Abstract
Objectives:
Isocyanate chemicals essential for polyurethane production are widely used industrially, and are increasingly found in consumer products. Asthma and other adverse health effects of isocyanates are well-documented and exposure surveillance is crucial to disease prevention. Hexamethylene diisocyanate (HDI)-specific serum immunoglobulin G (IgG) was evaluated as an exposure biomarker among workers at a US Air Force Air Logistics Center, which includes a large aircraft maintenance facility.
Methods:
HDI-specific IgG (HDI-IgG) titers in serum samples (n = 74) were measured using an enzyme-linked immunosorbent assay based upon the biuret form of HDI conjugated to human albumin. Information on personal protective equipment (PPE), work location/tasks, smoking, asthma history, basic demographics, and HDI skin exposure was obtained through questionnaire.
Results:
HDI-specific serum IgG levels were elevated in n = 17 (23%) of the workers studied. The prevalence and/or end-titer of the HDI-IgG was significantly (P < 0.05) associated with specific job titles, self-reported skin exposure, night-shift work, and respirator use, but not atopy, asthma, or other demographic information. The highest titers were localized to specific worksites (C-130 painting), while other worksites (generator painting) had no or few workers with detectable HDI-IgG.
Conclusions:
HDI-specific immune responses (IgG) provide a practical biomarker to aid in exposure surveillance and ongoing industrial hygiene efforts. The strategy may supplement current air sampling approaches, which do not assess exposures via skin, or variability in PPE use or effectiveness. The approach may also be applicable to evaluating isocyanate exposures in other settings, and may extend to other chemical allergens.
Keywords: biomarker, exposure, HDI, hygiene, occupational
INTRODUCTION
Isocyanates (R–N=C=O), the low-molecular weight chemicals essential to making polyurethane, are immunogenic, and remain a leading cause of occupational asthma, despite substantial efforts to reduce exposure (Redlich et al., 2007; Tarlo, 2008; Dykewicz, 2009). Once allergic sensitization to isocyanate occurs, asthmatic reactions may be triggered by exceedingly minute concentrations, well below established occupational exposure limits (Redlich and Karol, 2002). Most reported cases of isocyanate asthma occur among workers in end-user settings, where usage is often intermittent and challenging to monitor (Rudzinski et al., 1998; Redlich and Karol, 2002; Sennbro et al., 2004; Henneken et al., 2007). The general public can also be exposed to isocyanates from the growing use of isocyanate-based consumer products (such as do-it-yourself polyurethane spray foam and paints) or as bystanders during isocyanate application in residential or public areas (home/school) (Krone, 2004; Jan et al., 2008). Exposure may also occur from finished polyurethane products, under conditions where free isocyanates are (re)generated, e.g. heat/abrasion, cutting/sanding (Dalene et al., 1997; Boutin et al., 2006). The recognized dangers of isocyanate exposure have prompted the European Union to restrict the aromatic isocyanate, methylene diphenyl diisocyanate, in consumer products (European Parliament and Council on the Registration, Evaluation, Authorization and Restriction of Chemicals [REACH], 22 June 2009).
Exposure surveillance is crucial to disease prevention in environments where isocyanates are used (Lesage et al., 1992; Carlton and England, 2000; Whittaker and Reeb-Whitaker, 2009). Currently, the primary approach to isocyanate exposure surveillance and regulation is air sample analysis, via chemical derivatization and spectrometry (Myer et al., 1993; Streicher et al., 2002). However, the measurement of airborne isocyanate vapor and aerosols presents challenging sampling problems, especially in diverse end-user settings, where usage can be highly variable, dependent upon multiple factors, e.g. solvent usage, catalysts, isocyanate isomer composition, and pre-polymerization (Rudzinski et al., 1998; Carlton and England, 2000; Sennbro et al., 2004; Sparer et al., 2004; Henneken et al., 2007). Additionally, isocyanate skin exposure, which likely contributes to immune sensitization and the development of isocyanate asthma, is not detected through air sampling, and standardized methods for assessing skin exposure remain lacking (Vanoirbeek et al., 2004; Redlich and Herrick, 2008; Wisnewski et al., 2011). Thus, contemporary isocyanate exposure surveillance methods are limited and additional strategies are needed to help identify sources of ongoing exposure.
In the present study, we evaluate the possibility of biomonitoring isocyanate exposure, based on immunologic [immunoglobulin G (IgG)] responses to exposure. Isocyanate-specific IgG are not normally found in human serum (the chemicals are man-made and do not exist naturally), but are present with a relatively high prevalence among exposed workers (Welinder et al., 1988; Lushniak et al., 1998; Redlich et al., 2001; Pronk et al., 2007). The dose–response relationship between isocyanate exposure and isocyanate-specific serum IgG has been difficult to quantify, given the challenges of exposure monitoring; however, positive correlations have been reported from epidemiologic studies of isocyanate-exposed workers, based on timed task-derived personal exposure estimates (Pronk et al., 2007). Similar associations of allergen-specific IgG and exposure have been documented for other occupational allergens, e.g. rat allergens and wheat flour, among laboratory workers and bakers, respectively (Platts-Mills et al., 1987; Tiikkainen and Klockars, 1990; Cullinan, 1998).
The use of serum IgG as an exposure biomarker is a relatively under-appreciated concept in chemical exposure assessment; however, the approach is a well-established strategy for exposure surveillance of infectious agents (Re et al., 1988; Lee et al., 2001). Exposure-induced, antigen-specific IgG is an especially useful biomarker, when methods are not available or practical for directly measuring a given substance within the body (Francis et al. 1988; Re et al. 1988). Isocyanate-specific IgG may provide a similarly practical approach to assessing isocyanate exposure, given the obstacles/challenges of current isocyanate exposure monitoring methods.
