Abstract
The utility of different models to identify cancer stem cells continues to be a subject of intense debate. Here, we summarize recent efforts to characterize intra-tumoral heterogeneity of melanoma and delineate key questions for future studies. Within a developing or already established tumor microenvironment, we propose that continuous tumor maintenance is assured by specific subpopulations whose phenotype is not static but instead is dynamically regulated. These small and temporarily distinct subpopulations likely play critical roles in tumor progression. They are important therapeutic targets but only in the context of combination therapies that also eliminate the bulk of the tumor.
The evolving definition of cancer stem cells
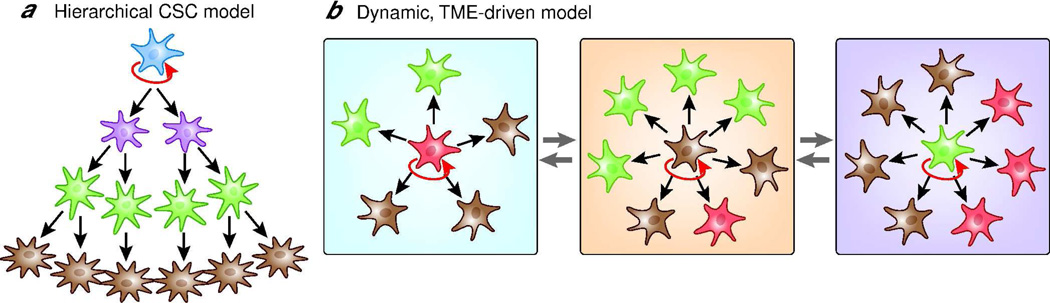
In the mid nineties, Lapidot et al. reported that only a small subpopulation of acute myeloid leukemia (AML) cells could establish tumors when transplanted into severe combined immunodeficiency (SCID) mice whereas the major population could not (Lapidot et al., 1994). The tumorigenic sub-population was named ‘AML-initiating cells’, and it was defined by the presence of the cell surface marker CD34 and the absence of CD38. Both markers also represent immature cells in normal human bone marrow, which raised the question of whether CD34+ CD38− cells were leukemic stem cells. Using NOD/SCID mice, which are more immunocompromised than SCID mice, it was shown that the CD34+ CD38− AML subpopulation generated differentiated leukemic progenitors, which have limited proliferative capacity, suggesting that AML leukemic clones are hierarchically organized (Bonnet and Dick, 1997). Although those findings were challenged by recent reports that AML-initiating cells are not restricted to the CD34+ CD38− subpopulation (Sarry et al., 2010; Taussig et al., 2010), subsequent studies in a variety of human solid tumors confirmed that only subsets of cells isolated from tumors are able to initiate growth in SCID or NOD/SCID mice. ‘Tumor-initiating’ subpopulations are generally characterized by cell surface markers, which are often shared with the stem cell populations in the normal tissue counterparts. In 2006, the field came to a consensus definition: a cancer stem cell (CSC) is capable of self-renewal, can give rise to all heterogeneous lineages of the parental tumor, and has the ability to establish and maintain continuous tumor growth (Clarke et al., 2006). In the CSC model, the direction of differentiation from stem cells to progenitors and terminally differentiated cells is hierarchically organized (Reya et al., 2001). The most critical aspect of the top-down model of CSC is that differentiated cells do not revert back to stem cells unless reprogramming events occur (Figure 1).
Figure 1.
(A) Hierarchal cancer stem cell model. Self-renewing (indicated by red arrows) CSC sustain the stem-cell population while giving rise to progenitor cells that are not capable of self-renewal. These progenitor cells can give rise to differentiating clones of varying dominance that contribute to overall tumor heterogeneity. Differentiated cancer cells do not revert back to CSC.
(B) Stochastic, tumor microenvironment-driven model. Cancer cells are clonally evolved, yet may contain phenotypically heterogeneous subpopulations. Virtually every single cell can self-renew and propagate tumors. The self-renewal capability of each cell is determined by distinct signals from the tumor microenvironment (TME, indicated by blue and orange rectangles).
