Abstract
The immune system develops in waves during ontogeny, being initially populated by cells generated from fetal hematopoietic stem cells (HSCs) and later by cells derived from adult HSCs. Remarkably, the genetic programs that control these two distinct stem cell fates remain poorly understood. We report that Lin28b is specifically expressed in mouse and human fetal liver and thymus, but not in adult bone marrow or thymus. We demonstrate that ectopic expression of Lin28 reprograms hematopoietic stem/progenitor cells (HSPCs) from adult bone marrow, endowing them with the ability to mediate multi-lineage reconstitution that resembles fetal lymphopoiesis, including increased development of B-1a, marginal zone B, gamma/delta (γδ) T cells, and natural killer T (NKT) cells.
Keywords: regenerative medicine, post-transcriptional regulation, microRNA, hematopoiesis, lymphocyte development, immune system, ontogeny
Hematopoietic stem cells give rise to all the erythroid, myeloid and lymphoid lineages and are used clinically in transplants to treat patients with a wide variety of blood and immune system disorders that include immunodeficiencies and malignancies. In mice, the major site of hematopoiesis transitions from the fetal liver (FL) to the fetal spleen (FS) and bone marrow (BM) during late embryogenesis. FL HSCs differ phenotypically and functionally from adult BM HSCs and a developmental switch is thought to occur within the first weeks of post-natal life (1–3). Adoptive transfer and fetal thymic organ culture studies established that FL HSPCs, unlike adult BM HSPCs, preferentially give rise to lymphocyte subsets that can be collectively referred to as innate-like lymphocytes. For example, CD5+ B cells, referred to as the B-1a subset, and certain γδ T cell subsets develop almost exclusively in the fetus and/or neonate (4–7). More recently, a fetal source of HSPCs was found to preferentially give rise to the B-1a and marginal zone (MZ) B cell lineages (8). In contrast, adult BM HSPCs are less efficient at reconstituting the B-1a, and fetal γδ-T cell subsets compared to conventional B and T lymphocyte pools. Although the development of these innate-like lymphocyte subsets requires recombination activating gene (RAG)-mediated V(D)J recombination at the T or B cell antigen receptor loci, their repertoire has been described as semi-invariant or oligo-clonal (9). Similar to cells of the innate immune system, they are often strategically localized in the body to respond rapidly to a limited set of conserved antigens. Despite being potent mediators of host defense, the molecular basis for their preference to develop in the fetus or neonate largely remains a mystery. The ability to generate B-1a B cells and Vγ3+ γδ-T cells (nomenclature used) is intrinsically programmed in the FL HSCs (5, 10, 11). Sox17 was identified as a transcription factor that is specifically expressed in and required for the maintenance of fetal but not adult HSCs (12). Thus, transcriptional control can distinguish the fetal from adult HSC fate. In this study, we show that a layer of post-transcriptional control involving Lin28b and let-7 microRNAs (miRNAs) contributes to the distinct differentiation potential of these two stem cell types.
Lin28b and the let-7 family of miRNAs are differentially expressed in lymphoid progenitors originating from fetal and adult HSCs
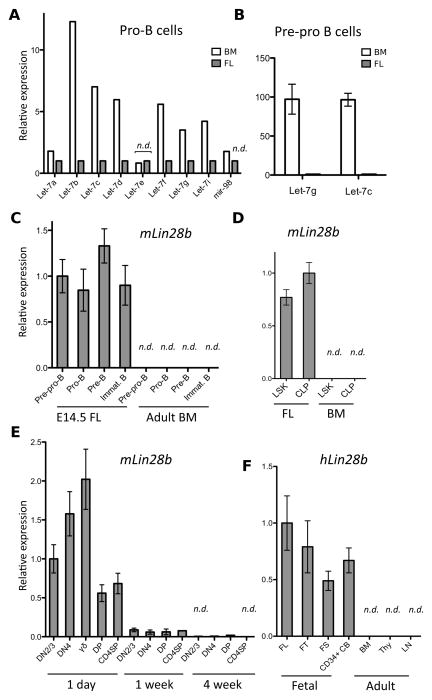
To identify the molecular differences between fetal and adult lymphoid progenitors, we performed global miRNA-expression profiling of FL and adult BM progenitor B (pro-B) cells (B220+CD43+IgM−veCD19+CD24+). This population was chosen because we could readily sort sufficient numbers of these progenitors to high purity and led to the observation that expression of the let-7 family of miRNAs was largely absent in FL pro-B cells. In the mouse genome, there are twelve let-7 paralogs that result in the expression of nine distinguishable mature miRNA isoforms (isomiRs) (Fig. S1). The let-7 miRNAs are broadly and highly expressed in the hematopoietic system (13). Surprisingly, the eight let-7 isomiRs that were detectable in adult BM pro-B cells were either significantly reduced in expression or undetectable in FL pro-B cells (Fig. 1A). From the same FACS sort, we had isolated the B220+CD43+IgM−ve CD19−veCD24−ve precursors to the pro-B cells (pre-pro-B cells). We determined that mature let-7g and let-7c isomiRs were abundantly expressed in pre-pro-B cells from adult BM but not from FL using quantitative RT-PCR (qRT-PCR) (Fig. 1B). Next, we examined if the observed global repression of let-7 miRNAs in fetal B-cell progenitors was mediated by endogenous Lin28 or Lin28b, encoded by two evolutionarily conserved paralogs, known to specifically block the biogenesis of let-7 miRNAs post-transcriptionally by binding to the terminal loop region of the let-7 primary or precursor miRNAs (pri- or pre-miRNAs) (14–17). We determined in FACS-sorted populations that Lin28b mRNA was abundant in all stages of FL B cell development (Fig. 1C) and also in FL lineage (Lin)−veSca-1+c-Kit+ (LSK) HSCs and Lin−ve c-Kitint Sca-1int IL7Rα+ common lymphoid progenitors (CLPs) (Fig. 1D) but absent the corresponding populations in adult BM. In contrast, Lin28 mRNA was not detectable in fetal HSPCs (Fig. S2A). These findings indicate that Lin28b expression is limited to HSPCs of fetal origin and could account for the observed reduction in mature let-7 expression.
