Abstract
Obesity is a public health crisis that has reached epidemic proportions. Although intensive behavioral interventions can produce clinically significant weight loss, their cost to implement, coupled with resource limitations, pose significant barriers to scalability. To overcome these challenges, researchers have made attempts to shift intervention content to the Internet and other mobile devices. This article systematically reviews the recent literature examining technology-supported interventions for weight loss and maintenance among overweight and obese adults. Thirteen studies were identified that satisfied our inclusion criteria (12 weight loss trials, 1 weight maintenance trial). Our findings suggest that technology interventions may be efficacious at producing weight loss. However, several studies are limited by methodologic shortcomings. There are insufficient data to evaluate their efficacy for weight maintenance. Further research is needed that employs state-of-the-art methodology, with careful attention being paid to adherence and fidelity to intervention protocols.
Keywords: Obesity, weight loss, weight maintenance, technology, systematic review
Introduction
More than 400 million adults worldwide and 90 million Americans are obese, reflecting a significant public health crisis [1–3]. Although losing 5% to 10% of body weight has been shown to reduce the risk of significant morbidity and premature mortality [3, 4], few individuals are able to adhere to weight loss behaviors with consistency.
Intensive behavioral interventions have been shown to produce clinically significant weight loss [5]. Components of such interventions include 1) self-monitoring of diet, physical activity, and body weight, 2) reducing energy intake, and 3) increasing energy expenditure [6–9]. Furthermore, intensive interventions often incorporate a variety of skills, including stimulus control, stress management, and problem solving, which bolster individuals’ ability to implement these behavioral changes across a variety of challenging contexts and situations. Unfortunately, these interventions are expensive, time consuming for both patients and providers, and often inaccessible, posing significant barriers to achieving population-level reach.
Researchers and clinicians have capitalized on the use of technologies, such as the Internet and mobile devices (eg, PDAs, smartphones, cellular phones), to deliver weight management interventions. Such platforms are attractive because they help overcome resource and access barriers encountered when delivering traditional face-to-face individual or group interventions. Consequently, these platforms may enhance our ability to produce significant and healthy change in larger segments of the obese population.
In 2010, the American Heart Association commissioned a systematic review of technology interventions for weight loss and maintenance [10]. It concluded that such interventions indeed hold promise; however, several caveats were noted: 1) successful technology interventions (eg, Internet, PDAs) contain elements of human contact (eg, e-mail support with behavioral coaches), 2) samples used in randomized control trials (RCTs) have been largely homogenous (ie, white and female), 3) researchers of several trials limited their analyses to participants with complete data, and 4) several trials reported high rates of attrition (20%–80%). Therefore, researchers were charged with the task of further study using more heterogeneous samples and employing state-of-the-art clinical trial methodology (eg, employing the intent-to-treat principle in all analyses and increased efforts to maximize participant retention). This article provides a review and update of technology-supported interventions for weight loss and weight maintenance among obese adults, focusing on the recent literature (2010–2011). The authors also provide comment on their experiences developing technology interventions for weight loss and provide directions for future research.
Methods
Search strategy
In September 2011, a comprehensive literature search was conducted of technology-supported interventions for weight loss or weight maintenance. At the outset, two lists of relevant keywords were generated that included both technology-related terms (eg, Internet, PDA) and weigh-trelated terms (eg, obese, weight loss). The two lists were intra-linked with “OR” and inter-linked with “AND” so that all candidate articles contained at least one relevant technology term and at least one weight-related term. For a full list of keywords used, please contact the corresponding author (M. Coons).
Our search was executed in the following databases: PubMed, Medline, EMBASE, PsychINFO, CINAHL, Cochrane CENTRAL, and IEEE Xplore. Searches were limited to articles published in 2010 and 2011, of adult humans, and published in English. Four authors (AD, AP, JB, JS) performed the literature search and initial abstract reviews. After all duplicate titles were excluded, 48 full-text articles were collected. Candidate articles were evaluated for inclusion by two investigator dyads (MC + CP and JB + JD). A third independent reviewer resolved any discrepancies between the dyads.
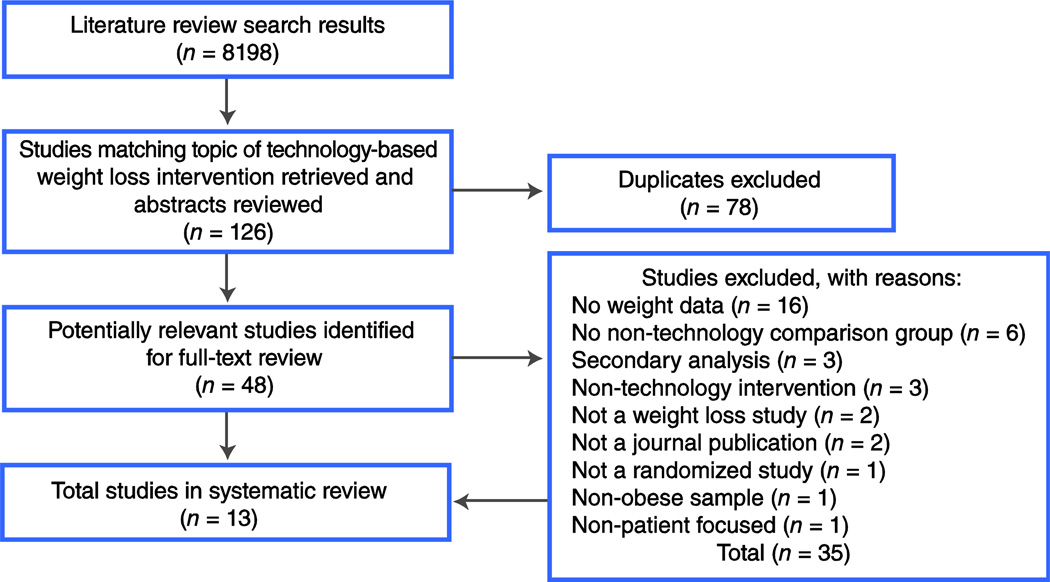
Studies were included in this present review if they satisfied all of the following criteria: 1) were a RCT (including at least one intervention and a comparison condition), 2) included a technology-supported intervention platform with participant interface (eg, Internet, PDA), 3) included weight loss outcome variable(s) (eg, weight, weight change, body mass index (BMI), waist circumference), 4) were published in a peer-reviewed journal, and 5) were published in English. Articles were excluded from our review if they were secondary analyses, conference presentations, dissertations, studies of pediatric or adolescent populations, or included specific subpopulations (eg, individuals with psychosis). Of the 48 full-text articles, 13 were included in the final review. For a detailed description of our search results, please refer to the consort diagram in Figure 1.
