Abstract
Myocardial cell death is initiated by excessive mitochondrial Ca2+ entry, causing Ca2+ overload, mitochondrial permeability transition pore (mPTP) opening and dissipation of the mitochondrial inner membrane potential (ΔΨm)1,2. However, the signaling pathways that control mitochondrial Ca2+ entry through the inner membrane mitochondrial Ca2+ uniporter (MCU)3–5 are not known. The multifunctional Ca2+ and calmodulin-dependent protein kinase II (CaMKII) is activated in ischemia reperfusion (I/R), myocardial infarction (MI) and neurohumoral injury, common causes of myocardial death and heart failure, suggesting CaMKII could couple disease stress to mitochondrial injury. Here we show that CaMKII promotes mPTP opening and myocardial death by increasing MCU current (IMCU). Mitochondrial-targeted CaMKII inhibitory protein or cyclosporin A (CsA), an mPTP antagonist with clinical efficacy in I/R injury6, equivalently prevent mPTP opening, ΔΨm deterioration and diminish mitochondrial disruption and programmed cell death in response to I/R injury. Mice with myocardial and mitochondrial-targeted CaMKII inhibition are resistant to I/R injury, MI and neurohumoral injury, suggesting pathological actions of CaMKII are substantially mediated by increasing IMCU. Our findings identify CaMKII activity as a central mechanism for mitochondrial Ca2+ entry and suggest mitochondrial-targeted CaMKII inhibition could prevent or reduce myocardial death and heart failure dysfunction in response to common experimental forms of pathophysiological stress.
Excessive activation of the multifunctional Ca2+ and calmodulin-dependent protein kinase II (CaMKII) by Ca2+ triggers myocardial death and heart failure7,8, while excessive CaMKII activity promotes multiple defects in myocardial Ca2+ homeostasis, including increased mitochondrial Ca2+ 9,10. CaMKII inhibition is protective against I/R, MI and neurohumoral toxicity, clinically-relevant forms of myocardial injury marked by disturbed intracellular Ca2+ homeostasis7,8,11, but the mechanisms for myocardial protection by CaMKII inhibition are uncertain. Excessive increases in mitochondrial Ca2+ lead to mitochondrial permeability transition pore (mPTP) opening and dissipation of the mitochondrial inner membrane potential (ΔΨm)1,2. We first asked if excessive activation of mitochondrial CaMKII could be a mechanism for myocardial dysfunction or death during I/R injury, because I/R injury occurs in the setting of increased mitochondrial Ca2+ and because I/R injury is alleviated by Ru36012,13, a selective inhibitor of the mitochondrial uniporter (MCU) current (IMCU) in rats12, and by CsA, an inhibitor of mPTP, in patients6. Here we show that mitochondrial-targeted CaMKII inhibition or treatment with CsA, an mPTP antagonist with clinical efficacy in I/R injury6, are both protective against mPTP opening, loss of ΔΨm, mitochondrial disruption and programmed cell death in response to I/R, MI or isoproterenol. The myocardial protective effects of mitochondrial CaMKII inhibition are multivalent and involve increasing mPTP Ca2+ tolerance and reduction in IMCU.
In order to test if CaMKII catalytic activity was the mechanism for Ca2+ to affect downstream responses to I/R injury, we developed mice with myocardial-delimited CaMKII inhibition by transgenic expression of a membrane-targeted CaMKII inhibitor, CaMKIIN, the most potent and specific CaMKII inhibitory protein14. We engineered CaMKIIN with a palmitoylation sequence to enhance partitioning into intracellular membranes. We identified CaMKIIN expression in isolated mitochondria of transgenic mice (Supplemental Fig. 1a,b). To determine if CaMKIIN transgenic mice were resistant to I/R injury by a Ca2+-regulated pathway we used isolated, perfused, working mouse hearts to directly measure myocardial mechanical responses to I/R injury under conditions designed to restrict glycolytic metabolism. WT hearts or hearts with transgenic CaMKIIN expression were perfused with a pyruvate-containing solution (at non-physiological levels) without glucose, so that ATP production relied on oxidative metabolism, and CsA, to prevent mPTP opening, or vehicle (Supplemental Fig. 2a–c). Left ventricular developed pressure (Supplemental Fig. 2b) and the first derivative of left ventricular developed pressure (Supplemental Fig. 3a) were reduced in WT vehicle-treated hearts after I/R injury, but were preserved after I/R injury in hearts with CaMKII inhibition or in WT hearts treated with CsA. Baseline recordings were similar between CaMKIIN-expressing, WT and WT with CsA (Supplemental Fig. 3b). The area of infarcted myocardium following I/R injury (Supplemental Fig. 2d,e) was 66 ± 3.3 percent of the area at risk for WT hearts and was reduced by half with CsA or CaMKIIN expression. The relative area of infarcted myocardium (Supplemental Fig. 2e) was inversely related to the extent of mechanical recovery (Supplemental Fig. 2c), suggesting that the beneficial effects of CsA and CaMKII inhibition ultimately derived from prevention of myocardial death in response to I/R injury. We measured caspase 9, a marker of mitochondrial-triggered apoptosis15. Caspase 9 was significantly reduced in the CaMKIIN transgenic hearts after I/R injury and in WT hearts treated with CsA (Supplemental Fig. 2f).
We next asked if transgenic expression of CaMKIIN protected mitochondria from I/R injury. Mitochondria are structurally dynamic organelles and loss of the highly ordered internal membrane cristae is an ultrastructural correlate of mPTP opening, loss of ΔΨm and apoptosis initiation16. We used transmission electron microscopy to examine mitochondrial ultrastructure and to quantify mitochondrial disruption (Supplemental Fig. 2g,h) after I/R injury. Mitochondria of vehicle-treated WT hearts suffered extensive disruption after I/R injury, while CsA significantly protected mitochondria in WT hearts. In contrast, mitochondria from CaMKIIN transgenic hearts were resistant to I/R injury, in the presence or absence of CsA (Supplemental Fig. 2h). These data show that infarct size, mitochondrial structural integrity, mitochondrial-triggered cell death and dysfunction are similarly improved by CsA or CaMKII inhibition, consistent with a concept where CsA and CaMKII both engage a mitochondrial pathway leading to mPTP opening during pathological stress.