Fig. 1.
Differential let-7 and Lin28b expression in fetal and adult lymphocyte progenitors. Mouse expression data represent RNA of FACS-sorted populations pooled from at least three adult or neonatal mice or 12 FLs. (A) Graph shows relative expression of mature let-7 isomiRs in FL and adult BM pro-B cells (B220+CD43+IgM−veCD19+CD24+) determined by NanoString global miRNA-expression profiling. (B) Graph shows qRT-PCR analysis of mature let-7g and let-7c expression in pre-pro-B cells (B220+CD43+IgM−veCD19−veCD24−ve) from FL and adult BM. (C) Graph shows qRT-PCR analysis of Lin28b mRNA expression in FACS-sorted B cell precursor subsets from the indicated organs. Pre-pro-B (B220+CD43+IgM−veCD19−veCD24−ve), pro-B (B220+CD43+IgMveCD19+CD24+), pre-B (B220+CD43−veIgM−veCD19+CD24+), immature B (B220+CD43veIgM+CD19+CD24+). (D) Graph shows qRT-PCR analysis of Lin28b mRNA in sorted FL and BM HSPC populations: LSK (Lin−veSca-1+c-Kit+), CLP (Lin−ve c-Kitint Sca-1int CD127+). (E) Graph shows qRT-PCR analysis of Lin28b mRNA expression in FACS-sorted thymocyte subsets from mice of the indicated age. DN2/3 (CD4−ve CD8veCD25+CD44int), DN4 (CD4−veCD8−veCD25−veCD44−ve), γδ-T (γδ-TCR+ CD3+), DP (CD4+CD8+CD3lo), CD4SP (CD4+CD8−veCD3+). All thymocyte subsets except for the γδ-T cells were also gated through a γδ-TCR−ve CD1dPBS57−ve gate. (F) Graph shows qRT-PCR analysis of human Lin28b mRNA expression in the indicated fetal and adult organs: FL, FT, FS, CD34+ CB, BM, thymus (Thy), lymph nodes (LN), spleen (SPL). Human samples contain commercially obtained RNA pooled from at least 3 donors each. For all panels, error bars represent standard error of triplicate experimental replicates. n.d. indicates not detectable or below background signal level.
Classic fetal thymus (FT) graft studies suggest that fetal HSPCs seed the thymus between 10 and 13 days of gestation, whereas cells derived from adult HSPCs begin to substantially dilute the first generation thymocytes at day 7 after birth, and have largely replaced them by two weeks of age (18). To test if Lin28b mRNA expression in the thymus correlates with the presence of fetal HSC-derived cells we performed qRT-PCR on various FACS-sorted thymocyte populations ranging from the double negative (DN)2/3 (CD4−veCD8−veCD25+CD44int) stage to the CD4+CD8−ve single positive (CD4SP) stage from 1 day, 1 week and 4 week-old mice. We observed abundant Lin28b transcripts in all the tested thymocyte subsets from 1 day-old thymi with the greatest amount observed in the γδ-TCR+CD3+ thymocytes (Fig. 1E). The abundance of endogenous Lin28b mRNA expression was dramatically decreased in thymocytes by 7 days after birth and was undetectable by 4 weeks of age. Declining Lin28b expression coincided with rising levels of mature let-7g (Fig. S2B). These data demonstrate that endogenous Lin28b mRNA expression declines in concordance with the decrease in the proportion of thymocyte progenitors of fetal origin in the postnatal mouse. Interestingly, Lin28b expression was not maintained in the thymus or peripheral lymphoid populations in sub-lethally irradiated adult Rag1−/− mice reconstituted with FL HSPCs (Fig. S2C). These findings suggest that Lin28b expression is turned on during fetal hematopoiesis but not sustained during ontogeny.
To determine if these observed fetal signatures in mouse HSPCs are conserved in humans, we compared Lin28b and let-7g expression in human FL, FT, FS and CD34+ umbilical cord blood (CB) cells, with that in adult BM, lymph node and thymus. As in mice, Lin28b mRNA expression was exclusively detected in fetal human hematopoietic tissues (Fig. 1F), whereas mature let-7g was more abundant in the adult (Fig. S2D). Taken together, these expression analyses reveal a previously unknown molecular difference between HSPCs of fetal and adult origin that is conserved between human and mice and prompted us to identify a heterochronic role of the Lin28b/let-7 axis in regulating hematopoiesis during mammalian ontogeny.
