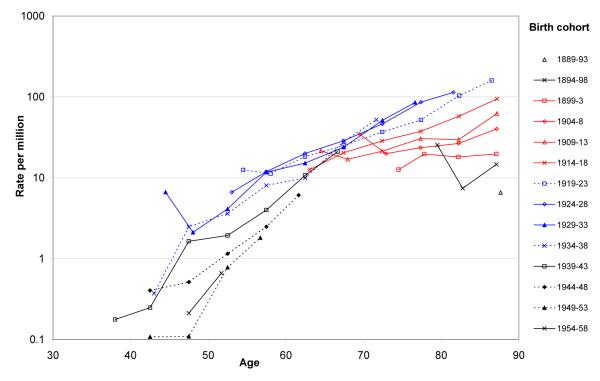
Figure 2 shows a corresponding plot for deaths during 1978-2008 from asbestosis without mention of mesothelioma. Except in the oldest birth cohorts (born before 1903), mortality again increased with age, although the pattern of increase between cohorts was not as consistent as for mesothelioma with some birth cohorts showing a bigger increase than others at very old ages. Again, a strong cohort effect was apparent, but in this case, with peak mortality in men born during 1924-38. Although more recent birth cohorts had lower mortality from asbestosis at each age, there was no indication that the reduction in age-specific mortality was larger later in the study period. For example, the ratio of mortality in the 1949-53 birth cohort to that in the 1944-48 birth cohort was lower at ages 40-44 and 45-49 years (deaths occurring during the late 1980s and early 1990s) than at older ages (deaths occurring in the late 1990s and 2000s).
Figure 2.
Death rates for asbestosis in Great Britain by age group for fourteen birth cohorts
Discussion
Our analysis indicates that as with mesothelioma, mortality from asbestosis continues to increase with age at least 20 years after most workers have retired and ceased occupational exposure. Furthermore, time trends in mortality from asbestosis in Great Britain, like those for mesothelioma, exhibit a clear cohort effect, although with the highest mortality in rather earlier birth cohorts (1924-38 for asbestosis as compared with 1939-43 for mesothelioma). However, despite increasingly stringent controls on exposure to asbestos in the workplace since 1970, there was no evidence of a consistent reduction in mortality across all cohorts in recent years (i.e. of a period effect). This suggests that mortality from asbestosis, even at old ages, has been determined largely by exposures early in working life, and that controls on exposure have had little impact on older workers who had already accumulated higher exposures during their first few decades of work.
It is likely that there were improvements in the ascertainment of asbestosis over the study period – for example, through the wider use of computerised tomography. However, artefacts of diagnosis and reporting would be expected to affect most or all age groups simultaneously and therefore could not account for a cohort effect of the type that we have demonstrated, although they may explain part of the observed increase in asbestosis rates with age within cohorts in which deaths are still being accrued. Nor can the observed patterns of mortality realistically be attributed to chance. Even at ages 45-49 years, mortality rates from asbestosis in all but the two most recent birth cohorts were based on more than 5 deaths, and for mesothelioma the numbers were higher.
A strong cohort effect in mortality from mesothelioma in Britain has been noted previously.(3) It can be explained if the cancer develops through DNA damage associated with increased cell turnover as a consequence of chronic inflammation induced by asbestos fibres retained in the periphery of the lung and in the peritoneum. If the level of inflammation at a given time depends on the total retained burden of fibres, then exposures accumulated early in life will have the greatest impact on risk. Thus, risk will be maximal in birth cohorts with the highest exposures at younger ages. Furthermore, because it often takes many years for a tumour to develop, this peak mortality will be apparent through to old age. Controls on asbestos exposure in the UK started to have major impact in the 1970s, when the birth cohort with highest death rates from mesothelioma would have been in their thirties.(4)
The continuing increase in mortality from asbestosis through to old age could be a consequence of later fatality in people who already had the disease at the time when they retired. There is evidence that the disease can continue to progress following cessation of exposure.(5) Moreover, when present, it may render individuals more susceptible to other diseases such as bronchopneumonia and heart failure, which eventually precipitate deaths for which the asbestosis is considered the underlying or a contributing cause.