Figure 1.
Consort diagram of search methods used in this article.
Data extraction
The following variables were extracted during the review process: sample characteristics (ie, sample size, demographics, retention rates), descriptions of all intervention and control conditions, weight-related outcome variables, and intervention results.
Results
A total of 48 abstracts were identified through our search. Thirteen studies satisfied our search criteria and were included in the present review (12 weight loss trials, 1 weight maintenance trial). These studies are summarized in Table 1.
Table 1.
Summary of weight loss and maintenance clinical trials
| Study (year)/ description |
Sample | Intervention/control | Outcome measures |
Analysis/results |
|---|---|---|---|---|
| Bennett et al. [11] (2010) 12-wk RCT of Internet behavioral weight loss intervention in primary care |
n = 101→85 47.5% Female 50% White Age 54.4 ± 8.1 yr Weight 97.3 ± 10.9 kg BMI 34.6 ± 3.2 kg/m2 Systolic BP 137 mm Hg Diastolic BP 76 mm Hg Waist circumference: not reported Retention rate: 84% |
Intervention: Targeted obesogenic behavior goals (diet + physical activity), self- monitoring, Motivational Interviewing (baseline + 6 wk) through website (n = 51→43) Control: Usual care (n = 50→42) |
Outcomes: Δ at 12 weeks in body weight, BMI, BP, waist circumference |
Intent-to-treat (BOCF): Weight loss: I = −2.28 ± 3.21 kg C = 0.28 ± 1.87 kg (M diff = −2.56 kg; 95% CI, −3.60 to 1.53) BMI: I = −0.94 ± 1.16 kg/m2 C = 0.13 ± 0.75 kg/m2 (M diff = −1.07 kg/m2; 95% CI, −1.49 to −0.64) No sig Δ in BP or waist circumference |
| Bennett et al. [22] (2011) 6-month Internet weight loss, health, and leadership intervention |
n = 145→83 64% Female 82% White Age 41.5 ± 10.3 yr Men: Weight 203.63 ± 9.23 lbs. BMI 29.89 ± 1.25 kg/m2 Waist circumference: 38.35 ± 1.01 % Body Fat: 24.24 ± 1.38 Women: Weight 160.30 ± 5.34 lbs. BMI 26.83 ± 0.88 kg/m2 Waist circumference: 33.59 ± 0.74 % Body Fat: 30.78 ± 1.01 Retention Rate: 57% |
Intervention: Managers spent 10 hours interacting with a program that included: education on healthy diet + activity habits, their role as health role models, improving workplace health, and other leadership components (n = 72→36 completed biometrics) Control: No access to web- based program (n = 73→47 completed biometrics) |
Outcomes: Δ at 6-months in body weight, waist circumference, BMI, body fat % |
Intent-to-treat (BOCF): Women Waist Circumference: I = 32.6 ± 0.80 in C = 33.88 ± 0.80 in (M diff = −1.26, P < 0.02) No significant between-group differences in waist circumference in men, and in weight, BMI, or % body fat in men or women |
| Burke et al. [18•] (2010) A 24-mo handheld behavioral weight loss intervention |
n = 210→192 PDA n = 68→64 85.3% Female 80.9 % White Age 46.7 ± 9.2 yrs BMI 34.9 ± 4.6 kg/m2 94.1% retention at 6 mo PDA + daily feedback (FB) n = 70→65 84.3% Female 78.6 % White Age 46.4 ± 9.5 yrs BMI 34.8 ± 4.6 kg/m2 92.9% retention at 6 mos Paper diary (PD) n = 72→63 84.7% Female 76.4 % White Age 47.4 ± 8.5 yr BMI 33.4 ± 4.5 kg/m2 Retention Rate: 91% |
All subjects received a 24-month behavioral weight loss intervention, including group sessions, daily self-monitoring of eating/exercise behaviors, daily dietary goals, and weekly exercise goals PD: Use paper diary for self-monitoring diet and exercise PDA: Use PDA with dietary & exercise software for electronic self- monitoring. PDA includes date- & time- stamp to measure self-monitoring adherence. PDA + FB: Use PDA self- monitoring diet and exercise, daily- automated messages tailored to diary entries |
Outcomes: % weight loss at 6-months, proportion achieving ≥ 5% weight loss |
Intent-to-treat (BOCF): % weight loss at 6-mo: PD: 5.3% ± 5.9% PDA: 5.5% ± 7.0% PDA + FB: 7.3% ± 6.6% (PDA + FB > PD or PDA, P < 0.12) Proportion of each group that achieved 5% wt. loss (compared to PDA + FB 63%): Paper diary: 46%, P < 0.05 PDA 49%, P < 0.05 Median % adherence to self-monitoring: PR 55% PDA 80% PDA+FB 90%, P <.01 |
| Harvey-Berino et al. [23] (2010) 6-month RCT of Internet behavioral weight loss intervention |
