Abstract
Oral cancer ranks in the top three of all cancers in India, which accounts for over thirty per cent of all cancers reported in the country and oral cancer control is quickly becoming a global health priority. This paper provides a synopsis of the incidence of oral cancer in India by focusing on its measurement in cancer registries across the country. Based on the International Classification of Disease case definition adopted by the World Health Organisation, and the International Agency for Research on Cancer, this review systematically examines primary and secondary data where the incidence or prevalence of oral cancer is known to be directly reported. Variability in age-adjusted incidence with crude incidence is projected to increase by 2030. Challenges focus on measurement of disease incidence and disease-specific risk behavior, predominantly, alcohol, and tobacco use. Future research should be aimed at improving quality of data for early detection and prevention of oral cancer.
1. High Burden of Oral Cancer in India
Oral cancer is a major problem in the Indian subcontinent where it ranks among the top three types of cancer in the country [1]. Age-adjusted rates of oral cancer in India is high, that is, 20 per 100,000 population and accounts for over 30% of all cancers in the country [2]. The variation in incidence and pattern of the disease can be attributed to the combined effect of ageing of the population, as well as regional differences in the prevalence of disease-specific risk factors [3].
Oral cancer is of significant public health importance to India. Firstly, it is diagnosed at later stages which result in low treatment outcomes and considerable costs to the patients whom typically cannot afford this type of treatment [4]. Secondly, rural areas in middle- and low-income countries also have inadequate access to trained providers and limited health services. As a result, delay has also been largely associated with advanced stages of oral cancer [5]. Earlier detection of oral cancer offers the best chance for long term survival and has the potential to improve treatment outcomes and make healthcare affordable [6]. Thirdly, oral cancer affects those from the lower socioeconomic groups, that is, people from the lower socioeconomic strata of society due to a higher exposure to risk factors such as the use of tobacco [7]. Lastly, even though clinical diagnosis occurs via examination of the oral cavity and tongue which is accessible by current diagnostic tools, the majority of cases present to a healthcare facility at later stages of cancer subtypes, thereby reducing chances of survival due to delays in diagnosis [8].
Public health officials, private hospitals, and academic medical centres within India have recognised oral cancer as a grave problem. Efforts to increase the body of literature on the knowledge of the disease aetiology and regional distribution of risk factors have begun gaining momentum. Oral cancer will remain a major health problem and efforts towards early detection, and prevention will reduce this burden. In light of this, the objective of this paper is to review and summarise existing literature on the descriptive epidemiology of oral cancer in India, focusing on the incidence of disease in the country.
2. Case Definition of Oral Cancer
Due to the heterogeneity of pathologies presented in oral cavity tumour research, as well as the intraoral cavity evaluation with respect to the subsites such as the oropharynx, the case definition for oral cancer has been further complicated. Due to this failure to specify and define oral cancer in peer-reviewed literature, meaningful comparisons for description and epidemiological purposes have proved to be a challenge. To minimise misclassification errors and for the purpose of this review, oral cancer is defined as the cancer of the lip, mouth, and tongue, to include the anatomic description of the oral cavity as reported in previous major population-based research reports [9]. This case definition is adopted, and conforms to the definitions of oral cavity cancer by the International Classification of Diseases (ICD) Coding scheme, WHO case definitions and IARC. Based on these criteria, oral cavity cancer is the 8th most frequent cancer in the world among males and 14th among females [6], the main risk factors being tobacco and alcohol use.
3. Search Strategy
A systematic search of the literature was accomplished using the Pubmed Database. Medical Subject headings and free text terms included the following.
“oral cancer” OR “mouth cancer” OR “tongue cancer.” The use of these terms generated a list of numerous MeSH entry terms which included subheadings of these main terms to include mouth neoplasms, oral neoplasms, cancer of the mouth, and head and neck cancers. All these variations of the term were added to the search, except head and neck cancers since, this did not meet the case definition criterion.
“Epidemiology” OR “Descriptive Statistics” OR “Incidence” OR “Prevalence” OR “Longitudinal” OR “Cohort” OR “Case Control” OR “Cross sectional.”
Free text terms included
-
(3) India OR South Asia.
All three search terms were stringed together to perform a targeted search with the following inclusion criteria.
Studies where incidence or prevalence of oral cancer was measured.
Population-based studies—Hospital-/Community-based registries.
Studies with standardised criteria for diagnosis of oral cancer.
Studies published in the English language.
The search strategy included all published studies referencing the incidence and, if possible, prevalence of oral cancer in various regions of India. Even though at first it was thought it would be important to limit the search to include only the most recent up-to-date studies from the past ten years, it was later decided that it would be important to include all studies in the initial review due to a dearth of available peer-reviewed literature on the specified case definition. The search revealed few frequency studies to include the associated risk factors for the disease, such as use of alcohol and tobacco. Even though a few of these studies were selected in the analysis, for the purpose of this paper, it was decided not to focus solely on these studies due to the heterogeneity in the case definitions of oral cancer.
Only studies presenting primary research were included as part of this review. All studies were included if they measured incidence of oral cavity cancer in a standardised manner. A number of studies generated through the search, presented primary data from national and regional cancer registries in India and were included in this review. Additionally, for the purpose of representativeness, information was also retrieved from an online repository of cancer registration data available through IARC, the cancer incidence in five continents series (CI5), volumes I–IX, the Plus data collection, and the Globocan Project; all of which are cancer incidence and mortality projects conducted under the auspices of the WHO.
4. Search Results
Out of the initial 416 articles generated from the first search, the abstracts were reviewed for determining inclusion based on the established criteria. Once review was completed, 28 studies were selected as relevant to the search. However, after reviewing full texts of all articles, 11 studies were determined to be types of studies which only measure the prevalence of the associated risk factors such as smoking or alcohol use without a direct measure of the incidence or prevalence of oral cancer. These 11 studies were excluded.
17 of these articles were included as studies based on primary data where the incidence or prevalence of oral cancer as per case definitions was known to be directly reported. Following an in-depth full-text review of the 17 studies, additional articles with secondary data were obtained via the references checks. These articles were only used as reference articles; no data was included in the analysis. A majority of these studies, namely 11were cross-sectional studies, 8 of which described data from national and regional cancer registries within the country (Box 1 and Table 1). In most cases, registry data was also utilised as baseline data on population characteristics. Three of the studies described data from population-based prospective cohort studies; one of the studies utilised a randomized control trial conducted using a population-based cancer registry, one of the studies utilised a mixed methods approach to include a combination of cross-sectional design, ten-year followup and an intervention study. Only one of the studies utilised a case-control method nested within a population-based cancer registry. No specific review articles were identified in the search.
Box 1.
List of cancer registries reporting incidence of oral cancer.
Table 1.
