TO THE EDITOR
Xeroderma pigmentosum complementation group E (XP-E) patients exhibit sunlight-induced lentiginous pigmentation without blistering on minimal sun exposure, yet they are prone to develop multiple skin cancers. Only eight XP-E patients have been reported (Bootsma et al., 1970; De Weerd-Kastelein et al., 1974; Kraemer et al., 1975; Nichols et al., 1996; Rapic et al., 1998; Itoh et al., 1999, 2000; Rapic-Otrin et al., 2003) with mutations in the DDB2 gene (Tang and Chu, 2002; Itoh, 2006), resulting in the loss of UV-damaged DNA-binding protein (UV-DDB) activity (Nichols et al., 2000; Rapic-Otrin et al., 2003) (Table 1). UV-DDB is a heterodimer of DDB1 (p127) and DDB2 (p48) (Keeney et al., 1994; Kazantsev et al., 1996) that binds with high affinity to DNA damaged by UV and is involved in initiation of global genome nucleotide excision repair (GG-NER) (Sugasawa, 2010).
Table 1.
Clinical features and DDB2 mutations
| Family | Cell line | Last reported age/sex |
Location | Clinical features | DDB2 mutations1 | References6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allele 1 | Allele 2 | ||||||||||
| cDNA | Amino acid | Size (aa)2 |
cDNA | Amino acid | Size (aa)2 |
||||||
| A | XP1GO | 45y/M | Germany | >400 BCCs and SCCs; 6 melanomas before age 30 |
c.914 C>A (exon 7) |
pThr305Asn | 427 | Homozygous | This paper | ||
| B | XP37BE3 | 45y/F | USA/the Netherlands |
>300 BCCs and SCCs, no melanomas |
c.818 G>A (exon 6) |
p.Arg273His | 427 | Homozygous | This paper | ||
| B | XP66BE3 | 43y/M | USA/the Netherlands |
6 Melanomas before age 40 |
c.818 G>A (exon 6) |
p.Arg273His | 427 | Homozygous | This paper | ||
| C | XP408BE4 | 53y/F | USA | >600 BCCs, SCCs, and 12 melanomas by age 50 |
c.1049 T>C (exon 8) |
p.Leu350Pro | 427 | c.1045_1047del (exon 8) |
p.Asn349del | 426 | This paper |
| C | GM013894 | 21y/F | USA | Multiple skin cancers | c.1049 T>C (exon 8) |
p.Leu350Pro | 427 | c.1045_1047del (exon 8) |
p.Asn349del | 426 | A |
| D | XP2RO5 | 34y/F | The Netherlands |
Skin cancer developed at age 14 |
c.818 G>A (exon 6) |
p.Arg273His | 427 | Homozygous | B, C, E | ||
| D | XP3RO5 | 29y/F | The Netherlands |
Skin cancer present | c.818 G>A (exon 6) |
p.Arg273His | 427 | Homozygous | B, C, E | ||
| E | XP82TO | 41y/F | Japan | No skin cancer | c.730 A>G (exon 6) |
p.Lys244Glu | 427 | Homozygous | B | ||
| F | XP23PV | 18y/M | Italy | 7 BCCs from age 16 to 18 |
c.703_1023del (del. exon 6 and 7) |
p.Leu235_Lys341del | 320 | Homozygous | A, D | ||
| G | XP25PV | 29y/F | Italy | 5 BCCs and 1 SCC from age 22 to 28 |
c.919 G>T (exon 7) c.918 G>A (exon 7) |
p.Asp307Tyr No change |
427 427 |
Homozygous Homozygous |
A, D | ||
| H | XP27PV | 35y/F | Italy | BCC, SCC, and melanoma |
1. c.730_733del (exon 6) 2. c.703_880 (del. exon 6) 3. c.703_1023del (del. exon 6 and 7) |
p.Lys244X p.Trp236Valfs*10 p.Leu235_Lys341del |
243 244 320 |
Homozygous Homozygous Homozygous |
A | ||
| I | Ops1 | 62y/F | Japan | 14 BCCs and 5 melanomas on face, 2 SCC extremities |
c.937 C>T (exon 7) |
p.Arg313X | 312 | Homozygous | F | ||
Abbreviations: BCC, basal cell carcinoma; cDNA, complementary DNA; DDB, DNA-binding protein; SCC, squamous cell carcinoma.