n = 481→462 93.0% Female 28.0% African American Age 46.6 ± 9.9 yr Weight 97.0 ± 17.7 kg BMI 35.7 ± 5.6 kg/m2 Retention Rate: 96% |
Intervention: 6-month behavioral weight loss program (Internet or Internet + face-to-face group formats) containing educational material, self-monitoring of diet intake and physical activity, graded goals for physical activity Control: 6-month behavioral weight loss program (face-to-face groups) containing identical content, using paper- based self-monitoring records I1: In-person (n = 158→150) I2: Internet (n = 161→159) I3: Hybrid (n = 162→153) |
Outcomes: 6- month Δ body weight, proportion achieving 5 and 7% weight loss |
Intent-to-treat (BOCF): Δ body weight at 6-months: I1 = −7.6 ± 6.2 kg I2 = −5.5 ± 5.6 kg I3 = −5.7 ± 5.5kg (I1 > I2 or I3, P < 0.01) 5% weight loss: I1 = 62.0% I2 = 52.2% I3 = 55.6% (I1 = I2 = I3, ns) 7% weight loss: I1 = 53.2% I2 = 37.3% I3 = 42.0% (I1 > I2, P < 0.01) |
| Maruyama et al. [20 (2010) 4-mo RCT of Internet behavioral weight loss in the workplace in Japan |
n = 101→87 100% Male ethnicity: not reported Intervention: Age 43.1 ± 7.7 yr Weight 75.4 ± 11.5 kg BMI 25.7 ± 3.7 kg/m2 Waist circ 89.2 ± 9.3 cm Control: Age 35.5 ± 8.1 yr Weight 75.8 ± 9.9 kg BMI 25.8 ± 3.3 kg/m2 Waist circ 90.4 ± 8.2 cm Retention Rate: 86% |
Intervention: 4-mo Internet dietary weight loss intervention by increasing healthy foods (eg, vegetables) and decreasing unhealthy foods (eg, fatty-meats); 4 monthly groups (10- min each) for assessment goal/setting plus two individual counseling sessions (n=52→39) Control: no treatment (n=47→24) |
Outcomes: 4- month Δ in weight, BMI, waist circumference |
Completer analysis: Weight: I:−2.14 ± 2.68 kg C: −0.8 ± 2.2 kg (I > C, P <0.01) BMI: I: −0.74 ± 0.94 kg/m2 C: −0.26 ± 0.69 kg/m2 (I > C, p < 0.01) Waist circumference: I: −1.43 ± 4.14 cm C: −0.63 ± 3.53 cm (I > C, P < 0.35) |
| McDoniel et al. [12] (2010) 12-week handheld behavioral weight loss intervention |
n = 111→ 80 Intervention: 34.0% Female 44.0% White Age 45.9 ± 10 yr BMI 37.9 ± 6.0 kg/m2 Weight 109.0 ± 21.9 kg Control: 35% Female 43.0% White Age 44.9 ± 11.2 yr BMI 36.2 ± 5.7 kg/m2 Weight 103.8 ± 20.8 kg Retention Rate: 72% |
Intervention: PDA to derive resting metabolic rate; BalanceLog® to track calories + activity; individualized goals for ~ 1 lb/week weight loss (n = 55→39) Control: Standard nutrition plan with 3-day food menu (~1200 kcal/day for women; ~1600 kcal/day for men); 30- day paper-pencil diaries for diet + activity + bodyweight; 1 session of MI @ 4- week f/u; automated e-mail weeks 5–12 for reminders (n = 56→41) |
Outcomes: Δ at 3-months in bodyweight, BP |
Intent-to-treat (LOCF): I = −3.5 ± 4.3 kg C = −3.7 ± 4.2 kg (I = C, ns) BP (systolic): I = −4.0 ± 11.8 mm Hg C = −4.0 ± 11.8 mm Hg (I = C, ns) BP (diastolic): I = −2 mm Hg C = 0 mm Hg (I = C, ns) |
| Morgan et al. [13•] (2011) 14-wk RCT of Internet behavioral weight loss intervention (Workplace POWER) |
n = 110→90 100% Male ethnicity: not reported Age 44.4 ± 8.6 yr Weight 94.9 ± 13.4 kg BMI 30.5 ± 3.6 kg/m2 Systolic BP 135.0 ± 14.9 mm Hg Diastolic BP 85.4 ± 9.2 mm Hg Waist circ: 100.7 ± 10 cm Retention Rate: 81% |
Intervention: 1 face-to-face session, access to weight loss website (www.calorieking.com) for self-monitoring diet and physical activity, and additional weight loss resources (n = 65→54) Control: Wait list (n = 45→36) |
Outcomes: Δ at 14 weeks in body weight, waist circumference, BMI, systolic and diastolic BP, resting HR, physical activity |
Intent-to-treat (BOCF): Weight loss at 14-weeks: I = −4 kg (−5.1, − 2.9) C = 0.3 kg (−0.1, 1.7) (I > C, P <.001) Waist circumference: I = −4.4 cm (− 5.5, −3.3) C = 1.5 cm (0.2, 2.9) (I > C, P < 0.001) BMI: I = −1.3 kg/m2 (− 1.6, −0.9) C = 0.1 kg/m2 (− 0.3, 0.6) (I > C, P < 0.001) Systolic BP: I = −7.3 mm Hg (− 10.6, −4.1) C = −1.3 mm Hg (−5.4, 2.7) (I > C, P = 0.02) Diastolic BP: I = −3.7 mmHg (− 5.9, −1.4) C = −2.5 mmHg (−5.3, 0.3) (I = C, ns) Resting HR: I = −6.2 bpm (− 8.5, −3.9) C = 1.7 (−1.3, 4.7) (I > C, P < 0.001) Physical Activity: I = 0.4 MET minutes (0.2, 0.5) C = 0.1 MET minutes (−0.1, 0.3) (I > C, P < 0.04) |