Summary of study design and sample characteristics of all studies included in this review of oral cancer in India.
| First author | Period of study | Study design | Age | City, state (region) | Sample size (n) |
|---|---|---|---|---|---|
| Manoharan et al. [3] | 2001–2005 | Cross-sectional study using a population-based cancer registry. Data collected on new cancer cases diagnosed among Delhi urban resident population. The sources for cancer registration are more than 162 government hospitals/centres and 250 private hospitals and nursing homes. | 0–80 | Delhi (urban) | 54,554 participants 28,262 males, 26,292 females |
|
| |||||
| Sunny et al. [10] | 1986–2000 | Cross sectional study using a population-based cancer registry Mumbai population-based cancer registry. | 0–80 | Mumbai, Maharashtra (urban) | 9,670 Participants with oral cancers registered, 6577 males, 3093 females |
|
| |||||
| Thorat et al. [11] | 1995 | Cross-sectional random sample of villages from Barshi rural cancer registry. House to house recruitment visits Eligibility: adult males were only included; interviewed for tobacco habits in 1995 and again in 2004-2005. | >14 years | Barshi (rural) and Mumbai (urban), Western Maharashtra | 5,319 enrolled out of a random sample of 6,673 enrolled |
|
| |||||
| Elango et al. [1] | 1986–1998 | Retrospective Study using data collected as part of a Cancer Registry of an urban and rural population reviewed over a 13 and 11 year period. Cancer registry data monitored by National Cancer Registry Programme of the Indian Council of Medical Research (ICMR). | 0–80 years | Chennai, Tamil Nadu (urban) & Barshi, Maharashtra (rural) | Urban registry recorded head and neck cancers. 6, 857 total; 4777 males, 2080 females. Rural registry recorded 325 total; 272 males, 53 females |
|
| |||||
| Sankaranarayanan et al. [2] | 1996–2004 | Cluster-randomized, controlled trial of oral cancer screening in southern India. Participants were arranged in 13 clusters and randomized to either an intervention group or a control group. Subjects in the intervention group received 3 rounds of screening consisting of oral visual inspection by trained health workers at 3-year intervals. | >35 years | Kerala, South India (urban) | 59,894 eligible subjects in the intervention group and 54,707 in the control group; 31.4% of the former group reported no tobacco or alcohol habits, compared with 44.1% of the latter |
|
| |||||
| Gupta et al. [12] | 1977–1982 | Case-control study conducted as a house to house survey interviewed for tobacco habits and examined for the presence of oral leukoplakia and precancerous lesions in a survey and then over a five-year period. Control group included the first 5 year results of a ten year followup study conducted in the same areas, but with different individuals within the population. Study was nested within another survey study using a cancer registry in Ernakulum, Srikakulam, and Bhavanagar. | >15 years | 3 locations; Ernakulum, Kerala, Srikakulam, AP and Bhavanagar, Gujarat (rural) | 36,471 participants selected based on use of tobacco chewing products and smoking. Followup rate: 97% |
|
| |||||
| Malaowalla et al. [13] | 1976 | Prospective cohort of industrial workers examined for oral lesions, and reexamined after a 2 year interval; biopsies conducted. | >35 years | Gujarat (urban) | 57,518 participants selected based on presence of oral lesions |
|
| |||||
| Swaminathan et al. [14] | 2003–2006 | Cross sectional study using Cancer registry including the registration of incident cancer cases occurring in the resident population carried out by active case finding from medical records at major hospitals in government and private sectors, nursing homes, consultants, radiation centers, pathology laboratories, imaging centers, and hospices. House visits were undertaken annually for each registered case for data completion. | 0–80 | Dindigul District, Tamil Nadu-South (rural) | 4516 incident cancers participants included in sample |
|
| |||||
| Mehta et al. [15] | Date of Publication 1989 (date of study unknown) | Cross-sectional study using house to house recruitment techniques conducted during a population-based survey of tobacco users. Participants excluded based on loss to followup. Baseline diagnosis was conducted in 1977 and 106 in nine annual followup examinations conducted through the course of the study. | 15–44 years | Ernakulam district, Kerala India (rural) | 182 participants selected on the basis of presence of tongue lesions and the use of tobacco. 16 excluded from analyses |
|
| |||||
| Gupta et al. [16] | 1977-1978 (Baseline conducted) | Prospective cohort, house to house recruitment, interviewed and examined in baseline survey to record details of tobacco use and examined for presence of oral cancer and reexamined annually for 10 years. Eligibility: any type of tobacco habit. | >15 years | Ernakulam district, Kerala India (rural) | 12,212 participants |
|
| |||||
| Khandekar et al. [4] | 1999-2000 | Cross-sectional study, hospital based, selected based on patient reporting to outpatient dental clinic department for oral complaints and provisional confirmation of clinical diagnosis. Patient interviews conducted. 117 initially included, 13 excluded due to insufficient information, and 24 declined biopsy. | Majority >51–60 years (Range: 1–71 years) | Nagpur, Maharashtra (urban) | 80 cases of oral cancer; registered with demographic characteristics and history of tobacco use |
|
| |||||
| Maudgal et al. [17] | Date of Publication 2010, (date of study unknown) | Cross-sectional study conducted at 12 organizations and community centres; children with suspected vulnerability to tobacco usage interviewed and screened by trained social workers for precancerous lesions. Children suspected with suspicious oral lesions sent for further evaluation at diagnostic cancer facility. | 3–21 years | Regions of Maharashtra and Assam (urban and rural) | 1700 participants checked for precancerous lesions |
|
| |||||
| Cancela et al. [9] | 1996–1999 | Prospective cohort study embedded into a cluster randomized oral cancer screening trial evaluating role of alcohol intake and oral cavity caner risk; participants completed baseline lifestyle questionnaire on frequency and duration of alcohol consumption, and followed up for oral cancer incidence and mortality in Trivandrum oral cancer screening study. | >35 years | Trivandrum, Kerala; (urban area) | 32,347 male participants |
|
| |||||
| Mehta et al. [18] | 1966 | Mixed Methods Study was conduced in 3 phases: first phase of the study consisted of a cross-section survey to determine the prevalence of oral cancer and precancerous lesions, second phase was a ten year followup survey to determine the incidence and natural history of oral precancer third phase was an intervention study aimed at persuading subjects to give up tobacco, and to measure the subsequent changes in the incidence and regression rate of oral precancer. | >15 years | Ernakulum district, Kerala, Bhavnagar district, Gujarat, and Srikakulam district, AP (rural) | 12 000 participants selected on the basis of tobacco use |
|
| |||||
| Van der Eb et al. [19] | 1991-1992 | Cross-sectional study, participants randomly selected to be interviewed. All persons were visited at home for an examination of the oral cavity and a detailed interview. Physical examination of the mouth was carried out before the detailed interview. Structured questionnaire was used for data collection Information about smoking status, diet, and access to mass media was obtained in each case and an examination of the oral cavity was performed. | >21 years | North Coastal Areas of AP (rural) | 480 participants |
|
| |||||
| Jayalekshmi et al. [20] | 1990–2005 | Cross-sectional study of all residents in the area using cancer registry; all the households were visited by trained interviewers. Information collected on sociodemographic factors, religion, family income in rupees, education, occupation, lifestyles, and other factors, using a 6-page standardised questionnaire, baseline information collected on lifestyle, including tobacco chewing, and sociodemographic factors during the period. | 30–84 years | Karunagappally, Kerala, India (rural) | 78,140 female-92 oral cancer cases |
|
| |||||
| Wahi [21] | 1964–1966 | Cross sectional study conducted in order to set up cancer registry at a local medical school in association with WHO, seven rural cancer detection clinics were setup and staffed by a team of specialists; an interview study was carried out for a 10% random sample of population using the cluster sampling method. | >35 years | Mainpuri district near Agra (rural) | 34,997 participants 600 cases of oral cancer registered; 346 confirmed |
5. Summary of Findings
Summary of the study designs and characteristics of all the studies included in this review is presented in Table 1. Data reported in these studies span the last thirty five years across a number of regions within India. A number of these studies utilised data from urban and rural cancer registries established at the national or regional level. Urban registries included Delhi, Mumbai and Chennai, and rural registries included Barshi, Dindigul, Mainpuri, Karunagapally, Ernakulum, Srikakulam, and Bhavanagar.