Ectopic Lin28 expression in adult BM HSPCs represses the let-7 family of miRNAs and allows multi-lineage reconstitution
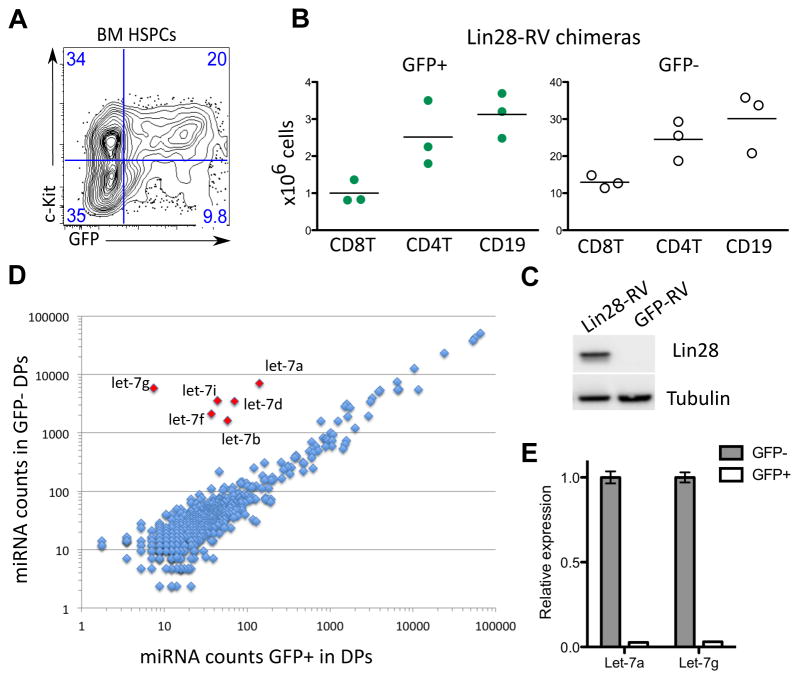
To test if ectopic expression of Lin28 can confer characteristics of fetal lymphopoiesis in adult mice, we used a retrogenic BM chimera system. Lin28 was chosen because an excellent antibody was available to confirm its expression. HSPCs from lineage-depleted adult BM of wild-type C57BL/6 mice were transduced with a Lin28-encoding retrovirus and transplanted into sub-lethally irradiated Rag1−/−recipients. Full-length mouse Lin28 cDNA was cloned into a mouse stem cell virus-based vector upstream of an internal ribosomal entry site followed by GFP (Lin28-RV) (19). Lineage-depleted C57BL/6 bone marrow cells from adult mice were transduced with Lin28-RV or control GFP-RV ex vivo and injected intravenously into sub-lethally irradiated Rag1−/− recipients (Fig. S3A). The initial transduction efficiency varied between 20–60% as determined by frequency of GFP+ cells 24 hours post-infection (Fig. 2A). HSPCs transduced with Lin28-RV mediated multi-lineage reconstitution with grossly normal proportions of T, B and myeloid cells in the peripheral lymphoid organs 6–8 weeks following transplantation in recipient mice hereafter referred to as Lin28-RV BM chimeras (Fig. 2B, S3). Ectopic Lin28 protein expression was confirmed by western blot in thymocytes of Lin28-RV BM chimeras (Fig. 2C). To assess the effect of ectopic Lin28 protein on miRNA expression, we performed global miRNA profiling of sorted GFP+ and GFP−ve pre-selection double positive (DP) thymocytes (CD3loCD4+CD8+) (Fig. S4A). Our results demonstrate that the impact of Lin28-RV on miRNA expression is dramatic and highly specific to the let-7 family members (Fig. 2D). Diminished expression of mature let-7a and let-7g was further validated by qRT-PCR (Fig. 2E). To assess the impact on the transcriptome, we performed deep sequencing of cDNA generated from poly-A+ RNA (RNA-seq) comparing sorted GFP+ and GFP−ve pre-selection DP thymocytes in Lin28-RV BM chimeras (Fig. S4). Statistical analysis using the Sylamer software demonstrated enrichment only of the complementary let-7 family heptameric seed sequence TACCTCA in 3′ UTRs of genes up-regulated in GFP+ DP thymocytes (Fig. S5) and not the 969 other heptameric seed sequences that were also tested. Out of the 175 up-regulated genes containing this seed motif and passing Bonferroni-corrected E-value threshold of 0.01 identified using Sylamer, we observed a higher than expected overlap of 47 and 67 genes with let-7 target genes phylogenetically predicted by PicTar and TargetScan respectively (Tables S1 and S2) (20, 21). Thus, Lin28-RV mediated a dramatic decrease in steady-state levels of mature let-7 miRNAs in our retrogenic system with minimal effects on other miRNAs, resulting in the specific global de-repression of putative let-7 target mRNAs. These data reveal that despite abundant let-7 expression throughout the adult hematopoietic system, we can readily knock-down their expression by Lin28-RV transduction without inhibiting multi-lineage reconstitution.
Fig. 2.
Lin28-mediated depletion of let-7 and multi-lineage reconstitution in Lin28-RV BM chimeras. (A) FACS plot shows representative frequency of GFP+ cells among lineage-depleted adult BM cells enriched in HSPCs 24 hours post-transduction with Lin28-RV. (B) Absolute numbers of GFP+ (green) and GFP−ve (white) CD19+ B and CD4+ and CD8+ T-lymphocyte subsets in lymph nodes of three Lin28-RV BM chimeras 6–8 weeks post-adoptive transfer are plotted. Data are representative of >5 independent reconstitution experiments. (C) Upper panel shows Lin28 western blot of total thymocyte lysate from GFP-RV and Lin28-RV BM chimeras. Lower panel shows tubulin western blot as a loading control. (D) Results of NanoString global miRNA-expression profiling analysis of FACS-sorted GFP+ and GFP−ve DP thymocytes (CD4+CD8+CD3lo) are shown. Normalized counts of individual mature miRNAs in each population are plotted on x and y-axis respectively (log scale). RNA was pooled from FACS-sorted populations of 3 individual Lin28-RV BM chimeras. (E) Graph shows qRT-PCR validation of mature let-7a and let-7g expression levels in GFP−ve and GFP+ DP thymocytes from Lin28-RV BM chimera. Error bars indicate standard error of triplicate experimental replicates.
