Abstract
Accumulated evidence suggests that nicotine induces analgesia, and endogenous pain regulatory mechanisms may be altered by chronic smoking. The extent to which individual differences in pain perception are related to smokers' ability to abstain from smoking has not been directly examined. Seventy-one smokers who were interested in quitting completed a pre-cessation laboratory session which included the cold pressor test (CPT). Pain ratings were collected during and after CPT. Also, mood changes, cardiovascular measures, and salivary cortisol samples were evaluated prior to, during, and after CPT. Participants attended 4 weekly follow-up assessment sessions after their quit day. Cox regression analysis revealed that higher pain ratings during and after CPT predicted greater risk for smoking relapse. These results remained significant after affective and physiological responses to CPT were controlled, suggesting that pain ratings prior to smoking cessation is potentially useful in identifying smokers who are at greater risk of early smoking relapse and may reflect underlying putative risk for nicotine dependence and relapse.
Keywords: pain, smoking, relapse, cortisol, cold pressor test
Introduction
Nicotine produces hypoalgesia (Kanarek & Carrington, 2004; Lane et al., 1995; Rau et al., 1993). Smokers may become vulnerable to physical discomfort during acute smoking withdrawal when nicotine effects are diminished. One recent study found that smokers who completed the cold pressor task reported increased smoking urges and showed shortened latency to smoke after the task (Ditre & Brandon, 2008). Also, individual differences in distress tolerance are related to risk for relapse (Brandon et al., 2003; Brown et al., 2009). Smokers who are sensitive to pain prior to a cessation attempt may become particularly responsive to distress-related symptoms during abstinence and vulnerable to early smoking relapse. However, whether pre-cessation pain ratings are associated with enhanced risk for smoking relapse has not been directly examined.
While experiments on rats suggest that nicotine’s antinociceptive effect is subject to tolerance (McCallum et al., 1999; Wewers et al., 1999), other studies indicate that withdrawal is associated with rapid reversal of this tolerance (Anderson et al., 2004; Mousa et al., 1988; Pomerleau et al., 1984; Silverstein, 1982). In one study, pain-attenuating effects of smoking a cigarette were greater in smokers deprived from smoking overnight (12 hours) relative to those who smoked ad libitum (Pauli et al., 1993). These results are consistent with the increased discomfort smokers experience during the early stages of abstinence (Drobes et al., 1994; Pomerleau, 1992). Since enhanced withdrawal symptoms are associated with early smoking relapse (al'Absi et al., 2004), absence of nicotine-related analgesia may represent a risk factor contributing to early smoking relapse.
Although nicotine’s analgesic effect is a fairly consistent finding in animals and humans, some exceptions do exist (Knott, 1990; Nesbitt, 1973; Silverstein, 1982; Sult & Moss, 1986). These inconsistent findings may have resulted from methodological differences such as smoking selection criteria, length of abstinence, and pain assessment procedures. For instance, pain reports obtained during the actual exposure to a noxious stimulus yield results different from reports obtained afterward, and subsequently group differences may depend on whether pain reports were obtained during or after exposure to the noxious stimulus (al'Absi et al., 2002; France et al., 1994). Some studies suggest that retrospective reports of pain are influenced by what the individual experienced at the end of the painful task (al'Absi et al., 1996; Fredrickson & Kahneman, 1993; Khaneman et al., 1993) and how the individual coped with the pain (Burns et al., 2004; Burns et al., 2007; Cioffi & Holloway, 1993). These suggest that the timing (e.g., simultaneous or retrospective) of pain assessment may have different patterns of prediction for smoking relapse.
The purpose of the current study was to examine the extent to which pain perception prior to smoking cessation predicted early relapse. Also, we tested if pain ratings during and after cold pressor test (CPT) had different prediction patterns of relapse. Measures on pain perception, mood changes, heart rate, blood pressure, and cortisol were collected prior to a cessation attempt and were evaluated as predictors of relapse over a 4-week period. We hypothesized that increased pain ratings during and after CPT would be associated with increased risk for early relapse.
Methods
Participants
Smokers were recruited through newspaper advertisements in the community and flyers in the university. Interested participants completed a phone interview that consisted of questions on current history of medical or psychiatric disorders, medication intake, and smoking behavior. Participants must have smoked an average of 10 cigarettes or more per day at least for 2 years, and a strong desire for quitting smoking (i.e., required to report the score of 4 (strong) or higher to the question "On a scale of 1 (not at all) to 5 (very strong), what is your desire to quit smoking at this time?"). Participants who passed the initial phone interview were invited to an on-site medical screening. In order to qualify for the study, participants must have been free of history of physical or psychiatric disorders, weighed within ± 30% of Metropolitan Life Insurance norms (i.e., body mass index between 18 and 30), consumed 2 or less alcohol beverages a day, and did not routinely use prescriptive medications except contraceptives. Participants then read and signed a consent form approved by the Institutional Review Board of the University of Minnesota. Participants were monetarily compensated for their participation.
Ninety-one participants (46 women and 45 men) completed the laboratory session including the pain assessment (described below) prior to their quit date of smoking. Seventy-one participants (33 women and 38 men) continued in the study and attended the post-cessation weekly sessions. Participants who completed the study and those who terminated after the laboratory session (n = 20) did not differ in age, body mass index, pain ratings during or after the cold pressor test (CPT), McGill Pain Questionnaire (MPQ; Melzack, 1975), withdrawal symptoms as assessed by Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1998; Hughes & Hatsukami, 1986), levels of nicotine dependence as measured by the Fagerström Test of Nicotine Dependence (FTND; Heatherton et al., 1991), distress, positive affect, craving, cortisol, heart rate, or blood pressure levels (Fs < 2.7, ps > .10; details of these measures are described below). The sample size (N = 71) was comparable or exceeds previous samples used in similar studies (e.g., al'Absi et al., 2003).