Various study designs were employed to obtain a sample reflective of the Indian population. Most of these studies were population-based cross-sectional studies utilising cancer registry data, with the exception of some studies. Gupta et al. [12] conducted a case-control study; however, this study was nested within a larger study utilising a rural based population registry. Sankaranarayanan et al. [22] utilised a community-based cluster-randomised controlled trial where participants were randomised to either an intervention group or a control group to test the effect of a screening programme on the oral cancer incidence and mortality. Mehta et al. [15] utilised a mixed methods approach conducted in different phases. Malaowalla et al. [13], Gupta et al. [23], and Cancela et al. [9] conducted population-based prospective cohort studies to examine the incidence of oral cancer tracked prospectively over a period of time.
Since most of the studies included national and regional surveys of a population, a wide range of ages were included; however, Khandekar et al. [4] selected participants in an older age group (>51–60 years) and Maudgal et al. [17] selected participants in a younger age group (range 3–21 years).
Summary of case definitions and comments on all studies included in this review is presented in Table 2. In India, cancer is not a notifiable disease. Hence, most of the studies utilised an active case finding approach to register incident cases of oral cancer. Sources of registration data included government hospitals, private health centres, nursing homes, shelters, nongovernmental organisations (NGOs), and community welfare centres. A number of survey methods were employed, including house-to-house recruitment, interviewing, and data abstraction from medical records. Standardised diagnostic criteria for the majority of the studies included the coding system devised by the WHO-ICD Classification for the definition of oral cancer. Mehta et al. [15] reported nonstandardised approach and utilised central papillary atrophy (CPA) of the tongue as a marker for oral lesions and precancer, determined by clinical examination conducted by the authors of the study.
Table 2.
Summary of the case definitions of oral cancer and analytical comments of all studies included in the review of oral cancer in India.
| First Author | Diagnostic Criteria | Comments |
|---|---|---|
| Manoharan et al. [3] | WHO-ICD Classification for different types of cancers including oral cancer | Age-adjusted (world population) incidence rates 116.9 per 100,000 for males and 116.7 per 100,000 for females. Leading sites among males lung (ASR: 13.8 per 100,000) followed by oral cavity (ASR: 11.4), prostate (ASR: 9.0), and larynx (ASR: 7.9). In females, breast (ASR: 30.2 per 100,000) most common site of cancer, followed by cervix uteri (ASR: 17.5), ovary (ASR: 8.5), and gallbladder (ASR: 7.4). |
|
| ||
| Sunny et al. [10] | WHO-ICD Classification for different types of cancers including oral cancer | Age-adjusted rates, linear regression model based on the logarithm of the observed incidence rates. Annual percentage. |
|
| ||
| Thorat et al. [11] | WHO (National Cancers Registry Project) ICD Classification | Incidence Rates for tobacco-dependent cancers. Prevalence of tobacco habits. |
|
| ||
| Elango et al. [1] | WHO-ICD Classification | Age-adjusted rates and age specific incidence rates were calculated. Cumulative risk and corresponding confidence intervals were also calculated. Amongst all cancers, Tongue and Oral Cavity Cancer was the predominant site in all groups, except in rural males. |
|
| ||
| Sankaranarayanan et al. [2] | WHO-ICD Classification | Of 3585 subjects in the intervention group referred, 52.4% were examined by physicians, 36 subjects with oral cancers, and 1310 with oral pre cancers were diagnosed. Of the 63 oral cancers recorded in the cancer registry, 47 were in the intervention group and 16 were in the control group, incidence rates of 56.1 and 20.3 per 100,000 person-years in the intervention and control groups. The program sensitivity for detection of oral cancer was 76.6% and the specificity 76.2%; the positive predictive value was 1.0% for oral cancer. In the intervention group, 72.3% of the cases were in Stages I-II, as opposed to 12.5% in the control Outcome measures were survival, case fatality, and oral cancer mortality. Oral cancer mortality in the study groups was analysed and compared by the use of cluster analysis. Age-standardized incidence rates were calculated, sensitivity and specificity for oral cancer screening were also calculated. Data on oral cancer incidence, stage distribution, survival, and mortality in the study groups were linked with the records at Trivandrum population-based cancer registry and municipal death registration systems. |
|
| ||
| Gupta et al. [12] | Interview, Clinical Mouth Examination and Standardised methods for diagnosis of Oral lesions; WHO-ICD Classification | Age-adjusted incidence rates were calculated. |
|
| ||
| Malaowalla et al. [13] | Standardized method to include case confirmation of OCC based on biopsies. Leukoplakia defined as precancerous oral lesion | Oral cancer prevalence rate recorded at 50 per 100,000 and after followup was conducted, 25/100,000 per year—85% of participants reported oral habits of some form—tobacco, and/or combination with chewing pan or supari. |
|
| ||
| Swaminathan et al. [14] | Standardized method to include Coding using WHO standard ICD Classification | Studied incidence pattern, of which, 1045 incident cancers registered in 2003 were followed up for estimating 5 year survival. Average annual age-standardized rate per 100,000 of all cancers higher among women (62.6) than men (51.9). Most common cancers for men stomach (5.6), mouth (4.2) and esophagus (3.7). (22.1) was ranked at the top among women followed by breast (10.9) and ovary (3.3). Cancer pattern was described using average annual incidence rates and survival experience was expressed by computing observed survival by actuarial method and age standardized relative survival (ASRS). |
|
| ||
| Mehta et al. [15] | Oral pre cancer lesions defined as Central Papillary Atrophy of the tongue, identified by clinical examinations. Diagnosis subjective (pink area devoid of pappillae was present in the centre of the dorsum of the tongue), Biopsies not conducted | Distribution of individuals with CPA according to age and sex. Association between CPA and tobacco use. Correlational Analyses was also conducted between tobacco consumption and palatal lesions. (98%) lesions occurred among bidi smokers. Clinically, 31% occurred in combination with bidi smoking associated lesions such as palatal erythemia (14%), leukoplakia (8%), or both (3%). 10 year follow up (mean 6.7 year) of the 182 lesions showed that the regression was highest (87%) among those who stopped their smoking habit and persistence among those who did not reduce their smoking habits. |
|
| ||
| Gupta et al. [16] | Oral mucous lesions as defined by presence of Oral Lichen planus or Oral Leukoplakia, WHO standardized criteria for Diagnosis | Age-adjusted incidence rates per 100,000 were calculated using person years method amongst those who stopped tobacco use. Incidence ratio of oral lichen planus to tobacco cessation habits (1.35) versus Oral leukoplakia to tobacco cessation (0.31). |
|
| ||
| Khandekar et al. [4] | TNM Classification of the American Joint Committee for Cancer staging; Histopathology case diagnosis of oral cancer as verrucous carcinoma, squamous cell carcinoma, and moderate to poorly differentiated squamous cell carcinoma | Statistical analyses conducted and limited to the use of percentages and proportions. |
