Abstract
The male-to-female sex ratio at birth is constant across world populations with an average of 1.06 (106 male to 100 female live births) for populations of European descent. The sex ratio is considered to be affected by numerous biological and environmental factors and to have a heritable component. The aim of this study was to investigate the presence of common allele modest effects at autosomal and chromosome X variants that could explain the observed sex ratio at birth. We conducted a large-scale genome-wide association scan (GWAS) meta-analysis across 51 studies, comprising overall 114 863 individuals (61 094 women and 53 769 men) of European ancestry and 2 623 828 common (minor allele frequency >0.05) single-nucleotide polymorphisms (SNPs). Allele frequencies were compared between men and women for directly-typed and imputed variants within each study. Forward-time simulations for unlinked, neutral, autosomal, common loci were performed under the demographic model for European populations with a fixed sex ratio and a random mating scheme to assess the probability of detecting significant allele frequency differences. We do not detect any genome-wide significant (P < 5 × 10−8) common SNP differences between men and women in this well-powered meta-analysis. The simulated data provided results entirely consistent with these findings. This large-scale investigation across ∼115 000 individuals shows no detectable contribution from common genetic variants to the observed skew in the sex ratio. The absence of sex-specific differences is useful in guiding genetic association study design, for example when using mixed controls for sex-biased traits.
INTRODUCTION
The male-to-female sex ratio at birth is very constant across world populations, ranging between 1.02 and 1.08 (102–108 male to 100 female live births), with an average of 1.06 for populations of European descent (1–3). The sex ratio at birth is mainly determined by factors influencing the primary sex ratio, which is the sex ratio at conception, and those influencing the survival of the embryo (4). Frequently reported primary sex ratio-determining factors include motility and survival time of X-bearing and Y-bearing sperm. A proportion of prenatal mortality can be attributable to immunological interaction between mother and embryo (4). Interestingly, more males are being born in spite of the fact that there is higher mortality of males than females during intrauterine life (4,5). In addition, the sex ratio is considered to be affected by numerous other biological (endogenous) and environmental (exogenous) factors, albeit their influence is generally thought to be of a small effect (1,6). These factors include gonadotropins and/or testosterone concentration at the time of conception, ovulation induction, parental age, parity, birth order, coital rates, infertility, parental illness, maternal malnutrition, smoking, exposure to certain chemicals, stress, war, socioeconomic status and many others (1,6–8). The variation in sex ratio was also observed in many animal and plant species (9). Studies of parasitoid wasps, particularly Nasonia vitropennis, identified several quantitative trait loci (QTL) associated with the sex ratio, pointing to a genetic contribution (9). In addition, many authors suggest that the human sex ratio also has a heritable component. Paternal effects have been proposed to play a role in the sex ratio, for example, men with more brothers tend to have more sons whereas men with more sisters tend to have more daughters (5,10,11). Based on population genetics modelling, Gellatly et al. (11) suggested that the sex ratio is determined by common inheritance of polymorphic autosomal genes that exert their effect through the male reproductive system. Another study of reproductive fitness in the Hutterite population suggested that genetic variants, both autosomal and X-linked, influence natural fertility in humans (12). Research of human births in two-child families observed that sexes of offspring do not follow a binomial model of inheritance where probability of having a boy equals probability of having a girl (13). This study also pointed to the lack of independence among sexes of children of the same parents (13). A couple of possible genetic mechanisms underlying this observation such as Y- and X-linked immunological incompatibilities between mother and embryo have been proposed (13,14).
In the present study, we test whether common variant genetic effects partly underlie the observed male-to-female sex ratio at birth. To address this, we investigate the presence of autosomal and chromosome X variant differences between men and women across 114 863 individuals through large-scale genome-wide association study (GWAS) meta-analysis. We also conduct a forward-time simulation study to assess the probability of observing significant allele frequency differences at autosomal markers between men and women. Our study has high power to detect loci with modest to small effect sizes.
RESULTS
GWAS meta-analysis results