The “ups-and-downs” of the CSC model in melanoma
Melanomas are composed of heterogeneous cell populations. The diversity in biological properties and molecular marker expression has been recognized for decades and gave rise to speculations on the existence of CSC subpopulations in melanoma. Following the pioneering studies on leukemia, several laboratories including our own attempted to confirm the existence of melanoma stem cells using cell surface markers (Fang et al., 2005; Frank et al., 2005; Monzani et al., 2007). The ATP-binding cassette (ABC) transporters compose a family of trans-membrane proteins that act as efflux pumps of a variety of substrates. A wide variety of normal stem cells including embryonic stem cells and hematopoietic stem cells generally express high levels of ABC transporters such as ABCB1 and ABCG2 for protection from potentially damaging influxes (Chaudhary and Roninson, 1991; Zhou et al., 2001). In 2005, Frank and co-workers reported that the ABC transporter ABCB5 in melanoma mediates doxorubicin resistance (Frank et al., 2005). Three years later, that group reported that ABCB5-positive melanoma cells from clinical samples also have increased tumor-initiating capacity when xenotransplanted into immunodeficient NOD/SCID mice (Schatton et al., 2008). In the ABCB5-positive population, tumor initiation was achieved with 105 cells, whereas in the ABCB5-negative population, 100-fold more tumor cells were required to form tumors suggesting that the CSC model also applied to melanoma. Also in 2008, Morrison’s group demonstrated that the frequency of melanoma-initiating cells is dramatically higher, as high as 1 in 4 tumor cells, regardless of whether or not the tumors were separated into different sub-populations (Quintana et al., 2008). The sum of the data indicates that the potential for tumor initiation is not restricted to a rare subpopulation but is a common feature of all or nearly all melanoma cells. This suggests that melanoma is not hierarchically organized and rather follows a stochastic model in which subpopulations change randomly depending on microenvironmental signals (Figure 1). On the other hand, very recently Weissman and co-workers (Boiko et al., 2010) supported the CSC model by describing the CD271-positive subpopulation as essential to the initiation of tumors in mice. CD271, a.k.a. low affinity nerve growth factor receptor (HUGO term: NGFR) or p75NTR, is a marker of neural crest stem cells (Stemple and Anderson, 1992) and is heterogeneously expressed by an average of 17% cells in most melanomas. In the Boiko et al. study, CD271-positive melanoma cells formed tumors in 72% of xenografts whereas CD271-negative cells were tumorigenic in only 7% of xenografts. CD271-positive but not CD271-negative melanoma cells also formed distant metastases. More recently, Ghadially’s group (Boonyaratanakornkit et al., 2010) isolated melanoma cells with a tumor-initiating property based on the activity of Aldehyde Dehydrogenase (ALDH), which is a functional marker for hematopoietic stem cells. It has been suggested that an ALDH-positive cell population is correlated with poor clinical prognosis in solid tumors such as breast or prostate cancers (Ma and Allan, 2010). In their study, 1 in 4 of ALDH-positive cells initiated tumors in NSG mice while only 1 in 600 ALDH-negative cells and 1 in 400 unsegregated cells did. Both the CD271 and the ALDH studies support the concept of CSC in melanoma where only a subset of cells drives progression. However, the most recent report from the Morrison laboratory contradicts the findings of the Frank and Weissman laboratories (Quintana et al., 2010). In their refined model system, Morrison and co-workers report that ABCB5-positive and ABCB5-negative melanoma cells are equally tumorigenic when injecting as few as 10 cells. No differences were found in the metastatic capacities of CD271-positive vs. CD271-negative melanoma cells. Both marker-positive and -negative cells reproduced intra-tumoral heterogeneity similar to the parental tumors and marker-negative subpopulations gave rise to marker-positive cells. These data imply that neither ABCB5 nor CD271 can be classified as CSC markers in Morrison’s experimental model.