| Morgan et al. [14] (2011) 12-month RCT of internet behavioral weight loss program for men (SHED-IT) |
n = 65→;46 100% Male ethnicity: not reported Age 35.9 ± 11.1 yr Weight 99.1 ± 12.8 kg BMI 30.6 ± 2.8 kg/m2 Systolic BP 134 ± 14 mm Hg Diastolic BP 84 ± 9 mm Hg Waist circ: 103.1 ± 7.5 cm Retention Rate: 85% at 3-month follow up, 71% at 12-month follow up |
Intervention: 1 face-to-face session, access to weight loss website (www.calorieking.com) for self-monitoring diet and physical activity (n =34→28→26) Control: In-person session and weight loss information booklet (n = 31→27→20) |
Outcomes: Δ at 3 and 12 months in body weight, BMI, BP, waist circumference |
Intent-to-treat (BOCF): Weight loss (3- months): I = −4. 8kg (95% CI = −6.4 to −3.3) C = −3.0 kg (95% CI = −4.5 to −1.4) (I > C, ns) Weight loss (12 months): I = Ȓ5.3 kg (95% CI = −7.5 to −3.0) C = −3.1 kg (95% CI = −5.4 to −0.7) (I > C, ns) BMI (3 months): I = −1.5 kg/m2 (95% CI = −2.0 to −1.0) C = −0.9 kg/m2 (95% CI = −1.4 to −0.5) (I > C, ns) BMI (12 months): I = −1.7 kg/m2 (95% CI = −2.4 to −1.0) C = −0.9 kg/m2 (95% CI = −1.7 to −0.2) (I > C, ns) Waist circumference (12 months): I = −5.8 cm (95% CI = −7.1 to −3.4) C = −4.4 cm (95% CI = −6.3 to −2.5) (I > C, ns) Waist circumference (12 months): I = −5.8 cm (95% CI = −7.9 to −3.6) C = −3.8 cm (95% CI = −6.1 to −1.6) (I > C, ns) Systolic BP (3 months): I = −6 mm Hg (95% CI = −10 to −1) C = −8 mm Hg (95% CI = −12 to −3) (I > C, ns) Systolic BP (12 months): I = −11 mm Hg (95% CI = −14 to −7) C = −6 mm Hg (95% CI = −10 to −2) (I > C, P < 0.04) Diastolic BP (3 months): I = −4 mm Hg (95% CI = −8 to − 1) C = −6 mm Hg (95% CI = −10 to −2) (I = C, ns) Diastolic BP (12 months): I = −6 mm Hg (95% CI = −10 to −2) C = −4 mm Hg (95% CI = −9 to − 1) (I = C, ns) |
| Shuger et al. [15] (2011) 9-month RCT of behavioral weight loss Intervention |
n = 197→123 82.0% Female 66.8% White Age 46.9 ± 10.8 yr Weight 92.8 ± 18.4 kg BMI 33.3 ± 5.2 kg/m2 Waist circ: 99.7 ± 13.9 cm % Body fat 38.4 ± 5.3 Mean energy expenditure 2209.4 ± 502 kcal/day Retention Rate: 62.4% |
Group Weight Loss (GWL) 14 GWL lessons (months 1–4) of intervention based on Active Living Every Day (ALED) and Healthy Eating Everyday (HEED) protocols. Weekly weigh-ins. Received 6 phone counseling sessions during months 5–9 (n = 49→28) Sensewear Armband (SWA) SWA provided minutes of PA, steps, energy expenditure. SWA worn daily for 16 hours per day. Data uploaded to a weight management website along with energy intake and weight. (n = 49→32) GWL+SWA (n = 49→37) Control (SC): Standard Care Self-directed weight loss manual (ALED and HEED); no further contact. (n = 50→26) |
Outcomes: Δ at 4 months (M4) and 9 months (M9) in weight, waist circumference, BMI, % body fat, energy expenditure |
Intent-to-treat (BOCF): Weight loss: GWL BL 101.84 (2.95) M4 100.74 (2.99) M9 99.98 (3.00) P < 0.05 SWA BL 101.15 (2.95) M4 98.48 (2.97) M9 97.60 (2.99) P < 0.001 GWL+SWA BL 100.32 (2.97) M4 96.83 (2.99) M9 93.73 (2.99)* P < 0.0001) SC BL 102.22 (2.97) M4 101.23 (3.03) M9 101.32 (3.05) P < 0.40 Waist circumference: SC = GWL = GWL + SWA, ns) Note: BMI change analogous to Body Weight Change at Each Time Point Body Fat % Change Significant at all Time Points (BL to 4mo and BL to 9mo) for all groups with no significant between group differences) |
| Thomas et al. [21] (2010) 6-month RCT of behavioral weight maintenance intervention. |
n = 55→49 81.7% Female 66.8% White Intervention: Age 43.2 ± 15.2 yr Weight 86 ± 38.2 kg BMI 33.1 ± 10 kg/m2 Control: Age 46.2 ± 12.0 yrs Weight 91.9 ± 39.7 kg BMI 32.7 ± 10 kg/m2 Retention Rate: 89% |
All participants lost > 5% of initial body weight during a dietetic-led weight loss program before randomization to one of two maintenance conditions. Intervention: Weekly email messages, monthly personalized messages with report of weight (n = 28→26) Control: No contact (n = 27→23) |
Outcomes: Δ at 6- months in weight, % weight loss maintained |
Completer analysis: Weight loss maintenance (median): I = 9.6 ± 10.9 kg C = 7.8 ± 5.9 kg (I = C, ns) % Weight loss maintenance (mean): I = 10.4 ± 5.1 kg C = 7.6 ± 4.0 kg (I = C, ns) |