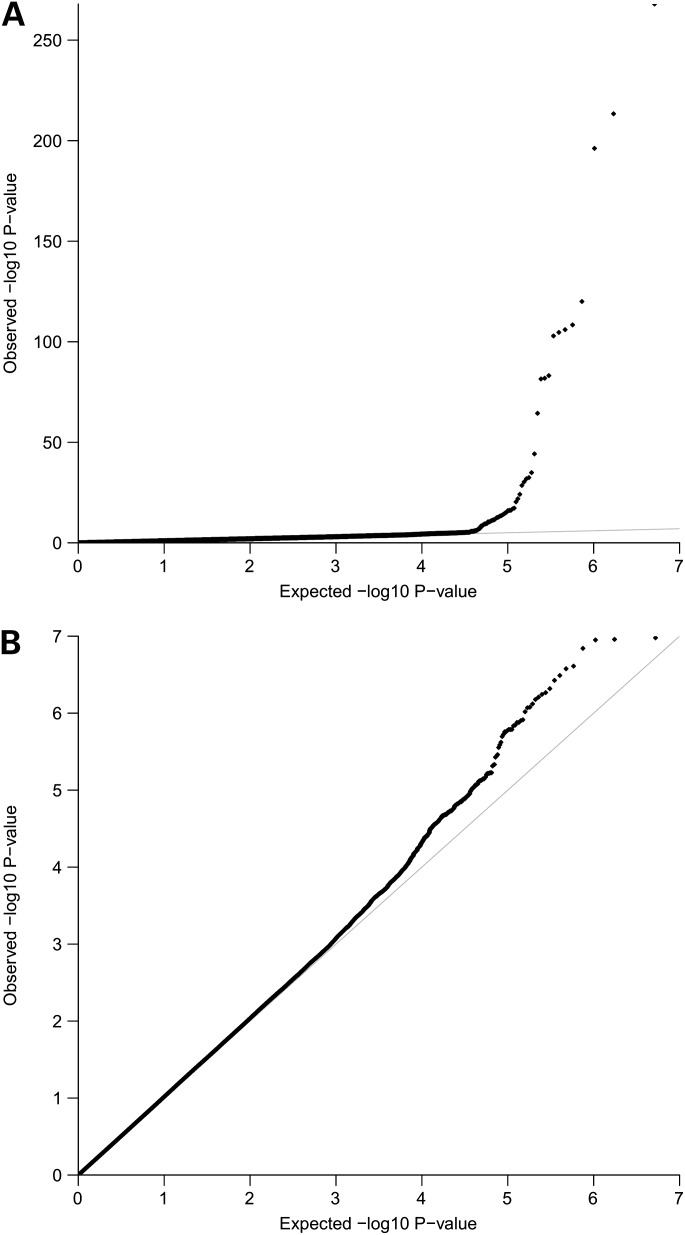
Initial meta-analysis results pointed to an excess of associations compared with the null distribution (Fig. 1A). We examined all genome-wide significant SNPs with effective sample size >10 000 to check for false positives due to genotyping error or other artefacts. We investigated three main diagnostic metrics: poor cluster plots in men or women (Supplementary Material, Fig. S1), sequence similarity on chromosome Y and exact Hardy–Weinberg equilibrium (HWE), P < 1.0 × 10−6 for men or women. Autosomal SNPs that lie in genomic regions that have sequence similarity on the Y chromosome may be incorrectly genotyped/called in men, but not in women, which may give rise to false-positive associations. This can be traced through several quality control (QC) checks in men: excess of heterozygosity, deviation from HWE and poor cluster plots in men and not in women. Some/all of these factors were observed for SNPs designated for exclusion from follow-up (all with highly significant association P-values). We excluded SNPs from the pseudoautosomal chromosome X boundary regions (within 55 kb on the short arm of chromosome X and 115 kb on the long arm that lie next to the non-pseudoautosomal regions) to guard against genotyping error in men (for example, caused by the presence of truncated copies of genes and/or mappings to multiple places across the genome). After exclusion of all poorly genotyped/called SNPs, we detect a single genome-wide significant association at a non-pseudoautosomal chromosome X variant (rs12689384, P = 2.66 × 10−13), which is an intronic variant within RBMX2. As this SNP was imputed in all studies driving the association, we checked cluster plots of all directly typed variants 500 kb upstream and downstream of the associated SNP in all studies, excluded SNPs with poor clustering, re-imputed the region and re-run the meta-analysis. The significance of rs12689384 dropped by five orders of magnitude (allele G, OR = 1.18, 95% CI [1.11–1.25], P = 3.27 × 10−8) but remained nominally genome-wide significant. However, there are several factors that reduce the credibility of this finding. First, 12 studies in total contributed summary statistics for this variant (for a total of 33 259 individuals), out of which six WTCCC1 studies drive the association (Supplementary Material, Table S1). This SNP is imputed in all WTCCC1 studies. Secondly, as shown in the regional association plot (Supplementary Material, Fig. S2), this SNP lacks support for association from neighbouring variants. The next most statistically strong association in the region is modest (P = 1.99 × 10−4) and observed at an SNP (rs2294956) which is in perfect linkage disequilibrium with rs12689384 (r2= 1, D’ = 1) based on HapMap CEU, on which imputation was based. This second SNP (rs2294956) is also imputed in the WTCCC1 studies, but in fact the meta-analysis includes data from over twice the sample size (31 studies, 67 162 individuals, Supplementary Material, Table S1). All studies contributing directly typed data for this variant (46 066 individuals with directly typed data) show no evidence for association, indicating that the signal observed at the imputed variant may be an artefact. We have therefore not considered this single associated SNP any further.
Figure 1.
QQ plots for 2 623 828 directly genotyped and imputed SNPs: (A) for all examined SNPs; (B) after exclusion of poorly genotyped/called SNPs.
The distribution of association P-values after meta-analysis QC was consistent with the null (Fig. 1B). Our study has 80% power to detect OR of 1.13 (at α = 5 × 10−8) for SNPs with minor allele frequency (MAF) >5%, assuming an additive model. We did not further examine SNPs with P-values above the genome-wide significance threshold since our study had sufficient power to detect associations of loci with small-to-modest effect sizes.
Simulation study results
Association analysis of 1 337 699 autosomal common and 135 988 autosomal low-frequency variants in the simulated case–control set matching the empirical study did not identify any differences in allele frequencies between men and women (α = 5 × 10−8). Quantile–quantile (QQ) plots for simulated common and low-frequency variants are shown in Supplementary Material, Figure S3.
DISCUSSION
This large-scale investigation across 114 863 individuals identified no detectable contribution from common genetic variants to the observed skew in sex ratio at birth. This study combined the data from 51 cohorts and has excellent power to detect small-to-modest effect sizes at common loci. The sample sizes contributing to the analysis of chromosome X SNPs were lower due to limited overlap of directly typed SNPs across platforms (in the absence of imputed data across all studies). However, power remains high at over 80% to detect small-to-modest effect sizes. From the phenotypic aspect, sex is a well-characterized trait representing an additional strength of this study, which is unlikely to suffer from phenotype misclassification.