Lin28 reprograms adult BM HSPCs to undergo fetal-like B-lymphopoiesis
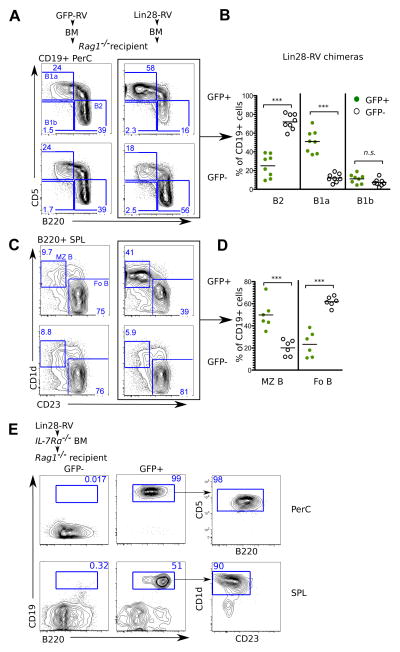
It has been reported that FL HSPCs preferentially differentiate into B-1a cells compared to adult HSPCs (6, 7). To test for the effect of Lin28-RV transduction on the development of B-1a cells in adult mice, the peritoneal cavities of Lin28 retrogenic BM chimeras were analyzed 6–8 weeks post-transplant for donor-derived B cells. Remarkably, Lin28-RV transduced adult HSPCs efficiently generated the B-1a subset, whereas untransduced and GFP-RV transduced adult HSPC controls were biased towards the generation of conventional B-2 cells (Fig. 3A, 3B and S6). In addition to the B-1a subset, growing evidence suggests that a significant component of the MZ B-cell compartment is also of fetal origin (8, 22, 23). Consistent with this notion, Lin28-RV transduced HSPCs reproducibly gave rise to an increased percentage of B220+CD1d+CD23−ve splenic MZ B cells compared to their untransduced (GFP−ve) or GFP-RV transduced controls (Fig. 3C and 3D). Similar results were obtained from hLin28b-RV BM chimeras (Fig. S7A and S7B). These data indicate that the Lin28b/let-7 axis may account for the long-known differences between fetal and adult fate bias in B cell development and that ectopic Lin28 or Lin28b expression in HSPCs is sufficient to confer fetal-like B-lymphopoieisis.
Fig. 3. Ectopic Lin28 expression in adult BM HSPCs confers fetal-like B cell development.
Plots depict flow cytometric analyses of (A) CD19+ peritoneal cavity (PerC) and (C) B220+ splenic (SPL) B-cell subsets in recipients of adult BM HSPCs transduced with the indicated retroviral particles. (B) Graph shows percentages of B-2 (CD19+ B220hi CD5−ve), B-1a (CD19+ B220lo CD5+) and B-1b (CD19+ B220lo CD5−ve) B-lymphocytes among the GFP+ and GFP−ve fractions of peritoneal cavity CD19+ B cells in eight independent BM chimeras. (D) Graph shows percentages of marginal zone (MZ) (B220+ CD1d+ CD23−ve) and follicular B-2 (FoB) cells (B220+ CD1d−ve CD23+) among GFP+ and GFP−ve splenic CD19+ B cells in six independent BM chimeras. (E) FACS analysis show the presence of B-cells in the peritoneal cavity and spleen of BM chimeras reconstituted with Lin28-RV transduced Il7rα−/−BM HSPCs. Data are representative of two independent BM chimeras. ***P value<0.0001 represent statistical significance calculated by t-test. n.s indicates not statistically significant.
Signaling through the IL-7 receptor α-chain (IL7Rα) has been reported to be required for adult B cell development but dispensable for fetal B-lymphopoiesis (1). To further examine the notion that ectopic Lin28 expression reprograms adult BM HSPCs to acquire fetal-like B-cell development we investigated whether Lin28-RV could restore B cell potential in adult Il7rα−/−BM HSPCs. Recipients reconstituted with Il7rα−/−HSPCs transduced with Lin28-RV were analyzed 7 weeks post-transplant for the presence of B cells in the peritoneal cavity and the spleen. In accordance with previous reports, control GFP−ve IL7Rα-deficient donor cells failed to give rise to any detectable population of mature CD19+B220+ B cells. In contrast, sizable populations of GFP+ Lin28-induced B cells were detected in peritoneal cavities and spleens of Lin28-RV BM chimeras (Fig. 3E). Reminiscent of fetal lymphopoiesis, we observed an over-representation of the B-1a and MZ B cell lineages. These results demonstrate that Lin28 acts as a potent reprogramming factor in adult HSPCs to confer fetal-like aspects of B cell development that include independence from IL7Rα-mediated signaling.