GenBank reference sequence NC_000011.8 for genomic sequence, NM_000107.1 for cDNA, and NP_000098.1 for protein.
Predicted size.
Siblings.
Same patient, cultures established at different ages (see text for details).
Second cousins.
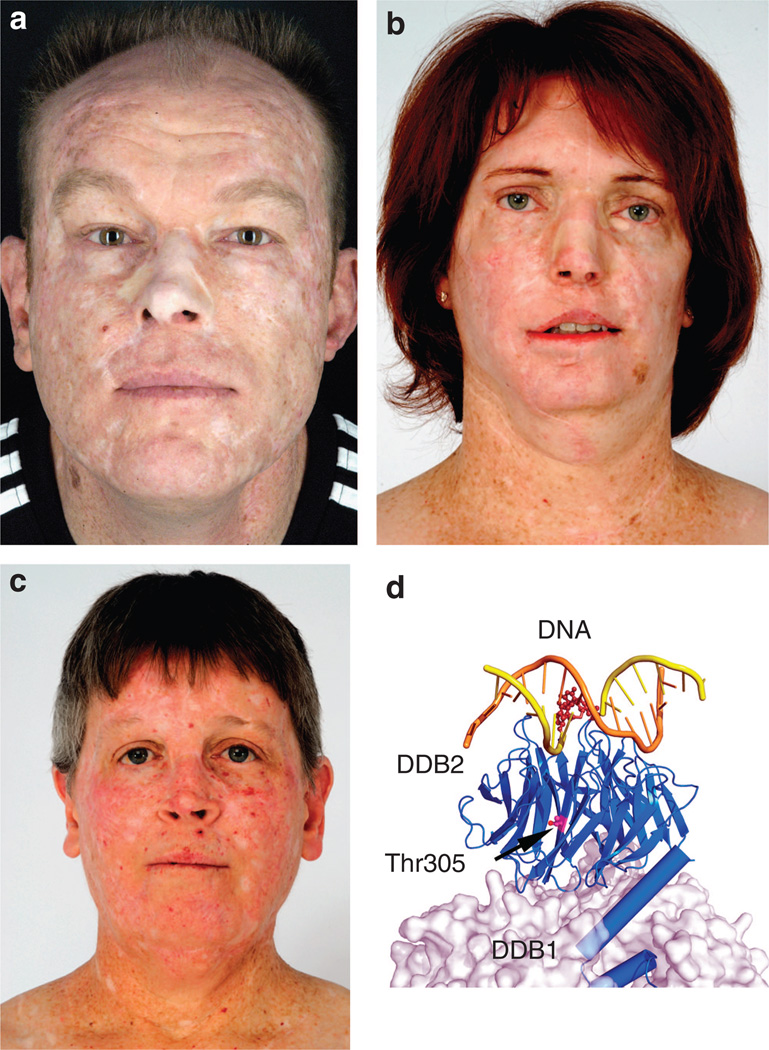
We identified four adult XP-E patients from three kindreds with large numbers of skin cancers (Table 1). Patients’ written, informed consent was obtained. The Declaration of Helsinki guidelines were followed and all necessary institutional approvals were obtained. Patient XP1GO, 45 years old, in family A from Germany never experienced a blistering sunburn (Figure 1a). Diagnosed with XP at age 22, he works as a train conductor. His first tumor was removed at age 12. He had >400 basal cell carcinomas (BCCs) and squamous cell carcinoma (SCCs) and 6 melanomas treated by age 30, and now he develops ~20 skin cancers per year. He has no neurological abnormalities. Patient XP37BE is a 45-year-old Caucasian female of Dutch ancestry in family B living in the western United States (Figure 1b). She denies ever having a blistering sunburn. She developed a keratoacanthoma on her face at 7 years and was diagnosed with XP. XP37BE has had >300 BCC and SCC skin cancers but no melanomas. She has no neurological abnormalities. Patient XP66BE is a 43-year-old brother of XP37BE. He was diagnosed with XP at age 4 at the same time his older sister was diagnosed and exhibits similar clinical symptoms, yet, milder because of improved sun protection. Patient XP408BE is a 53-year-old Caucasian female in family C from the eastern United States (Figure 1c). She had no sunburns and tanned easily, but did experience significant photophobia. At age 14, she was found to have multiple skin cancers (BCCs and SCCs) on her face and a diagnosis of XP was made. She has no XP neurological abnormalities.