Apparatus and Measures
A Dinamap oscillometric monitor (Critikon, Tampa, FL) was used to measure cardiovascular responses such as heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP). We also assessed cortisol concentrations, since cortisol is an important index of stress (al'Absi et al., 1997) and cold pressor pain (al’Absi et al., 2002), and is linked to smoking relapse (al'Absi et al., 2005). For measurement of cortisol, participants were asked to provide 1–2 ml of saliva using cotton dental rolls held in their mouth until saturated, and the rolls were collected into a plastic tube (Salivette® tubes, Sarstedt, Rommelsdorf, Germany). Samples were stored in −70°C until assayed. Cortisol assays were performed in duplicate using a time-resolved fluorescence immunoassay with a cortisol-biotin conjugate as a tracer (Dressendorfer et al., 1992). This assay has a sensitivity of 0.4 nmol/l. The inter- and intra-assay coefficients of variation were less than 10 and 12%, respectively. To test the validity of self-report abstinence, MicroCO™ monitors (Micro Direct Inc., Auburn, Maine) were used for the measurement of expired carbon monoxide (CO).
CPT was conducted to assess pain perception. CPT consisted of a 1-gallon container that was filled with a ice-water slurry (0 – 1 °C). To evaluate pain intensity during and after CPT, a visual and numerical rating scale was used. This scale had a range from 0 (not at all painful) to 100 (extremely painful), with additional labels on 25 (somewhat painful), 50 (moderately painful), and 75 (very painful). An enlarged poster of this scale (50 cm length and 20 cm width) was placed in front of the participant during CPT to use while rating the pain. Participants were asked to place their dominant hand into the ice water up to the wrist and rate their pain every 15 seconds throughout 90-second exposure to CPT and 90-second recovery period. Also, a short version of the MPQ (Melzack, 1975) was used to assess participants’ global pain experience. Participants were asked to recall the most intense pain they felt during the study.
To assess withdrawal symptoms, a modified version of MNWS (Hughes & Hatsukami, 1998; Hughes & Hatsukami, 1986) was used. MNWS includes items such as irritability, anger, anxiety, difficulty concentrating, restlessness, depressed or sad mood, and hunger. Levels of withdrawal severity were calculated without using the item of craving. We changed the wording of "craving" to "desire to smoke" and analyzed this item separately in light of evidence suggesting that patterns of changes in craving are distinct from other withdrawal symptoms (Hughes & Hatsukami, 1998).
Mood states and physical symptoms were assessed by a form which has been used in previous studies (al'Absi et al., 2004; al'Absi et al., 1998). Positive affect consisted of items such as cheerfulness, contentness, calmness, controllability, and interest. Negative affect consisted of anxiety, irritability, impatience, and restlessness. Withdrawal-related physical symptoms included items of headache, sweating, tremor, stomachache, drowsiness, fatigue, and coughing. Positive affect, negative affect, and physical symptoms were included in one form with each item having a 7-point scale anchored by the end points, “Not at All” and “Very Strong.”
Forms regarding demographic information, smoking history, and levels of nicotine dependence (as assessed by FTND; Heatherton et al., 1991) and were also collected.
Procedure
Participants who were smoking at their regular pace were tested individually, with each session beginning approximately at 9:00 am. After reading and signing the consent form, a Dinamap blood pressure (BP) cuff was placed. Measures of carbon monoxide (CO) and time since participant last smoked were then obtained. Participants were allowed to smoke prior to the session but not during the entire laboratory session.
The participant was asked to sit upright with legs uncrossed and feet on the floor for 45 minutes (i.e., baseline period). At the beginning of the period, the participant was given a battery of questionnaires including demographic information, smoking history, and FTND. The participant also completed the MNWS and the form on affect and physical symptoms. Then, HR, SBP, and DBP were collected every 3 minutes. Three saliva samples were obtained over the 45 minute resting period.
After the baseline period, the participant listened to audiotaped instructions and was asked to insert his or her dominant hand into the ice water up to the wrist. During the 90-second CPT, the participant rated pain at 15-second intervals using the numerical scale (explained above). Two HR and BP readings were completed during the task (i.e., one minute after hand immersion and immediately after removing the hand from the ice water). After reporting 6 pain ratings, the participant was asked to remove his or her hand from the cold water and to rest it on a towel-covered armchair. Post-CPT pain ratings were taken at 15-second intervals for the next 90 seconds. After CPT, the participant completed the MPQ, the MNWS, the form on positive and negative affect and physical symptoms, and provided a saliva sample. During a 15-minute recovery phase, HR and BP were obtained every 3 minutes and the fifth saliva sample was collected at the end. The forms on withdrawal symptoms, affect, and physical symptoms were also completed. Participants were debriefed and scheduled for subsequent sessions.
Participants who agreed to continue the study were asked to set a quit date within 2 to 3 weeks from their laboratory session. Prior to quitting, participants attended a brief educational session focusing on the negative impact of smoking and benefits from cessation. All participants were asked to abstain from smoking for a minimum of 24 hours. Each participant then attended 4 weekly follow-up support sessions with a licensed psychologist. During the sessions, the participant provided samples of expired CO and saliva to verify abstinence, and completed questionnaires on withdrawal symptoms, affect, and smoking behavior. The participant was encouraged to reduce the amount of smoking (if the participant had relapsed) or maintain abstinence.
Data Analysis
The current study included data obtained during the precessation laboratory session and postcessation smoking behavior. For the analysis of pain ratings mean scores were obtained from 6 ratings during and after CPT, respectively. For cardiovascular measures (i.e., HR, SBP, and DBP), average scores were calculated for each period. Cortisol data were log-transformed to meet assumption of normality. Smokers were classified as relapsers if they smoked everyday for at least a week. This guideline has been recommended by Society for Nicotine and Tobacco Research and is widely used in studies of smoking cessation (Hughes et al., 2003). Smokers were identified as abstainers if they were able to maintain abstinent for 4 weeks. Abstinence was determined based on self report and biochemical verifications.