In the present study, we evaluated HDI-specific IgG (HDI-IgG) responses in a group of US Air Force (USAF) aircraft maintenance workers, to help assess the effectiveness of ongoing HDI industrial hygiene efforts at their workplace. Aircraft maintenance centers, like many isocyanate end-user settings, present substantial challenges with regard to exposure surveillance, including the complex mixtures of aerosols/vapors, variable timing of chemical use, opportunity for skin exposure, and reliance upon personal protective equipment (PPE) (LaPuma and Bolch, 1999; Carlton and England, 2000; England et al., 2000). In this setting, we demonstrate the practical application of isocyanate-specific IgG as an exposure biomarker, and its potential utility in assisting industrial hygiene efforts.
METHODS
Workers and workplace
Workers at Hill Air Force Base (AFB) of the USAF were invited to attend an informational session conducted by Yale investigators, to explain the study’s purpose and recruit volunteers. Seventy-four subjects volunteered to participate and provided informed consent, then completed a questionnaire, donated 10 ml of blood, and were assigned a unique number to de-identify questionnaire data and blood test results. All work was performed in accordance with the USAF and Yale University rules regarding the ethical conduct of clinical research and protection of human subjects.
HDI-specific IgG
Serum was analyzed for HDI-IgG by an enzyme-linked immunosorbent assay (ELISA), using Nunc MaxiSorp™ microtiter plates (VWR International, West Chester, PA) coated with 1 μg per well of albumin that was conjugated with the biuret form of HDI (see below), as previously described (Wisnewski et al., 2004; Campo et al., 2007). A peroxidase-conjugated anti-human IgG secondary and the substrate 3,3’,5,5’ tetramethyl benzidine were both from Pharmingen (San Diego, CA). HDI-IgG was quantitated as an end-titer, i.e. the maximal dilution that yields a positive ELISA test result, defined as an optical density value >3 standard deviations (SDs) above the mean value obtained with pooled sera of unexposed subjects (n = 12). An HDI-IgG titer >1:8 was considered significantly elevated based upon previously published studies with >1000 different individuals (Redlich et al., 2001; Sparer et al., 2004; Wisnewski et al., 2004; Campo et al., 2007; Pronk et al., 2007).
HDI-conjugated albumin
The biuret of HDI (Bayer, Pittsburgh, PA) was diluted 2-fold in acetone (JT Baker Company; Phillipsburg, NJ) to reduce viscosity, and then diluted another 500-fold with rapid stirring, into a 5 mg*ml-1 solution of human albumin (Sigma Chemical Company, St Louis, MO), so that the final reaction mixture contained 0.1% (v/v) HDI biuret. After 2 h at room temperature, the reaction mixture was 0.2 μm filtered, dialyzed four times against 20-mM phosphate buffered saline, pH 7.2, refiltered, aliquoted, and stored at –20°C.
Data analysis
The results of the serum HDI-antibody analyses and questionnaires were entered into a secure database. HDI-IgG titers were log-transformed and geometric means and SDs were used for analyses. To determine if there were differences in characteristics between groups (positive and negative titers, job categories) with regard to continuous variables, Student’s t-tests were utilized. A pooled variance was used to determine significance, unless the group variances were significantly different at α = 0.05, in which case Satterthwaite’s method was used to calculate variance. Fisher’s Exact test, or in some cases, Cochran–Mantel–Haenszel or chi-squared analyses were used to determine differences between groups with regard to categorical variables. All analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Demographics of study participants
Seventy-four, out of ∼400 Hill AFB workers with potential exposure to isocyanate-containing products, volunteered to participate. The majority of participants were from the 309th Maintenance Wing (MXW), and had a number of different job titles including aircraft painter, aircraft corrosion control, missile equipment/generator corrosion control, and maintenance/vehicle mechanic. The remaining participants, from the 388th Fighter Wing (FW), were all classified as aircraft structural maintenance workers.
Demographic information on ethnicity, age, length of employment, smoking status, and asthma history are summarized in Table 1. The population was predominantly male (89.2%), Caucasian (86.4%), and non-smoking (73%), with a mean age of 37.7 years, and an average 8.1 years employment at Hill AFB. Workers from the 309th MXW were slightly older (mean age 41 versus 26 years) and included more women (13.6 versus 0%), more night shift workers (8.5 versus 0%), more asthmatics (8.5 versus 0%), and more smokers (30.5 versus 13.3%).
Table 1.
Demographics of the study population.