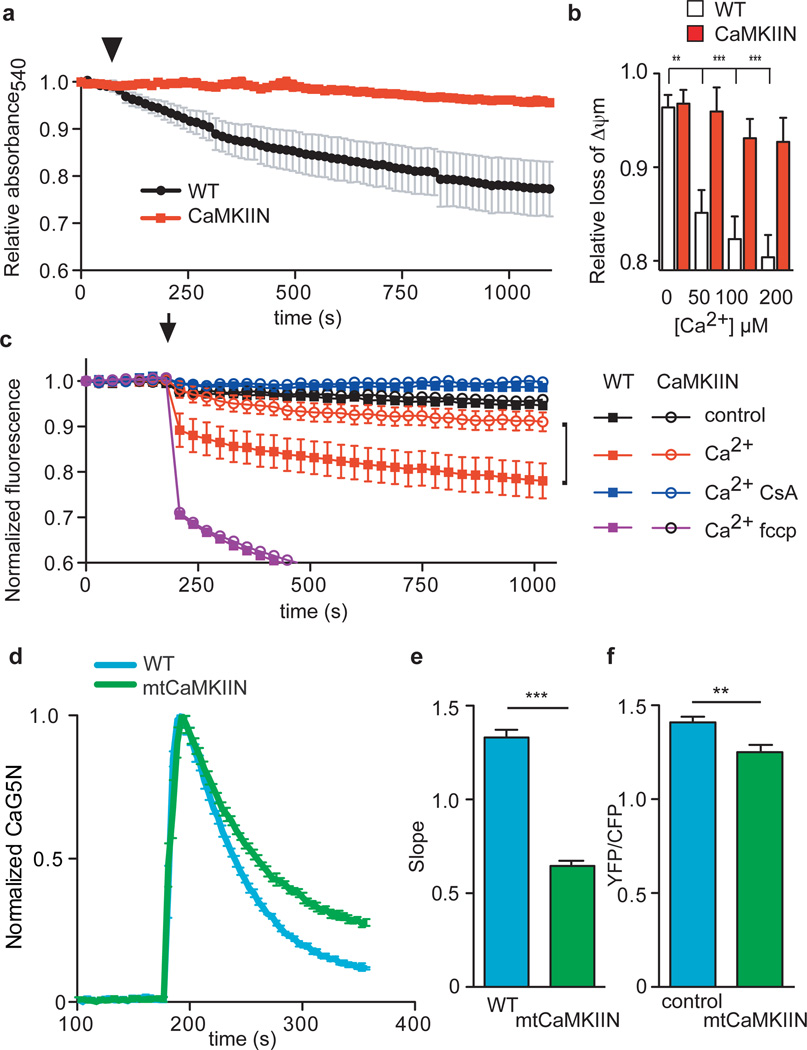
In order to better understand the protective effects of mitochondrial CaMKIIN expression, we measured Ca2+-induced injury in isolated mitochondria. Loss of mitochondria function by mPTP opening can be measured in isolated mitochondria in response to supraphysiological increases in mitochondrial Ca2+ 17. Decreases in light scattering is a correlate of mPTP opening18. We found that mitochondria with CaMKIIN expression were resistant to Ca2+-triggered increases in light scattering compared to WT controls (Fig. 1a). We corroborated our findings with Ca2+-induced mitochondria light scattering by a similar assay where a decrease in fluorescence corresponds to a loss of ΔΨm. We loaded mitochondria isolated from WT and CaMKIIN transgenic mice with a voltage-sensitive fluorescent indicator, JC-116, to test whether CaMKIIN expression affected ΔΨm responses to Ca2+. Mitochondria isolated from WT hearts were significantly more sensitive to ΔΨm loss in response to a lethal Ca2+ challenge compared to mitochondria with CaMKIIN expression (Fig. 1b). CsA treatment preserved the ΔΨm during exogenous Ca2+ challenge in mitochondria isolated from WT, while mitochondria derived from CaMKIIN transgenic hearts were protected even in the absence of CsA (Fig. 1c).
Figure 1.
Isolated mitochondria with transgenic, membrane-targeted CaMKII inhibition (CaMKIIN) are resistant to Ca2+ (200 µM Ca2+ free) challenge. a. Mitochondria mPTP Ca2+-dependent (arrow) opening reflected by a decrease in light scattering corresponding to an increase in mitochondrial volume, n = 3/genotype. b. Increasing [Ca2+] promotes loss of ΔΨm more in mitochondria isolated from WT than CaMKIIN mouse hearts (**p < 0.001, ***p < 0.0001, n = 3 hearts/group with duplicate measurements). c. Inner mitochondrial membrane potential (ΔΨm) measurements in isolated mitochondria using a fluorescent reporter, JC-1. Reduced signal from baseline after addition of Ca2+ (200 µM free Ca2+, at arrow) indicates loss of ΔΨm (p < 0.03 for all time points after the Ca2+ challenge) between WT and CaMKIIN treated with Ca2+ alone (indicated with black bracket), red squares versus open red circles, n = 5 hearts/group with duplicate measurements. d. Normalized traces showing rate of mitochondrial Ca2+ uptake in saponin-permeabolized cardiomyocytes after the addition of Ca2+ (arrow, 100 µM free Ca2+). Mitochondrial Ca2+ uptake was monitored in cells by loss of Ca2+ Green-5N fluorescence. The rate of decline in fluorescence reflects the rate of mitochondrial Ca2+ uptake. e. Summary data show the rate of mitochondrial Ca2+ uptake. Nonlinear regression fits for mitochondria Ca2+ uptake between WT and CaMKIIN cardiomyocytes (***p < 0.0001, n = 6/genotype). f. Summary data show reduced Ca2+ uptake in mitochondria, measured using a cameleon mitochondrial-targeted Ca2+ indicator, from HeLa cells expressing mtCaMKIIN compared to myc-expressing controls (**p = 0.003, n = 23 cells/group). Data represent mean ± s.e.m.