Information regarding demographics and smoking were analyzed by one-way ANOVA with smoking status (abstinent and relapsed) as a grouping variable. A series of one-way analysis of covariance (ANCOVA) was conducted to examine whether the abstinent and the relapsed groups differed in pain perception. In this analysis, gender was included as a covariate rather than an independent factor, due to the small sample size. FTND was also controlled for due to a marginally significant group difference found in the present study (described below).
Cardiovascular measures were analyzed using 2 (smoking status: abstinent and relapsed) × 4 (time: baseline, during the CPT, immediately after the CPT, recovery) repeated measures ANOVAs with smoking status as a between-subject factor and time as a within-subject factor. Similarly, cortisol data were analyzed using a 2 (smoking status: abstinent and relapsed) × 5 (time: three samples during the baseline, one sample immediately after the CPT, one sample after recovery) repeated measures ANOVA. Self-report measures (i.e., MNWS, craving, negative affect, positive affect, and physical symptoms) were analyzed using 2 (smoking status: abstinent and relapsed) × 3 (time: baseline, after the CPT, recovery) repeated measures ANOVA. Expired CO was analyzed by a 2 (smoking status: abstinent and relapsed) × 5 (session: precessation, postcessation week 1, 2, 3, 4) repeated measures ANOVA with smoking status as a between-subject factor and session as a within-subject factor. Wilk's criterion was used for these analyses and Bonferroni correction was applied for post-hoc analysis.
To examine our primary hypothesis, we conducted Cox regression analysis to examine the extent to which pain ratings predicted time to relapse during follow-up period. In the Cox regression model, B is the regression coefficients that represent the relative effect of an independent variable (e.g., pain ratings) on the survival function. Hazard ratio (HR) reflects the probability of the occurrence of a targeted event (i.e., smoking relapse) with each one-point increase in the independent variable (e.g., pain ratings). Potential confounders (i.e., gender and FTND) were entered as covariates. SPSS 17.0 was used for the data analyses. Due to occasional missing data, variations existed between sample size and degrees of freedom for the reported results.
Results
Demographic and Smoking Variables
Nineteen smokers (27%) relapsed by the first week post-quit, 30 (42%) smokers relapsed by the end of the second week, and 36 smokers (51%) returned to smoking by the 4-week follow up. As shown in Table 1, abstinent and relapsed groups did not differ in age, body mass index, education, caffeine intake, average number of hours of sleep, number of daily cigarettes, duration of smoking, previous quit attempts, or motivation to quit (ps > .24). The relapsed group had a marginally higher FTND score than the abstinent group (F (1, 69) = 3.45, p = .07).
Table 1.
Participant Characteristics. Note. Entries show mean (standard error); BMI = body mass index; FTND = Fagerström Test of Nicotine Dependence; ppm = parts per million.
| Abstinent | Relapsed | ||
|---|---|---|---|
| Age (years) | 36.7 (2.4) | 35.4 (2.4) | |
| BMI (kg/m2) | 25.1 (0.7) | 25.1 (0.7) | |
| Education (years) | 14.4 (0.5) | 14.3 (0.5) | |
| Caffeine (servings per day) | 4.7 (0.5) | 3.9 (0.5) | |
| Sleep (hours per day) | 7.2 (0.2) | 6.9 (0.2) | |
| Cigarettes (per day) | 18.5 (1.2) | 20.5 (1.2) | |
| Duration (years) | 11.8 (2.0) | 12.9 (1.9) | |
| Previous quit attempts | 5.7 (2.2) | 6.6 (2.2) | |
| Motivation to quit | 5.9 (0.2) | 6.1 (0.2) | |
| FTND | 5.1 (0.4) | 6.0 (0.3) | |
| Carbon monoxide (ppm) | |||
| Prequit baseline | 22.6 (1.7) | 24.2 (1.9) | |
| Postquit week 1 | 4.6 (1.1) | 12.0 (1.3) | |
| Postquit week 2 | 5.4 (1.2) | 13.9 (1.4) | |
| Postquit week 3 | 5.1 (1.2) | 14.6 (1.4) | |
| Postquit week 4 | 5.3 (1.2) | 16.3 (1.3) | |
There was a significant group × session interaction in expired CO (F (4, 56) = 3.64, p = .01). This was followed by conducting a one-way ANOVA at each period using Bonferroni adjustment. As expected, pre-quit baseline CO levels were comparable between abstinent and relapsed groups (p = .41), while the levels at each post-quit period were higher among the relapsers than the abstainers (Fs (1, 69) > 24, ps < .001; see Table 1). CO levels in the abstinent group did not exceed 6 ppm during follow-up assessments, suggesting compliance with the smoking abstinence restriction.