| Variable | All workers, n (%) | 309th MXW, n (%) | 388th FW, n (%) |
| Total workers | 74 (100.0) | 59 (100.0) | 15 (100.0) |
| Gender | |||
| Male | 66 (89.2) | 51 (86.4) | 15 (100.0) |
| Female | 8 (10.8) | 8 (13.6) | 0 (0.0) |
| Ethnicity | |||
| White | 64 (86.5) | 52 (88.1) | 12 (80.0) |
| Hispanic | 7 (9.5) | 6 (10.1) | 1 (6.7) |
| African American | 2 (2.7) | 0 (0.0) | 2 (13.3) |
| Other | 1 (1.35) | 1 (1.7) | 0 (0.0) |
| Age (years) | |||
| Mean ± SD | 37.7 ± 13.3 | 40.6 ± 13.1 | 26 ± 6.2 |
| Range | 20–65 | 24–65 | 20–40 |
| Years worked at Hill AFB | |||
| Mean ± SD | 8.1 ± 7.2 | 8.5 ± 7.6 | 6.5 ± 5.1 |
| Range | 0–35 | 0–35 | 0–20 |
| Shift | |||
| Day | 55 (74.3) | 45 (76.3) | 10 (66.7) |
| Swing | 14 (18.9) | 9 (15.3) | 5 (33.3) |
| Night | 5 (6.8) | 5 (8.5) | 0 (0.0) |
| Current smoker | |||
| Yes | 20 (27.0) | 18 (30.5) | 2 (13.3) |
| Allergic history (n = 73) | |||
| Yes | 30 (41.1) | 26 (44.1) | 4 (26.7) |
| Current asthma | |||
| Current | 5 (6.8) | 5 (8.5) | 0 (0.0) |
PPE use
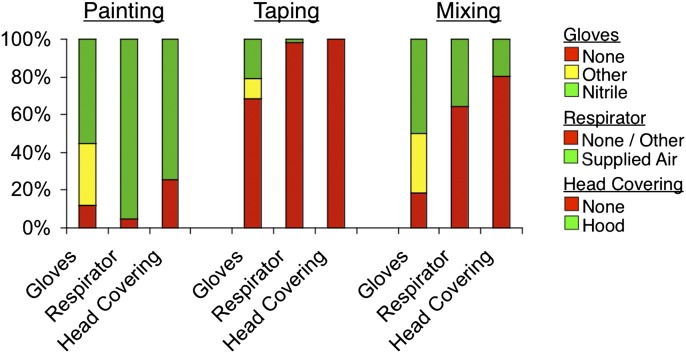
PPE use during different tasks with potential HDI exposure (spray painting, mixing, taping, sandblasting) was derived from questionnaire data, and is summarized in Fig. 1. As shown, nearly all workers reported using optimal respiratory PPE when painting (i.e. 91% reporting using supplied air respirators); however, less protection was worn for other tasks with potential HDI exposure (taping, mixing). Workers from the 388th FW reported being more likely to wear hood covering when painting (P < 0.05), nitrile gloves when taping (P < 0.05), and a respirator when mixing paint (P < 0.01), compared with workers from the 309th MXW (Supplementary Table 1 is available at Annals of Occupational Hygiene online).
Fig. 1.
Frequency of PPE usage by task. The percentage of time workers’ used different PPE, when performing different tasks is displayed on the y-axis. Data were derived from questionnaires.
Respiratory tract and skin exposure assessment
Extensive air sample analyses from Hill and other USAF bases, including measurements of HDI levels during spray painting and similar tasks, are publically available (Poitrast and Carpenter, 1990; LaPuma and Bolch, 1999; Carlton and England, 2000; England et al., 2000; England et al., 2000a; LaPuma, 2001; DeCamp and Murphy, 2006). HDI monomer concentrations generally range from 0.31 to 3.51 μg/m3, well below the permissible exposure level (34 μg/m3) defined by the Occupational Safety and Health Administration (OSHA), based on an 8-h time-weighted average. HDI oligomer levels typically range from <0.01 to 0.36 mg/m3, below the action level (0.5–1 mg/m3) recommended by some individual state governments (oligomeric HDI is not regulated by OSHA) (LaPuma and Bolch, 1999; LaPuma, 2001; Streicher et al., 2002).
Skin exposure to HDI was qualitatively evaluated through questionnaire, and found to be relatively common (Table 2). Over 95% of the workers reported skin exposure to paints, most commonly on the hands and arms, which correlated with the amount of time spent painting and taping (data not shown).
Table 2.
Frequency with which workers noticed paint on skin, and time spent on different job tasks (n = 73).a
| Hours workers spent on job tasks (mean ± SD) | |||||
| Skin exposure | Number of workers (%) | Taping | Painting | Mixing | Sandblasting |
| Frequently | 28 (37.8) | 11.8 ± 10.5 | 9.6 ± 8.0 | 3.2 ± 3.2 | 3.5 ± 6.5 |
| Occasionally | 42 (56.8) | 8.9 ± 7.6 | 8.5 ± 7.7 | 3.3 ± 4.8 | 2.3 ± 4.8 |
| Never | 3 (4.1) | 2.3 ± 3.2 | 4.2 ± 5.2 | 2.3 ± 3.2 | 1.7 ± 2.1 |
Data derived from questionnaires; no data from one worker.
HDI-specific IgG
Of the seventy-four workers who participated in this study, seventeen (23%) had measurable HDI-specific serum IgG levels, with a mean titer >1:100, and individual titers ranging from 1:20 to 1:2560 (Supplementary Tables 2 and 3 are available at Annals of Occupational Hygiene online). Of the seventeen subjects with HDI-specific serum IgG, sixteen (94%) were from the 309th MXW, while only 1 (<7%) was from the 388th FW.