| Touger-Decker et al. [16] (2010) 12-week Internet behavioral weight loss intervention |
n = 137→95 93.0% Female 46.0% White Age 46.5 ± 10.5 yr Weight 201.8 ± 47.0 lbs Retention Rate: 69% |
All participants from academic medical center received 12- week weight loss intervention (diet + activity + pedometer) by Registered Dietician Intervention: Received content via Internet (WebCT) + access to email and chat room support (n = 68→48) Control: Received 12-week face-to-face group nutrition education sessions led by a Registered Dietician (n = 69→47) |
Outcomes: Δ at 12 and 26 weeks in weight, energy intake |
Completer Analysis: Bodyweight at 12 weeks I = 182.1 ± 44.9 lbs C = 208.9 ± 49.9 lbs (I = C, ns) Bodyweight at 26 weeks I = 181.4 ± 45.0 lbs C = 207.7 ± 50.0 lbs (I = C, ns) Energy intake at 12 weeks I = 1278 ± 260 kcal/d C = 1368 ± 327 kcal/d (I = C, ns) Energy intake at 26 weeks I = 1479 ± 770 kcal/d C = 1465 ± 482 kcal/d (I = C, ns) |
| van Wier et al. [17] (2011) 6-mo RCT of behavioral weight loss intervention |
n = 1386→792 33.0% Female Intervention: Phone (Ph): Age 43 ± 8.8 yr Weight 93.6 ± 14.0 kg BMI 29.5 ± 3.5 kg/m2 Waist circ 102.4 ± 9.7 cm Internet (Int): Age 43 ± 8.4 yr Weight 92.9 ± 14.4 kg BMI 29.6 ± 3.4 kg/m2 Waist circ 101.5 ± 9.9 cm Control (C): Age 43 ± 8.7 yr Weight 93.0 ± 13.4 kg BMI 29.6 ± 3.7 kg/m2 Waist circ 101.3 ± 9.1 cm Retention rate: 57% |
Intervention: Phone (Ph): Self-help brochures plus 10- module behavioral weight loss intervention (workbook) plus telephone counseling Internet (Int): Self- help brochures plus 10-module behavioral weight loss intervention (tailored, interactive Website) plus email counseling Control (C): Self-help brochures only |
Outcomes: Δ at 12 and 24 months in body weight, waist circumference |
Intent-to-treat (multiple imputation): 6-month weight loss: Ph: −1.6 kg (95% CI = −2.2− 1.0) I: −0.7 kg (CI = − 1.2−.01) C: not reported (Ph and I > C) 24-month weight: Ph: 92.1 ± 13.7 kg Int: 91.1 ± 14.4 kg C: 92.0 ± 13.2 kg (Ph = I = C, ns) Completer analysis: 24-month weight loss: Ph: 90.0 ± 13.3 kg Int: 89.6 ± 13.9 kg C: 90.6 ± 12.9 kg (I > C, P < 0.005) 24-month waist circumference: Ph: 99.8 ± 10.1 cm Int: 99.4 ± 10.5 cm C: 99.5 ± 9.7 cm (Ph = I = C, ns) |
| Wing et al. [19] (2010) 12-week RCTs of behavioral weight loss interventions |
Study 1 n = 179→168 83.0% Female 88% White Age 46.5 ± 10.1 yr BMI 33.8 ± 6.3 kg/m2 Weight 92.0 ± 19.2 kg Study 2 n = 128→112 90.0% Female 88% White Age 46.9 ± 9.7 yr BMI 33.9 ± 5.6 kg/m2 Weight 92.0 ± 17.8 kg |
Study 1 Intervention: Standard Shape Up Rhode Island campaign + weekly multimedia lessons based on Diabetes Prevention Program Control: Standard Shape Up Rhode Island weight loss campaign (directory of weight loss websites of comparable content) Study 2 Intervention: 1 session before start of Shape Up RI campaign + shortened multimedia lessons like in study1 + Participants instructed to self-monitor daily weight, calories, fat, physical activity, and steps via Shape Up RI website. Control: Standard Shape Up RI weight loss campaign (directory of weight loss websites of comparable content) |
Outcomes: Δ at 12 weeks in weight loss, % of initial weight, proportion losing > 5% of initial weight |
Study 1 Weight loss at 12 weeks Intent to treat I = −1.9 ± 2.8 C = −1.3 ± 2.9 (C = I, ns) Completers I = 2.0 ± 2.8 C = 1.4 ± 2.9 (C = I, ns) % of initial weight Intent to treat I = 2.1 ± 3.0 C = 1.5 ± 3.2 (C = I, ns) Completers I = 2.2 ± 3.1 C = 1.6 ± 3.3 (C = I, ns) Proportion losing > 5% Intent to treat C = 11.1 I = 16.9 (C = I, ns) Completers C = 11.6 I = 18.3 (C = I, ns) Study 2 Weight loss at 12 weeks Intent to treat I = −3.1 ± 3.7 C = −1.2 ± 2.5 (I > C, P < 0.01) Completers I = −3.5 ± 3.8 C = −1.4 ± 2.7 (I > C, P < .01) % of initial weight Intent to treat I = −3.6 ± 4.4 C = −1.4 ± 3.0 (I > C, P < 0.01) Completers I = −4.0 ± 4.4 C = −1.6 ± 3.2 (I > C, P < 0.01) Proportion losing > 5% Intent to treat I = 36.6 C = 11.1 (I > C, P < .01) Completers I = 40.5 C = 13.2 (I > C, P < 0.01) |
Significant difference from SC M9 (P < 0.05).