Effects of CPT
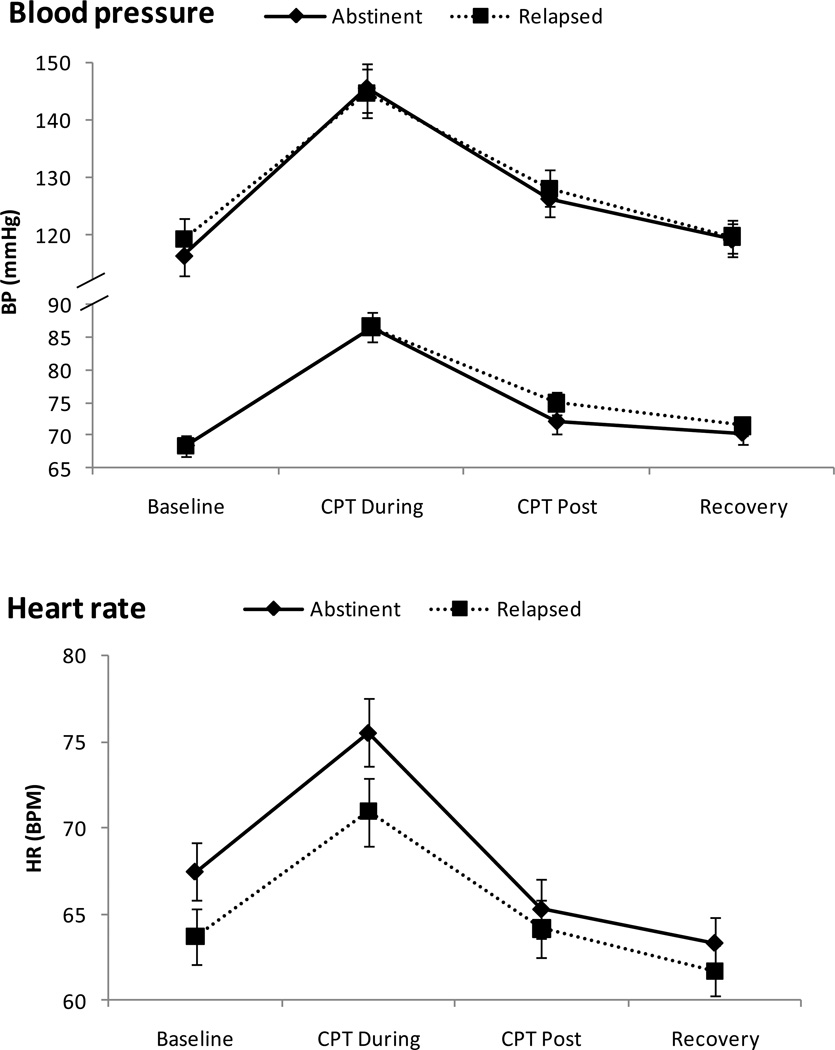
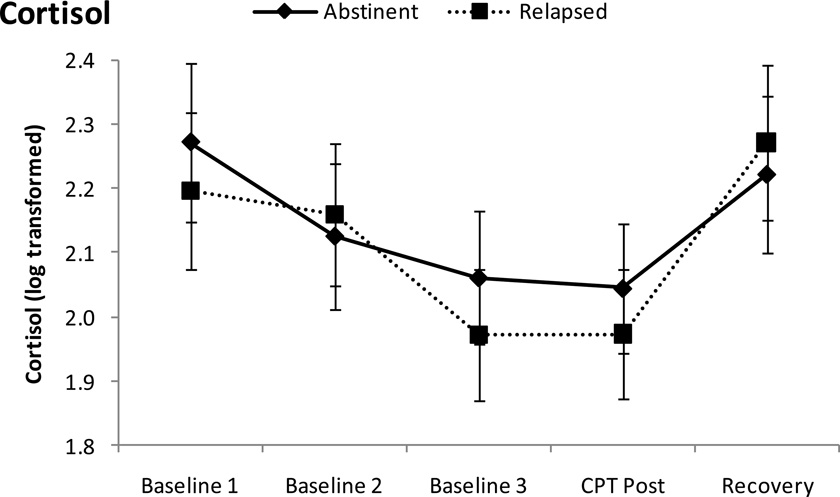
As shown in Figure 1, there were significant changes over time in HR, SBP, and DBP (Fs (3, 65) > 26, ps < .001). Multiple comparison tests revealed that levels of these measures were higher during CPT than those during baseline (ps < .001). Cortisol concentrations also changed during the session (F (4, 64) = 10.62, p < .001), showing declines during rest baseline (i.e., the third sample was lower than the first sample; p < .001) and increases following CPT (i.e., the last sample was higher than the third sample; p < .05). There were no differences in the cardiovascular measures and cortisol levels between the abstinent and the relapsed groups (ps > .18).
Figure 1.
Mean Cardiovascular and adrenocortical measures to the cold pressor test (CPT). Line bars indicate standard error of the mean. Note. BP = blood pressure, HR = heart rate, BPM = beats per minute.
Withdrawal symptoms, craving, negative affect, and positive affect also changed over time (Fs (2, 68) > 4, ps < .05; see Table 2). Multiple comparisons found that withdrawal symptoms, craving, and negative affect increased following exposure to CPT (i.e., recovery period) and positive affect decreased at this time as compared to levels immediately after CPT (ps < .05). There were no group differences in withdrawal symptoms, craving, or negative affect (ps > .26), with one exception of a trend indicating that the relapsed group reported less positive affect than the abstinent group (F (1, 69) = 3.81, p = .06).
Table 2.
Craving, State Mood, and Pain Measures. Note. Entries show mean (standard error); MNWS = Minnesota Nicotine Withdrawal Scale; CPT = cold pressor test; MPQ = McGill Pain Questionnaire.