|
| ||
| Maudgal et al. [17] | Clinical checkup was carried out to detect and treat precancerous lesions in tobacco using children. Oral cancer signs included submucous fibrosis, erythoplakia, leukoplakia, melanoplakia, buccal mucosa, and further biopsy at cancer specialty hospital; WHO-ICD Classification | Addresses tobacco habits of sample of marginalized children in urban and rural areas of India and report on all variant factors, detection of precancerous oral lesions. Very descriptive, no statistical analyses; (23% presented with precancerous oral lesions) and 1004 surveyed for tobacco habits and awareness (253 Tobacco users and 79% males). |
|
| ||
| Cancela et al. [9] | Oral Cavity Cancer was defined by ICD 10 codes: C02 (parts of tongue), C03 (gum), C04 (floor of mouth), C05 (palate), and C06 (other parts of mouth) | Age Standardized incidence rate and Mortality attributed to oral cavity cancer was calculated. Cox regression model utilised and adjusted for age, religion, education, occupation, BMI, standard of living index, chewing habits, smoking habits, and vegetable and fruit intake. Hazard ratios were also calculated. 134 developed oral cancer; analysed to estimate risk of oral cancer incidence and mortality according to drinking patterns. HR increased by 49% (95 CI = 1–121%) among current drinkers and 90% (95% CI = 13–218%) among past drinkers. |
|
| ||
| Mehta et al. [15] | Each subject seen by a dental surgeon, who carried out a full clinical examination of the mouth to diagnose oral cancer. Unspecified criteria for case definition | Statistical Analyses conducted included the Regression rate on leukoplakia. After one year, proportions of subjects who had discontinued tobacco use were found to be 2% in Ernakulam, 1% in Bhavnagar, and 5% in Srikakulam. 1% to 16% of participants reduced their tobacco use overall. Bhavnagar and Ernakulum regression rate of leukoplakia was significantly higher among those who had stopped or reduced their tobacco consumption. |
|
| ||
| Van der Eb et al. [19] | Oral cancer definition largely based on previous literature, palatal lesions, hyperpigmentation, Nicotine excrescences, preleukoplakia, Leukoplakia palatii, Palatal keratosis, and atrophic areas, carcinoma of the hard palate | Data analysis by cross-tabulation and stratification. Direct standardisation Statistical significance assessed using 95% confidence intervals. Prevalence rate of all palatal lesions was 55%. The prevalence rates of the separate lesions, leukoplakia palatii, palatal keratosis and palatal cancer, were 9.8%, 18.1% and 1.9%. Premalignant lesions strongly associated with reverse smoking and also associated with conventional chutta smoking. Reverse smoking induced significantly more lesions than conventional chutta smoking, and it was a major determinant of subsequent palatal cancer. |
|
| ||
| Jayalekshmi et al. [20] | Oral cancer cases were identified by the Karunagappally Cancer Registry, reported in CI5, volume. VII–IX. Active registration method; visiting all health-care facilities in the taluka | Poisson regression analysis of grouped data was completed. Age at starting tobacco chewing was not significantly related to oral cancer risk. oral cancer incidence was strongly related to daily frequency of tobacco chewing. |
|
| ||
| Wahi [21] | Case definition includes both the cancer of the oral cavity and oropharynx | Examines factors associated with the occurrence of cancer by region, age, sex, and prevalence of risk factors such as smoking and chewing. |
6. Measurement of Disease Incidence
A majority of the studies reported the calculation of incidence rates as a measure of disease occurrence. Incidence is defined by the number of new cases of oral cancer, which occur in a defined population of disease-free individuals, over a specified period of time. The incidence rate of oral cancer is generally expressed for 100,000 population—over one year (or a range of years). IARC, in its series on the cancer Incidence in Five Continents, utilised incidence rates for a defined period [26]. Age- and sex-specific incidence rates are calculated to provide an estimate of the risk of oral cancer in defined groups in India. Figure 1 shows the age specific incidence rates for oral cancer between 1983 and 2002; by gender and location (based on 4 cancer registries) in India. An increasing trend based on age; however, lower incidence recorded amongst females as compared to males is indicative of gender differences in the lifestyle and behavioural patterns associated with incidence of oral cancer.
Figure 1.
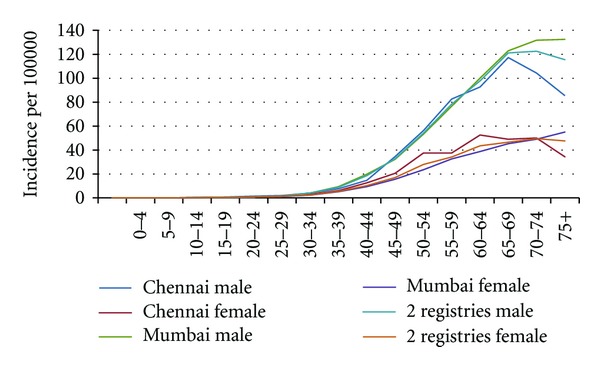
Age specific incidence rates lip, oral cavity cancer (includes: pharynx) 1983–2002. source: Ferlay et al. [26], IARC: 2010.
Additionally, incidence of oral cancer is age specific. Thus, for comparison of incidence rates in different areas or for the same area, over a period of time, it is necessary to adjust the rates for variations in the proportion of population in different age groups. The generally adopted procedure is that of direct standardisation, which applies the age- and sex-specific incidence rates of the area under consideration to world-standard population, to derive the number of cancer cases expected to occur in the standard population. Such age-standardised (or adjusted) incidence rates are useful in international or secular comparisons.
Table 3 summarizes the age-standardised (or adjusted) incidence rates per 100,000 population for oral cancer reported in reviewed literature by location in India and time period under study. Different studies reported a range of age-adjusted incidence rates (per 100,000 population) for oral cancer. A 4-fold variation in these rates suggests methodological differences in the regional registration of oral cancer. The extent to which multiple sources of case ascertainment resulted in measurement bias is unclear; however, data suggests the occurrence of under reporting or over reporting at different sites.
Table 3.
Age standardized incidence rates per 100,000 population comparison—By location, time period and gender.
| Author | Location | Year | M | F |
|---|---|---|---|---|
| CI5 Data, IARC | Mumbai | 1973–1975 | 16.3 | 10.3 |
| ICMR | Mumbai, Madras, Bangalore | 1982–1984 | 11 | 10.5 |
| ICMR | Trivandrum (Kerala) | 1982–1984 | 24.2 | 11.5 |
| Sunny et al. | Mumbai (Maharashtra) | 1986–2000 | 12.6 | 7.3 |
| Manoharan et al. | Delhi | 2001–2005 | 11.4 | 3.7 |
Manoharan et al. [3] reported variable age-standardised incidence rates across various geographical regions within India for a defined period of time (2004-2005). Data for Kolkata only includes 2005. Variations in case registration technique such as medical record abstraction by trained medical social workers may have contributed to sample selection bias. Additionally, patient interviews were utilized to obtain information highly prone to recall bias, explaining some variation.
As can be seen in Table 3, age-standardised incidence rates when stratified by sex were lower in females than males in the reported articles and data repositories across the different time frames. This is consistent with our earlier comparison in Figure 1. In Figures 2 and 3, variation in age-standardised incidence rates per 100,000 population by location and time period in males is reported in the literature.
Figure 2.
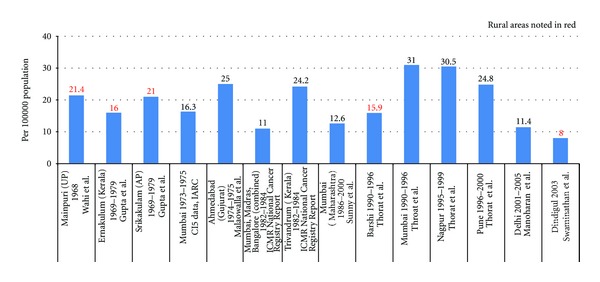
Age standardized incidence rates per 100,000 population for oral cancer reported in reviewed literature—by location and time period (in males only).