Our results, within the power constraints of our study, indicate that sex-specific selection against particular autosomal genetic variants is not a plausible explanation for the observed male-to-female sex ratio at birth and argue against the hypothesis that incompatible genotypes at common variants between the autosomes and sex chromosomes could lead to miscarriage, thus generating sex-specific genetic differences. We performed forward-time simulations of 1.3 million independent autosomal, common, neutral loci, conditioning on the male-to-female sex ratio at birth, in a cohort matching the original study sample. The lack of any significant allele frequency differences between men and women was in keeping with the findings of the GWAS meta-analysis for autosomal SNPs. We also tested the effects of low-frequency variants in the simulated data, and found no evidence for association with the observed sex ratio at birth. However, we cannot rule out the effects of rare, structural or chromosome Y variants since these were not analysed in our study.
Sex chromosome loci may be relevant for the sex ratio determination due to their expression in the reproductive system, their role in spermatogenesis, sperm morphogenesis and movement and male–female fertility in general (15,16). Therefore, we performed a comprehensive chromosome X analysis involving two main chromosome X regions: pseudoautosomal and non-pseudoautosomal. There are two pseudoautosomal regions (PAR1 and PAR2), which are homologous on X and Y chromosomes, and for which men and women carry two alleles per SNP, whereas for the non-pseudoautosomal region men carry only one allele per SNP. We investigated allele frequency differences between men and women in both chromosome X regions and we observed the association of one non-pseudoautosomal SNP (rs12689384) just below the genome-wide significance level. For various reasons expanded in the Results section, we believe that this variant may be an imputation artefact and have thus not taken it forward to further studies.
The investigated dataset consisted of more women (61 094) than men (53 769). Our meta-analysis incorporated summary statistics deriving from 51 collaborating studies and the vast majority of these studies (36 studies) are population based. The main difference in the sex ratio is driven by these population based studies and the reasons for having fewer men can be heterogeneous and study specific. Most likely the main reasons are the generally recognized lower male response to take part in epidemiological population-based studies (17) and/or sex differences in longevity where women have a higher expected lifespan (18). Fifteen of the 51 contributing studies are disease- rather than population-based and the sex ratio in these studies approximately corresponds to the disease sex ratio in the population. We were driven by the rationale that the sex ratio at birth is constant throughout time and across all world populations, meaning that common variants are more likely to underlie the observed sex ratio at birth. Therefore, in the case of a higher male death rate, we would still have enough power to detect common variant differences due to a very large sample set. However, there are scenarios where this sampling difference between men and women might cause bias, for example there may be a genetic variant that is influencing both the sex ratio and longevity in men. In that case, higher male death rate would cause the removal of this specific genetic variant, thus masking the signal.
Our results have important implications for genetic association study design, for example regarding the selection of control sets for sex-biased traits such as prostate cancer in men or anorexia nervosa in women. The use of single-sex controls for sex-specific diseases generally decreases the sample size and power of a study. Our findings demonstrate that mixed sex controls can be used as an appropriate set in studies of sex-specific traits, when focusing on common loci. As one additional implication for genetic association study analyses, our study stresses the importance of careful pre- and post-analysis QC. QQ plots of our initial meta-analysis results showed high deviation from the null, yet, after QC we observe no inflation of signal. A robust and thorough QC pipeline is necessary to verify any positive association signals, especially in meta-analyses where many studies contribute data that were genotyped (and phenotyped) in many different settings.
We conclude that common genetic variants do not play a role in defining male-to-female sex ratio at birth. In this large-scale meta-analysis of ∼115 000 individuals, we found no allele frequency differences at common loci between men and women. Simulated data of autosomal neutral variants support these findings. Our results can be useful in informing GWAS study design, especially when using mixed controls for sex-biased traits.
MATERIALS AND METHODS
Study samples
We conducted genome-wide meta-analysis across 51 studies, comprising overall 114 863 individuals (61 094 women and 53 769 men) of European ancestry. The characteristics of samples from contributing studies are presented in Supplementary Material, Table S2.
Ethics statement
Each study obtained ethical approval from their respective research ethics committee and all participants gave signed informed consent in accordance with the Declaration of Helsinki.
Genotyping, imputation and QC
All samples were genotyped using commercially available Illumina (Illumina, Inc., San Diego, CA, USA) or Affymetrix (Affymetrix, Inc., Santa Clara, CA, USA) platforms. Imputation of missing genotypes was based on HapMap Phase II genotypes for the European population (CEU). QC of directly typed and imputed variants was conducted separately in each study. Study-specific information on genotyping platforms, imputation methods and QC metrics is presented in Supplementary Material, Table S3. QC checks included tests for relatedness among samples within individual studies.
Genome-wide association analysis of autosomal variants
Case–control association analysis of autosomal SNPs was conducted under the additive model, for directly typed and imputed variants, within each study. Women were coded as cases and men as controls. Association analyses of imputed variants took genotype uncertainty into account, with the exception of the QIMR study which conducted analysis on best-guess genotypes. Where necessary, the first three genotype-based principal components were used as covariates. Studies with related individuals additionally adjusted analyses for family relatedness using linear mixed models. Study-specific association analysis software is presented in Supplementary Material, Table S3.
Chromosome X analysis
Each contributing study performed two separate chromosome X analyses, including pseudoautosomal and non-pseudoautosomal regions. Association analyses were performed, as per autosomes, under the additive model. Overall, 42 studies performed analysis of pseudoautosomal region, 11 of these imputed data using HapMap Phase II, all others used directly typed variants only. For non-pseudoautosomal region, 46 studies performed association analysis, 12 of these used HapMap Phase II imputed data whereas others used directly typed variants only. Study-specific chromosome X imputation/association analysis software is presented in Supplementary Material, Table S3.