| Period | Abstinent | Relapsed | |
|---|---|---|---|
| MNWSa | Baseline | 6.2 (0.9) | 7.4 (0.9) |
| After CPT | 6.2 (1.0) | 7.0 (1.0) | |
| Recovery | 7.0 (1.0) | 8.4 (1.0) | |
| Cravinga | Baseline | 2.3 (0.3) | 2.7 (0.3) |
| After CPT | 2.3 (0.4) | 2.9 (0.4) | |
| Recovery | 2.8 (0.3) | 3.3 (0.3) | |
| Negative Affecta | Baseline | 2.6 (0.5) | 3.1 (0.5) |
| After CPT | 2.2 (0.5) | 2.6 (0.5) | |
| Recovery | 2.5 (0.6) | 3.6 (0.6) | |
| Positive Affectab | Baseline | 22.9 (1.1) | 20.3 (1.1) |
| After CPT | 22.2 (1.2) | 18.8 (1.2) | |
| Recovery | 21.0 (1.2) | 17.8 (1.2) | |
| Physical Symptomsa | Baseline | 6.1 (0.8) | 6.4 (0.8) |
| After CPT | 6.0 (0.9) | 7.3 (0.9) | |
| Recovery | 5.1 (0.7) | 5.2 (0.7) | |
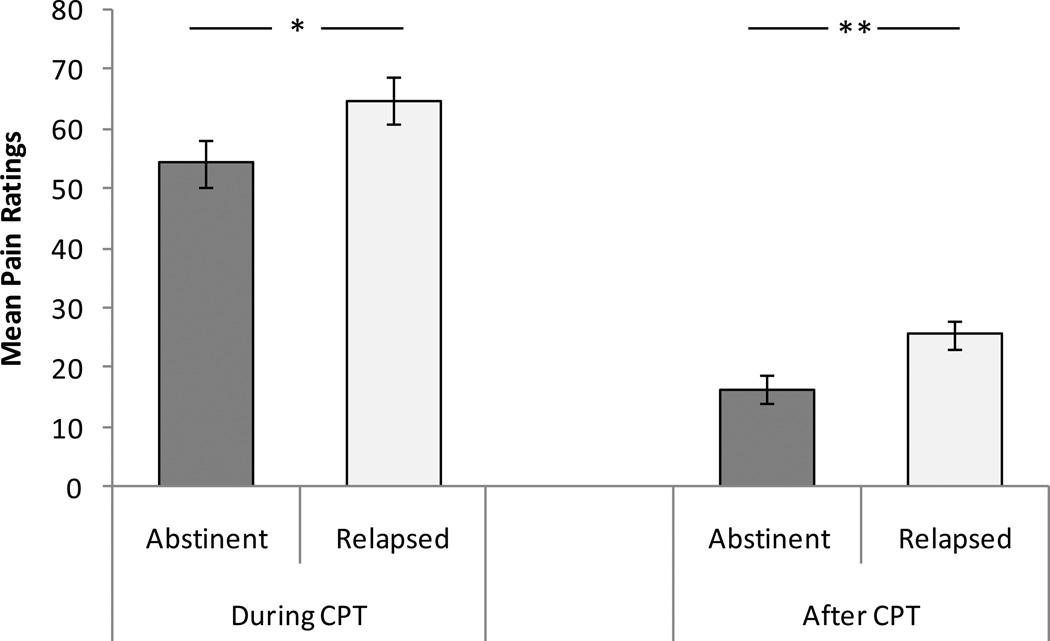
| Pain Measures | Intensity (During)* | 54.3 (3.8) | 64.9 (3.8) |
| Intensity (Post)** | 16.4 (2.4) | 25.6 (2.4) | |
| MPQ-Total | 14.7 (1.4) | 16.8 (1.4) |
Effect of time was significant at p < .05 level.
Effect of group was p = .06.
p < .05.
p < .01.
Pain Measures and Relapse
Analysis of variance covarying for FTND and gender revealed a significant difference between relapsers and successful abstainers in the pain rating after CPT, with the relapsed group reporting higher pain than the abstinent group (F (1, 67) = 8.17, p < .01; see Figure 2). Pain ratings during CPT were also higher in relapsers than abstainers, F (1, 67) = 4.21, p < .05). MPQ total score did not differ between the two groups (p > .29 ; see Table 2).
Figure 2.
Mean pain ratings during and after CPT. Line bars indicate standard error of the mean. Note. * p < .05. ** p < .01.
Separate Cox regression analysis was conducted on the pain measures to test our primary hypothesis. For each analysis, we entered FTND and gender in the first step and the pain measure (i.e., during, after CPT, or MPQ) in the second step (see Table 3). In the analysis examining the effects of pain ratings (during or after CPT), the overall model for the first step was significant (χ2 = 5.96, p < .05). FTND was predictive of time to relapse (B = .20, HR = 1.23, p < .05). In the second step, the addition of pain ratings significantly improved the overall model (χ2 > 4.50, ps < .05). Pain ratings during (B = .02, HR = 1.02, p < .05) and after (B = .03, HR = 1.03, p < .05) CPT predicted smoking relapse after controlling for FTND and gender. These results indicate that a one-point increase in the pain rating during CPT raised the risk of smoking relapse by 2%, and a unit increase in the post-CPT rating elevated the probability by 3%. Although one-point increase in FTND score was associated with 23% increase in the likelihood of smoking relapse, it should be noted that the range of scores was different between the pain rating (0 to 100) and FTND (0 to 10). Scores on MPQ did not predict relapse (p > .25).
Table 3.
Results of Cox regression models examining the association between pain measures and smoking relapse. The omnibus model of the second step significantly improved when pain ratings (during or after) CPT was added to the first model including gender and nicotine dependence (χ2 > 4.5, ps < .05). In addition, the pain ratings (during or after) predicted smoking relapse after controlling for other variables (ps < .05). Note. χ2 = chi-square test for the overall model; Δ χ2 = chi-square test for the change from the previous step; B = regression coefficients; SE = standard error; HR = hazard ratio; CI = confidence interval; CPT = cold pressor task; FTND = Fagerström Test of Nicotine Dependence; MPQ = McGill Pain Questionnaire.
| χ2 | Δ χ2 | B | SE | HR | 95% CI | ||
|---|---|---|---|---|---|---|---|
|
Pain ratings (during CPT) |
|||||||
| Step 1 | 5.96* | ---- | |||||
| Gender | −.03 | .34 | 0.97 | 0.50 – 1.88 | |||
| FTND | .20 | .08 | 1.23* | 1.05 – 1.44 | |||
| Step 2 | 10.49* | 4.54* | |||||
| Pain | .02 | .01 | 1.02* | 1.001 – 1.03 | |||
|
Pain ratings (after CPT) |
|||||||
| Step 1 | 5.96* | ---- | |||||
| Gender | −.03 | .34 | 0.97 | 0.50 – 1.88 | |||
| FTND | .20 | .08 | 1.23* | 1.05 – 1.44 | |||
| Step 2 | 11.97** | 6.18* | |||||
| Pain | .03 | .01 | 1.03* | 1.01 – 1.05 | |||
| MPQ | |||||||
| Step 1 | 5.29 | ---- | |||||
| Gender | .07 | .34 | 1.08 | 0.55 – 2.10 | |||
| FTND | .20 | .08 | 1.22* | 1.04 – 1.43 | |||
| Step 2 | 6.78 | 1.31 | |||||
| MPQ | .02 | .02 | 1.02 | 0.98 – 1.07 | |||
p < .05.
p < .01.