Ectopic Lin28 expression favors the development of innate-like T cells
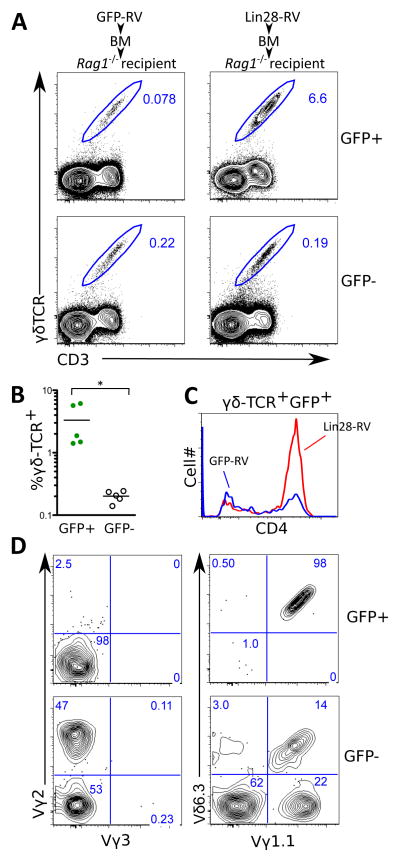
Adult thymocytes mainly differentiate into αβ-T cells but also Vγ2, Vγ1.1 and Vγ5 expressing γδ-T cell subsets while fetal thymocytes preferentially rearrange the Vγ3 and Vγ4 TCR gene segments (24). In addition, one sub-population of Vγ1.1+ T cells is the CD4SP+ Vγ1.1+Vδ6.3+ innate-like T cell subset and has been described to develop perinatally originating primarily from late embryonic precursors (25). Strikingly, we observed a over 15-fold increase in the percentage of total γδ-TCR+ CD3+ cells among GFP+ thymocytes in Lin28-RV BM chimeras compared to either the GFP−ve thymocytes in the same mouse or GFP+ thymocytes in the GFP-RV BM chimeras (Fig. 4A and 4B). The Lin28-induced γδ T cells are primarily CD4SP (Fig. 4C), contributing to an expansion of the overall CD4SP thymocyte compartment (Fig. S8A), and do not express the adult-specific Vγ2-TCR chain or the fetal-specific the Vγ3-TCR chain. The latter is expected since the development of Vγ3+ T cells is known to require both fetal HSCs and a fetal thymus environment (5). Remarkably, we identified Lin28-induced γδ-T cells to be almost exclusively carrying the Vγ1.1+Vδ6.3+ innate-like γδ-TCR that normally arise perinatally (Fig. 4D). Both the innate-like CD4+Vγ1.1+Vδ6.3+ and invariant Vα14+Jα18+ NKT (iNKT) cells are known to express the PLZF transcription factor, and the development of PLZF+ CD4 T-cells is a physiological process in humans during fetal and perinatal stages of ontogeny (26). Indeed, we were able to demonstrate an increased representation of PLZF+γδ-TCR+CD3+ thymocytes in Lin28-RV BM chimeras (Fig. S8B). The ability of Lin28 to induce γδ T cell development correlates with the finding that γδ-TCR+ thymocytes naturally express the highest levels of Lin28b in neonatal mice (Fig. 1E). In line with our findings regarding B cell development, these data support the ability of Lin28 to confer fetal-like lymphopoiesis in the γδ T cell lineage.
Fig. 4. Ectopic Lin28 expression in adult BM HSPCs promotes the development of Vγ1.1+Vδ6.3+ T cells.
(A) Flow cytometric analysis to enumerate γδ-TCR+ CD3+ cells in thymi of GFP-RV and Lin28-RV BM chimeras. (B) Graph shows the percentages of γδ-TCR+ CD3+ thymocytes among GFP+ and GFP−ve cells in the thymi of five independent Lin28-RV BM chimeras. *P value<0.05 represent statistical significance calculated by t-test. (C) Flow cytometric analysis shows distribution of surface CD4 expression among GFP+ γδ-TCR+ CD3+ thymocytes in the Lin28-RV (red line) and GFP-RV BM (blue line) chimeras. (D) Flow cytometric clonotype analysis of γγ-TCR+ CD3+ thymocytes in the GFP+ and GFP−ve fractions of the Lin28-RV BM chimeras.
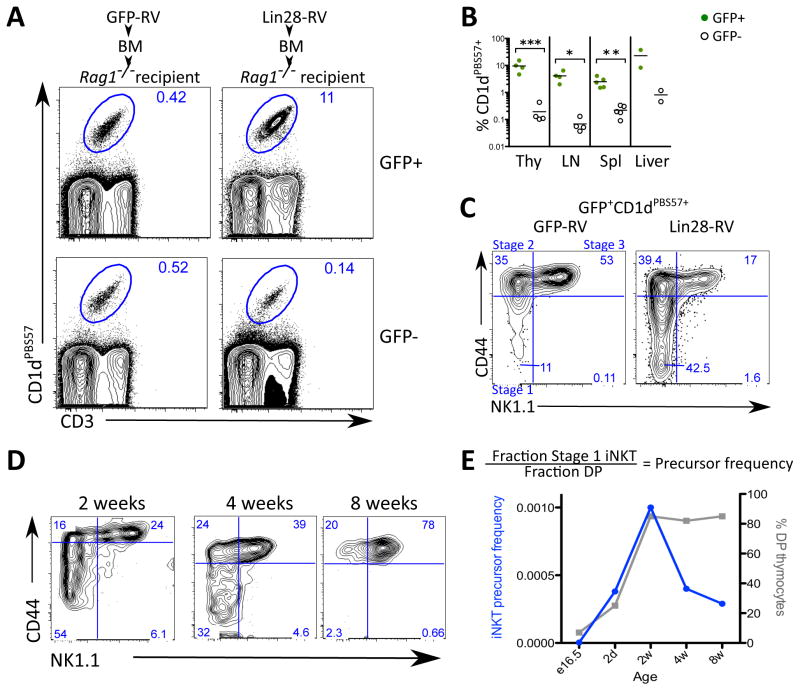
Although the invariant NKT (iNKT) cell lineage has not been directly demonstrated to preferentially arise from fetal precursors, several groups have described a transient burst of rapidly proliferating iNKT cell precursors around day 12 after birth (27, 28), consistent with a biased ontogenic window of development. Our discovery of an increased PLZF+ thymocyte population in thymi of Lin28-RV BM chimeras prompted us to further evaluate the effect of Lin28 on iNKT cell development. We observed a 100-fold increase in the percentage of iNKT (CD1dPBS57+ CD3+) cells among GFP+ thymocytes in the Lin28-RV BM chimeras compared to the GFP−ve thymocytes within the same mouse or a 30-fold increase compared to the GFP+ thymocytes in the GFP-RV BM chimeras (Fig. 5A, Fig. 5B). This dramatic increase in thymic iNKT cells contributes to a Lin28-induced expansion of the overall CD4SP and DN thymocyte compartments (Fig. S8A) as well as the PLZF+ thymocyte pool (Fig. S8B). A dramatic increase in the frequency of GFP+ iNKT cells was also observed in the spleen, lymph node and liver of Lin28-RV BM chimeras (Fig. 5B), translating to higher than expected iNKT cell absolute numbers in the spleen (Fig. S8C). Positive selection of iNKT cells relies on thymocyte-thymocyte interactions mediated by CD1d (29). To determine whether CD1d is required for the development of Lin28-induced iNKT cells, we reconstituted Rag1−/− recipients with Cd1d−/− HSPCs transduced with Lin28-RV. No iNKT cells were detectable in these recipients consistent with the notion that their positive selection requires CD1d (Fig. S9).