Ten trials tested Internet interventions and 3 trials tested handheld devices (eg, PDAs, armband with tri-axial accelerometer). Regardless of the technology platform, intervention components included 1) education about diet, physical activity, and weight management; 2) self-monitoring of diet, physical activity, and weight parameters (eg, weight, BMI, waist circumference); and 3) goal setting for diet and physical activity. Furthermore, several trials included elements of motivational enhancement [11, 12] and some provided social support through e-mail or Internet chat room contact with both coaches and peers [13•, 14–17].
Six of the 12 weight loss trials reported significantly greater weight loss among individuals randomized to technology interventions compared to controls [13•, 17, 18•, 19, 20]. However, researchers of one trial conducted analyses exclusively on participants with complete data [20], and one trial lost 43% of their sample due to attrition [17]. Of the remaining four positive trials that analyzed data using the intent-to-treat principle (ie, imputation of missing weight loss outcomes using either the last observation carried forward or baseline observation carried forward), three studies tested Internet-mediated interventions [11, 13•, 19] and one study tested a mobile platform [18]. Components of these successful interventions included self-monitoring (diet, physical activity, and weight), goal setting for calorie intake and physical activity, and feedback on current diet and activity behaviors relative to daily and weekly goals. Interestingly, these successful trials were implemented in a variety of settings, including the workplace [13•], primary care 11], academic medical center [18•], and the community [19]. Furthermore, the proportion of individuals using technology interventions that achieved clinically meaningful weight loss (defined as achieving ≥ 5% of initial body weight) ranged from 37% [19] to 63% [18•].
Unfortunately, only one weight maintenance trial was included in our review [21]. These authors reported no significant differences in weight between Internet intervention and control conditions. However, this trial was underpowered to detect a significant group×time interaction (n = 55), the intervention lacked sufficient intensity by being limited to monthly e-mail messages, and did not include key weight management interventions (eg, self-monitoring, goal setting, and feedback).
Five trials reported no significant difference in weight loss between intervention and control conditions [12, 14–16, 22]; however, all of these trials reported within-group weight loss. Surprisingly, one trial reported significantly greater weight loss in the control condition (ie, face-to-face group weight loss) compared to the intervention conditions (ie, Internet alone or hybrid conditions) [23]. The authors of this trial attribute these findings to differences in perceived social support, with higher levels being noted in the face-to-face conditions compared to the Internet conditions. Further examination of these negative trials revealed that several included control conditions that contained potent intervention components (eg, face-to-face weight loss groups) that may have undermined the ability to detect significant between-group differences at post-treatment follow-up. Furthermore, only two of the trials reported data on adherence to the intervention [14, 15]. Absence of these data in other negative trials challenges our ability to determine whether the lack of significant weight loss between groups was a result of treatment inefficacy or an artifact of non-adherence.
Reporting of adherence across trials was variable and differed by how adherence was defined (eg, session attendance, number of logins to intervention websites, self-monitoring behaviors). Of the studies reviewed, seven reported data on adherence to the intervention [11, 13•, 14, 17–19, 23]. Four studies reported a comparison of adherence between groups. Two studies reported no significant differences between groups on adherence measures [19, 23]. One study reported better adherence in the technology group [18•] and another reported better adherence in the comparison group (eg, 34% of participants completed all sessions in the phone intervention group versus 18% in the technology group) [17]. Three studies only reported adherence within the technology groups [11, 13•, 14]. Of these three trials, adherence to the technology-supported interventions was poor, ranging from 28% [13•] to 41.2% [14] participant participation rates. Only one study reported any data on participant satisfaction and usability of the technology [14]; participants reported that they were highly satisfied with the website, finding it to be enjoyable and usable.
Discussion
The results of our review suggest that technology-supported interventions using Internet-based and mobile platforms may be efficacious in producing weight loss among overweight and obese adults. Four of the trials reviewed showed that technology interventions (ie, Internet, PDA) produced significantly greater weight loss compared to controls in a variety of treatment settings, including the workplace, primary care, academic medical center, and the community. However, several issues were identified that influence our conclusions. First, six of the trials reported attrition rates > 20% (ranging from 25% to 43%) [12, 14–17, 22], which compromises the validity of the trial outcomes and power to detect significant between group differences. Second, three of the trials included in this review conducted completer analyses, rather than employing the intent-to-treat principle [16, 20, 21]. Although this practice lends insight into the effect of the intervention on those who complete it, this undermines the effect of randomization, limiting the conclusions that can be drawn. Third, of the five negative trials that showed no significant difference in weight loss between groups, only two of these trials paid adequate attention to adherence to intervention components (eg, the extent to which individuals engaged with the technology to receive, process, and enact its content). Overall, only half of the trials reviewed reported adherence data, and the manner in which adherence was both defined and reported was variable across studies. Over half of the studies reviewed failed to report data on adherence to the interventions. Of those that did, a few of the trials reported low adherence rates in the technology groups. In the absence of adherence data or efforts to ensure fidelity to the interventions, we are unable to determine whether the results are attributed to an ineffective intervention, or non-adherence to treatment.