To further explore the data, we also conducted stepwise Cox regression model to examine the extent to which CPT-related variables (i.e., pain ratings, withdrawal symptoms, craving, positive and negative affect, physical symptoms, and cardiovascular and hormonal measures) were predictive of time to relapse. Separate analysis was conducted on pain ratings collected during and after CPT. FTND and gender were also included in the analysis. In both models, pain ratings and FTND were the only significant predictors of relapse (ps < .05).
Discussion
The results of the current study clearly demonstrated that pain ratings during and after CPT prior to cessation of smoking were associated with early smoking relapse. The findings also support the hypothesis that individuals who are low in distress tolerance are at risk for early relapse (Brandon et al., 2003; Brown et al., 2009). In addition, the results of our study extend previous findings (Ditre & Brandon, 2008) by examining individual differences in pain perception prior to smoking cessation, and in that context using pain measures as predictors of early smoking relapse.
Our findings that pain ratings prior to smoking cessation was predictive of relapse after statistically controlling for nicotine dependence, smoking withdrawal, craving, cardiovascular, and adrenocortical measures suggest that the link between pain perception and smoking relapse may not be mediated by these CPT-related response measures. In other words, the findings more specifically reflect that enhanced pain ratings during and after CPT are a unique predictor of increased risk for subsequent relapse, suggesting that pain perception and withdrawal tolerance may have different mechanisms or pathways to smoking relapse. This is partially supported by evidence suggesting specific neurochemical modulation of endogenous pain mechanisms by chronic administration of nicotine (Kanarek & Carrington, 2004; Lane et al., 1995; Rau et al., 1993).
Studies have reported that chronic exposure to drugs, including nicotine, is associated with beta endorphin deficiency (Girdler et al., 2005; Scanlon et al., 1992) that persists for several weeks following withdrawal (Rasmussen, 1998; Rasmussen et al., 2000). Also, mice that were chronically treated with nicotine exhibited reduced analgesic effects to morphine (Zarrindast et al., 1999), suggesting that tolerance may develop in the endogenous opioid system due to chronic use of nicotine. Since pain is regulated by endogenous opioid system, it is possible that habitual smoking desensitizes opioid-pain modulating functions that may influence severity of pain and withdrawal symptoms during abstinence. This hypothesis is consistent with findings that nicotine's analgesic effect is subject to tolerance (McCallum et al., 1999; Wewers et al., 1999) but withdrawal is related to rapid reversal of this tolerance (Anderson et al., 2004; Mousa et al., 1988; Pomerleau et al., 1984; Silverstein, 1982). Therefore, the relationship between pain perception and smoking relapse may be mediated by nicotine-induced neuroplasticity. However, more research is warranted to define such model.
In the current study, relapsed smokers reported higher pain during and after CPT than abstinent smokers. This association was clearer with the post-CPT ratings, suggesting that delayed, rather than simultaneous, assessment of pain may enhance sensitivity of these measures in identifying individual differences in pain perception. Studies have suggested that certain coping strategies (e.g., suppression, acceptance) to experimental pain influences one’s appraisal of subsequent pain experience (Cioffi & Holloway, 1993; Masedo & Rosa, 2007). Although effects of coping style were not specifically assessed, extensive research has been published on coping with withdrawal symptoms during abstinence among smokers (Drobes et al., 1994; Gulliver et al., 1995; O'Connell et al., 2007; Shiffman, 1984). Poor coping with physical discomfort and absence of nicotine effects during abstinence may exacerbate withdrawal symptoms that would increase the likelihood of early relapse (Hajek et al., 2010; Schmitz et al., 1993).
In this study CPT was associated with increased cardiovascular and HPA activity, however, responses to CPT were comparable between abstinent and relapsed participants. The results seem to be inconsistent with the growing literature showing that attenuated responses to stress may contribute to enhanced intensity of withdrawal symptoms and craving, and risk for relapse (al'Absi et al., 2005; Shaw & al'Absi, 2008). It should be noted, however, that whereas these studies examined stress responses in smokers who were abstinent at least for 24 hours, participants in this study were smoking at their own pace until they reported to our laboratory. Nicotine stimulates secretion of various neurotransmitters in the brain (Benowitz, 2010) that would produce stress-like symptoms (e.g., activation of HPA axis; Pickworth & Fant, 1998; Pomerleau & Pomerleau, 1991). Thus, it is possible that when smokers are smoking ad lib, alterations in the central system due to habitual smoking may be masked by neurochemical effects of nicotine. Therefore, influences of nicotine on cardiovascular and adrenocortical systems that account for smoking relapse may not appear until after quitting smoking (al'Absi et al., 2004; al'Absi et al., 2005). The association between pain perception prior to a cessation attempt and smoking relapse may be mediated by neurobiological system other than cardiovascular or HPA activity such as endogenous opioid system.
Also in the present study, abstinent and relapsed participants differed in pain perception even though two groups exhibited comparable cardiovascular and hormonal reactivity patterns in response to CPT. To further investigate the mechanisms of nicotine-related analgesia and smoking and relapse, future study should include stress-induced analgesia (SIA) because evidence suggests that SIA is mediated by endogenous systems such as opioid functions, baroreceptor reflex, noradrenergic activity, and HPA axis (Bruehl & Chung, 2004; Butler & Finn, 2009; Dworkin et al., 1994; Girdler et al., 2005). This is particularly important in light of findings that smokers who exhibited attenuated beta endorphin (Shaw & al'Absi, 2008) and HPA measures (al'Absi et al., 2005; Frederick et al., 1998; Ussher et al., 2006) during abstinence were at high risk of early relapse. The extent to which nicotine-related central and peripheral modulation influences smokers' pain perception and withdrawal symptoms before and after smoking cessation may help better understand psychobiological mechanisms of smoking and relapse.