GWAS meta-analysis
We performed fixed and random effects meta-analysis to synthesize summary statistics results across contributing studies to identify autosomal and chromosome X common SNP differences between men and women. For meta-analysis purposes, we used GWAMA (19). Prior to meta-analysis, we excluded SNPs with MAF lower than 0.05 and SNPs with low imputation accuracy scores. Specifically, we used a cut-off of rsq_hat < 0.3 for genotypes imputed with MACH (20), BEAGLE (21) and PLINK (22) software and a cut-off of proper info score <0.5 for IMPUTE (23) software. Overall, 2 623 828 directly genotyped and imputed SNPs passed QC criteria and were included in the meta-analysis. The genomic control (GC) inflation factor (lambda) was calculated and applied to correct the results for each study separately prior to the meta-analysis. The meta-analysis results were also corrected for overall lambda GC. The average GC inflation factor across studies was 1.005 for directly genotyped SNPs, 0.97 for imputed SNPs and 1.007 overall, suggesting little population stratification. To determine the effective number of individuals for each study, we calculated effective number of cases (N_eff_case) and multiplied it by 2. N_eff_case was derived using the formula N_eff_case = 2 × N_case × N_ctrl/(N_case + N_ctrl), where N_case and N_ctrl is the number of cases (women) and controls (men), respectively. We investigated evidence of heterogeneity using the I2 statistic (24). Genome-wide significance was set to 5 × 10−8. We created QQ plots to visualize meta-analysis association results. The power of our study was determined using QUANTO (25).
Simulation study
To exclude the possibility that our null results for autosomal variants are due to either sampling bias or data quality and to examine the probability of having false positives within the power constraints of our study, we sought a theoretical corroboration of our empirical results by conducting association analysis in an ‘ideal’ unbiased simulated dataset. Simulated genetic data were produced by means of forward-time simulation (26–28) under a model of a single population with two bottlenecks according to Schaffner et al. (29) with two exceptions: recent exponential growth of population size (instead of instantaneous changes) and final effective population size of 106 (instead of 105), as this has been shown to be the case for the European population (30). Demographic model parameters are given in Supplementary Material, Table S4. The generation time was assumed to be 25 years, and the mutation rate per site per generation was 1.5 × 10−8 (29). We applied a fixed sex ratio and a random mating scheme (i.e. parents are randomly selected irrespective of their genotype) validated by different genetic and demographic models (27). We set a probability of having a male offspring to 0.5122, which corresponds to a male-to-female ratio of 1.05. Simulations were run for 17 000 generations after which we randomly sampled women and men matching the original study for sample size (women = 61 094; men = 53 769).
We simulated unlinked, neutral, autosomal common variants with initial MAF of 0.02 in the founder population (27,31). The total number of simulated loci was 56 502 900, out of which 2.4% were common (MAF > 0.05). We performed allele-based chi-squared association tests on the 1 337 699 common loci. This figure matches the estimated number of independent SNPs in HapMap CEU samples of around 1 million (32). We additionally performed allele-based Fishers exact association tests on 135 988 low-frequency variants (MAF 0.01–0.05). Supplementary Material, Figure S4 shows the MAF spectrum for simulated data compared with the 1000 Genomes Project Pilot 3 CEU (2n = 60) data.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
ACKNOWLEDGMENTS
The authors are grateful to David Clayton, Shaun Purcell and Reedik Magi for their helpful contribution.
WTCCC: This study makes use of data generated by the Wellcome Trust Case Control Consortium. A full list of the investigators who contributed to the generation of the data is available in the Supplementary Material and at www.wtccc.org.uk. Funding for the project was provided by the Wellcome Trust under award 076113.
AGES: The researchers are indebted to the participants for their willingness to participate in the study. This study has been funded by NIH contract N01-AG-1-2100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). The study is approved by the Icelandic National Bioethics Committee, VSN: 00-063.
ARIC: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research.
BLSA: The BLSA was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. A portion of that support was through a R&D contract with MedStar Research Institute.
CoLaus: The authors express their gratitude to the participants in the Lausanne CoLaus study and to the investigators who have contributed to the recruitment. We would like to thank Drs Vincent Mooser and Dawn Waterworth from GlaxoSmithKline for helpful comments and for their continuous support for the CoLaus project. Part of the computation has been performed on the Vital-IT cluster (www.vital-it.ch). We are grateful to Dr. Toby Johnson and Pr. Sven Bergmann for statistical discussions. The CoLaus study was supported by research grants from GlaxoSmithKline; the Faculty of Biology and Medicine of Lausanne, Switzerland; and the Swiss National Science Foundation (grant no:33CSCO-122661). Gérard Waeber and Peter Vollenweider received an unrestricted grant from GSK to build the CoLaus study.
CROATIA-Korcula: We would like to acknowledge the staff of several institutions in Croatia that supported the field work, including but not limited to The University of Split and Zagreb Medical Schools and Croatian Institute for Public Health. The SNP genotyping for the CROATIA-Korcula cohort was performed in Helmholtz Zentrum München, Neuherberg, Germany. The CROATIA-Korcula study was funded by grants from the Medical Research Council (UK), and Republic of Croatia Ministry of Science, Education and Sports research (108-1080315-0302).
CROATIA-Split: We would like to acknowledge the staff of several institutions in Croatia that supported the field work, including but not limited to The University of Split and Zagreb Medical Schools and Croatian Institute for Public Health. The SNP genotyping for the CROATIA-Split cohort was performed by AROS Applied Biotechnology, Aarhus, Denmark. The CROATIA-Split study is funded by grants from the Medical Research Council (UK) and Republic of Croatia Ministry of Science, Education and Sports research grants to I.R. (108-1080315-0302).
CROATIA-Vis: We would like to acknowledge the staff of several institutions in Croatia that supported the field work, including but not limited to The University of Split and Zagreb Medical Schools, Institute for Anthropological Research in Zagreb and Croatian Institute for Public Health. The SNP genotyping for the CROATIA-Vis cohort was performed in the core genotyping laboratory of the Wellcome Trust Clinical Research Facility at the Western General Hospital, Edinburgh, Scotland, UK. The CROATIA-Vis study was funded by grants from the Medical Research Council (UK), European Commission Framework 6 project EUROSPAN (Contract No. LSHG-CT-2006-018947) and Republic of Croatia Ministry of Science, Education and Sports research grants (108-1080315-0302).