We detected CaMKII associated with mitochondria and mitoplasts, inner mitochondrial membrane vesicles from osmotically-shocked isolated cardiac mitochondria (Supplemental Fig. 4c,d), similar to previous studies19,20. However, CaMKII may contribute to Ca2+ overload and myocardial death by actions at extra-mitochondrial Ca2+ homeostatic proteins21. Therefore we developed transgenic mice with CaMKIIN expression limited to myocardial mitochondria (mtCaMKIIN) to test if CaMKII inhibition targeted to mitochondria affected mitochondria susceptibility to excess Ca2+. Unlike CaMKIIN transgenic mice, hearts expressing mtCaMKIIN have expression restricted to mitoplasts (Supplemental Fig. 1b). Mitochondria isolated from mtCaMKIIN hearts showed no difference in baseline metabolic function compared to WT littermates (Supplemental Fig. 5). We measured mitochondrial Ca2+ uptake with a membrane-impermeable indicator, Ca2+ Green-5N4 and found cardiomyocytes from mtCaMKIIN transgenic mouse hearts had significantly slower mitochondrial Ca2+ uptake, measured as a rate of decrease in Ca2+ Green-5N fluorescence, than mitochondria of WT cardiomyocytes (Fig. 1d,e). We interrogated the overall Ca2+ capacity of myocardial mitochondria with serial additions of Ca2+, and found that mtCaMKIIN expression increased the mitochondrial Ca2+ capacity prior to mPTP opening (Supplemental Fig. 6a,b). We next measured mitochondrial Ca2+ concentration changes in HeLa cells in response to ATP-evoked Ca2+ release using a GFP-based Ca2+ indicator, cameleon22, designed to monitor Ca2+ concentration within the mitochondria. Our results show reduced ATP-triggered Ca2+ entry into mitochondria in HeLa cells co-transfected with mitochondrial-directed cameleon and mtCaMKIIN compared with control cells without mtCaMKIIN expression (Fig. 1f & Supplemental Fig. 7a,b). These data suggest that the benefits of CaMKII inhibition to protect against mitochondrial stress derive from reducing mitochondria Ca2+ entry and enhancing mPTP Ca2+ tolerance.
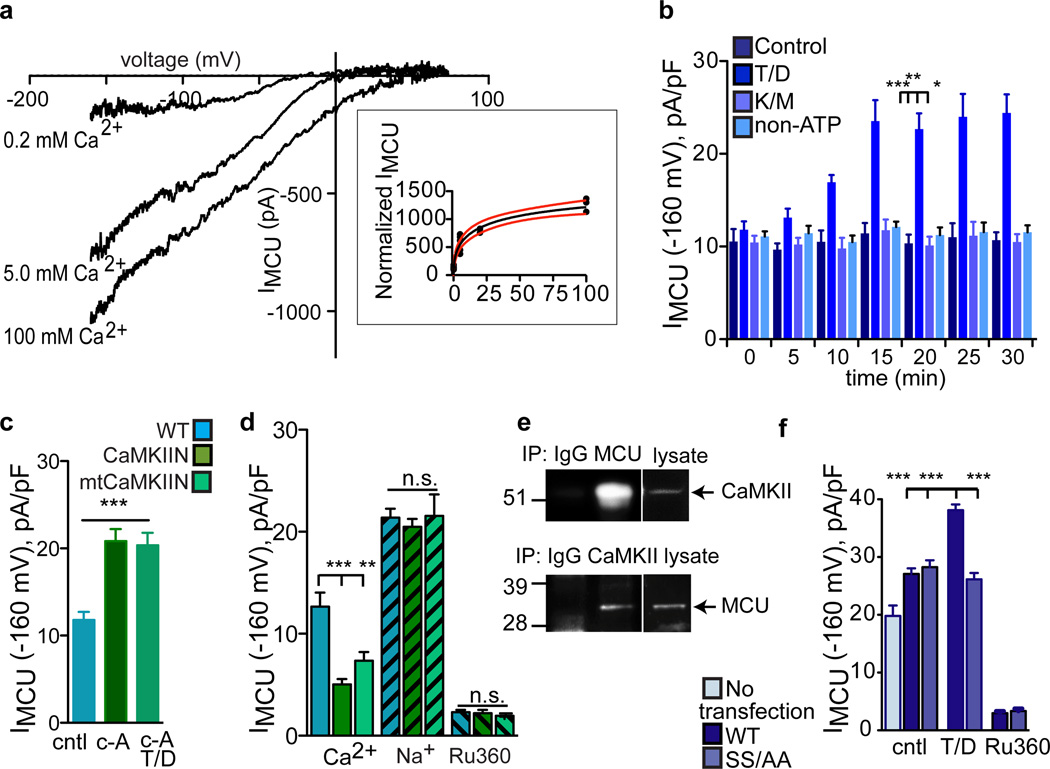
The MCU is a major Ca2+ entry pathway through the mitochondrial inner membrane3–5 and isolated cardiomyocytes23 and perfused hearts12,13 are resistant to opening and cell death after Ca2+ challenge in the presence of IMCU inhibitor drugs. To examine potential effects of CaMKII on the MCU, we performed voltage (patch) clamp studies on mitoplasts. We measured an Ru360-sensitive mitochondrial Ca2+ current, that was activated by Ca2+ (K½ = 23 mM), consistent with known properties of IMCU3 (Fig. 2a & inset). Furthermore, CsA had no effect on IMCU (Supplemental Fig. 8a), indicating that the beneficial actions of CaMKII inhibition on mPTP were distinct from the effects of CaMKII on the MCU. IMCU was increased by addition of a constitutively active, monomeric CaMKII mutant (T287 to D, T/D) to the pipette solution (equivalent to the mitochondrial matrix) in the presence of ATP (Fig. 2b & Supplemental Fig. 8b). Addition of T/D CaMKII did not increase IMCU in the presence of a non-hydrolyzable ATP analog, adenosine 5’-(β, γ-imido) triphosphate tetralithium salt (Fig. 2b), or in the absence of ATP (not shown). Furthermore, a catalytically incompetent CaMKII mutant (K43 to M, K/M) failed to increase IMCU in the presence of activating ATP and calmodulin (Fig. 2b & Supplemental Fig. 8b). These findings indicate that the catalytic activity of CaMKII was necessary to increase IMCU. Inhibiting serine/threonine phosphatases with calyculin A increased IMCU (Fig. 2c & Supplemental Fig. 8c), suggesting that endogenous and exogenous CaMKII were capable of increasing IMCU under appropriate experimental conditions. We next measured IMCU from