Figure 3.
Age standardized incidence rates per 100,000 population (in males only). 2004-2005 reported in Manoharan et al. [3].
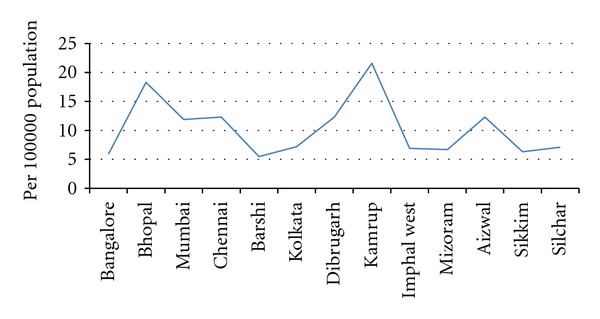
As can be seen Figure 4, age-standardised incidence rates were compared across the rural males in selected studies. These rates were identified through the rural population-based cancer registries. Variation in the data calls into question the robustness of cancer registration information utilized in the methodology of these studies especially in agrarian based rural parts of India where lack of transportation inhibits patients from seeking care.
Figure 4.
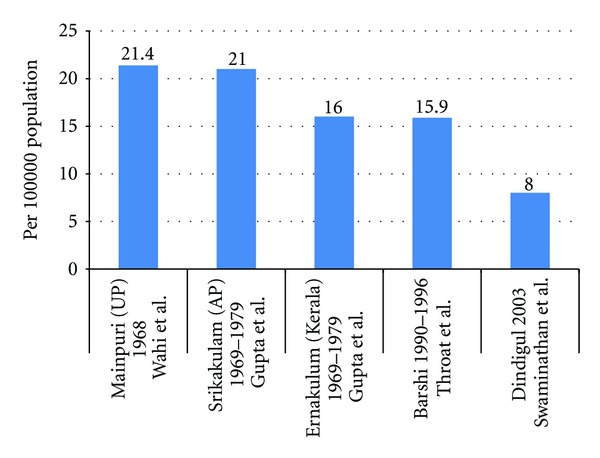
Age standardized incidence rates per 100,000 population comparison—rural males only.
7. Cancer Registry Data—Comparisons from IARC Sources
These IARC projects provide estimates of the incidence of, and mortality from major type of cancers for all countries of the world. GLOBOCAN only includes data for 2008. Data on India was extracted from population-based cancer registries. Figure 5 shows the age-standardised incidence and mortality rates for all types of cancer. Note that oral cancer ranks third amongst all types of cancer.
Figure 5.
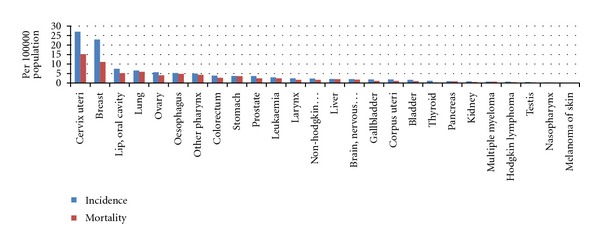
Incidence and mortality rates (age standardised) by cancer type in India—(sexes combined) data extracted from Globocan, 2008 data.
As can be seen in Figure 6 and consistent with earlier estimates, when stratified by sex, males have a higher age-standardised incidence and mortality rate than females.
Figure 6.
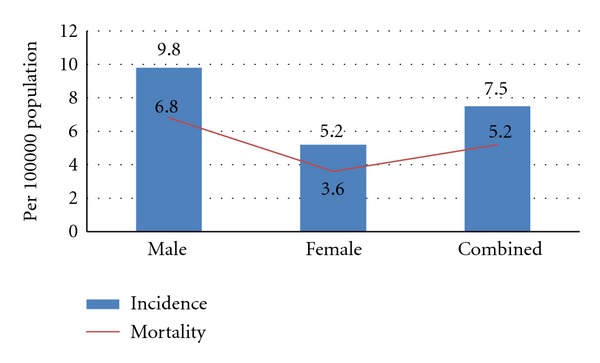
Incidence and mortality rates (age standardized) lip and oral cancer in India—by sex extracted from Globocan, 2008 data.
The CI5 series compares age-standardised incidence rates in India by time, location and gender and registries summarised in Table 4. Tables 5, 6, 7 describe a comparisonof incidence and trends consistent with earlier findings.
Table 4.
Summary of Indian cancer registries reported by IARC, CI5 Series.
| CI5 I-IX registries | |||
|---|---|---|---|
| Registry/population | Volume | Time period | |
| India, Mumbai (Bombay) | 2 | 1964 | 1966 |
| India, Mumbai (Bombay) | 3 | 1968 | 1972 |
| India, Mumbai (Bombay) | 4 | 1973 | 1975 |
| India, Poona | 4 | 1973 | 1977 |
| India, Bangalore | 5 | 1982 | 1982 |
| India, Chennai (Madras) | 5 | 1982 | 1982 |
| India, Mumbai (Bombay) | 5 | 1978 | 1982 |
| India, Nagpur | 5 | 1980 | 1982 |
| India, Poona | 5 | 1978 | 1982 |
| India, Ahmedabad | 6 | 1983 | 1987 |
| India, Bangalore | 6 | 1983 | 1987 |
| India, Chennai (Madras) | 6 | 1983 | 1987 |
| India, Mumbai (Bombay) | 6 | 1983 | 1987 |
| India, Bangalore | 7 | 1988 | 1992 |
| India, Barshi | 7 | 1988 | 1992 |
| India, Chennai (Madras) | 7 | 1988 | 1992 |
| India, Karunagappally | 7 | 1991 | 1992 |
| India, Mumbai (Bombay) | 7 | 1988 | 1992 |
| India, Trivandrum | 7 | 1991 | 1992 |
| India, Ahmedabad | 8 | 1993 | 1997 |
| India, Bangalore | 8 | 1993 | 1997 |
| India, Chennai (Madras) | 8 | 1993 | 1997 |
| India, Delhi | 8 | 1993 | 1996 |
| India, Karunagappally | 8 | 1993 | 1997 |
| India, Mumbai (Bombay) | 8 | 1993 | 1997 |
| India, Nagpur | 8 | 1993 | 1997 |
| India, Poona | 8 | 1993 | 1997 |
| India, Trivandrum | 8 | 1993 | 1997 |
| India, Chennai (Madras) | 9 | 1998 | 2002 |
| India, Delhi | 9 | 1998 | 2002 |
| India, Karunagappally | 9 | 1998 | 2002 |
| India, Mumbai (Bombay) | 9 | 1998 | 2002 |
| India, Nagpur | 9 | 1998 | 2002 |
| India, Poona | 9 | 1998 | 2002 |
| India, Trivandrum | 9 | 1998 | 2002 |
Table 5.
Age standardized incidence rate—1993 to 1997.
| Oral cavity and pharynx cancer | Age groups 0–85+ | |
|---|---|---|
| Location in India | Male | Female |
| India, Ahmedabad | 29.6 | 7.5 |
| India, Bangalore | 15.2 | 11.2 |
| India, Chennai (Madras) | 21.9 | 10.4 |
| India, Delhi | 18* | 6.4* |
| India, Karunagappally | 16.4 | 6.4 |
| India, Mumbai (Bombay) | 22.5 | 10 |
| India, Nagpur | 23.4 | 8.2 |
| India, Poona | 19.3 | 9.4 |
| India, Trivandrum | 21.4 | 9.1 |
*Includes data only upto 1996.