What causes the discrepancy? – The devil is in the detail
Limiting dilution assays in immunocompromised mice are a widely approved method to demonstrate that a selected marker enriches for tumor stemness. Although all three groups applied this technique, there are considerable differences between each experimental system. To study the ABCB5-positive subpopulation, Frank’s group used NOD/SCID mice that have impaired T and B cell lymphocyte development, yet still maintain NK cell activity, which interferes with engraftment efficiency. In contrast, Morrison’s group used the more immunocompromised NSG (NOD/SCID IL2Rγnull) mouse model (Ito et al., 2002), which lacks NK cell activity in addition to T and B cell depletion. Furthermore, the latter group co-injected tumor cells with Matrigel®, which consists of collagen type IV, laminin and other matrix proteins, to mimic the extracellular environment in the tumor tissue and Matrigel® may act to stimulate angiogenesis and/or growth (Mortarini et al., 1995). For the CD271 study, Weissman’s group injected CD271-positive/negative subpopulations in T-, B- and NK-deficient Rag2−/−γc−/− mice with Matrigel®, which have an equivalent efficiency of tumor engraftment compared to Morrison’s NSG-Matrigel® model. It is noteworthy that they also tested the tumor initiation capability of each subpopulation in the mouse-human chimera model, which has normal human skin grafted onto the backs of immunodeficient mice. In this physiologically more relevant model, 2×104 CD271-positive melanoma cells, but not CD271-negative cells formed tumors presumably without Matrigel®. In a very recent paper, Civenni et al. showed that both CD271-positive and CD271-negative cells form tumors in NSG mouse, but CD271-negative cells failed in initiating tumors in nude or NOD/SCID mice (Civenni et al., 2011). For the ALDH study, the Ghadially group utilized the NSG-Matrigel® model. However, they used the regular Matrigel® (product 354234, BD Biosciences, San Jose, CA), while the Morrison group used high protein Matrigel® (product 354248, BD Biosciences). Each group also used different methods to isolate melanoma cells from the tumor mass, which is likely critical for the yield of cells. Weissman’s group digested minced tumors with collagenases (isoforms I and II) and thermolysin at 37°C for 3 h with pipetting every 30 min. In contrast, Morrison’s group placed the minced tissue in collagenase IV for only 20 min at 37°C followed by short incubation with low concentrations of trypsin, which is a milder digestion process than Weissman’s method (Shackleton, 2010). Similarly to Weissman’s protocol, Frank’s group also digested the tumor pieces in collagenase for 3 h at 37°C. Ghadially’s group incubated minced tumors in collagenase and hyaluronidase for 2 h at 37°C. Civenni et al. incubated the tissue from metastatic melanoma lesions in collagenase III and dispase for 1h at 37°C with simultaneous mincing to obtain single-cell suspensions. Taking into account such significant procedural differences, one may question whether the ABCB5/CD271/ALDH-positive cells are simply more capable of surviving harsh conditions during the digestion irrespective of any tumor propagating ability. However, selective ablation of ABCB5+ cells by anti-ABCB5 antibodies reduced tumor growth and formation in xenografts on nude mice from unsegregated tumors (Schatton et al., 2008). We observed that the expression of ABCB5 and the in vivo lung colonization in NSG mice are both decreased in melanoma cells when matricellular protein tenascin C is disrupted (Fukunaga-Kalabis et al., 2010), suggesting that matricellular proteins cooperate with ABCB5 to stimulate tumor growth and metastasis. Recently, Frank and co-workers added another level of complexity by demonstrating that the ABCB5-positive subpopulation suppresses T-cell activation, suggesting an immune-evasive capacity of this subpopulation (Schatton et al., 2010). This observation needs to be validated in different mouse models such as “humanized mice”, which have reconstituted human immune system components (Ishikawa et al., 2005; Strowig et al., 2009) or in mouse genetic models similar to the CSC studies from the Bosenberg laboratory (Held et al., 2010).
Does tumor initiation reflect the true character of tumor stemness?