CaMKIIN and mtCaMKIIN heart mitochondria to test if CaMKIIN expression in mitoplasts affected Ca2+ uptake. IMCU was lower in CaMKIIN-expressing mitoplasts using Ca2+ as the charge carrier (Fig. 2d & Supplemental 8d). In contrast, when Na+ was substituted for Ca2+, a condition that does not support CaMKII activation, the differences in WT and CaMKIIN-expressing mitoplasts were no longer apparent, indicating that CaMKIIN was unlikely to affect MCU expression. Ru360 nearly and equivalently eliminated IMCU in all genotypes, suggesting that MCU antagonist sensitivity was not affected by CaMKIIN (Fig. 2d & Supplemental Fig. 8a). CaMKII and MCU co-immunoprecipitated from myocardial mitochondria lysate (Fig. 2e), suggesting that CaMKII is associated with MCU in heart. CaMKII enhances Ca2+ currents through sarcolemmal voltage-gated Ca2+ channels (CaV1.2)24 and intracellular Ca2+-gated ryanodine receptor Ca2+ release channels25 by phosphorylation of defined serines/threonines. We next identified two putative CaMKII target sites on MCU, serines 57 and 92, and mutated them to alanines (SS/AA), to test for a direct effect of CaMKII on MCU. We over-expressed WT and SS/AA MCU in HEK cells and measured IMCU from isolated mitoplasts. Constitutively active, monomeric CaMKII (T/D) added to the pipette solution failed to increase IMCU in mitochondria from cells transfected with SS/AA MCU compared to WT MCU transfections (Fig. 2f & Supplemental Fig. 8e). In contrast, CaMKII added to the bath solution failed to affect IMCU recorded from WT or SS/AA over-expressing mitoplasts (not shown). These studies, using a CaMKII-resistant SS/AA MCU mutant, suggest that the N-terminus of MCU is oriented in the mitochondrial matrix4. Taken together, these data show that CaMKII interacts with MCU and promotes mitochondrial Ca2+ entry, likely by catalyzing phosphorylation of serines 57 and 92, consistent with the concept that MCU is an important CaMKII target in mitochondria.
Figure 2.
CaMKII agonist actions on IMCU require serines 57 and 92. a. IMCU is a Ca2+-dependent conductance. Inset shows IMCU in 0.2, 5 and 100 mM bath [Ca2+] fit with the Hill equation (standard slope). V½ = 23.8 mM, R2= 0.955 and h = 0.057. Red lines show the 95% confidence intervals (runs test p = 0.743). b. Summary data and time course for IMCU recorded with 0.2 mM Ca2+ after obtaining a high resistance seal and mitoplast membrane rupture (time 0) allowing dialysis of CaMKII T/D. Replacing ATP with non-hydrolyzable ATP (non-ATP) analog (both at 0.1 mM) does not allow a CaMKII-dependent increase in IMCU (all mutant CaMKII at 0.5 µM); T/D and ATP; T/D non-ATP; kinase inactive CaMKII (K/M), CaM and ATP, n = 6 (WT), 7 (T/D), 6 (K/M) and 5 (non-ATP), *p < 0.01, **p < 0.001, ***p < 0.0001 at 20 min. c. Summary data for IMCU recorded with 0.2 mM Ca2+ after addition of 100 nM calyculin A (c-A); c-A with T/D CaMKII (0.5 µM), ATP. n = 7 (control), 9 (C-a) and 7 (C-a with T/D), *p < 0.0001. d. Summary data for IMCU from WT, CaMKIIN and mtCaMKIIN mitochondria. Na+ current (150 mM) recorded in the absence of bath (intermembrane space equivalent) Ca2+ or Ru360 (10 nM). **p < 0.001, ***p < 0.0001, n = 7 (cntl groups), 8 (T/D, WT), 9 (T/D, SS/AA) and 6 (Ru360 groups). e. MCU and CaMKII co-immunoprecipitate from mitochondrial lysate. f. Summary data for IMCU recorded from HEK cell mitoplasts with and without transfection of WT or SS/AA MCU mutants. HEK mitoplast Ca+ currents were inhibited by Ru360 (10 nM). For Ca2+ n = 10 (WT and CaMKIIN) and 7 (mtCaMKIIN); Na+ n = 15 (WT), 13 (CaMKIIN) and 12 (mtCaMKIIN) and for Ru360 n = 8 (WT and CaMKIIN), 5 (mtCaMKIIN), ***p < 0.0001. Data represent mean ± s.e.m, except for inset (see a).