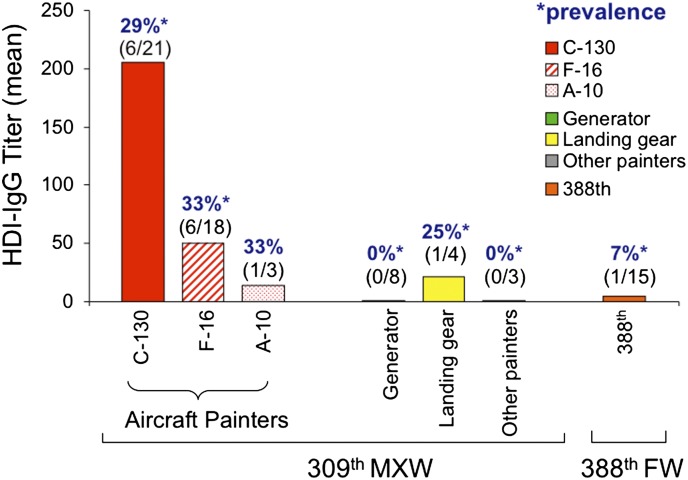
Within the 309th MXW, the prevalence of HDI-IgG was highest among Aircraft Painters (30.8%) compared with other painters, and was similar (28.6–33.3%) among workers on different aircraft types, e.g. F-16, A-10, and C-130 painters (Fig. 2). The lowest prevalence of HDI-IgG was found among the 309th MXW Generator Painters (0%), who, despite reporting the highest number of hours painting per week, primarily undertook tasks with more limited opportunities for exposure. In quantitative terms, the highest titers of HDI-IgG (mean >1:200) were observed among C-130 painters. Notably, three of five (60%) of the night shift workers (all C-130 painters) had elevated levels of HDI-specific serum IgG, with a mean titer >1:800. Together, the data identify workers in specific work zones (C-130 painting), which may benefit most from increased industrial hygiene efforts.
Fig. 2.
HDI-IgG titers among workers at different worksites (wing/organization). The reciprocal of the mean titers of HDI-IgG for each subgroup of three or more workers (different color bars) was determined by ELISA and is depicted on the y-axis. Negative titers <1:4 were assigned a value of 1. Work locations are shown in key. The prevalence (%) and number of positive workers/total number in that location are given above each bar.
Skin exposure, PPE use, and other factors associated with HDI-IgG
Further analyses were performed to identify additional factors that might be associated with HDI exposure, based on correlation with HDI-specific serum IgG titers. Within the entire study population, significant associations of HDI-specific serum IgG were observed with skin exposure and respirator use, but not demographics, total IgE, or self-reported atopy/allergies (Tables 3 and 4). Re-analysis of only the 309th MXW Aircraft Painters, presumably a more homogeneous worker group with greater potential for exposure, also identified a significant association between HDI-IgG and skin exposure, but not respirator use, or other PPE (Supplementary Table 4 is available at Annals of Occupational Hygiene online). In fact, 70.6% (12/17) of the workers with elevated HDI-IgG titers wore supplied air respirators, which provide optimal protection against respiratory tract exposure. Together, the data suggest that immune sensitization can occur despite the use of recommended respirators (and other PPE), and provide further evidence for the potential contribution of skin exposure toward this process.
Table 3.
Comparison of workers with elevated or normal HDI-IgG titers.
| HDI-IgG+ (n = 17) | HDI-IgG− (n = 57) | |
| Titer (mean ± SD) | 120.3 ± 5.0 | 1.0 ± 0 |
| Years worked (mean ± SD) | 11.23 ± 9.9 | 7.1 ± 5.9 |
| Age (mean ± SD) | 40.4 ± 15.5 | 36.9 ± 12.6 |
| Sex, n | ||
| Male | 16 (94.1) | 50 (87.7) |
| Female | 1 (5.9) | 7 (12.3) |
| Hours spent on tasks | ||
| Taping | 9.1 ± 10.1 | 10.0 ± 8.5 |
| Painting | 8.1 ± 7.2 | 8.9 ± 8.0 |
| Mixing | 2.9 ± 2.6 | 3.4 ± 4.5 |
| Sandblasting | 0.6 ± 2.4 | 2.4 ± 5.9 |
| Brush/roll | 1.2 ± 2.5 | 1.3 ± 2.4 |
| Other | 2.6 ± 6.3 | 2.8 ± 5.1 |
| Frequency of skin exposurea,b | ||
| Frequently | 10 (58.8) | 18 (32.1) |
| Occasionally | 6 (35.3) | 36 (64.3) |
| Never | 1 (5.9) | 2 (3.6) |
| Currently smokingc | ||
| Yes | 1 (5.9) | 19 (33.3) |
| PPE use | ||
| Glove type | ||
| Nitrile | 12 (70.6) | 38 (66.7) |
| Latex | 3 (17.6) | 12 (21.1) |
| Other | 1 (5.9) | 4 (7.0) |
| None | 1 (5.9) | 3 (5.3) |
| Respirator typed,e | ||
| Supplied air respirator | 12 (70.6) | 54 (94.7) |
| Cartridge respirator | 3 (17.6) | 1 (1.8) |
| None | 2 (11.8) | 2 (3.5) |
| Head covering | ||
| Hood | 11 (64.7) | 44 (77.2) |
| No hood | 6 (35.9) | 13 (22.8) |
| Total IgE | ||
| ≥50 | 2 (11.8) | 8 (14.0) |
Based on questionnaire, data for one worker was missing.
People who have elevated HDI-IgG are more likely to notice paint on their skin frequently (p<0.05), care less likely to be current smokers (p=0.03), dand are less likely to be wearing supplied air respirators (P < 0.02).
All workers who reported wearing respirators also reported having undergone appropriate fit testing.
Table 4.
HDI-IgG status and skin exposure among workers who paint (n = 67).
| Frequency of skin exposurea | HDI-IgG | Titer | Range |
| No. elevated/total (%) | Mean ± SD | ||
| Frequently | 10/27 (37.0)b | 91.0 ± 262.6 | 1–1280 |
| Occasionally | 5/38 (13.2) | 146.7 ± 578.9 | 1–2560 |
| Never | 0/2 (0) | 1.0 | 0.0 |
Reported frequency of paint on skin for workers who paint based on questionnaire.
Painters who notice paint on their skin frequently (versus occasionally or never) are more likely to have elevated HDI-IgG (P < 0.05).