ECHRS-Spain: Spanish Ministry of Science and Innovation grant MTM2008-02457; Fondo de Investigaciones Santarias grants 97/0035-01, 99/0034-01 and 99/0034-02; Hospital Universitario de Albacete; Consejeria de Sanidad; FWO (Fund for Scientific Research) grant G.0402.00; University of Antwerp; the Flemish Health Ministry; Sociedad Española de Neumología y Cirugía Torácica (SEPAR), Public Health Service grant R01 HL62633-01; Consell Interdepartamental de Recerca i Innovació Tecnològica (CIRIT) grant 1999SGR 00241; Red Respira Instituto de Salud Carlos III (ISCIII).
EGCUT: EGCUT received financial support from FP7 programs (ENGAGE, OPENGENE), targeted financial support from Estonian Government SF0180142s08, Estonian Research Roadmap through Estonian Ministry of Education and Research, Center of Excellence in Genomics (EXCEGEN) and University of Tartu (SP1GVARENG). The work of K.F. was supported by Estonian Science Foundation grant EstSF ETF9353. We acknowledge EGCUT technical personnel, especially Mr V. Soo and S. Smit. Data analyzes were carried out in part in the High Performance Computing Center of University of Tartu.
ENGAGE: This research was supported through funds from The European Community's Seventh Framework Programme (FP7/2007-2013), ENGAGE Consortium, grant agreement HEALTH-F4-2007-201413.
EPIC-Obesity: The EPIC Norfolk Study is funded by program grants from the Medical Research Council UK and Cancer Research UK.
FENLAND: We are grateful to all the volunteers for their time and help, and to the General Practitioners and practice staff for help with recruitment. We thank the Fenland Study co-ordination team and the Field Epidemiology team of the MRC Epidemiology Unit for recruitment and clinical testing. Inês Barroso and spouse own stock in Incyte Ltd and GlaxoSmithKline.
FUSION: We would like to thank the many Finnish volunteers who generously participated in our study. The Center for Inherited Disease Research performed the GWA genotyping. National Institutes of Health DK062370.
GerMIFS: Supported by the Deutsche Forschungsgemeinschaft and the German Federal Ministry of Education and Research (BMBF) in the context of the German National Genome Research Network (NGFN-2 and NGFN-plus), the FP6 and FP7 EU funded integrated projects Cardiogenics (LSHM-CT-2006-037593) and ENGAGE (201413), and the bi-national BMBF/ANR funded project CARDomics (01KU0908A). Supported by the DZHK (Deutsches Zentrum für Herz-Kreislauf-Forschung – German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research).
HBCS: Helsinki Birth Cohort Study has been supported by grants from the Academy of Finland (Grant No. 120315 and 129287 to EW, 1129457 and 1216965 to KR, 120386 and 125876 to JGE), the Finnish Diabetes Research Society, Folkhälsan Research Foundation, Novo Nordisk Foundation, Finska Läkaresällskapet, the European Science Foundation (EuroSTRESS), the Wellcome Trust (Grant No. 89061/Z/09/Z and 089062/Z/09/Z), Samfundet Folkhälsan, Finska Läkaresällskapet and the Signe and Ane Gyllenberg foundation.
InCHIANTI: We thank the Intramural Research Program of the NIH, National Institute on Aging who are responsible for the InCHIANTI samples. We also thank the InCHIANTI participants. The InCHIANTI study baseline (1998-2000) was supported as a ‘targeted project’ (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 (2001-2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI Follow-ups 2 and 3 studies (2004-2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002); supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland. JRBP is funded by a Sir Henry Wellcome Postdoctoral Fellowship (092447/Z/10/Z).
INGI-CARLANTINO and INGI-FVG: The study was funded Regione FVG (L.26.2008). We thank Laura Esposito, Angela D'Eustacchio and Emmanouil Athanasakis for technical support. We are very grateful to the municipal administrators for their collaboration on the project and for logistic support. We would like to thank all participants to this study.
INGI-VB: We thank the inhabitants of the VB that made this study possible, the local administrations, the Tortona and Genova archdiocese and the ASL-22, Novi Ligure (Al) for support. We also thank Cinzia Sala and Clara Camaschella for data collection supervision and organization of the clinical data collection, Fiammetta Viganò for technical help, Corrado Masciullo and Massimiliano Cocca for building the analysis platform. The research was supported by funds from Compagnia di San Paolo, Torino, Italy; Fondazione Cariplo, Italy and Ministry of Health, Ricerca Finalizzata 2008 and Telethon, Italy to DT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
KORA: The KORA authors acknowledge the contribution of Peter Lichtner, Gertrud Eckstein, Guido Fischer and all members of the Helmholtz Center Munich genotyping staff for generating the SNP data, as well as all members of field staffs who were involved in the planning and conduction of the KORA Augsburg studies. The KORA research platform was initiated and financed by the Helmholtz Center Munich, German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria. Part of this work was financed by the German National Genome Research Network (NGFN-2 and NGFNPlus: 01GS0823). Our research was supported within the Munich Center of Health Sciences (MC Health) as part of LMUinnovativ.
NBS: We thank the participants from the Municipality of Nijmegen for their continued support to the Nijmegen Biomedical Study. The study was partly funded by an investment grant of the Radboud University Nijmegen Medical Centre.