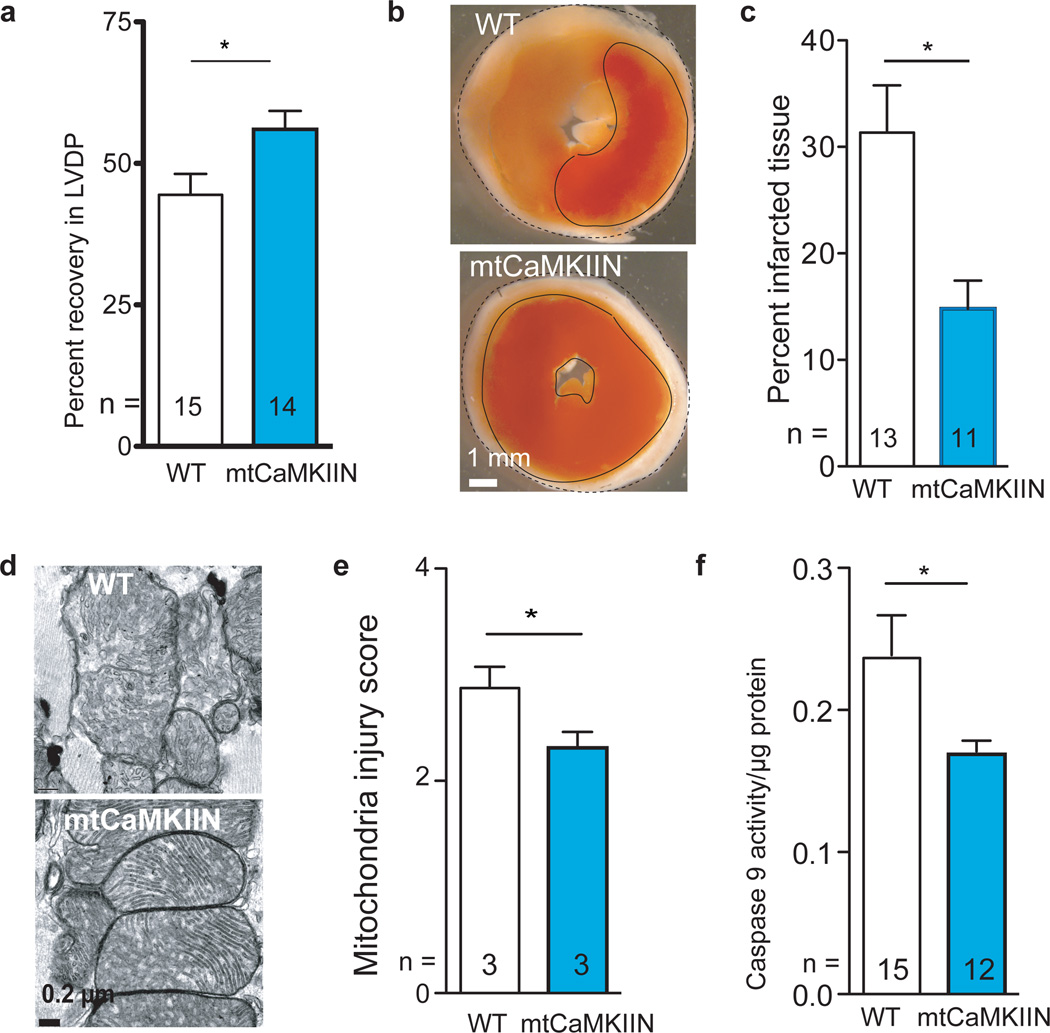
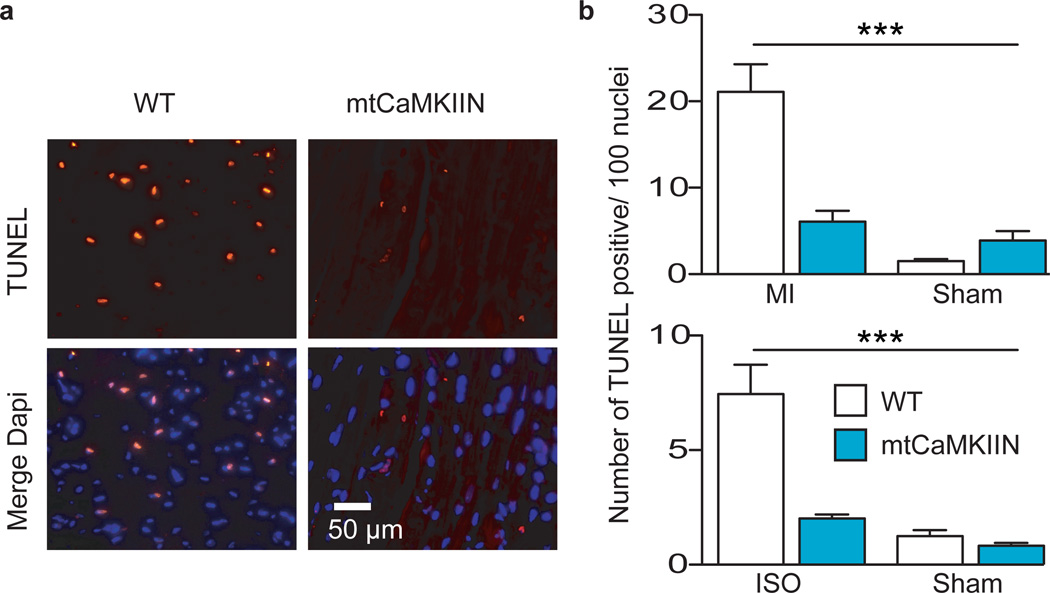
We next used our mtCaMKIIN mice to determine if mitochondrial CaMKII inhibition was sufficient to explain the protective effects of CaMKII inhibition on I/R injury in isolated perfused hearts in the absence of glucose (Supplemental Fig. 2)7,11. We found that hearts from mtCaMKIIN mice had preserved mechanical function (Fig 3a), reduced MI size (Fig 3b,c), preserved mitochondrial integrity (Fig. 3d,e) and reduced caspase 9 activity (Fig. 3f) compared to WT littermate control hearts after I/R injury. Baseline left ventricular developed pressures were similar between WT and mtCaMKIIN hearts (Supplemental Fig. 9). We found no difference in left ventricular mechanical recovery (p = 0.976) between WT and mtCaMKIIN hearts treated with CsA, and CsA added no further protection for mtCaMKIIN hearts subjected to I/R injury (p = 0.946). Neurohumoral toxicity is a major cause of adverse outcomes after MI, including heart failure26, and β adrenergic receptor antagonist drugs are a therapeutic mainstay for reducing myocardial death and dysfunction after ischemic injury and MI26. CaMKII is activated by isoproterenol in heart and CaMKII inhibition protects against isoproterenol-mediated myocardial injury7,8. The protective effects of mtCaMKIIN expression were also evident in vivo, because mtCaMKIIN mice showed fewer TUNEL-stained nuclei than WT controls 5 h after a MI (Fig 4a,b) or 30 min after isoproterenol treatment (Fig. 4b). Thus, mitochondrial targets appear to be essential for the cardioprotective benefits of CaMKII inhibition in a diverse set of clinically-relevant injury models.