The only other variable, in the present study, found to be associated with HDI-specific serum IgG was (non)smoking status. The negative association between smoking and HDI-IgG is intriguing in light of the inverse relationship between smoking and isocyanate asthma reported in limited prior studies; however, the implications of such findings remain unclear and will require further analysis to better understand (Mapp et al., 1988; Chan-Yeung, 1990).
DISCUSSION
We have used a relatively novel HDI exposure biomonitoring approach, based on the detection of chemical-specific serum IgG, to assist HDI exposure surveillance efforts at a USAF Air Logistics Center, including a large aircraft maintenance facility. HDI-specific serum IgG levels were found to be elevated in 23% of the study participants, despite extensive industrial hygiene controls and regular PPE use. HDI-IgG titers were significantly (P < 0.05) associated with specific job titles, self-reported skin exposure, night shift work, and respirator use, but not with atopy, asthma, or asthma symptoms. Specific workers, work sites, and practices that would likely benefit most from improved industrial hygiene efforts were identified.
The data demonstrate the potential use of HDI serology, as a strategy to complement contemporary exposure surveillance efforts. As a biomarker, HDI-IgG provides exposure information distinct from that obtained through traditional air sampling approaches, which typically is performed intermittently for selected workers or work areas. Importantly, HDI-IgG biomonitoring may detect exposures missed by air sampling, such as skin exposure, and may account for potential variability in PPE use and/or effectiveness. Serum IgG responses may also detect highly variable (e.g. short-term/high dose or accidental) exposures and/or those limited to distinct locations or tasks, which non-continuous air sampling might miss due to timing or placement of monitoring equipment. Thus, HDI-IgG offers a unique modality for monitoring isocyanate exposures, and can provide information that synergizes with that obtained though current exposure monitoring methods.
The practicality and feasibility of biomonitoring HDI-specific serum IgG by ELISA is a major strength toward its potential incorporation into exposure surveillance programs. HDI-IgG ELISAs, which have recently been improved by using oligomeric (biuret) HDI-albumin as an antigenic basis, can be performed relatively quickly (∼6 h) using small volumes of blood (∼100 μl), in high-throughput fashion, with widely available laboratory equipment (Campo et al., 2007; Wisnewski, 2007). This contrasts with other exposure biomarkers, such as chemically bound self-proteins (adducts) or urinary metabolites, which typically require specialized chromatography and mass spectrometry equipment for detection and quantitation (Sepai et al., 1995; Johannesson et al., 2004). Additionally, the typical half-life of serum IgG (∼1 month) likely provides a stable integrated marker of exposure over the past weeks to months; unlike urine metabolites, whose short half-life (∼2h) captures only a ‘snap-shot’ of exposure, and creates critical time constraints that limit practical utility (Liu et al., 2004).
The present data, along with other recent isocyanate serology and animal studies, refute the hypothesis that isocyanate-specific IgG participates in asthma pathogenesis or represents a biomarker of isocyanate asthma, which had been suggested by limited prior clinical investigations (Cartier et al., 1989; Grammer et al., 1990; Bernstein et al., 1997; Park et al., 1999; Redlich et al., 2001; Herrick et al., 2000; Wisnewski et al., 2004; Matheson et al., 2005; Pronk et al., 2007). These earlier investigations focused primarily on individuals with proven cases of isocyanate asthma, and generally contained limited numbers of healthy exposed workers for comparison. In retrospect, significant association between isocyanate asthma and isocyanate-specific IgG observed by different investigators, in different studies, may reflect higher exposure among affected workers, which may have increased their risk for developing isocyanate asthma. Such data further underscore the importance of exposure surveillance as a critical aspect of isocyanate asthma prevention efforts.
The present finding that 23% of the subjects had elevated HDI-IgG titers, despite extensive industrial hygiene efforts and use of PPE, challenges common perceptions regarding the effectiveness of such practices and current air monitoring approaches of surveillance. However, the findings are consistent with limited, but not widely appreciated, data on auto body spray painters, demonstrating isocyanate exposure underneath nitrile gloves, respirators, and protective clothing (Bello et al., 2008). Notably, the prevalence and titer of HDI-IgG among aircraft workers in the present study is similar to that reported in the auto body repair industry, where it has also been significantly associated with self-reported skin exposure (Redlich et al., 2001). Together, with extensive use of air supply respirators/engineering controls, and available air sample data during similar Air Force paint operations, the present findings further support skin as an important route of occupational isocyanate exposure (LaPuma and Bolch, 1999; LaPuma, 2001; Streicher et al., 2002).
The findings in this report should be interpreted with an understanding of the practical obstacles that commonly hinder such occupational investigations. First, exposure assessment was based on questionnaire data, rather than quantitative measurements, which was beyond the scope of this project, and would have been prohibitively expensive and time consuming. While historical isocyanate air sampling data were available for this AFB, and documented HDI concentrations typically well below the OSHA PEL, the task-based nature of the data limited direct comparison of airborne exposure levels between different subgroups of workers (e.g. 309th MXW versus the 388th FW). For isocyanate skin exposure, quantitative methods remain unvalidated and impractical, necessitating reliance on more qualitative estimates, such as that obtained through self-report questionnaire, as used here. Thus, while elevated HDI-IgG titers were associated with specific jobs and self-reported skin exposure, it was not possible to define dose–response relationships, or to know for sure whether the response resulted from airborne exposures, skin exposures, or both. Epidemiology studies that incorporate quantitative individual and area, air and skin exposure estimates (e.g. including urine metabolites and serum adducts), will be needed to better define dose–response relationships and the contribution of isocyanate skin exposure. The potential influence of factors other than exposure (genetics, environmental co-exposures), on the human IgG response to HDI, as with other antigens, will likely require further empirical investigation.