NESDA: Neuroscience Campus Amsterdam; EMGO+ Institute for Health and Care Research; NIMH R01 MH059160; Geestkracht program of ZonMW (10-000-1002); matching funds from universities and mental health care institutes involved in NESDA. Genotyping was funded by the Genetic Association Information Network (GAIN) of the Foundation for the US National Institutes of Health, and analysis was supported by grants from GAIN and the NIMH (MH081802).
NTR: We thank the twins and their families for their participation.
NTR1: Funding was obtained from the Netherlands Heart Foundation (90.313, 86.083 and 88.042), the Netherlands Organization for Scientific Research (NWO: MagW/ZonMW): Genetic basis of anxiety and depression (904-61-090); Genetics of individual differences in smoking initiation and persistence (NWO 985-10-002); Resolving cause and effect in the association between exercise and well-being (904-61-193); Twin family database for behaviour genomics studies (480-04-004); Twin research focusing on behaviour (400-05-717); Genetic determinants of risk behaviour in relation to alcohol use and alcohol use disorder (Addiction-31160008); Genotype/phenotype database for behaviour genetic and genetic epidemiological studies (911-09-032); Spinozapremie (SPI 56-464-14192); CMSB: Center for Medical Systems Biology (NWO Genomics); NBIC/BioAssist/RK/2008.024); BBMRI –NL: Biobanking and Biomolecular Resources Research Infrastructure; the VU University: Institute for Health and Care Research (EMGO+) and Neuroscience Campus Amsterdam (NCA); the European Science Foundation (ESF): Genomewide analyses of European twin and population cohorts (EU/QLRT-2001-01254); European Community's Seventh Framework Program (FP7/2007-2013): ENGAGE (HEALTH-F4-2007-201413); the European Science Council (ERC) Genetics of Mental Illness (230374); Rutgers University Cell and DNA Repository cooperative agreement (NIMH U24 MH068457-06); Collaborative study of the genetics of DZ twinning (NIH R01D0042157-01A); the Genetic Association Information Network, a public–private partnership between the NIH and Pfizer Inc., Affymetrix Inc. and Abbott Laboratories.
NTR2: Funding is acknowledged from the Netherlands Organization for Scientific Research (NWO: MagW/ZonMW): Twin family database for behaviour genomics studies (480-04-004); Twin research focusing on behaviour (400-05-717); Genetic determinants of risk behaviour in relation to alcohol use (Addiction-31160008); Genotype/phenotype database for behaviour genetic and genetic epidemiological studies (911-09-032); Spinozapremie (SPI 56-464-14192); CMSB: Center for Medical Systems Biology (NWO Genomics); NBIC/BioAssist/RK/2008.024); BBMRI –NL: Biobanking and Biomolecular Resources Research Infrastructure; European Science Foundation (ESF): Genome-wide analyses of European twin and population cohorts (EU/QLRT-2001-01254); European Community's Seventh Framework Program (FP7/2007-2013): ENGAGE (HEALTH-F4-2007-201413); the European Science Council (ERC) Genetics of Mental Illness (230374); Rutgers University Cell and DNA Repository cooperative agreement (NIMH U24 MH068457-06).
ORCADES: ORCADES DNA extractions were performed at the Wellcome Trust Clinical Research Facility in Edinburgh. We would like to acknowledge the invaluable contributions of Lorraine Anderson and the research nurses in Orkney, the administrative team in Edinburgh and the people of Orkney. ORCADES was supported by the Chief Scientist Office of the Scottish Government, the Royal Society and the European Union framework program 6 EUROSPAN project (contract no. LSHG-CT-2006-018947).
PREVEND: PREVEND genetics is supported by the Dutch Kidney Foundation (Grant E033), The Netherlands Heart Foundation (Grant 2006B140, 2006T003), National Institutes of Health (grant LM010098, HL65234, HL67466, RR018787) and the EU project grant GENECURE (FP-6 LSHM CT 2006 037697). P.vd.H is supported by NWO VENI grant 91676170 and Dutch Inter University Cardiology Institute Netherlands (ICIN).
QIMR: We thank the twins and their families for their participation. We also thank Dixie Statham, Ann Eldridge, Marlene Grace, Kerrie McAloney (sample collection); Anjali Henders, Megan Campbell, Lisa Bowdler and Steven Crooks (sample and DNA processing); Scott Gordon, David Smyth, Harry Beeby, and Daniel Park (IT support). We also acknowledge David Duffy, Peter Visscher, Margaret Wright, Pamela Madden and Wendy Slutske for their funding contributions. Genotype imputation was carried out on the Genetic Cluster Computer. Funding was provided by the Australian National Health and Medical Research Council (NHMRC grants 241944, 339462, 389927, 389875, 389891, 389892, 389938, 442915, 442981, 496739, 552485, 552498), the Australian Research Council (ARC grants A7960034, A79906588, A79801419, DP0770096, DP0212016, DP0343921), the FP-5 GenomEUtwin Project (QLG2-CT-2002-01254), and the U.S. National Institutes of Health (NIH grants AA07535, AA10248, AA13320, AA13321, AA13326, AA14041, MH66206). The Genetic Cluster Computer is financially supported by the Netherlands Scientific Organization (NWO 480-05-003). G.W.M. was supported by an NHMRC Fellowship (619667), D.R.N. (FT0991022) and S.E.M. (FT110100548) were supported by an ARC Future Fellowship.
RAINE: The authors are grateful to the Raine Study participants and their families and to the Raine Study research staff for cohort coordination and data collection. The authors gratefully acknowledge the NH&MRC for their long term contribution to funding the study over the last 20 years and also the following Institutions for providing funding for Core Management of the Raine Study: The University of Western Australia (UWA), Raine Medical Research Foundation, UWA Faculty of Medicine, Dentistry and Health Sciences, The Telethon Institute for Child Health Research and Women and Infants Research Foundation. The authors gratefully acknowledge the assistance of the Western Australian DNA Bank (National Health and Medical Research Council of Australia National Enabling Facility). The authors also acknowledge the support of the National Health and Medical Research Council of Australia (Grant ID 403981 and ID 003209) and the Canadian Institutes of Health Research (Grant ID MOP-82893).