Figure 3.
mtCaMKIIN hearts are resistant to I/R injury. a. LVDP recovery following I/R as a percentage of the baseline value (*p = 0.026). b. Representative TTC stained heart sections. The dark red staining represents living myocardium and the solid black outlines form boundaries demarcating viable from dead tissue. c. Summary data from TTC stained hearts showing relative area of infarct normalized to WT, measured as in Supplemental Fig. 2e, *p = 0.006. d. Representative TEM images from hearts treated as in panel a. e. Summary mitochondria injury scores for TEM studies by the criteria used in Supplemental Fig. 2h. (*p = 0.003, more than 500 mitochondria from at least 10 random fields were counted/genotype). f. Caspase 9 activity from hearts treated as in panel a (*p = 0.033, n = number of hearts/genotype). Data represent mean ± s.e.m.
Figure 4.
mtCaMKIIN hearts are resistant to apoptosis in vivo. a. Representative images of TUNEL-stained nuclei from WT and mtCaMKIIN heart transverse sections 5 h after MI. Dapi stain shows total number of nuclei. Scale bar indicates 50 µm. b. Upper panel - Summary data for the number of TUNEL-stained nuclei from WT and mtCaMKIIN heart sections 5 h after MI (3 hearts/genotype, 10 images/heart). Lower panel - Summary data for the number of TUNEL-stained nuclei from WT and mtCaMKIIN heart sections 30 min after isoproterenol (ISO) treatment (15 mg/kg, 3 hearts/genotype, 10 images/heart, ***p < 0.0001). Data represent mean ± s.e.m.
Our findings identify CaMKII as a modulator of mitochondrial Ca2+ homeostasis and a crucial component of a final pathway in heart disease due to ischemia and neurohumoral toxicity, by showing that CaMKII increases IMCU and that CaMKII inhibition reduces IMCU and the mPTP opening. Our study suggests that CaMKII inhibition could reduce adverse responses, including death, to common forms of pathological myocardial stress by multivalent actions on mitochondria, including IMCU inhibition and blunting Ca2+ responsiveness of mPTP.
Methods
Transgenic mice
Animals were maintained in the University of Iowa Animal Facility and treated in accordance with Institutional Animal Care and Use Committee guidelines (PHS Animal Welfare Assurance, A3021-01). CaMKIIN transgenic mice (C57BL/6 background) were generated as described14. Targeting of βCaMKIIN (accession NM_033259.2) to mitochondria in transgenic mice was achieved by fusion of the cox8a N-terminus. The N-terminal 28 amino acids of cox8a were added to the 5’ of HA-βCaMKIIN sequence by PCR using the 5’ primer: CGGTCGACATGTCCGTCCTGAC and the 3’ primer ATGTCGACTTCTCCCGGCGGCA (Integrated DNA Technologies) with SalI sites at the extreme ends. The product (~100 base pairs) was purified from agarose gel (Quiagen gel extraction kit) and was digested with the restriction enzyme, SalI. The resulting DNA product was ligated into the SalI site 5’ of the HA sequence of pα-MHC-script-Hgh-HA-tagged CaMKIIN vector. Mouse embryonic stem cells were injected with the linearized DNA (digested with NotI) in the University of Iowa Transgenic Mouse Core Facility and implanted into B6D2 pseudo-pregnant females. Insertion of the transgene into the mouse genome was confirmed by PCR analysis (not shown) using the forward primer, cgataaacttatgtccgagatccta and reverse primer, gtcatcctcgatcaccactctct, producing a product of 196 bases. Mice were backcrossed to F4 generation or greater into the C57 background. Transgenic and control mice of either gender were sacrificed at the age of 2-3 months.
Isolated perfused working mouse hearts
Langendorff-perfused mouse hearts were used to measure left ventricular function and myocardial viability, as described27. Mice were anesthetized with avertin and hearts were quickly excised and rinsed in iced Tyrode’s solution containing (in mM): 137 NaCl, 5.4 KCl, 0.5 MgCl2, 0.16 NaH2PO4, 3 NaHCO3, 5 HEPES-NaOH, and Heparin 1 mg/10 ml, which was previously adjusted to a pH of 7.4 with NaOH at room temperature. All chemicals were from sigmaaldrich.com, unless otherwise noted. Excess tissue was dissected away. Hearts were perfused retrogradely through the aorta for 1–2 minutes at room temperature with Hanks’ balanced salt solution (GIBCO, Cat No. 14025-092) and mounted on a modified Langendorff apparatus (HSE-HA perfusion systems, Harvard Apparatus) for retrograde aortic perfusion at a constant pressure of 80 mm Hg with carbogen (95% O2, 5% CO2) and Krebs–Henseleit bicarbonate (KHB) solution consisting of (in mM): 25 NaHCO3, 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 2.5 CaCl2, 0.5 Na-EDTA, 2 pyruvate, with pH equilibrated to 7.4 with 5 µM CsA or DMSO for control. Hearts were perfused for 10 min to ensure metabolic equilibrium followed by 20 or 30 min of global ischemia then 50 min of reperfusion. Measurements were recorded from each heart for the entire time of perfusion.
Heart infarct size and caspase 9 activity assay
Following the I/R protocol shown in Supplemental Figure 2, hearts were immediately frozen. Three-0.5 mm sections were cut from frozen hearts, labeled with 5% (wt/vol) 2,3,5-triphenyltetrazolium chloride (TTC) in PBS buffer and fixed in 10% formalin. Caspase 9 activity assays were performed on tissue lysate following manufacturers protocol (Invitrogen.com) and normalized to protein concentration (µg/ml).
Transmission electron microscopy
Hearts were fixed in 2.5 % gluteraldehyde and 0.1 M Na cacodylate buffer overnight at 4°C, washed 3 X 20 min in 0.1 M Na cacodylate buffer, pH 7.2, fixed in 4 % OsO4, washed in 0.1 M Na cacodylate buffer then dH2O, 2.5 % uranyl acetate, EtOH series to dehydrate then EtOH and Spurr’s with final solution 100% Spurr’s. Hearts were embedded in Spurr’s at 60°C for 24 – 48 h. Ultramicrotomy at 90 nm and samples collected on 200 mesh for formvard grids for staining with uranyl and lead. Stained sections were examined with a JEOL JEM-1230 and digital images were collected with a Gatan UltraScan 1000 2k×2k CCD camera.