The declining mortality from asbestosis at all ages in cohorts born after 1938 is most easily explained by lower cumulative exposures to asbestos as a consequence of regulatory controls imposed from the 1970s. However, it is of note that such controls had no perceptible impact on the mortality of earlier birth cohorts, even though many of them would still have been in employment through to the 1990s. This suggests that it is cumulative exposure before 45 years of age that is most relevant to risk, perhaps because it contributes to the development of pathology over a longer period. This would apply if the rate of progression of asbestosis at a given time were a function of the total exposure that had been accumulated up to that time.
There remains a question as to why the birth cohorts with highest mortality from asbestosis differ from that with highest mortality from mesothelioma, and why peak asbestosis mortality rates were maintained across three successive birth cohorts while the peak of mesothelioma affected a single five-year cohort (in which asbestosis mortality had started to fall). This seems likely to reflect the changing prevalence of different intensities of exposure to asbestos. It is known that the relative frequency of deaths from pleural mesothelioma and asbestosis differs markedly by occupation, with proportionately higher mortality from asbestosis in occupations with the heaviest exposures to the mineral, and it has been proposed that this reflects differences in the exposure-response relationship for the two diseases – the risk of death from asbestosis increasing more steeply with higher exposures.(6) It may be that the highest prevalence of heavy exposure to asbestos (particularly crocidolite and amosite) occurred in the cohorts of men born during 1924-38, whereas the prevalence of lower exposures was maximal in those born somewhat later.
Our findings suggest that in time, mortality in Great Britain from both asbestosis and mesothelioma can be expected to decline at even the oldest ages, the reduction for asbestosis occurring a little earlier than that for mesothelioma. However, because death rates from both diseases increase steeply with age and largely reflect exposures early in working life, it will be many years before we can be confident that controls on occupational exposure to asbestos have fully eliminated the risks associated with the mineral. Meanwhile, there is a need for continued vigilance to ensure that exposures are kept as low as is reasonably practicable, and that exposure limits are never exceeded.
Key points.
Our findings indicate that in Britain, mortality from asbestosis, even at old ages, has been determined largely by exposures early in working life
Controls on exposure appear to have had little impact on older workers who had already accumulated higher exposures during their first few decades of work.
Because death rates from both mesothelioma and asbestosis increase steeply with age and largely reflect exposures early in working life, it will be many years before we can be confident that controls on occupational exposure to asbestos have fully eliminated the risks associated with the mineral.
Abbreviations/Definitions used in Manuscript
- HSE
Health and Safety Executive
- ONS
Office for National Statistics
- NRS
National Records of Scotland
Footnotes
Competing Financial Interests Declaration: All authors declare that they have no actual or potential competing financial interest.
References
- 1.Coggon D, Harris EC, Brown T, Rice S, Palmer KT. Work-related mortality in England and Wales, 1979-2000. Occup Environ Med. 2010;67:816–22. doi: 10.1136/oem.2009.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElvenny D, Darnton A, Price MJ, Hodgson JT. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med. 2005;55:79–87. doi: 10.1093/occmed/kqi034. [DOI] [PubMed] [Google Scholar]
- 3.Peto J, Hodgson JT, Matthews FE, Jones JR. Continuing increase in mesothelioma mortality in Britain. Lancet. 1995;345:535–9. doi: 10.1016/s0140-6736(95)90462-x. [DOI] [PubMed] [Google Scholar]
- 4.Rake C, Gilham C, Hatch J, et al. Occupational, domestic and environmental mesothelioma risks in the British population: a case-control study. Br J Cancer. 2009;100:1175–83. doi: 10.1038/sj.bjc.6604879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sluis-Cremer, GK. Asbestos disease at low exposures after long residence times. Annals of New York Academy of Science. 1991;643:182–193. doi: 10.1111/j.1749-6632.1991.tb24461.x. [DOI] [PubMed] [Google Scholar]
- 6.Coggon D, Inskip H, Winter P, Pannett B. Differences in occupational mortality from pleural cancer, peritoneal cancer and asbestosis. Occup Environ Med. 1995;52:775–7. doi: 10.1136/oem.52.11.775. [DOI] [PMC free article] [PubMed] [Google Scholar]