Unequivocally, self-monitoring of diet and physical activity is paramount to successful weight loss and maintenance. However, until recently, behavioral weight loss interventions have been challenged with self-monitoring tools that are cumbersome and prone to inaccuracies [24]. In particular, dietary self-monitoring using paper diaries is onerous for individuals to complete and maintain with consistency. Furthermore, individuals tend to under-report calorie intake and over-report physical activity [25, 26].Technologies such as smartphones and other devices hold considerable promise to minimize such barriers and ensure the accuracy of the data [18]. Efforts to streamline the self-monitoring process may promote adherence to well-established weight loss behaviors. In our experience, user interfaces, regardless of their platform (Internet, mobile) need to be intuitive and engaging to ensure their use. Fortunately, the use of accelerometers overcomes the recall biases inherent in self-reporting of physical activity. With wireless communication abilities, such as Bluetooth transmitters, individuals are now able to receive objective information on their activity levels in real-time. When interfacing with smartphones, we are able to provide individuals with an integrated system to self-monitor their diet and activity and provide real-time feedback for decision support.
Through ongoing research in our laboratory, we are examining whether handheld technologies can improve adherence to, and outcomes of, intensive behavioral weight loss treatments. E-Networks Guiding Adherence to Goals in Exercise and Diet (ENGAGED) is an integrated smartphone weight loss intervention platform that implements a modification of the Diabetes Prevention Program [8]. Participants are provided with an Android smartphone that is equipped with a customized software application that links with a comprehensive food database and Bluetooth enabled tri-axial accelerometer. Individuals are asked to use the application to electronically self-monitor their dietary intake and weight and to wear their accelerometers to objectively measure participation in moderate/vigorous-intensity physical activity. Our software application provides real-time feedback on diet (ie, calories and fat) and physical activity (ie, minutes/week of moderate-vigorous intensity) through graphic and color-coded visualizations. Our interface is designed to provide “in the moment” decision support, connect participants to behavioral coaches and peers, and persuasively motivate healthy diet and activity choices.
The ENGAGED study was developed based on the Control Systems Theory (CST) of self-regulation. CST posits that behavior change occurs when an individual becomes aware of the discrepancy between their goals and current behaviors. Individuals are then motivated to reduce this discrepancy by making the necessary changes to their behavior [27]. With our ENGAGED mobile interface, participants are provided with daily and weekly goals for calorie and fat intake as well as physical activity. These goals are intended to produce 5% to 10% weight loss over the course of the 12-month intervention. Our persuasive graphics display discrepancies between current diet and activity behaviors and daily and weekly goals. Participants are asked to reduce and minimize the discrepancies to promote adherence to the intervention, which in turn, facilitates weight loss. In light of research showing that social networks influence weight [28], the ENGAGED application also contains a virtual social network that links participants to a behavioral coach and a weight loss support group. Individuals can use this tool to solicit or provide social support, fostering frequent communication among teammates and behavioral coaches to promote adherence to behavioral goals and outcomes. The ENGAGED technology is currently being tested in our laboratory in an RCT sponsored by the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH).
At present, many commercially produced software applications are available to consumers on a number of mobile platforms including Android, Apple (iPhone, iPad), and Blackberry. However, many of these products were designed for commercial distribution. Although some commercial weight loss programs have received empirical support, many of the mobile applications that are based on these programs have not yet been tested using well-designed clinical trials. Although only three studies were identified during this review that tested mobile intervention platforms [12, 15, 18•], we anticipate a proliferation in this area of research in the coming years. Smartphones have become ubiquitous across a variety of demographics [29], reducing the access barriers that have been typically encountered over this past decade. The development of efficacious and mobile weight loss intervention platforms will help to ensure the population-level reach that is needed to produce clinically significant weight loss in larger segments of the obese population.
Future Directions
Despite our enthusiasm for the studies reviewed here, research is needed to address a variety of outstanding issues. First, although a limited number of well-designed positive trials included diverse samples and were tested in a variety of treatment settings, more studies enrolling diverse samples are needed to verify the results of positive trials. Second, although electronic tools to self-monitor dietary intake overcome many of the barriers encountered when individuals maintain paper diaries, self-monitoring of dietary intake still relies on self-report. Consequently, efforts to develop objective measures of dietary intake will provide the most accurate information to ensure fidelity to weight loss interventions. These efforts are currently underway in several laboratories across the United States and abroad. For example, some researchers are developing technologies that capture real time images of foods that individuals consume using smartphones. Individuals take photographs of their food before and after meals, and these images are uploaded via smartphone to a remote server. These images are then analyzed using a software program to determine macronutrient composition. Although this technology remains under development, it holds the potential to both facilitate the process of dietary self-monitoring, and provide a more objective measure of dietary intake. Lastly, the intervention platforms reviewed in this article represent older-generation technologies including the Internet and PDAs. Smartphones hold the potential to revolutionize the delivery of behavioral weight loss interventions because they are mobile, streamline the self-monitoring process, enable the objective measurement of weight loss behaviors, and link individuals virtually to behavioral coaches and peers for social support. Future investigation of smartphone platforms, such as the ENGAGED system, lends excitement to forthcoming research.
Conclusions
The results of our review suggest that technology-enhanced interventions may be effective in producing clinically significant weight loss among overweight and obese adults. Such interventions, regardless of their technology platform, include well-established weight loss behaviors including self-monitoring (of diet, physical activity, and weight), goal setting (to reduce calorie intake and increase calorie expenditure), feedback on weight loss behaviors, and social support from coaches or peers. Clinicians looking to implement or recommend technology-enhanced interventions to their overweight and obese patients should ensure that programs include these established treatment components.