The findings of the current study are limited by a number of factors. First, there was no non-smoking control group. Inclusion of this group could have helped in better interpret the non-significant cardiovascular and adrenocortical results observed in the study. However, because the primary purpose was to examine whether pain perceptions in smokers predicted relapse, we believe that the lack of a control group did not seriously limit the impact of the findings. Second, other biomarkers such as beta endorphin should be included to clarify the specific mechanism of pain sensitivity, withdrawal symptoms, and smoking relapse. Third, our sample was not large enough to carefully examine sex differences. Since sex differences in pain regulatory mechanisms may be altered by chronic smoking (Girdler et al., 2005), moderating effects of sex on pain regulation and relapse needs to be tested. Lastly, perceived effectiveness of brief support sessions might have influenced the outcome of smoking cessation. Future study should examine this possibility. Nonetheless, this study used a comprehensive pain assessment method, collected both physiological, hormonal, and self-report measures, carefully assessed smoking status over an extended follow-up period, and prospectively assessed predictors of smoking relapse.
In conclusion, the current study found that pain ratings during and after CPT prior to cessation of smoking predicted early smoking relapse. The findings suggest that pain perception is a potentially useful marker in identifying smokers who are at greater risk of early smoking relapse. Future research should investigate mechanisms mediating the relationship between pain perception and smoking relapse including the role of endogenous opioid dysregulation.
Research Highlights.
We tested whether pain ratings prior to smoking cessation predicted early relapse.
Pain ratings were related to increased risk for early smoking relapse.
The finding remained significant after controlling for stress-related variables.
Pain perception may be helpful in identifying smokers at risk for early relapse.
Acknowledgments
We thank Koji Fujiwara for assistance with data analysis. This research was supported in part by a grand to Dr. al’Absi from the National Cancer Institute (CA 88272). Dr. al’Absi was also supported by grants from the National Institute on Drug Abuse (DA013435 and DA 016351).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest regarding this manuscript.
References
- al'Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Buchanan T, Lovallo WR. Pain perception and cardiovascular responses in men with positive parental history for hypertension. Psychophysiology. 1996;33:655–661. doi: 10.1111/j.1469-8986.1996.tb02361.x. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug and Alcohol Dependence. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Lovallo WR, McKey B, Sung BH, Whitsett TL, Wilson MF. Hypothalamic-pituitary-adrenocortical responses to psychological stress and caffeine in men at high and low risk for hypertension. Psychosomatic Medicine. 1998;60:521–527. doi: 10.1097/00006842-199807000-00021. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology Biochemistry and Behavior. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Anderson KL, Pinkerton KE, Uyeminami D, Simons CT, Carstens MI, Carstens E. Antinociception induced by chronic exposure of rats to cigarette smoke. Neuroscience Letters. 2004;366:86–91. doi: 10.1016/j.neulet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. New England Journal of Medicine. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. Journal of Abnormal Psychology. 2003;112:448–456. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, et al. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine and Tobacco Research. 2009;11:493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neuroscience & Biobehavioral Reviews. 2004;28:395–414. doi: 10.1016/j.neubiorev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Burns JW, Bruehl S, Caceres C. Anger management style, blood pressure reactivity, and acute pain sensitivity: evidence for "Trait × Situation" models. Annals of Behavioral Medicine. 2004;27:195–204. doi: 10.1207/s15324796abm2703_7. [DOI] [PubMed] [Google Scholar]
- Burns JW, Quartana PJ, Bruehl S. Anger management style moderates effects of emotion suppression during initial stress on pain and cardiovascular responses during subsequent pain-induction. Annals of Behavioral Medicine. 2007;34:154–165. doi: 10.1007/BF02872670. [DOI] [PubMed] [Google Scholar]
- Butler RK, Finn DP. Stress-induced analgesia. Progress in Neurobiology. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Cioffi D, Holloway J. Delayed costs of suppressed pain. Journal of Personality and Social Psychology. 1993;64:274–282. doi: 10.1037//0022-3514.64.2.274. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH. Pain as a motivator of smoking: effects of pain induction on smoking urge and behavior. Journal of Abnormal Psychology. 2008;117:467–472. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Meier EA, Tiffany ST. Assessment of the effects of urges and negative affect on smokers' coping skills. Behaviour Research and Therapy. 1994;32:165–174. doi: 10.1016/0005-7967(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Dworkin BR, Elbert T, Rau H, Birbaumer N, Pauli P, Droste C, et al. Central effects of baroreceptor activation in humans: attenuation of skeletal reflexes and pain perception. Proceedings of National Academy of Science of the United States of America. 1994;91:6329–6333. doi: 10.1073/pnas.91.14.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France C, Adler P, France J, Ditto B. Family history of hypertension and pain during blood donation. Psychosomatic Medicine. 1994;56:52–60. doi: 10.1097/00006842-199401000-00007. [DOI] [PubMed] [Google Scholar]
- Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biological Psychiatry. 1998;43:525–530. doi: 10.1016/S0006-3223(97)00423-X. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Kahneman D. Duration Neglect in Retrospective Evaluations of Affective Episodes. Journal of Personality and Social Psychology. 1993;65:45–55. doi: 10.1037//0022-3514.65.1.45. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Gulliver SB, Hughes JR, Solomon LJ, Dey AN. An investigation of self-efficacy, partner support and daily stresses as predictors of relapse to smoking in self-quitters. Addiction. 1995;90:767–772. doi: 10.1046/j.1360-0443.1995.9067673.x. [DOI] [PubMed] [Google Scholar]