See [24].
Table 6.
Age standardized incidence rates—1998 to 2002.
| Oral cavity and pharynx cancer | Age Groups 0–85+ | |
|---|---|---|
| Location in India | Male | Female |
| India, Chennai (Madras) | 20.8 | 10 |
| India, Delhi | 17 | 5.6 |
| India, Karunagappally | 20.4 | 8.9 |
| India, Mumbai (Bombay) | 19 | 8 |
| India, Nagpur | 19 | 7.5 |
| India, Poona | 15.6 | 9.5 |
| India, Trivandrum | 21.6 | 7.9 |
See [25].
Table 7.
Comparison of age standardized incidence rates per 100,000 by time, location and gender.
| Males | Females | |||
|---|---|---|---|---|
| 1998–2002 | 1993–1997 | 1998–2002 | 1993–1997 | |
| Chennai (Madras) | 20.8 | 21.9 | 10 | 10.4 |
| Delhi | 17 | 18 | 5.6 | 6.4 |
| Karunagappally | 20.4 | 16.4 | 8.9 | 6.4 |
| Mumbai (Bombay) | 19 | 22.5 | 8 | 10 |
| Nagpur | 19 | 23.4 | 7.5 | 8.2 |
| Poona | 15.6 | 19.3 | 9.5 | 9.4 |
| Trivandrum | 21.6 | 21.4 | 7.9 | 9.1 |
Bold data only include rates upto 1996.
See [25].
8. Incidence and Trends of Oral Cancer in India
Oral cancer is a heterogeneous group of cancers arising from different parts of the oral cavity, with different predisposing factors, prevalence, and treatment outcomes. It is the sixth most common cancer reported globally with an annual incidence of over 300,000 cases, of which 62% arise in developing countries.
There is a significant difference in the incidence of oral cancer in different regions of the world, with the age-adjusted rates varying from over 20 per 100,000 population in India, to 10 per 100,000 in the U.S.A, and less than 2 per 100,000 in the Middle East [27].
In comparison with the U.S. population, where oral cavity cancer represents only about 3% of malignancies, it accounts for over 30% of all cancers in India. The variation in incidence and pattern of oral cancer is due to regional differences in the prevalence of risk factors.
9. Variability in Incidence
Age-adjusted incidence of oral cancer is highly variable in India. The population-based cancer registry data, as well as the literature reviewed in our search demonstrate the nationwide incidence can be as high as 20 per 100,000 population, which varied considerably based on study designs, sampling methodology and case ascertainment, as well as by age, gender and location. Variations in age-specific incidence rates also increased with age, which drops at the age of seventy, a trend which is consistent in multiple studies.
Studies reporting active case finding as a mode of ascertainment may only include those individuals registered in different parts of the country. Underregistration may have been magnified in rural areas. As a result, registration rate may not reflect the true incidence in these areas. This may very well be the case in other parts of India.
Although the definition of oral cancer in most studies reviewed was standardised as per the WHO-ICD Classification system, the diagnosis of oral cancer is dependent on the clinical examination conducted by staff or in some studies the authors themselves. Such examinations are prone to numerous variations based on practice in different parts of the country, resulting in inaccurate coding of data, and unreliable registration information.
In a majority of the studies, data were collected through one-time community or hospital-based cross-sectional surveys; however, no studies refer to continuous available data. Such data would assist in understanding the trends in cancer occurrence and variation according to demographic or life style characteristics of the population to determine further aetiological factors influencing oral cancer.
10. Aetiological Factors
High incidence of oral cancer in India can be attributed to a number of aetiological factors. Although not a focus of this paper, the limited studies reported the use of tobacco (smoking or chewing) or alcohol intake associated with oral cancer. Seven studies discussed the associations between use of tobacco and oral cancer incidence. Mehta et al. [15] reported the regression rate of leukoplakia as significantly higher among those who had stopped or reduced tobacco consumption in rural populations in Kerala, Andhra Pradesh and Gujarat. Gupta et al. [12] reported an association between the cessation of tobacco habits and a drop in the incidence of leukoplakia implying reduced risk for oral cancer after cessation of tobacco use. Khandekar et al. [4] reported tobacco consumption habits among subjects that included chewing (in the form of betel quid, or khaini) and smoking (bidis and cigarettes) as the common cause of oral cancer. Based on the TNM classification, 48% of these oral cancer cases presented in later stages, that is, III and IV. Mehta et al. [15] reported occurrence of CPA of the tongue as a marker for oral precancerous lesions among users of bidis in rural parts of India with 98% lesions occurring in this group. A ten-year followup showed that regression was the highest amongst those who stopped the smoking habit. Jayalekshmi et al. [20] reported a significant association between oral cancer incidence and daily frequency of tobacco chewing (P < 0.001, which increased 9.2 fold among women chewing tobacco 10 times or more a day, with the highest risk during the first twenty years of chewing).
One study discussed the association between alcohol use and oral cancer. Cancela et al. [9] reported a significant association between risk between alcohol intake and development of oral cancer in Kerala males among current- and past-drinkers. They quoted an increased hazard ratio (HR) of 49% (95% CI = 1–121%) among current drinkers and 90% (95% CI = 13–218%) among past drinkers. A significant dose response relationship between intake frequencies, duration, and risk was observed.
11. Tobacco Use
Tobacco use and alcohol are known risk factors for cancers of the oral cavity. Estimates indicate 57% of all men and 11% of women between 15–49 years of age use some form of tobacco. Besides smoking, use of smokeless tobacco is also widely prevalent as noted in Box 2, the use of Betel quid, also referred to as pan consist of pieces of areca nut, processed or unprocessed tobacco, aqueous calcium hydroxide (slaked lime), and some spices wrapped in the leaf of piper betel vine leaf. This is very common and is accepted socially and culturally in many parts of India. Additionally, gutka, zarda, kharra, mawa, and khainni are all dry mixtures of lime, areca nut flakes, and powdered tobacco custom mixed by vendors. In recent years, commercially available sachets of premixed areca nut, lime, condiments with or without powdered tobacco have become very popular, particularly among younger Indians. Typically, the pan or gutka is kept in the cheek and chewed or sucked for 10–15 minutes, with some users keeping it in overnight.
Box 2.
Definition of tobacco products as reported in peer-reviewed literature.
Acquisition of the tobacco habit typically occurs early in life through imitation of a family member or peers. Various studies carried out across the country report that at least a third of school students less than 15 years of age have used one form or another of tobacco. However, with improved public health education, the prevalence of these risk factors is decreasing around the globe, including in India [1].
Oral cancer incidence from 1990 to 2005 reveals the benefit of public health interventions such as screening demonstrating potential significant reductions in oral cancer incidence. A comparison of oral cancer incidence in India and USA has shown a similar downward trend in both countries. However, the reduction is much more dramatic in India, where there is a much higher prevalence of oral cancer.
Recently, the trend has also been observed towards increased incidence of oral cancer among young adults. This increase in incidence is observed in patients with tongue cancer. In an analysis of 482 consecutive patients presenting with head and neck cancer to a tertiary care cancer center in India, 135 out of the 286 (47%) oral cavity cancer patients did not have any known risk habits (tobacco or alcohol use, unpublished data from a blog).