Other ‘real-world’ limitations of the present investigation include the cross-sectional nature of the study, the relatively small number of participants, and uncertainty regarding what fraction of each group of workers (e.g. F-16 or C-130 Painters) participated; thus, we cannot assess how representative the findings are of the larger group of workers at Hill AFB. Selection biases related to study recruitment and participation could also have influenced results; however, our experience is that workers that practice better industrial hygiene are typically more likely to participate, possibly leading to an underestimate of isocyanate exposures in the larger workforce or industry. Finally, the study was focused on evaluating the practical utility of using HDI-IgG as a biomarker of isocyanate exposure in a group of healthy Air Force workers, and not at identifying health outcomes. The impact of immune-based exposure surveillance on disease prevention will require further investigation.
In summary, we applied an immunologic assay, based on HDI-specific serum IgG responses, to biomonitor HDI exposure at a major US aircraft maintenance facility. The assay is particularly applicable for detecting unsuspected or difficult to quantitate exposures, such as skin exposure, or sporadic, variable airborne exposures, that can easily be missed by current air sample-based exposure surveillance approaches. The technique may also be useful for evaluating the efficacy of PPE. Thus, isocyanate IgG biomonitoring may complement current isocyanate exposure monitoring, and provide supplemental guidance for industrial hygiene efforts. The approach may also be useful to monitor potential isocyanate exposures in other settings, or exposure to other chemical allergens.
CONCLUSIONS
The findings highlight the practicality and potential utility of using isocyanate-specific IgG responses as an exposure biomarker. Isocyanate IgG biomonitoring may help identify exposure via skin, or other sources not detected by current air monitoring methods, and should help focus and improve current industrial hygiene efforts. The data further suggest that skin exposure may contribute to immunologic sensitization and thus may represent a critical component of isocyanate industrial hygiene efforts toward asthma prevention.
SUPPLEMENTARY DATA
Supplementary data can be found at http://annhyg.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences (ES016728 and ES018021 to A.V.W. and K24-ES000355 to C.A.R.) and United States Air Force.
Disclaimer —The authors declare they have no actual or potential competing financial interests.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Helen H. Lee for clinical assistance. The interpretation of the data and conclusions are those of the study’s authors, and do not necessarily represent those of the USAF. The information contained within this manuscript was cleared for public release by the Department of the Air Force on 9 September 2011 (SAF/PA case # 2011-506).
References
- Bello D, Redlich CA, Stowe MH, et al. Skin exposure to aliphatic polyisocyanates in the auto body repair and refinishing industry: II. A quantitative assessment. Ann Occup Hyg. 2008;52:117–24. doi: 10.1093/annhyg/mem066. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Munson J, Lummus ZL, et al. T-cell receptor V beta gene segment expression in diisocyanate-induced occupational asthma. J Allergy Clin Immunol. 1997;99:245–50. doi: 10.1016/s0091-6749(97)70104-0. [DOI] [PubMed] [Google Scholar]
- Boutin M, Dufresne A, Ostiguy C, et al. Determination of airborne isocyanates generated during the thermal degradation of car paint in body repair shops. Ann Occup Hyg. 2006;50:385–93. doi: 10.1093/annhyg/mei075. [DOI] [PubMed] [Google Scholar]
- Campo P, Wisnewski AV, Lummus Z, et al. Diisocyanate conjugate and immunoassay characteristics influence detection of specific antibodies in HDI-exposed workers. Clin Exp Allergy. 2007;37:1095–102. doi: 10.1111/j.1365-2222.2007.02745.x. [DOI] [PubMed] [Google Scholar]
- Carlton GN, England EC. Exposures to 1,6-hexamethylene diisocyanate during polyurethane spray painting in the U.S. Air Force. Appl Occup Environ Hyg. 2000;15:705–12. doi: 10.1080/10473220050110121. [DOI] [PubMed] [Google Scholar]
- Cartier A, Grammer L, Malo JL, et al. Specific serum antibodies against isocyanates: association with occupational asthma. J Allergy Clin Immunol. 1989;84:507–14. doi: 10.1016/0091-6749(89)90364-3. [DOI] [PubMed] [Google Scholar]
- Chan-Yeung M. Occupational asthma. Chest. 1990;98((Suppl. 5)):148S–61S. doi: 10.1378/chest.98.5_supplement.148s. [DOI] [PubMed] [Google Scholar]
- Cullinan P. Occupational asthma, IgE and IgG. Clin Exp Allergy. 1998;28:668–70. doi: 10.1046/j.1365-2222.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- Dalene M, Skarping G, Lind P. Workers exposed to thermal degradation products of TDI- and MDI-based polyurethane: biomonitoring of 2,4-TDA, 2,6-TDA, and 4,4'-MDA in hydrolyzed urine and plasma. Am Ind Hyg Assoc J. 1997;58:587–91. doi: 10.1080/15428119791012522. [DOI] [PubMed] [Google Scholar]
- DeCamp D, Murphy J. 24 April 2006. Consultative Letter, IOH-RS-BR-CL-2006-0045, Isocyanate exposures during polyurethane spray painting operations of a F-16 Thunderbird, Nellis AFB NV. Department of the US Air Force.