The field is reaching a consensus that in principle every 1 in 4 melanoma cells harbors the potential to recapitulate tumor growth in a murine environment if the chosen host is permissive enough (e.g. NSG mouse). However, we have to ask whether the concept of tumor initiation in xenotransplantation models is a good model for determining tumor stemness, i.e. the maintenance of continuous tumor growth (Adams and Strasser, 2008). In most xenograft models, human melanoma cells are injected under the skin where they are surrounded by fascia. In patients, the malignant cells are surrounded by stromal, inflammatory and immune cells, including fibroblasts, endothelial cells, macrophages, T cells and B cells. Thus, tumor initiation by the injection of a few tumor cells into the subcutaneous space measures the ability of adjusting to a foreign microenvironment and host-derived growth factors rather than continuous tumor maintenance (Adams and Strasser, 2008). In this respect, congenic transplantation of murine lymphoma cells revealed that tumor initiation is not necessarily restricted to a minor population (Kelly et al., 2007).
Looking for alternative concepts, our laboratory has been asking whether tumor stemness could be better reflected by an assay measuring the long-term maintenance of an already established tumor. In different melanoma cell lines, we identified a slow-cycling subpopulation of melanoma cells representing only 1–5% of all cells that had stem-like properties (Roesch et al., 2010). These cells were characterized by high expression of JARID1B (HUGO term: KDM5B), a member of the H3K4 demethylase family, which is critical in regulating gene expression and cellular identities and is an epigenetic marker for embryonic stem cells (Christensen et al., 2007; Iwase et al., 2007; Klose et al., 2007; Yamane et al., 2007). When separated from JARID1B-negative cells, JARID1B-positive, non-proliferating melanoma cells gave rise to a rapidly proliferating progeny that reconstituted the parental heterogeneity of JARID1B-positive and -negative cells. Stable knockdown of JARID1B led to an initial acceleration of tumor growth followed by exhaustion as determined by serial xenotransplantation in NSG mice, suggesting that JARID1B plays an essential role in continuous melanoma growth. In contrast to studies in the Frank, Weissman, Ghadially and Morrison laboratories, we used a combination of serial xenotransplantations plus stable enforcement of a distinct (in this case “non-stem like”) cell phenotype to see how tumors that had been fully established can exhaust over time. Notably, when we performed conventional xenotransplantation with “spontaneous” JARID1B-positive vs. JAR1D1B-negative phenotypes, there was no significant difference in tumor initiation capacity between both populations. Single melanoma cells of either population were equally tumorigenic. Since JARID1B-negative cells also gave rise to JAR1D1B-positive cells in vitro and in vivo, we assume that stemness in melanoma does not follow a static but rather a dynamic model. Likely, a select number of genes keeps melanoma cells in a long-living status for continuous repopulation of the tumor. The Settleman laboratory recently pointed to a new direction of clinical importance: JARID1A, a close homologue of JARID1B, is required for drug resistance in non-small cell lung cancer cells (Sharma et al., 2010), which suggests that slow-cycling cells survive most conventional and targeted therapies and that this sub-population requires specific targeting.
Conclusions: Microenvironmental cues switch “stemness”
Collectively, many recent studies on melanoma stem cells suggest that intra-tumoral heterogeneity exists but the phenotypes are dynamic, not static. Tumor maintaining subpopulations likely continually arise and disappear. The big question is how this switching is controlled. Interestingly, conventional melanoma culture conditions allow a higher dynamic of the JARID1B phenotype compared to human embryonic stem cell culture conditions (Roesch et al., 2010), suggesting soluble factors as possible contributors to the regulation of this equilibrium. Additionally, hypoxia is a significant stimulator for the expression of JARID1B (Roesch et al., 2010; Xia et al., 2009). Overall, the tumor microenvironment plays a major role for the turnover and fate of CSC. In agreement with this view, Hoek and Goding recently framed the “phenotype-switching model” of melanoma on the basis of gene expression profiling and functional analyses (Hoek and Goding, 2010). In this model, melanomas contain MITF-high Brn-2 low “proliferative” and MITF-low Brn-2 high “invasive, stem-like” subpopulations. These phenotypes are distinct only temporarily and each subpopulation has the potential to adopt the other phenotype in response to exogenous growth factors such as TGF-β. Stromal cell-secreted factors such as HGF can restore the colon CSC phenotype in more differentiated tumor cells (Vermeulen et al., 2010) suggesting that the phenotypes of subpopulations can fluctuate depending on the signals from the tumor microenvironment. Therefore, the development of effective therapies against melanoma stemness remains a major challenge. Targeting CSC and responsible pathways may lead to an abrogation of melanoma stemness. In the future, we expect that the field will develop therapies that target the majority of malignant cells, using for example the BRAF inhibitor PLX4032, as well as the minor subpopulation(s). Most likely, the small non-proliferating or very slowly proliferating JARID1B-positive sub-population is a prime target for developing novel therapies that act in synergy with those targeting the major population of cells.