12. Global Burden of Disease
Approximately 12% of deaths worldwide occur due to cancer, and in about twenty years, it is projected to increase from about 6 to 10 million [26].
In the USA, cancer incidence and associated mortality have declined due to improved infrastructure of the health systems that include improved health education and awareness translating to improved prevention, earlier detection and availability of treatment options [28].
However, even though cancer has been previously thought to be a disease of the western world, more than half of all cancers occur in developing and under-developed countries becoming one of the leading causes of death and disability [29]. Consequently, cancer control is quickly becoming a global health priority. In 2009, global health and cancer care community leaders formed a global task force focused on the expansion of access to cancer care and control in developing countries and with a charter to propose, implement, and evaluate strategies to reduce the global burden of disease attributed to cancer [30].
13. Limitations
This review does not come without limitations. Firstly, a number of studies may not have been found using the identified search strategy. Secondly, mortality and survival from oral cancer in India have not been described and criteria used to identify studies may have resulted in published studies only where results are known to be significant or where incidence rates are high. As a result, bias from not including mortality and survival and publication bias may lead to overestimating the true incidence. Finally, the search strategy was also limited to studies published in English, leaving out local language-based Indian journals.
14. Projections
Cancer is not uncommon in India, where the number of people living with the disease is estimated to be around 2.5 million, with over 0.8 million new cases and 0.55 million deaths occurring each year [31]. According to the International Agency for Research on cancer (IARC), a group chartered by the World Health Organization to conduct research and develop scientific strategies for cancer prevention and control; cancers of the oral cavity, lungs, oesophagus, stomach, cervix, and breast are some of the most commonly occurring forms in both the male and female population of India.
Oral cancer in particular will continue to be a major problem. In Figure 7, crude incidence projections by Globocan demonstrate that oral cancer crude incidence will increase in India by 2020 and 2030 in both sexes.
Figure 7.
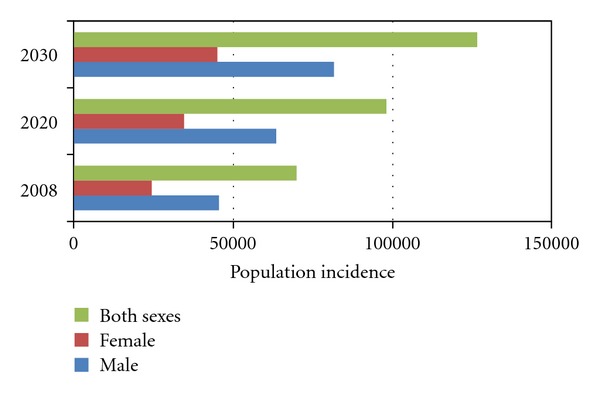
Crude incidence projections for lip/oral cavity cancer (2008 to 2030). Data extracted from Globocan, 2008 data. Population forecasts were extracted from the United Nations, World Population prospects, the 2008 revision. Numbers are computed using age-specific rates and corresponding populations for 10 age-groups.
Variability in the age-adjusted incidence rates of oral cancer in different regions of India has increased over the years. Although this review does not provide substantial evidence or information on aetiological factors such as smoking or chewing tobacco and the use of alcohol which increases ones risk of developing oral cancer, the specific focus on these factors will provide opportunities for future research aimed at prevention and control of the disease.
15. Screening and Early Detection
Despite the fact that the oral cavity is accessible for visual examination, and that oral cancers and premalignant lesions have well-defined clinical diagnostic features, oral cancers are typically detected in their advanced stages. In fact, in India, 60–80% of patients present with advanced disease as compared to 40% in developed countries. Consistent with patients presenting for medical care with more advanced disease in India compared with developed countries, overall survival is also reduced. Early detection would not only improve the cure rate, but it would also lower the cost and morbidity associated with treatment.
It is imperative that cost-effective oral cancer screening and awareness initiatives be introduced in high-risk populations such as those found in India. Several large population-based oral cancer screening programs have been carried out, either as opportunistic screenings or as population-wide screenings. Although these studies have confirmed the effectiveness of screening to detect oral cancer and precancerous lesions, only recently has a study from India demonstrated that oral cancer screening by trained health workers can lower mortality of the disease—especially in individuals with a history of tobacco use [32]. In this randomised, controlled trial of almost 192,000 people, carried out over an eight-year period, there was a significant reduction in mortality in the intervention arm (29.9 cases per 100,000) versus the control arm (45.4 cases per 100,000), due to detection of oral cancer at an early stage.
A cost-effectiveness analysis revealed that an oral cancer visual inspection by trained health workers can be carried out for under U.S. $6 per person. The incremental cost per life-year saved was U.S. $835 for the all-screened population and U.S. $156 in the high-risk population (individuals with a tobacco habit) [33].
Mouth self-examination could further reduce the cost of the screening and increase awareness in high-risk communities in India. Such a simple and cost-effective strategy has the potential to have a significant impact on the awareness of oral cancer in the broader community.
16. Future Challenges
Despite the fact that oral cancer and consequences can be prevented, treated, and controlled, there exists a significant gap in the Indian public's knowledge, attitudes, and behaviours. Efforts must be made to introduce a suite of preventive measures that has the potential to significantly reduce the burden and to help bridge the gap between research, development and public awareness. Knowledge dissemination to help people adopt behaviour patterns to improve their health and decisions making process and to provide required public health education and training to promote lifestyle modifications are key to confronting the challenge.
The greatest threat of the oral cancer burden exists among the lower socioeconomic strata. This segment of the population is the most vulnerable because of higher exposure to the risk factor—tobacco—which complicates the situation further. They have the most limited access to education, prevention and treatment. These disparities should be addressed to push for provision of easy, accessible, detection, and treatment services. Prevention through action against risk factors, especially tobacco will be key to reducing the burden amongst these groups.
Acknowledgments
Author would like to acknowledge Professor Martin Roland, Professor of Health Services Research and Dr. John Powles from the Department of Public Health and Primary Care at the University of Cambridge for mentorship and support, Dr. Rohan D'Souza, Specialist Registrar at Epsom and St. Helier Hospitals NHS Trust, London, United Kingdom, and Dr. Hermann Brenner, German Cancer Research Center, Heidelberg, Germany for comments during the review process. The author also declares that there are no potential conflict of interests, including financial interests, relationships, and affiliation relevant to the subject of the manuscript in any way. No funding was sought in the creation of this work.
Abbreviations
- AP:
Andhra Pradesh
- ASRS:
Age-standardised relative survival
- ASR:
Age-standardised rate
- CI5:
Cancer incidence in 5 continents
- CPA:
Central papillary atrophy
- DACR:
Dindigul Ambilikkai cancer registry
- GLOBOCAN:
IARC Global Cancer Project
- HW:
Health workers
- HR:
Hazard ratio
- IARC:
International Agency for Research on Cancer
- ICMR:
Indian Council of Medical Research
- ICD:
International Classification of Diseases
- MeSH:
Medical subject headings
- NGO:
Nongovernmental organisation
- OCC:
Oral cavity cancer
- TNM:
Tumour node metastasis
- UP:
Uttar Pradesh
- USA:
United States of America
- WHO:
World Health Organisation.