Figure 1. Clinical features and DDB2 crystal structure.
(a) XP1GO in family A is a 45-year-old German train conductor with a history of >400 skin cancers including basal cell carcinomas (BCCs), squamous-cell carcinomas (SCCs), and melanomas. His face shows multiple surgical scars and grafts. (b) XP37BE in family B is a 45-year-old woman with a history of >300 non-melanoma skin cancers. She had removal of part of her jaw from SCC. Her face shows multiple surgical scars and grafts. Lentiginous hyperpigmentation is present on her neck. (c) XP408BE in family C is a 53-year-old woman with a history of >600 skin cancers including BCCs, SCCs, and melanomas. She has multiple surgical scars and telangiectasias in sun-exposed areas. (d) Crystal structure of DDB1–DDB2 complexed with a 6–4 photoproduct. DDB2, shown as a blue ribbon diagram, is stabilized by DDB1, shown as a semitransparent molecular surface. The undamaged DNA strand is shown in brown, and the damaged strand in yellow, with the 6–4 photoproduct shown in dark brown. The p.Thr305 residue (magenta and red) (arrow) lies buried within the DDB2 protein and not near the DNA or DDB1 interfaces. (Image courtesy of Dr Wei Yang, modified from Chu and Yang, 2008.) Patients gave written permission for the use of their photographs.
All cells were either established at the Human Genetic Mutant Cell Repository, the NCI Repository, or in the Department of Dermatology, Goettingen, Germany. Plasmid host cell reactivation assay was performed for cellular DNA repair capacity measurement (Emmert et al., 2000). The cells were transfected with a UV-treated plasmid containing a reporter (luciferase) gene (pCMVLuc). Compared with normal and XP variant cells, XP1GO, XP37BE, XP66BE, and XP408BE/GM01389 cells had a reduced level of luciferase expression whereas severe XP-B control cells had an even lower level (data not shown). To determine the complementation group we co-transfected the UV-irradiated pCMVLuc with plasmids that carry cloned wild-type XP complementary DNA (cDNA). Only co-transfection of the DDB2 cDNA resulted in markedly enhanced reporter gene activities (data not shown).
Human primary XP-E fibroblasts have been reported to show abnormally low or undetectable levels of p53 and its downstream-regulated proteins (Hwang et al., 1999; Itoh et al., 2003; Itoh, 2006). In agreement with this observation, the intensities of p53 and p21 bands were reduced ~60–80% and 40–60%, respectively, in untreated XP37BE, XP66BE, and XP408BE/GM01389 cells (ECL Western blotting; Amersham, Piscataway, NJ) (data not shown).
Sequence analysis (NC_000011.8 for genomic sequence, NM_000107.1 for cDNA, and NP_000098.1 for protein) revealed a, to our knowledge previously unreported, homozygous C-to-A transversion (c.914 C > A) in exon 7 in the DDB2 gene of XP1GO. This missense mutation resulted in a p.Thr305Asn substitution (Table 1). His parents and brother were heterozygous for this mutation. The restriction enzyme BtgI cuts the normal but not the mutant sequence.
XP37BE and XP66BE showed homozygous G-to-A transitions in exon 6 of DDB2. This missense mutation (c.818 G > A) resulted in p.Arg273His and was also found in their mother and father but not their unaffected brother (Table 1). This mutation inactivates a HhaI restriction site. This mutation was previously reported in XP2RO and XP3RO cells from the Netherlands (Bootsma et al., 1970; De Weerd-Kastelein et al., 1974; Kraemer et al., 1975; Nichols et al., 1996).