RS: We thank Pascal Arp, Mila Jhamai, Marijn Verkerk, and Lisbeth Herrera for their help in creating the GWAS database. The authors are grateful to the study participants, the staff from the Rotterdam Study and the participating general practitioners and pharmacists. We would like to thank Dr. Tobias A. Knoch, Karol Estrada, Luc V. de Zeeuw, Anis Abuseiris and Rob de Graaf as well as their institutions the Erasmus Computing Grid, Rotterdam, The Netherlands, and especially the national German MediGRID and Services@MediGRID part of the German D-Grid for access to their grid resources. The generation and management of GWAS genotype data for the Rotterdam Study is supported by the Netherlands Organisation of Scientific Research NWO Investments (nr. 175.010.2005.011, 911-03-012). This study is funded by the Research Institute for Diseases in the Elderly (014-93-015; RIDE2), the Netherlands Genomics Initiative (NGI) – Netherlands Consortium of Healthy Aging (NCHA) project nr. 050-060-810, and funding from the European Commission (HEALTH-F2-2008-201865, GEFOS; HEALTH-F2-2008-35627, TREAT-OA). The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII) and the Municipality of Rotterdam. German MediGRID and Services@MediGRID are part of the German D-Grid and are both funded by the German Bundesministerium fuer Forschung und Technology under grants #01 AK 803 A-H and # 01 IG 07015 G.
SHIP: We thank all staff members and participants of the SHIP study, as well as all of the genotyping staff for generating the SHIP SNP data set. The genetic data analysis workflow was created using the Software InforSense. Genetic data were stored using the database Caché (InterSystems). SHIP is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grants no. 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania, and the network ‘Greifswald Approach to Individualized Medicine (GANI_MED)’ funded by the Federal Ministry of Education and Research (grant 03IS2061A). Genome-wide data have been supported by the Federal Ministry of Education and Research (grant no. 03ZIK012) and a joint grant from Siemens Healthcare, Erlangen, Germany and the Federal State of Mecklenburg- West Pomerania. The University of Greifswald is a member of the ‘Center of Knowledge Interchange’ program of the Siemens AG.
SORBS: We thank all those who participated in the study. Sincere thank is given to Peter Kovacs who was significantly involved in the planning and procedure of the Sorbs study. We also thank Knut Krohn (Microarray Core Facility of the Interdisciplinary Centre for Clinical Research, University of Leipzig) for the genotyping support and Inga Prokopenko and Nigel W. Rayner (WTCHG, University of Oxford, UK) for the excellent analytical and bioinformatics support. This work was supported by grants from the Interdisciplinary Centre for Clinical Research at the University of Leipzig (B27 to A.T.) from the German Diabetes Association (to A.T.), a Travel Grant from BIF (to A.T.) and by the DHFD, Diabetes Hilfs- und Forschungsfonds Deutschland. The work of Vasiliki Lagou was funded through the ENGAGE (European Network for Genetic and Genomic Epidemiology) Consortium, the European Community's Seventh Framework Programme (HEALTH-F4-2007-201413).
TwinsUK: We thank the staff from the TwinsUK, the DNA Collections and Genotyping Facilities at the Wellcome Trust Sanger Institute for sample preparation; Quality Control of the Twins UK cohort for genotyping (in particular Amy Chaney, Radhi Ravindrarajah, Douglas Simpkin, Cliff Hinds, and Thomas Dibling); Paul Martin and Simon Potter of the DNA and Genotyping Informatics teams for data handling; Le Centre National de Génotypage, France, led by Mark Lathrop, for genotyping; Duke University, North Carolina, USA, led by David Goldstein, for genotyping; and the Finnish Institute of Molecular Medicine, Finnish Genome Center, University of Helsinki, led by Aarno Palotie. The authors declare they have no conflicts of interest. The study was funded by the Wellcome Trust; European Community's Seventh Framework Programme (FP7/2007-2013)/grant agreement HEALTH-F2-2008-ENGAGE and the European Union FP-5 GenomEUtwin Project (QLG2-CT-2002-01254) and Framework 6 Project EUroClot. The study also receives support from the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas’ NHS Foundation Trust in partnership with King's College London.
YFS: The Cardiovascular Risk in Young Finns study (YFS) is supported by the Academy of Finland (grant no. 117797, 121584 and 126925), the Social Insurance Institution of Finland, University Hospital Medical funds to Tampere, and Turku University Hospitals, the Finnish Foundation of Cardiovascular Research Emil Aaltonen Foundation (T.L), and Tampere Tuberculosis Foundation.
Conflict of Interest statement. None declared.
FUNDING
V.B. is supported by Unity Through Knowledge Fund CONNECTIVITY PROGRAM (‘Gaining Experience’ Grant 2A), The National Foundation for Science, Higher Education and Technological Development of the Republic of Croatia (BRAIN GAIN—Postdoc fellowship) and the Wellcome Trust (098051). E.Z. is supported by the Wellcome Trust (098051). Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust (098051).