References
- 1.Elango JK, Gangadharan P, Sumithra S, Kuriakose MA. Trends of head and neck cancers in urban and rural India. Asian Pacific Journal of Cancer Prevention. 2006;7(1):108–112. [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. The Lancet. 2005;365(9475):1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 3.Manoharan N, Tyagi BB, Raina V. Cancer incidences in rural Delhi—2004–05. Asian Pacific Journal of Cancer Prevention. 2010;11(1):73–78. [PubMed] [Google Scholar]
- 4.Khandekar PS, Bagdey PS, Tiwari RR. oral cancer and Some epidemiological factors: a hospital based study. Indian Journal of Community Medicine. 2006;31(3):157–159. [Google Scholar]
- 5.Kumar S, Heller RF, Pandey U, Tewari V, Bala N, Oanh KTH. Delay in presentation of oral cancer: a multifactor analytical study. National Medical Journal of India. 2001;14(1):13–17. [PubMed] [Google Scholar]
- 6.Fritz. International Classification of Diseases For Oncology. 3rd edition. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 7.Conway DI, Petticrew M, Marlborough H, Berthiller J, Hashibe M, Macpherson LMD. Socioeconomic inequalities and oral cancer risk: a systematic review and meta-analysis of case-control studies. International Journal of Cancer. 2008;122(12):2811–2819. doi: 10.1002/ijc.23430. [DOI] [PubMed] [Google Scholar]
- 8.Allgar VL, Neal RD. Sociodemographic factors and delays in the diagnosis of six cancers: analysis of data from the ’National Survey of NHS Patients: cancer’. The British Journal of Cancer. 2005;92(11):1971–1975. doi: 10.1038/sj.bjc.6602623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancela MDC, Ramadas K, Fayette JM, et al. Alcohol intake and oral cavity cancer risk among men in a prospective study in Kerala, India. Community Dentistry and Oral Epidemiology. 2009;37(4):342–349. doi: 10.1111/j.1600-0528.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- 10.Sunny L, Yeole BB, Hakama M, et al. Oral cancers in Mumbai, India: a fifteen years perspective with respect to incidence trend and cumulative risk. Asian Pacific Journal of Cancer Prevention. 2004;5(3):294–300. [PubMed] [Google Scholar]
- 11.Thorat RV, Panse NS, Budukh AM, Dinshaw KA, Nene BM, Jayant K. Prevalence of tobacco use and tobacco-dependent cancers in males in the Rural Cancer Registry population at Barshi, India. Asian Pacific Journal of Cancer Prevention. 2009;10(6):1167–1170. [PubMed] [Google Scholar]
- 12.Gupta PC, Mehta FS, Pindborg JJ. Intervention study for primary prevention of oral cancer among 36,000 Indian tobacco users. The Lancet. 1986;1(8492):1235–1238. doi: 10.1016/s0140-6736(86)91386-3. [DOI] [PubMed] [Google Scholar]
- 13.Malaowalla AM, Silverman S, Mani NJ. Oral cancer in 57,518 industrial workers in Gujarat, India. A prevalence and followup study. Cancer. 1976;37(4):1882–1886. doi: 10.1002/1097-0142(197604)37:4<1882::aid-cncr2820370437>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Swaminathan R, Selvakumaran R, Esmy PO, et al. Cancer pattern and survival in a rural district in South India. Cancer Epidemiology. 2009;33(5):325–331. doi: 10.1016/j.canep.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Mehta FS, Bhonsle RB, Murti PR, Daftary DK, Gupta PC, Pindborg JJ. Central papillary atrophy of the tongue among bidi smokers in India: a 10-year study of 182 lesions. Journal of Oral Pathology and Medicine. 1989;18(8):475–480. doi: 10.1111/j.1600-0714.1989.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 16.Gupta PC, Murti PR, Bhonsle RB, Mehta FS, Pindborg JJ. Effect of cessation of tobacco use on the incidence of oral mucosal lesions in a 10-yr follow-up study of 12,212 users. Oral diseases. 1995;1(1):54–58. doi: 10.1111/j.1601-0825.1995.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 17.Maudgal S, More N, Raval S. Study on tobacco use and awareness among marginalized children. Indian journal of cancer. 2010;47:14–18. doi: 10.4103/0019-509X.63867. [DOI] [PubMed] [Google Scholar]
- 18.Mehta FS, Gupta MB, Pindborg JJ. An intervention study of oral cancer and precancer in rural Indian populations: a preliminary report. Bulletin of the World Health Organization. 1982;60(3):441–446. [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Eb MM, Leyten EMS, Gavarasana S, Vandenbroucke JP, Meera Kahn P, Cleton FJ. Reverse smoking as a risk factor for palatal cancer: a cross-sectional study in rural Andhra Pradesh, India. International Journal of Cancer. 1993;54(5):754–758. doi: 10.1002/ijc.2910540508. [DOI] [PubMed] [Google Scholar]
- 20.Jayalekshmi PA, Gangadharan P, Akiba S, Nair RRK, Tsuji M, Rajan B. Tobacco chewing and female oral cavity cancer risk in Karunagappally cohort, India. The British Journal of Cancer. 2009;100(5):848–852. doi: 10.1038/sj.bjc.6604907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahi PN. The epidemiology of oral anc oropharyngeal cancer. A report of the study in Mainpuri district, Uttar Pradesh, India. Bulletin of the World Health Organization. 1968;38(4):495–521. [PMC free article] [PubMed] [Google Scholar]
- 22.Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. The Lancet. 2005;365(9475):1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 23.Gupta PC, Mehta FS, Pindborg JJ, et al. A primary prevention study of oral cancer among Indian villagers. Eight-year follow-up results. IARC scientific publications. 1990;(103):149–156. [PubMed] [Google Scholar]
- 24.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer Incidence in Five Continents. Vol. 8. Lyon, France: IARC; 2002. (IARC Scientific Publications no 155). [Google Scholar]
- 25.Curado MP, Edwards B, Shin HR, et al., editors. Cancer Incidence in Five Continents. Vol. 9. Lyon, France: IARC; 2007. (IARC Scientific Publications no 160). [Google Scholar]
- 26.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN, 2003, 2008, 2010 cancer Incidence and Mortality Worldwide. IARC CancerBase. (10)
- 27.Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Research. 1998;18(6 B):4779–4786. [PubMed] [Google Scholar]
- 28.Jemal A, Thun MJ, Ries LAG, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. Journal of the National Cancer Institute. 2008;100(23):1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sloan F, et al. Cancer Control Opportunities in Low and Middle Income Countries. Washington, DC, USA: Institute of Medicine of the National Academies, National Academies Press; 2007. [PubMed] [Google Scholar]
- 30.Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: a call to action. The Lancet. 2010;376(9747):1186–1193. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 31.Nandakumar A, Gupta PC, Gangadharan P, Visweswara RN, Parkin DM. Geographic pathology revisited: development of an atlas of cancer in India. International Journal of Cancer. 2005;116(5):740–754. doi: 10.1002/ijc.21109. [DOI] [PubMed] [Google Scholar]
- 32.Daftary DK. Temporal role of tobacco in oral carcinogenesis: a hypothesis for the need to prioritize on precancer. Indian journal of cancer. 2010;47(supplement 1):105–107. doi: 10.4103/0019-509X.63863. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian S, Sankaranarayanan R, Bapat B, et al. Cost-effectiveness of oral cancer screening: results from a cluster randomized controlled trial in India. Bulletin of the World Health Organization. 2009;87(3):200–206. doi: 10.2471/BLT.08.053231. [DOI] [PMC free article] [PubMed] [Google Scholar]