The cells from patient XP408BE had compound heterozygous mutations in exon 8. One allele showed a T-to-C transversion (c.1049 T > C) resulting in p.Leu350Pro, and the other allele had a three-base deletion (c.1045_1047del) resulting in p.Asn349del (Table 1). These two mutations were identical to the mutations previously reported in cell line GM01389 (Nichols et al., 2000). We measured 15 single-nucleotide polymorphisms (SNPs) in the DDB2 gene to determine the relationship between these two cell lines (XP408BE and GM01389). All 15 SNPs were identical in both cells (data not shown). CODIS DNA fingerprinting of highly polymorphic short tandem repeats (STRs) was then performed (Azari et al., 2007). All 13 CODIS core STR loci were detected and were identical in both cell lines (data not shown). Thus, the likelihood that the cells are not identical is approximately one in one billion. Indeed, the patient recalled having a skin biopsy for fibroblast culture when she was 21 years old.
Figure 1d shows the crystal structure of DDB2 stabilized by DDB1 and contacting the damaged DNA extensively (Chu and Yang, 2008; Scrima et al., 2008). The heterozygous DDB2 mutations (Leu350Pro and Asn349del) in XP408BE impair DDB1 binding (DDB1–DDB2 interface mutations). In contrast, the Arg273His mutation in XP37BE and XP66BE directly interferes with DNA binding (DNA-binding mutation). The new mutation, p.Thr305Asn in XP1GO cells, is located in the WD domain near a known Asp307Tyr mutation. This mutation has been reported to disrupt damage detection and complex formation with DDB1 (Rapic et al., 1998; Rapic-Otrin et al., 2003).
The diagnosis of XP-E can be considered in adults with freckle-like pigmentation without blistering on minimal sun exposure who have many skin cancers.
ACKNOWLEDGMENTS
This research was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, and by the Deutsche Krebshilfe and the Deutsche Forschungsge-meinschaft (to SE). We thank Dr Wei Yang, NIDDK, NIH, for discussion of the crystal structure of DDB2, the Clinical Molecular Profiling Core facility of the Genetics Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD, for performing the CODIS analysis, and the patients for their participation.
Abbreviations
- BCC
basal cell carcinoma
- cDNA
complementary DNA
- GG-NER
global genome nucleotide excision repair
- SCC
squamous cell carcinoma
- SNP
single-nucleotide polymorphism
- STR
short tandem repeat
- UV-DDB
UV-damaged DNA-binding protein
- XP-E
xeroderma pigmentosum complementation group E
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Azari S, Ahmadi N, Tehrani MJ, et al. Profiling and authentication of human cell lines using short tandem repeat (STR) loci: report from the National Cell Bank of Iran. Biologicals. 2007;35:195–202. doi: 10.1016/j.biologicals.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Bootsma D, Mulder MP, Cohen JA, et al. Different inherited levels of DNA repair replication in xeroderma pigmentosum cell strains after exposure to ultraviolet irradiation. Mutat Res. 1970;9:507–516. doi: 10.1016/0027-5107(70)90035-7. [DOI] [PubMed] [Google Scholar]
- Chu G, Yang W. Here comes the sun: recognition of UV-damaged DNA. Cell. 2008;135:1172–1174. doi: 10.1016/j.cell.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weerd-Kastelein EA, Keijzer W, Bootsma D. A third complementation group in xeroderma pigmentosum. Mutat Res. 1974;22:87–91. doi: 10.1016/0027-5107(74)90013-x. [DOI] [PubMed] [Google Scholar]