REFERENCES
- 1.Mackenzie C.A., Lockridge A., Keith M. Declining sex ratio in a first nation community. Environ. Health Perspect. 2005;113:1295–1298. doi: 10.1289/ehp.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaba A.J. Sex ratio at birth and racial differences: why do black women give birth to more females than non-black women? Afr. J. Reprod. Health. 2008;12:139–150. [PubMed] [Google Scholar]
- 3.James W.H. The human sex ratio. Part 1: a review of the literature. Hum. Biol. 1987;59:721–752. [PubMed] [Google Scholar]
- 4.Chahnazarian A. Determinants of the sex ratio at birth: review of recent literature. Soc. Biol. 1988;35:214–235. doi: 10.1080/19485565.1988.9988703. [DOI] [PubMed] [Google Scholar]
- 5.Curtsinger J.W., Ito R., Hiraizumi Y. A two-generation study of human sex-ratio variation. Am. J. Hum. Genet. 1983;35:951–961. [PMC free article] [PubMed] [Google Scholar]
- 6.Dodds L., Armson B.A. Is Canada's sex ratio in decline? CMAJ. 1997;156:46–48. [PMC free article] [PubMed] [Google Scholar]
- 7.James W.H. Further evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels around the time of conception. Hum Reprod. 2004;19:1250–1256. doi: 10.1093/humrep/deh245. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M., Fukuda K., Shimizu T., Andersen C.Y., Byskov A.G. Parental periconceptional smoking and male: female ratio of newborn infants. Lancet. 2002;359:1407–1408. doi: 10.1016/S0140-6736(02)08362-9. [DOI] [PubMed] [Google Scholar]
- 9.Pannebakker B.A., Watt R., Knott S.A., West S.A., Shuker D.M. The quantitative genetic basis of sex ratio variation in Nasonia vitripennis: a QTL study. J. Evol. Biol. 2011;24:12–22. doi: 10.1111/j.1420-9101.2010.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trichopoulos D. Evidence of genetic variation in the human sex ratio. Hum. Biol. 1967;39:170–175. [PubMed] [Google Scholar]
- 11.Gellatly C. Trends in population sex ratios may be explained by changes in the frequencies of polymorphic alleles of a sex ratio gene. Evol. Biol. 2009;36:190–200. [Google Scholar]
- 12.Kosova G., Abney M., Ober C. Colloquium papers: heritability of reproductive fitness traits in a human population. Proc. Natl Acad. Sci. USA. 107(Suppl 1):1772–1778. doi: 10.1073/pnas.0906196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlton M.A., Stansfield W.D. Making babies by the flip of a coin? Am. Statist. 2005;59:180–182. [Google Scholar]
- 14.Stansfield W.D., Carlton M.A. Human sex ratios and sex distribution in sibships of size 2. Hum. Biol. 2007;79:255–260. doi: 10.1353/hub.2007.0035. [DOI] [PubMed] [Google Scholar]
- 15.Burgoyne P.S. The role of Y-encoded genes in mammalian spermatogenesis. Sem. Cell Dev. Biol. 1998;9:423–432. doi: 10.1006/scdb.1998.0228. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y.E., Vibranovski M.D., Landback P., Marais G.A., Long M. Chromosomal redistribution of male-biased genes in mammalian evolution with two bursts of gene gain on the X chromosome. PLoS Biol. 2010;8:pii: e1000494. doi: 10.1371/journal.pbio.1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarty C., Wilke R., Giampietro P., Wesbrook S., Caldwell M. Marshfield Clinic Personalized Medicine Research Project (PMRP): design, methods and recruitment for a large population-based biobank. Person. Med. 2005;2:49–79. doi: 10.1517/17410541.2.1.49. [DOI] [PubMed] [Google Scholar]
- 18.May R.C. Gender, immunity and the regulation of longevity. Bioessays. 2007;29:795–802. doi: 10.1002/bies.20614. [DOI] [PubMed] [Google Scholar]
- 19.Magi R., Morris A.P. GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics. 2010;11:288. doi: 10.1186/1471-2105-11-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Ding J., Abecasis G. Mach 1.0: rapid haplotype reconstruction and missing genotype inference. Am. J. Hum. Genet. 2006;79:S2290. [Google Scholar]
- 21.Browning S.R., Browning B.L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 2007;81:1084–1097. doi: 10.1086/521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchini J., Howie B., Myers S., McVean G., Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauderman W.J. Candidate gene association analysis for a quantitative trait, using parent-offspring trios. Genet. Epidemiol. 2003;25:327–338. doi: 10.1002/gepi.10262. [DOI] [PubMed] [Google Scholar]
- 26.Peng B., Amos C.I., Kimmel M. Forward-time simulations of human populations with complex diseases. PLoS Genet. 2007;3:e47. doi: 10.1371/journal.pgen.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng B., Kimmel M. Simulations provide support for the common disease-common variant hypothesis. Genetics. 2007;175:763–776. doi: 10.1534/genetics.106.058164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng B., Kimmel M. simuPOP: a forward-time population genetics simulation environment. Bioinformatics. 2005;21:3686–3687. doi: 10.1093/bioinformatics/bti584. [DOI] [PubMed] [Google Scholar]
- 29.Schaffner S.F., Foo C., Gabriel S., Reich D., Daly M.J., Altshuler D. Calibrating a coalescent simulation of human genome sequence variation. Genome Res. 2005;15:1576–1583. doi: 10.1101/gr.3709305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coventry A., Bull-Otterson L.M., Liu X., Clark A.G., Maxwell T.J., Crosby J., Hixson J.E., Rea T.J., Muzny D.M., Lewis L.R., et al. Deep resequencing reveals excess rare recent variants consistent with explosive population growth. Nat. Commun. 2010;1:131. doi: 10.1038/ncomms1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reich D.E., Lander E.S. On the allelic spectrum of human disease. Trends Genet. 2001;17:502–510. doi: 10.1016/s0168-9525(01)02410-6. [DOI] [PubMed] [Google Scholar]
- 32.Pe'er I., Yelensky R., Altshuler D., Daly M.J. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet. Epidemiol. 2008;32:381–385. doi: 10.1002/gepi.20303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.