Fig. 5. Ectopic Lin28 expression in adult BM HSPCs promotes the development of iNKT cells.
(A) Plots depict flow cytometric analysis of thymic iNKT cells defined by by double staining of CD1d tetramer loaded with PBS57 (CD1dPBS57+) and anti-CD3 in GFP-RV and Lin28-RV BM chimeras. (B) Graphs show the percentages of iNKT cells among the GFP+ and GFP−ve cells in the indicated organs from Lin28-RV BM chimeras. *P<0.05 **P<0.01 ***P<0.005. Indicated P-values represent statistical significance calculated by t-test. (C) Plots depict flow cytometric analysis of developmental stages 1–3 within the iNKT cell compartment among GFP+CD1dPBS57+CD3+ thymocytes in GFP-RV (left) and Lin28-RV BM chimeras (right). Stage 1 (CD44−veNK1.1−ve), 2 (CD44+NK1.1−ve) and 3 (CD44+NK1.1+) (D) Plots depict flow cytometric analysis of developmental stages 1–3 iNKT cells in the thymi of intact wild-type C57BL/6 mice of the indicated age. (E) Precursor frequency analysis show iNKT cell potential as a function of age. Left y-axis (blue line) indicates the ratio of stage 1 to DP thymocytes in intact C57BL/6 mice of the indicated age (equation shown above graph). Right y-axis (grey line) indicates the percentage of DP thymocytes.
Since it is not clear whether iNKT cells develop early during ontogeny, we performed flow cytometric analysis to resolve the proposed developmental stages of the iNKT cell lineage subsequent to the DP thymocyte stage: 1 (CD44−veNK1.1−ve), 2 (CD44+NK1.1−ve) and 3 (CD44+NK1.1+) (27). The composition of the Lin28-induced thymic CD3+CD1dPBS57+ population resembled that of a 2-week-old thymus, characterized by a marked increase in the percentage of immature stage 1 iNKT cells (Fig. 5C and 5D). Since DP thymocytes are precursors of stage 1 iNKT cells, we quantified the efficiency with which DP thymocytes in wild-type C57BL/6 mice of varying ages give rise to stage 1 iNKT by calculating the ratio of stage 1 iNKT cells to DP thymocytes. Consistent with previous reports, our results indicate a peak in iNKT cell potential between 2–4 weeks of age followed by a sharp decline (Fig. 5D and 5E). Thus, our data point towards a higher iNKT cell potential in DP thymocytes of embryonic or neonatal HSPC origin.
Increased numbers of thymic PLZF+ innate-like T cells can produce large amounts of IL-4 and thereby promote thymic development of Eomesodermin (Eomes)+ memory-like CD8 T cells, characterized by high surface expression of CD44 and low surface expression of CD24 (30–32). This phenomenon is not normally detectable in adult wild-type C57BL/6 mice due to the infrequency of PLZF+ thymocytes in this strain (31, 33). However, we observed a significant and global increase in memory-like CD8 T cells among both GFP+ and GFP−ve thymocytes in Lin28-RV BM chimeras but not in the GFP-RV control chimeras (Fig. S10). This result demonstrates the ability of Lin28-induced PLZF+ innate-like T cells to support the differentiation of memory-like CD8 T cells within the thymus and is consistent with the well-characterized IL4-mediated bystander effect that is not cell intrinsic (34).
Ectopic Lin28 confers fetal-like signatures to adult LSK HSPCs
It has been reported that the potential to develop into innate-like lymphocytes is intrinsically programmed in HSCs (5, 11). Therefore, we performed cell cycle and gene expression analyses to look for Lin28-induced fetal characteristics in the BM HSCs of Lin28-RV chimeras. Cell cycle analysis of Lin28-RV transduced BM HSPCs in culture compared to controls did not reveal any significant differences (Fig. S11B). However, this is an artificial system that is highly mitogenic for HSPCs. Furthermore, a number of surface markers including CD34, CD93 (AA4.1), and CD11b are preferentially expressed on FL HSCs, likely due to their heightened proliferative state (35–40). We did not detect any Lin28-induced changes in the expression of these markers, nor that of ESAM, CD48, and Tie-2 within the LSK HSC compartment in Lin28-RV chimeras (Fig. S12A). Together, our results suggest that ectopic Lin28 does not grossly affect the cell intrinsic proliferative capacity or the overall composition of the adult LSK HSC compartment and that increased proliferation alone cannot account for fetal-like developmental potential of HSPCs.