Mitochondrial isolation and subcellular fractionation
Isolation of mitochondria was performed on ice. Freshly harvested mouse hearts were injected with 1.0 % protease XXIV in MSE buffer (in mM, 5 MOPS, 70 sucrose, 2 EGTA, 220 mannitol, pH 7.2 with KOH) then homogenized. HEK cells were washed then homogenized. Nuclei and unbroken cells were pelleted by centrifugation at 600 Xg for 10 min twice. The crude mitochondrial and cytosolic fraction was obtained from the supernatant by centrifugation at 8,500 Xg for 20 min. The pellet was further purified with a 40% percoll gradient, washed twice in MSE then resuspended in 200 µl MSE with protease inhibitors. For mitoplast preparation, mitochondria were resuspended in hypotonic solution (in mM, 5 sucrose, 5 HEPES and 1 EGTA (pH 7.2 with KOH) for 5 min to remove outer membrane. After sedimenting for 5 min at 4,000Xg mitoplasts were resuspended in (in mM) 750 KCl, 100 HEPES and 1 EGTA (pH 7.2 with KOH).
MCU current recording (whole-mitoplast configuration)
Signals were measured with an Axon 200B patch-clamp amplifier controlled by a personal computer using a Digidata 1320A acquisition board driven by pClamp 8.0 software (Axon Instruments). For formation of gigaohm seals and initial break-in to the whole-cell voltage clamp configuration, mitoplasts were perfused with solution containing (mM): 150 Na gluconate, 10 Hepes and 0.2 CaCl2 (pH 7.4, adjusted with NaOH). Bath solutions with different Ca2+ concentrations were made by adding the proper of volume of 1 M CaCl2. Pipettes, after filling with solution (mM) Na gluconate, 5 NaCl, 135 sucrose, 10 HEPES and 1.5 EGTA (pH7.2 with NaOH) had an access resistance of 25–35 MOhm3. After rupturing of mitoplast membranes the capacitance was from 5 to 9 pF. A ramp voltage command protocol from -160 to 80 mV, for 2 seconds, was applied from a holding potential of 0 mV to evoke currents. Reagents and enzyme added (as noted in text) were 100 µM ATP, 100 µM non-hydrolysable ATP, 2 µM CaM, 0.5 µM CaMKII (WT and mutants; T/D and K/M), 10 nM Ru360 and 5 µM CsA.
Mitochondrial ΔΨm measurement
Isolated mitochondria were loaded with JC-1 following manufacturers instructions (Invitrogen.com) and placed in a 96 well black-walled plate with 0.2 µg/ml JC-1 in buffer with DMSO, 0.2 µM CsA or 2.5 µM carbonyl cyanide p-[trifluoromethoxy]-phenyl-hydrazone (FCCP). At the indicated time point 200 µM CaCl2 was added to the suspension. Fluorescent measurements were taken according to the manufacturer’s protocol. Light scattering was assessed after adding CaCl2 (free Ca2+ was calculated using the Maxchelator at http://www.stanford.edu/~cpatton) by decreased absorbance over 30 min at 540 nm using a F200 96-well plate reader (Tecan) in swelling buffer (in mM, 200 sucrose, 0.002 EGTA, 5 succinate, 0.0002 rotenone, 20 Tris, 20 Hepes, 1 KH2PO4 and 1 µg/ml oligomycin, pH 7.2), with a use gain of 100. Data were normalized to the initial measurement and plotted using Prism Graph.
Ca2+ retention assay
CaG5N was used to monitor extramitochondrial Ca2+ in saponin-permeabilized, isolated adult mouse cardiomyocytes. The ventricular cardiomyocytes were isolated from adult mice as described28. Recordings of cardiomyocyte mitochondrial Ca2+ uptake were performed in a 96 well plate with 100 µl of respiration buffer (in mM, 100 KAsp, 20 KCl, 10 HEPES, 5 glutamate, 5 malate, and 5 succinate pH 7.3) supplemented with 100 µM blebbistatin, 5 µM thapsigargin, 0.005% saponin and 1 µM Ca2+ green-5N (CaG5N, Invitrogen.com) with freshly isolated cardiomyocytes. CaG5N fluorescence was monitored at excitation, 485 nm; emission, 535 nm after adding CaCl2 (100 µM free Ca2+ at 3 min intervals at 30°C). Cyclosporin A (5 µM) and Ru360 (0.1 µM) were used to block the mitochondrial permeability transition pore and Ca2+ uniporter, respectively.
MI and Isoproterenol-treated mice
Assays are described in previous publications8.
Transmission immuno-electron microscopy
Isolated mitochondria fixed in EM grade 4% paraformaldehyde with 0.05% glutaraldehyde in PBS pH 7.4 for 1 h at 4°C were processed and labeled with antibodies at 1:250 (CaMKII, CoxIV, rabbit IgG and HA).
Immunoprecipitation, Western and activity assays
Immunoprecipitation was performed over 1 h with mitochondrial lysate (200 µg protein) and protein A magnetic beads. SDS-PAGE and Western blotting studies used lysate of heart subcellular fractions. Protein content was determined with a Bradford assay. Samples (25 µg) were separated on NuPAGE 4–12% Bis-Tris gels (Invitrogen.com) and transferred to PVDF membranes. After blocking with Odyssey blocking buffer (LI-COR Biosciences), the membranes were incubated with the following primary antibodies (dilutions): CaMKII (1:500), SERCA2 (1:250), calnexin (1:250), CoxIV (1:500) ATP5a (1:500), MCU (1:500), nucleolin (1:100), mfn1 (1:500) and HA (1:1000). Appropriate secondary antibodies were used and bands visualized. Activity assays29 were done using mitochondria lysate with biotin capture membrane (Promega).
Mitochondrial respiration
State 3 and state 4 respiration were measured by polarography in a 600-µl incubation chamber with stir bar and oxygen electrode as we have accomplished in past studies30. The ADP/O ratio was calculated as molar ADP added divided by molar oxygen consumed during conversion to ATP as we described previously30. Respiratory coupling was assessed by simultaneous determination of respiration and membrane potential as described30.
Statistical analysis
Statistical analysis was performed using the analysis of variance (ANOVA or Student’s t-test, as appropriate). Post hoc pairwise comparisons were performed using Bonferroni’s multiple-comparison test (Prism graph), as appropriate. Other tests were done as noted in the text using Prism graph. Results were given as means ± s.e.m. A p value < 0.05 was considered significant. n.s. = not significant.