- Hajek P, Taylor T, McRobbie H. The effect of stopping smoking on perceived stress levels. Addiction. 2010;105:1466–1471. doi: 10.1111/j.1360-0443.2010.02979.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Kanarek RB, Carrington C. Sucrose consumption enhances the analgesic effects of cigarette smoking in male and female smokers. Psychopharmacology (Berl) 2004;173:57–63. doi: 10.1007/s00213-003-1699-0. [DOI] [PubMed] [Google Scholar]
- Khaneman D, Fredrickson BL, Schreiber CA, Redelmeier DA. When More Pain is Preferred to Less: Adding a Better End. American Psychological Society. 1993;4:401–405. [Google Scholar]
- Knott VJ. Effects of cigarette smoking on subjective and brain evoked responses to electrical pain stimulation. Pharmacology Biochemistry and Behavior. 1990;35:341–346. doi: 10.1016/0091-3057(90)90166-f. [DOI] [PubMed] [Google Scholar]
- Lane JD, Lefebvre JC, Rose JE, Keefe FJ. Effects of cigarette smoking on perception of thermal pain. Experimental & Clinical Psychopharmacology. 1995;3:140–147. [Google Scholar]
- Masedo AI, Rosa EM. Effects of suppression, acceptance and spontaneous coping on pain tolerance, pain intensity and distress. Behaviour Research and Therapy. 2007;45:199–209. doi: 10.1016/j.brat.2006.02.006. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Caggiula AR, Epstein LH, Saylor S, Ploskina T, Sved AF. Mecamylamine blocks the development of tolerance to nicotine in rats: implications for the mechanisms of tolerance. Psychopharmacology (Berl) 1999;141:332–338. doi: 10.1007/s002130050842. [DOI] [PubMed] [Google Scholar]
- Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Aloyo VJ, Van Loon GR. Tolerance to tobacco smoke- and nicotine-induced analgesia in rats. Pharmacology Biochemistry and Behavior. 1988;31:265–268. doi: 10.1016/0091-3057(88)90344-9. [DOI] [PubMed] [Google Scholar]
- Nesbitt PD. Smoking, physiological arousal, and emotional response. Journal of Personality and Social Psychology. 1973;25:137–144. doi: 10.1037/h0034256. [DOI] [PubMed] [Google Scholar]
- O'Connell KA, Hosein VL, Schwartz JE, Leibowitz RQ. How does coping help people resist lapses during smoking cessation? Health Psychology. 2007;26:77–84. doi: 10.1037/0278-6133.26.1.77. [DOI] [PubMed] [Google Scholar]
- Pauli P, Rau H, Zhuang P, Brody S, Birbaumer N. Effects of smoking on thermal pain threshold in deprived and minimally-deprived habitual smokers. Psychopharmacology. 1993;111:472–476. doi: 10.1007/BF02253538. [DOI] [PubMed] [Google Scholar]
- Pickworth W, Fant R. Endocrine effects of nicotine administration, tobacco and other drug withdrawal in humans. Psychoneuroendocrinology. 1998;23:131–141. doi: 10.1016/s0306-4530(97)00075-9. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Nicotine and the central nervous system: biobehavioral effects of cigarette smoking. American Journal of Medicine. 1992;93:2S–7S. doi: 10.1016/0002-9343(92)90619-m. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Research on stress and smoking: progress and problems. British Journal of Addiction. 1991;86:599–603. doi: 10.1111/j.1360-0443.1991.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Turk DC, Fertig JB. The effects of cigarette smoking on pain and anxiety. Addictive Behaviors. 1984;9:265–271. doi: 10.1016/0306-4603(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen D. Effects of chronic nicotine treatment and withdrawal on hypothalamic proopiomelandocrine gene expression and neuroendocrine regulation. Psychoneuroendocrinology. 1998;23:245–259. doi: 10.1016/s0306-4530(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal-1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcoholism: Clinical and Experimental Research. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Rau H, Schweizer R, Zhuang P, Pauli P, Brody S, Larbig W, et al. Cigarette smoking, blood lipids, and baroreceptor-modulated nociception. Psychopharmacology. 1993;110:337–341. doi: 10.1007/BF02251290. [DOI] [PubMed] [Google Scholar]
- Scanlon MN, Lazar-Wesley E, Grant KA, Kunos G. Proopiomelanocortin messenger RNA is decreased in the mediobasal hypothalamus of rats made dependent on ethanol. Alcoholism: Clinical and Experimental Research. 1992;16:1147–1151. doi: 10.1111/j.1530-0277.1992.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Rosenfarb IS, Payne TJ. Cognitive and affective responses to successful coping during smoking cessation. Journal of Substance Abuse. 1993;5:61–72. doi: 10.1016/0899-3289(93)90123-s. [DOI] [PubMed] [Google Scholar]
- Shaw D, al'Absi M. Attenuated beta endorphin response to acute stress is associated with smoking relapse. Pharmacology Biochemistry and Behavior. 2008;90:357–362. doi: 10.1016/j.pbb.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Coping with temptations to smoke. Journal of Consulting and Clinical Psychology. 1984;52:261–267. doi: 10.1037//0022-006x.52.2.261. [DOI] [PubMed] [Google Scholar]
- Silverstein B. Cigarette smoking, nicotine addiction, and relaxation. Journal of Personality and Social Psychology. 1982;42:946–950. doi: 10.1037//0022-3514.42.5.946. [DOI] [PubMed] [Google Scholar]
- Sult SC, Moss RA. The effects of cigarette smoking on the perception of electrical stimulation and cold pressor pain. Addictive Behaviors. 1986;11:447–451. doi: 10.1016/0306-4603(86)90026-2. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, Evans P, Steptoe A, McEwen A, Clow A, et al. Reduction in cortisol after smoking cessation among users of nicotine patches. Psychosomatic Medicine. 2006;68:299–306. doi: 10.1097/01.psy.0000204926.27215.a1. [DOI] [PubMed] [Google Scholar]
- Wewers ME, Dhatt RK, Snively TA, Tejwani GA. The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Research. 1999;822:107–113. doi: 10.1016/s0006-8993(99)01095-1. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Khoshayand MR, Shafaghi B. The development of cross-tolerance between morphine and nicotine in mice. European Neuropsychopharmacology. 1999;9:227–233. doi: 10.1016/s0924-977x(98)00030-3. [DOI] [PubMed] [Google Scholar]