To characterize the effect of ectopic Lin28 on the long-term competence of HSCs, we performed FACS and qRT-PCR analysis of known long-term reconstituting (LT) HSC markers on GFP+ and GFP−ve LSK HSCs from aged Lin28-RV and GFP-RV BM chimeras 10 months following transplantation. CD150 surface expression marks long-term (LT) HSCs (41) and Mecom (Evi1) and Tal1 encode for transcription factors critical in regulating HSC quiescence and long-term competence (42, 43). The presence of GFP+ Lin28-RV transduced LSK HSCs 10 months post-adoptive transfer along with comparable expression of surface CD150 protein, Evi1 and Tal1 mRNA between Lin28-RV and control LSK HSCs suggest that ectopic Lin28 does not interfere with LT-HSCs (Fig. S12).
Hmga2 and Igf2 have been reported to be targets of the Lin28/Let-7 axis (44–46). Consistent with Lin28b being critical in conferring fetal HSPC identity, both Hmga2 and Igf2 are highly expressed in fetal but not adult HSCs (47). To assess if ectopic Lin28 confers fetal-like gene expression profile in adult BM HSCs we performed qRT-PCR of FACS-sorted LSK HSCs and detected marked enhancement in the expression of both genes in the GFP+ fraction from Lin28-RV BM chimeras (Fig. S12B). Collectively, these results demonstrate the ability of ectopic Lin28 expression to confer fetal-like gene expression signatures in LSK HSCs.
In summary, these results identify Lin28b expression as a key molecular feature distinguishing fetal from adult BM HSPCs in mice and humans, shedding new understanding on a longstanding question in the field of lymphocyte development (Fig. S13). We demonstrate that ectopic expression of Lin28 in adult murine BM HSPCs dramatically skews the lymphocyte repertoire towards the production of innate-like lymphocyte subsets. Thus, we propose that Lin28b is a master regulator gene for fetal HSPC identity that is capable of reprograming adult BM HSPCs to acquire fetal-like characteristics in a cell autonomous manner. As a result, Lin28b is a switch that turns on the development of major subsets of innate-like lymphocytes. Together, these findings highlight how post-transcriptional regulation of gene expression can be pivotal in determining cell fate decisions.
Our findings have potential clinical implications for improving the reconstitution of innate-like lymphocyte populations upon adult BM transplantation. Most HSC transplants utilize adult BM and it is not known whether B-1 and MZ B cells develop following hematopoietic reconstitution. Due to the scarcity and ethical limitations of human fetal HSCs, they have not been seriously considered for clinical use. Umbilical cord blood is used clinically, but typically contains too few HSCs to reconstitute an adult (48). In the mouse, innate-like B-1 cell subsets play a role in T-independent host defense against pathogens such as Streptococcus pneumoniae (49); GM-CSF producing, protective innate response activator B cells differentiate from B-1a cells during sepsis (50); Vδ6.3+γδ-T cells provide critical protection against Listeria monocytogenes (51). Furthermore, the ability of iNKT cells to produce large amounts of cytokines including IFNγ, IL-4, and TNF allows them to modulate a broad spectrum of diseases including cancer, graft rejection, autoimmunity and infectious diseases (52). Thus, given the growing awareness of the ability of innate-like lymphocytes to influence immunity, it is of clinical interest to determine if fetal-like HSCs can more effectively achieve reconstitution of the full continuum of the innate and adaptive immune system.
Supplementary Material
Acknowledgments
We thank W.E. Paul for his generous support and advice throughout; T. Bender, J. Daniel, B.J. Fowlkes, R. Germain, M. Lenardo, W.E. Paul, and M. Schlissel for critical reading of this manuscript and constructive suggestions; K. Laky and B.J. Fowlkes for valuable advice and antibodies; NIH Tetramer Core Facility for reagents; M. Holt for NKT cell discussions; P. Burr for sequencing; J. Edwards and C. Eigsti for cell sorting; M. Foster for animal care; R. Zahr for technical support; N. Bartonicek (EMBL-EBI, Hinxton, UK) for providing the RefSeq 3′ UTR library for Sylamer analysis. We gratefully acknowledge the high performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health (biowulf.nih.gov) and NIAID Office of Cyber Infrastructure and Computational Biology HPC cluster (hpcweb.niaid.nih.gov) required for massively parallel sequencing analyses. The data reported in this paper are tabulated in the main text and in the Supporting Online Materials. The RNA-seq and NanoString data are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo) under the accession numbers GSE34854, GSE35081 and GSE35107. We apologize that we could not cite all the relevant references due to space limitations. The Integrative Immunobiology Unit is supported by the Intramural Research Program of the NIAID, NIH.
Footnotes
The authors declare no competing financial interests.