Acknowledgements
This work was supported by grants from the NCI (CA076674, CA047159, CA093372)
Abbreviations
- AML
Acute myeloid leukemia
- CSC
Cancer stem cell
- SCID
Severe combined immune-deficient
- NOD
Non-obese diabetic
- NSG
NOD/SCID IL2Rγnull
- JARID1B
KDM5B/PLU-1/RBP2-H1
- H3K4
Histone 3 lysine 4
Footnotes
Conflict of Interest
The authors state no conflict of interest.
REFERENCES
- Adams JM, Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 2008;68:4018–4021. doi: 10.1158/0008-5472.CAN-07-6334. [DOI] [PubMed] [Google Scholar]
- Boiko AD, Razorenova OV, van de Rijn M, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit JB, Yue L, Strachan LR, et al. Selection of tumorigenic melanoma cells using ALDH. J Invest Dermatol. 2010;130:2799–2808. doi: 10.1038/jid.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Christensen J, Agger K, Cloos PA, et al. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell. 2007;128:1063–1076. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Civenni G, Walter A, Kobert N, et al. Human CD271-Positive Melanoma Stem Cells Associated with Metastasis Establish Tumor Heterogeneity and Long-Term Growth. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-3997. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- Frank NY, Margaryan A, Huang Y, et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–4333. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- Fukunaga-Kalabis M, Martinez G, Nguyen TK, et al. Tenascin-C promotes melanoma progression by maintaining the ABCB5-positive side population. Oncogene. 2010;29:6115–6124. doi: 10.1038/onc.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held MA, Curley DP, Dankort D, et al. Characterization of melanoma cells capable of propagating tumors from a single cell. Cancer Res. 2010;70:388–397. doi: 10.1158/0008-5472.CAN-09-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek KS, Goding CR. Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res. 2010;23:746–759. doi: 10.1111/j.1755-148X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- Iwase S, Lan F, Bayliss P, et al. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–1088. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, et al. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Yan Q, Tothova Z, et al. The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell. 2007;128:889–900. doi: 10.1016/j.cell.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Ma I, Allan AL. The Role of Human Aldehyde Dehydrogenase in Normal and Cancer Stem Cells. Stem Cell Rev. 2010 doi: 10.1007/s12015-010-9208-4. Epub ahead of print Nov 20. 2010. [DOI] [PubMed] [Google Scholar]
- Monzani E, Facchetti F, Galmozzi E, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Mortarini R, Gismondi A, Maggioni A, et al. Mitogenic activity of laminin on human melanoma and melanocytes: different signal requirements and role of beta 1 integrins. Cancer Res. 1995;55:4702–4710. [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Foster HR, et al. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18:510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarry JE, Murphy K, Perry R, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest. 2010;121:384–395. doi: 10.1172/JCI41495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Schutte U, Frank NY, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M. Melanoma stem cells--are there devils in the detail? Pigment Cell Melanoma Res. 2010;23:693–694. doi: 10.1111/j.1755-148X.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- Strowig T, Gurer C, Ploss A, et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. 2009;206:1423–1434. doi: 10.1084/jem.20081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig DC, Vargaftig J, Miraki-Moud F, et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(-) fraction. Blood. 2010;115:1976–1984. doi: 10.1182/blood-2009-02-206565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, De Sousa EMF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- Xia X, Lemieux ME, Li W, et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci U S A. 2009;106:4260–4265. doi: 10.1073/pnas.0810067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K, Tateishi K, Klose RJ, et al. PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol Cell. 2007;25:801–812. doi: 10.1016/j.molcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]