- Dykewicz MS. Occupational asthma: current concepts in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2009;123:519–28. doi: 10.1016/j.jaci.2009.01.061. ; quiz 529–30. [DOI] [PubMed] [Google Scholar]
- England E, Key-Schwartz R, Lesage J, et al. Comparison of sampling methods for monomer and polyisocyanates of 1,6-hexamethylene diisocyanate during spray finishing operations. Appl Occup Environ Hyg. 2000;15:472–8. doi: 10.1080/104732200301250. [DOI] [PubMed] [Google Scholar]
- England EC, Carlton GN, Krevonick PL. The impact of ventilation systems on worker exposures during advanced composite material and fiberglass repair operations on military aircraft. Appl Occup Environ Hyg. 2000a;15:391–6. doi: 10.1080/104732200301313. [DOI] [PubMed] [Google Scholar]
- European Parliament and Council on the Registration Evaluation, Authorization and Restriction of Chemicals (REACH) 22 June 2009. Commission Regulation (EC) No. 552/2009, Amending Regulation (EC) No. 1907/2006 of the European Parliament and Council on the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) as regards Annex XVII. Available at http://ec.europa.eu/environment/chemicals/reach/reviews_en.htm#annex17. Accessed 11 November 2011.
- Francis HL, Mann J, Colebunders RL, et al. Serodiagnosis of the acquired immune deficiency syndrome by enzyme-linked immunosorbent assay compared to cellular immunologic parameters in African AIDS patients and controls. Am J Trop Med Hyg. 1988;38:641–6. doi: 10.4269/ajtmh.1988.38.641. [DOI] [PubMed] [Google Scholar]
- Grammer LC, Harris KE, Malo JL, et al. The use of an immunoassay index for antibodies against isocyanate human protein conjugates and application to human isocyanate disease. J Allergy Clin Immunol. 1990;86:94–8. doi: 10.1016/s0091-6749(05)80128-9. [DOI] [PubMed] [Google Scholar]
- Henneken H, Vogel M, Karst U. Determination of airborne isocyanates. Anal Bioanal Chem. 2007;387:219–36. doi: 10.1007/s00216-006-0901-8. [DOI] [PubMed] [Google Scholar]
- Jan RL, Chen SH, Chang HY, et al. Asthma-like syndrome in school children after accidental exposure to xylene and methylene diphenyl diisocyanate. J Microbiol Immunol Infect. 2008;41:337–41. [PubMed] [Google Scholar]
- Johannesson G, Sennbro CJ, Willix P, et al. Identification and characterisation of adducts between serum albumin and 4,4'-methylenediphenyl diisocyanate (MDI) in human plasma. Arch Toxicol. 2004;78:378–83. doi: 10.1007/s00204-004-0555-2. [DOI] [PubMed] [Google Scholar]
- Krone CA. Diisocyanates and nonoccupational disease: a review. Arch Environ Health. 2004;59:306–16. [PubMed] [Google Scholar]
- LaPuma PT. Validation for a recirculation model. Appl Occup Environ Hyg. 2001;16:443–54. doi: 10.1080/10473220120601. [DOI] [PubMed] [Google Scholar]
- LaPuma PT, Bolch WE. The impact of recirculating industrial air on aircraft painting operations. Appl Occup Environ Hyg. 1999;14:682–90. doi: 10.1080/104732299302305. [DOI] [PubMed] [Google Scholar]
- Lee MS, Chien LJ, Yueh YY, et al. Measles seroepidemiology and decay rate of vaccine-induced measles IgG titers in Taiwan, 1995-1997. Vaccine. 2001;19:4644–51. doi: 10.1016/s0264-410x(01)00239-0. [DOI] [PubMed] [Google Scholar]
- Lesage J, Goyer N, Desjardins F, et al. Workers' exposure to isocyanates. Am Ind Hyg Assoc J. 1992;53:146–53. doi: 10.1080/15298669291359410. [DOI] [PubMed] [Google Scholar]
- Liu Y, Berode M, Stowe MH, et al. Urinary hexane diamine to assess respiratory exposure to hexamethylene diisocyanate aerosol: a human inhalation study. Int J Occup Environ Health. 2004;10:262–71. doi: 10.1179/oeh.2004.10.3.262. [DOI] [PubMed] [Google Scholar]
- Lushniak BD, Reh CM, Bernstein DI, et al. Indirect assessment of 4,4'-diphenylmethane diisocyanate (MDI) exposure by evaluation of specific humoral immune responses to MDI conjugated to human serum albumin. Am J Ind Med. 1998;33:471–7. doi: 10.1002/(sici)1097-0274(199805)33:5<471::aid-ajim6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Matheson JM, Johnson VJ, Vallyathan V, et al. Exposure and immunological determinants in a murine model for toluene diisocyanate (TDI) asthma. Toxicol Sci. 2005;84:88–98. doi: 10.1093/toxsci/kfi050. [DOI] [PubMed] [Google Scholar]
- Mapp CE, Boschetto P, Dal Vecchio L, et al. Occupational asthma due to isocyanates. Eur Respir J. 1988;1:273–9. [PubMed] [Google Scholar]
- Myer HE, O'Block ST, Dharmarajan V. A survey of airborne HDI, HDI-based polyisocyanate and solvent concentrations in the manufacture and application of polyurethane coatings. Am Ind Hyg Assoc J. 1993;54:663–70. doi: 10.1080/15298669391355206. [DOI] [PubMed] [Google Scholar]