Supplementary Material
Acknowledgments
The authors wish to acknowledge discussions with Dr. Amy Lee, Dr. Beverly Davidson, Dr. Kevin Campbell and Dr. Curt Sigmund (University of Iowa). This work was funded by AHA 0635357N (M.A.J.); NIH R01 HL090905 (L.-S.S); NIH R01 HL079031, R01 HL62494, R01 HL70250 and R01 HL113001 and supported by a grant from the Fondation Leducq for the Alliance for CaMKII Signaling (M.E.A.); NIH R01 HL084583 and R01 HL083422 and Pew Scholars Trust (P.J.M.).
Footnotes
Contributions
M.A.J. designed the project, carried out experimental work and wrote the manuscript. O.M.K. and L-S.S. carried out experimental work, analysed data and participated in data interpretation. C.A., Z.G., E.D.L., carried out experimental work and interpreted data. J.L., B.J.H., D.D.H., B.D.F., B.C. and J.Y. carried out experimental work. S.S. provided critical materials and wrote the manuscript. S.A.M. and T.D.S. interpreted data. P.J.M. supervised the research. W.I.S. supervised the research and wrote the manuscript. M.E.A. conceived the project, supervised the research and wrote the manuscript.
Competing financial interests
Dr. Anderson is a named inventor on patents claiming to treat heart disease by CaMKII inhibition. He is a founder and advisor to Allosteros Therapeutics. At present there is no income from these activities.
Contributor Information
Mei-ling A. Joiner, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA
Olha M. Koval, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA
Li Jingdong, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
B. Julie He, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA; Department of Molecular Physiology and Biophysics Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
Chantal Allamargot, University of Iowa Central Microscopy Research Facility, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
Zhan Gao, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
Elizabeth D. Luczak, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA
Duane D. Hall, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA
Brian D. Fink, Iowa City Veterans Affairs Medical, Iowa City, IA 52246, USA
Biyi Chen, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
Jinying Yang, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
Steven A. Moore, Department of Molecular Physiology and Biophysics Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA Department of Pathology, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
Thomas D. Scholz, Department of Pediatrics, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA
Stefan Strack, Department of Pharmacology, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
Peter J. Mohler, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA
William I. Sivitz, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA Iowa City Veterans Affairs Medical, Iowa City, IA 52246, USA.
Long-Sheng Song, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
Mark E. Anderson, Department of Internal Medicine and Cardiovascular Center, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA Department of Molecular Physiology and Biophysics Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA.
References
- 1.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE. Calcium Signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 4.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piot C, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl.J. Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 7.Erickson JR, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, et al. Calmodulin kinase II inhibition protects against myocardial cell apoptosis in vivo. Am.J. Physiol. Heart Circ. Physiol. 2006;291:H3065–H3075. doi: 10.1152/ajpheart.00353.2006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, et al. The {delta}C Isoform of CaMKII Is Activated in Cardiac Hypertrophy and Induces Dilated Cardiomyopathy and Heart Failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 10.Odagiri K, et al. Local control of mitochondrial membrane potential, permeability transition pore and reactive oxygen species by calcium and calmodulin in rat ventricular myocytes. Journal of Molecular and Cellular Cardiology. 2009;46:989–997. doi: 10.1016/j.yjmcc.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 12.García-Rivas GJ, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. British Journal of Pharmacology. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez JA, et al. Mitochondria regulate inactivation of L-type Ca2+ channels in rat heart. The Journal of Physiology. 2001;536:387–396. doi: 10.1111/j.1469-7793.2001.0387c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh MV, et al. Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. J. Clin. Invest. 2009;119:986–996. doi: 10.1172/JCI35814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakem R, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 16.Olichon Al, et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, et al. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halestrap AP. What is the mitochondrial permeability transition pore? Journal of Molecular and Cellular Cardiology. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Timmins JM, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. The Journal of Clinical Investigation. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma V, Abraham T, So A, Allard M, McNeill J. Functional effects of protein kinases and peroxynitrite on cardiac carnitine palmitoyltransferase-1 in isolated mitochondria. Molecular and Cellular Biochemistry. 2010;337:223–237. doi: 10.1007/s11010-009-0303-2. [DOI] [PubMed] [Google Scholar]
- 21.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. Journal of Molecular and Cellular Cardiology. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyawaki A, et al. Fluorescent indicators for Ca2+based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 23.Saotome M, et al. Mitochondrial membrane potential modulates regulation of mitochondrial Ca2+ in rat ventricular myocytes. American Journal of Physiology - Heart and Circulatory Physiology. 2005;288:H1820–H1828. doi: 10.1152/ajpheart.00589.2004. [DOI] [PubMed] [Google Scholar]
- 24.Koval OM, et al. CaV1.2 b-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc. Natl. Acad. Sci. USA. 2010;107:4996–5000. doi: 10.1073/pnas.0913760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wehrens XHT, Lehnart SE, Reiken SR, Marks AR. Ca2+/Calmodulin-Dependent Protein Kinase II Phosphorylation Regulates the Cardiac Ryanodine Receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 26.Feldman D, Carnes C, Abraham W, Bristow M. Mechanisms of disease: beta-adrenergic receptors--alterations in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med. 2005;2:475–483. doi: 10.1038/ncpcardio0309. [DOI] [PubMed] [Google Scholar]
- 27.Li J, et al. Calmodulin kinase II inhibition enhances ischemic preconditioning by augmenting ATP-sensitive K+ current. Channels. 2007;1:387–394. doi: 10.4161/chan.5449. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Colbran RJ, Anderson ME. Calmodulin kinase is a molecular switch for cardiac excitation, contraction coupling. Proc. Natl. Acad. Sci. USA. 2001;98:2877–2881. doi: 10.1073/pnas.051449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He BJ, et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herlein JA, Fink BD, O'Malley Y, Sivitz WI. Superoxide and Respiratory Coupling in Mitochondria of Insulin-Deficient Diabetic Rats. Endocrinology. 2009;150:46–55. doi: 10.1210/en.2008-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.