Glossary
- BMI
body mass index
- BOCF
best observation carried forward
- BP
blood pressure
- f/u
follow-up
- HR
heart rate
- LOCF
last observation carried forward
- ns
not significant
- RCT
randomized control trial
Footnotes
Disclosure
No conflicts of interest relevant to this article were reported.
References
- 1. [Accessed November 15, 2011];Obesity and Overweight Factsheet. World Health Organization Web site. http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity related health risk factors, 2001. JAMA. 2003;289:76. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect on weight loss. Atheroscler Thromb Vasc Biol. 2006;26:968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 5.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 6.Alhassan S, Kim S, Bersamin A, King A, Gardner C. Dietary adherence and weight loss success among overweight women: Results from the A to Z weight loss study. Int J Obes. 2008;32:985–991. doi: 10.1038/ijo.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker RC, Kirschenbaum DS. Self-monitoring may be necessary for successful weight control. Behav Ther. 1993;24:377–394. [Google Scholar]
- 8.Diabetes Prevention Program Research Group. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wing R, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:2225–2255. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 10.Rao G, Burke LE, Spring BJ, et al. New and emerging weight management strategies for busy ambulatory settings: A scientific statement from the American Heart Association. Circulation. 2010;124(10):1182–1203. doi: 10.1161/CIR.0b013e31822b9543. [DOI] [PubMed] [Google Scholar]
- 11.Bennett GG, Herring SJ, Puleo E, Stein EK, Emmons KM, Gillman MW. Web-based Weight Loss in Primary Care: A Randomized Controlled Trial. Obesity. 2010;18:308–313. doi: 10.1038/oby.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDoniel SO, Wolskee P, Shen J. Treating obesity with a novel hand-held device, computer software program, and Internet technology in primary care: The SMART motivational trial. Patient Educ Couns. 2010;79:185–191. doi: 10.1016/j.pec.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 13. Morgan PJ, Collins CE, Plotnikoff RC, et al. Efficacy of a workplace-based weight loss program for overweight male shift workers: The Workplace POWER (Preventing Obesity Without Eating like a Rabbit) randomized controlled trial. Prev Med. 2011;52:317–325. doi: 10.1016/j.ypmed.2011.01.031.. This trial established the efficacy of an online weight loss program (Calorie King™), which contains self-monitoring tools for diet, physical activity, and weight, and an online social network. The Calorie King™ program is available to the public.
- 14.Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. 12-Month Outcomes and Process Evaluation of the SHED-IT RCT: An Internet-Based Weight Loss Program Targeting Men. Obesity. 2011;19:142–151. doi: 10.1038/oby.2010.119. [DOI] [PubMed] [Google Scholar]
- 15.Shuger SL, Barry VW, Sui X, et al. Electronic feedback in a diet- and physical activity-based lifestyle intervention for weight loss: a randomized controlled trial. Int J Behav Nutr Phys Act. 2010;8:41. doi: 10.1186/1479-5868-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touger-Decker R, Denmark R, Bruno M, O’Sullivan-Maillet J, Lasser N. Workplace Weight Loss Program; Comparing Live and Internet Methods. J Occup Environ Med. 2010;52(11):1112–1118. doi: 10.1097/JOM.0b013e3181f9ee8c. [DOI] [PubMed] [Google Scholar]
- 17.van Wier MF, Dekkers JC, Hendriksen IJM, et al. Effectiveness of Phone and E-Mail Lifestyle Counseling for Long Term Weight Control Among Overweight Employees. J Occup Environ Med. 2011;53(6):680–686. doi: 10.1097/JOM.0b013e31821f2bbb. [DOI] [PubMed] [Google Scholar]
- 18. Burke LE, Conroy MB, Sereika SM, et al. The Effect of Electronic Self-Monitoring on Weight Loss and Dietary Intake: A Randomized Behavioral Weight Loss Trial. Obesity. 2011;19:338–344. doi: 10.1038/oby.2010.208.. This well-designed and well-implemented clinical trial established the efficacy of a behavioral weight loss intervention using a handheld device.
- 19.Wing RR, Crane MM, Thomas JG, Kumar J, Weinberg B. Improving Weight Loss Outcomes of Community Interventions by Incorporating Behavioral Strategies. Am J Public Health. 2010:e1–e7. doi: 10.2105/AJPH.2009.183616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruyama C, Kimura M, Okumura H, Hayashi K, Arao T. Effect of a worksite-based intervention program on metabolic parameters in middle-aged male white-collar workers: A randomized controlled trial. Prev Med. 2010;51:11–17. doi: 10.1016/j.ypmed.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Thomas D, Vydelingum V, Lawrence J. E-mail contact as an effective strategy in the maintenance of weight loss in adults. J Hum Nutr Diet. 2010;24:32–38. doi: 10.1111/j.1365-277X.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 22.Bennett GG, Broome KM, Schwab-Pilley A, Gilmore P. A Web-Based Approach to Address Cardiovascular Risks in Managers. J Occup Environ Med. 2011;53(8):911–918. doi: 10.1097/JOM.0b013e3182258bd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey-Berino J, West D, Krukowski R, et al. Internet delivered behavioral obesity treatment. Prev Med. 2010;51:123–128. doi: 10.1016/j.ypmed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufforz MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 25.Livingstone MBE, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133(3) suppl:895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 26.Prince SA, Adamo KB, Hamel ME, et al. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carver C, Scheier M. On the Self-regulation of Behavior. New York, NY: Cambridge University Press; 1998. [Google Scholar]
- 28.Christakis N, Fowler J. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 29. [Accessed November 15, 2011];Smartphone Adoption and Usage Report. PEW Research Center Web site. http://pewinternet.org/Reports/2011/Smartphones.aspx.