REFERENCES AND NOTES
- 1.Kikuchi K, Kondo M. Developmental switch of mouse hematopoietic stem cells from fetal to adult type occurs in bone marrow after birth. Proc Natl Acad Sci U S A. 2006 Nov 21;103:17852. doi: 10.1073/pnas.0603368103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowie MB, et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci U S A. 2007 Apr 3;104:5878. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzenberg LA. Toward a layered immune system. Cell. 1989 Dec 22;59:953. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 4.Ikuta K, Weissman IL. The junctional modifications of a T cell receptor gamma chain are determined at the level of thymic precursors. J Exp Med. 1991 Nov 1;174:1279. doi: 10.1084/jem.174.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikuta K, et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990 Sep 7;62:863. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- 6.Hardy RR, Hayakawa K. A developmental switch in B lymphopoiesis. Proc Natl Acad Sci U S A. 1991 Dec 15;88:11550. doi: 10.1073/pnas.88.24.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantor AB, Stall AM, Adams S, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A. 1992 Apr 15;89:3320. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimoto M, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011 Jan 25;108:1468. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendelac A, Bonneville M, Kearney J. Autoreactivity by design: innate B and T lymphocytes. Nature reviews Immunology. 2001;1:177. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 10.Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986 Jun 6;45:733. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- 11.Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc Natl Acad Sci U S A. 2011 Aug 16;108:13700. doi: 10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007 Aug 10;130:470. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuchen S, et al. Regulation of microRNA expression and abundance during lymphopoiesis. Immunity. 2010 Jun 25;32:828. doi: 10.1016/j.immuni.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heo I, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009 Aug 21;138:696. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008 Apr 4;320:97. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piskounova E, et al. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J Biol Chem. 2008 Aug 1;283:21310. doi: 10.1074/jbc.C800108200. [DOI] [PubMed] [Google Scholar]
- 17.Piskounova E, et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147:1066. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jotereau F, Heuze F, Salomon-Vie V, Gascan H. Cell kinetics in the fetal mouse thymus: precursor cell input, proliferation, and emigration. J Immunol. 1987 Feb 15;138:1026. [PubMed] [Google Scholar]
- 19.Ranganath S, et al. GATA-3-dependent enhancer activity in IL-4 gene regulation. J Immunol. 1998 Oct 15;161:3822. [PubMed] [Google Scholar]
- 20.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005 Jan 14;120:15. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Krek A, et al. Combinatorial microRNA target predictions. Nat Genet. 2005 May;37:495. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(−/)− mice. J Exp Med. 2001 Oct 15;194:1141. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. J Exp Med. 2008 Sep 1;205:2043. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annual review of immunology. 1993;11:637. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 25.Grigoriadou K, Boucontet L, Pereira P. Most IL-4-producing gamma delta thymocytes of adult mice originate from fetal precursors. J Immunol. 2003 Sep 1;171:2413. doi: 10.4049/jimmunol.171.5.2413. [DOI] [PubMed] [Google Scholar]
- 26.Lee YJ, et al. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. J Exp Med. 2010 Jan 18;207:237. doi: 10.1084/jem.20091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002 Apr 19;296:553. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 28.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001 Oct;2:971. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 29.Bendelac A, et al. CD1 recognition by mouse NK1+ T lymphocytes. Science (New York, NY) 1995;268:863. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 30.Alonzo ES, Sant’Angelo DB. Development of PLZF-expressing innate T cells. Curr Opin Immunol. 2011 Apr;23:220. doi: 10.1016/j.coi.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010 Aug;11:709. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics. Immunity. 2010 Aug 27;33:203. doi: 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai D, et al. KLF13 sustains thymic memory-like CD8(+) T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. J Exp Med. 2011 May 9;208:1093. doi: 10.1084/jem.20101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinreich MA, et al. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009 Jul 17;31:122. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tajima F, Deguchi T, Laver J, Zeng H, Ogawa M. Reciprocal expression of CD38 and CD34 by adult murine hematopoietic stem cells. Blood. 2001;97:2618. doi: 10.1182/blood.v97.9.2618. [DOI] [PubMed] [Google Scholar]
- 36.Randall T, Weissman I. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood. 1997;89:3596. [PubMed] [Google Scholar]
- 37.Rebel V, et al. A comparison of long-term repopulating hematopoietic stem cells in fetal liver and adult bone marrow from the mouse. Experimental hematology. 1996;24:638. [PubMed] [Google Scholar]
- 38.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci U S A. 1995 Oct 24;92:10302. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordan C, McKearn J, Lemischka I. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990;61:953. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- 40.Ito T, Tajima F, Ogawa M. Developmental changes of CD34 expression by murine hematopoietic stem cells. Experimental hematology. 2000;28:1269. doi: 10.1016/s0301-472x(00)00535-x. [DOI] [PubMed] [Google Scholar]
- 41.Kiel M, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 42.Lacombe J, et al. Scl regulates the quiescence and the long-term competence of hematopoietic stem cells. Blood. 2010;115:792. doi: 10.1182/blood-2009-01-201384. [DOI] [PubMed] [Google Scholar]
- 43.Kataoka K, et al. Evi1 is essential for hematopoietic stem cell self-renewal, and its expression marks hematopoietic cells with long-term multilineage repopulating activity. The Journal of experimental medicine. 2011;208:2403. doi: 10.1084/jem.20110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikeda K, Mason P, Bessler M. 3′UTR-truncated Hmga2 cDNA causes MPN-like hematopoiesis by conferring a clonal growth advantage at the level of HSC in mice. Blood. 2011;117:5860. doi: 10.1182/blood-2011-02-334425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayr C, Hemann M, Bartel D. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science (New York, NY) 2007;315:1576. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polesskaya A, et al. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes & development. 2007;21:1125. doi: 10.1101/gad.415007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiel M, Iwashita T, Yilmaz O, Morrison S. Spatial differences in hematopoiesis but not in stem cells indicate a lack of regional patterning in definitive hematopoietic stem cells. Developmental biology. 2005;283:29. doi: 10.1016/j.ydbio.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 48.Brown J, Boussiotis V. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clinical immunology (Orlando, Fla) 2008;127:286. doi: 10.1016/j.clim.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005 Jul;23:7. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Rauch P, et al. Innate Response Activator B Cells Protect Against Microbial Sepsis. Science (New York, NY) 2012 doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belles C, et al. Bias in the gamma delta T cell response to Listeria monocytogenes. V delta 6.3+ cells are a major component of the gamma delta T cell response to Listeria monocytogenes. J Immunol. 1996 Jun 1;156:4280. [PubMed] [Google Scholar]
- 52.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d− dependent NKT cells. J Clin Invest. 2004 Nov;114:1379. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.