- Park HS, Kim HY, Nahm DH, et al. Specific IgG, but not specific IgE, antibodies to toluene diisocyanate-human serum albumin conjugate are associated with toluene diisocyanate bronchoprovocation test results. J Allergy Clin Immunol. 1999;104:847–51. doi: 10.1016/s0091-6749(99)70297-6. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA, Longbottom J, Edwards J, et al. Occupational asthma and rhinitis related to laboratory rats: serum IgG and IgE antibodies to the rat urinary allergen. J Allergy Clin Immunol. 1987;79:505–15. doi: 10.1016/0091-6749(87)90369-1. [DOI] [PubMed] [Google Scholar]
- Poitrast BJ, Carpenter D. Sample collection, analysis, and respirator use with isocyanate paints/AFOEHL Report 90-030CA00156BPA. Air Force Occupational and Health Laboratory. Brooks AFB, TX: Human Systems Division; 1990. [Google Scholar]
- Pronk A, Preller L, Raulf-Heimsoth M, et al. Respiratory symptoms, sensitization, and exposure response relationships in spray painters exposed to isocyanates. Am J Respir Crit Care Med. 2007;176:1090–7. doi: 10.1164/rccm.200702-215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re MC, Furlini G, Baldassarri B, et al. Serological study of subjects with seroconversion to human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1988;7:144–8. doi: 10.1007/BF01963067. [DOI] [PubMed] [Google Scholar]
- Redlich CA, Bello D, Wisnewski AV. Health effects of isocyanates. In: Rom WN, editor. Environmental and occupational medicine. 2007. Philadelphia, PA: Lippincott Williams & Wilkins. 502–15. [Google Scholar]
- Redlich CA, Herrick CA. Lung/skin connections in occupational lung disease. Curr Opin Allergy Clin Immunol. 2008;8:115–19. doi: 10.1097/ACI.0b013e3282f85a31. [DOI] [PubMed] [Google Scholar]
- Redlich CA, Karol MH. Diisocyanate asthma: clinical aspects and immunopathogenesis. Int Immunopharmacol. 2002;2:213–24. doi: 10.1016/s1567-5769(01)00174-6. [DOI] [PubMed] [Google Scholar]
- Redlich CA, Stowe MH, Wisnewski AV, et al. Subclinical immunologic and physiologic responses in hexamethylene diisocyanate-exposed auto body shop workers. Am J Ind Med. 2001;39:587–97. doi: 10.1002/ajim.1058. [DOI] [PubMed] [Google Scholar]
- Rudzinski WE, Yin J, Norman SH, et al. Determination of hexamethylene-based isocyanates in spray-painting operations. Part 1. Evaluation of a polyurethane foam sponge sampler. Analyst. 1998;123:2079–83. doi: 10.1039/a803942i. [DOI] [PubMed] [Google Scholar]
- Sennbro CJ, Ekman J, Lindh CH, et al. Determination of isocyanates in air using 1-(2-methoxyphenyl)piperazine-impregnated filters: long-term sampling performance and field comparison with impingers with dibutylamine. Ann Occup Hyg. 2004;48:415–24. doi: 10.1093/annhyg/meh035. [DOI] [PubMed] [Google Scholar]
- Sepai O, Henschler D, Sabbioni G. Albumin adducts, hemoglobin adducts and urinary metabolites in workers exposed to 4,4′-methylenediphenyl diisocyanate. Carcinogenesis. 1995;16:2583–7. doi: 10.1093/carcin/16.10.2583. [DOI] [PubMed] [Google Scholar]
- Sparer J, Stowe MH, Bello D, et al. Isocyanate exposures in autobody shop work: the SPRAY study. J Occup Environ Hyg. 2004;1:570–81. doi: 10.1080/15459620490485909. [DOI] [PubMed] [Google Scholar]
- Streicher RP, Reh CM, Key-Schwartz R, et al. Selecting isocyanate sampling and analytical methods. Appl Occup Environ Hyg. 2002;17:157–62. doi: 10.1080/104732202753438234. [DOI] [PubMed] [Google Scholar]
- Tarlo SM. Occupational exposures and adult asthma. Immunol Allergy Clin North Am. 2008;28:563–76. doi: 10.1016/j.iac.2008.03.002. , viii. [DOI] [PubMed] [Google Scholar]
- Tiikkainen U, Klockars M. Clinical significance of IgG subclass antibodies to wheat flour antigens in bakers. Allergy. 1990;45:497–504. doi: 10.1111/j.1398-9995.1990.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Vanoirbeek JA, Tarkowski M, Ceuppens JL, et al. Respiratory response to toluene diisocyanate depends on prior frequency and concentration of dermal sensitization in mice. Toxicol Sci. 2004;80:310–21. doi: 10.1093/toxsci/kfh155. [DOI] [PubMed] [Google Scholar]
- Welinder H, Nielsen J, Bensryd I, et al. IgG antibodies against polyisocyanates in car painters. Clin Allergy. 1988;18:85–93. doi: 10.1111/j.1365-2222.1988.tb02847.x. [DOI] [PubMed] [Google Scholar]
- Whittaker SG, Reeb-Whitaker C. Characterizing the health and safety needs of the collision repair industry. J Occup Environ Hyg. 2009;6:273–82. doi: 10.1080/15459620902775609. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV. Developments in laboratory diagnostics for isocyanate asthma. Curr Opin Allergy Clin Immunol. 2007;7:138–45. doi: 10.1097/ACI.0b013e3280895d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Stowe MH, Cartier A, et al. Isocyanate vapor-induced antigenicity of human albumin. J Allergy Clin Immunol. 2004;113:1178–84. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Xu L, Robinson E, et al. Immune sensitization to methylene diphenyl diisocyanate (MDI) resulting from skin exposure: albumin as a carrier protein connecting skin exposure to subsequent respiratory responses. J Occup Med Toxicol. 2011;6:6. doi: 10.1186/1745-6673-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.