- Emmert S, Kobayashi N, Khan SG, et al. The xeroderma pigmentosum group C gene leads to selective repair of cyclobutane pyrimidine dimers rather than 6-4 photoproducts. Proc. Natl Acad Sci USA. 2000;97:2151–2156. doi: 10.1073/pnas.040559697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BJ, Ford JM, Hanawalt PC, et al. Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc Natl Acad Sci USA. 1999;96:424–428. doi: 10.1073/pnas.96.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T. Xeroderma pigmentosum group E and DDB2, a smaller subunit of damage-specific DNA binding protein: proposed classification of xeroderma pigmentosum, Cockayne syndrome, and ultraviolet-sensitive syndrome. J Dermatol Sci. 2006;41:87–96. doi: 10.1016/j.jdermsci.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Itoh T, Linn S, Ono T, et al. Reinvestigation of the classification of five cell strains of xeroderma pigmentosum group E with reclassification of three of them. J Invest Dermatol. 2000;114:1022–1029. doi: 10.1046/j.1523-1747.2000.00952.x. [DOI] [PubMed] [Google Scholar]
- Itoh T, Mori T, Ohkubo H, et al. A newly identified patient with clinical xeroderma pigmentosum phenotype has a non-sense mutation in the DDB2 gene and incomplete repair in (6-4) photoproducts. J Invest Dermatol. 1999;113:251–257. doi: 10.1046/j.1523-1747.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- Itoh T, O’Shea C, Linn S. Impaired regulation of tumor suppressor p53 caused by mutations in the xeroderma pigmentosum DDB2 gene: mutual regulatory interactions between p48(DDB2) and p53. Mol Cell Biol. 2003;23:7540–7553. doi: 10.1128/MCB.23.21.7540-7553.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev A, Mu D, Nichols AF, et al. Functional complementation of xeroderma pigmentosum complementation group E by replication protein A in an in vitro system. Proc Natl Acad Sci USA. 1996;93:5014–5018. doi: 10.1073/pnas.93.10.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S, Eker AP, Brody T, et al. Correction of the DNA repair defect in xeroderma pigmentosum group E by injection of a DNA damage-binding protein. Proc Natl Acad Sci USA. 1994;91:4053–4056. doi: 10.1073/pnas.91.9.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer KH, De Weerd-Kastelein EA, Robbins JH, et al. Five complementation groups in xeroderma pigmentosum. Mutat Res. 1975;33:327–340. doi: 10.1016/0027-5107(75)90208-0. [DOI] [PubMed] [Google Scholar]
- Nichols AF, Itoh T, Graham JA, et al. Human damage-specific DNA-binding protein p48. Characterization of XPE mutations and regulation following UV irradiation. J Biol Chem. 2000;275:21422–21428. doi: 10.1074/jbc.M000960200. [DOI] [PubMed] [Google Scholar]
- Nichols AF, Ong P, Linn S. Mutations specific to the xeroderma pigmentosum group E Ddb-phenotype. J Biol Chem. 1996;271:24317–24320. doi: 10.1074/jbc.271.40.24317. [DOI] [PubMed] [Google Scholar]
- Rapic OV, Kuraoka I, Nardo T, et al. Relationship of the xeroderma pigmentosum group E DNA repair defect to the chromatin and DNA binding proteins UV-DDB and replication protein A. Mol Cell Biol. 1998;18:3182–3190. doi: 10.1128/mcb.18.6.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapic-Otrin V, Navazza V, Nardo T, et al. True XP group E patients have a defective UV-damaged DNA binding protein complex and mutations in DDB2 which reveal the functional domains of its p48 product. Hum Mol Genet. 2003;12:1507–1522. doi: 10.1093/hmg/ddg174. [DOI] [PubMed] [Google Scholar]
- Scrima A, Konickova R, Czyzewski BK, et al. Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell. 2008;135:1213–1223. doi: 10.1016/j.cell.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa K. Regulation of damage recognition in mammalian global genomic nucleotide excision repair. Mutat Res. 2010;685:29–37. doi: 10.1016/j.mrfmmm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Tang J, Chu G. Xeroderma pigmentosum complementation group E and UV-damaged DNA-binding protein. DNA Repair (Amst) 2002;1:601–616. doi: 10.1016/s1568-7864(02)00052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]