Abstract
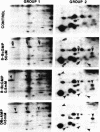
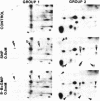
The effects of sodium nitroprusside, 8-bromo cyclic GMP, 8-bromoguanosine 5′-monophosphate, 8-bromo cyclic AMP, dibutyryl cyclic AMP, and isoproterenol on incorporation of 32P into proteins in intact rat thoracic aorta were studied. Aortas were incubated in [32P]orthophosphate in order to label endogenous adenosine triphosphate. Agents were then added for various times and the tissues were homogenized and fractionated (100,000 × g for 60 min) into soluble and particulate fractions. Soluble and particulate fractions were subjected to isoelectric focusing followed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and autoradiographs were made. Nitroprusside induced a concentration-dependent increase in incorporation of 32P into nine proteins and a decrease in 32P incorporation into two proteins. Some of these proteins appeared in both the soluble and particulate fractions of homogenates; others appeared only in the soluble fraction. The pattern of 32P incorporation was identical after 2- or 15-min exposure to nitroprusside and was mimicked by exposure to 50-500 μM 8-bromo cyclic GMP. 8-Bromoguanosine 5′-monophosphate did not alter 32P incorporation. Dibutyryl cyclic AMP at 50 μM had no effect upon 32P incorporation whereas a higher concentration (0.5 mM) caused increased or decreased 32P incorporation into some, but not all, of the same proteins. 8-Bromo cyclic AMP (5 mM) produced only small changes in 32P incorporation. The pattern of 32P incorporation induced by a relatively high concentration of isoproterenol 0.1 mM was similar but not identical to that seen with 0.5 mM dibutyryl cyclic AMP. The present study indicates that the incorporation of 32P into endogenous proteins of intact rat aorta can be regulated by nitroprusside. These effects can be mimicked by cyclic GMP analogues and only partially by cyclic AMP analogues or isoproterenol. Presumably, these effects of nitroprusside are mediated through a cyclic GMP-dependent process (protein kinase or phosphatase) which may play a role in the relaxant properties of nitroprusside and cyclic GMP.
Keywords: cyclic GMP, cyclic AMP, relaxation, smooth muscle, isoproterenol
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Aswad D. W., Greengard P. A specific substrate from rabbit cerebellum for guanosine 3':5'-monophosphate-dependent protein kinase. I. Purification and characterization. J Biol Chem. 1981 Apr 10;256(7):3487–3493. [PubMed] [Google Scholar]
- Aswad D. W., Greengard P. A specific substrate from rabbit cerebellum for guanosine 3':5'-monophosphate-dependent protein kinase. II. Kinetic studies on its phosphorylation by guanosine 3':5'-monophosphate-dependent and adenosine 3':5'-monophosphate-dependent protein kinases. J Biol Chem. 1981 Apr 10;256(7):3494–3500. [PubMed] [Google Scholar]
- Barron J. T., Bárány M., Bárány K., Storti R. V. Reversible phosphorylation and dephosphorylation of the 20,000-dalton light chain of myosin during the contraction-relaxation-contraction cycle of arterial smooth muscle. J Biol Chem. 1980 Jul 10;255(13):6238–6244. [PubMed] [Google Scholar]
- Brandwein H. J., Lewicki J. A., Murad F. Reversible inactivation of guanylate cyclase by mixed disulfide formation. J Biol Chem. 1981 Mar 25;256(6):2958–2962. [PubMed] [Google Scholar]
- Casnellie J. E., Greengard P. Guanosine 3':5'-cyclic monophosphate-dependent phosphorylation of endogenous substrate proteins in membranes of mammalian smooth muscle. Proc Natl Acad Sci U S A. 1974 May;71(5):1891–1895. doi: 10.1073/pnas.71.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E., Ives H. E., Jamieson J. D., Greengard P. Cyclic GMP-dependent protein phosphorylation in intact medial tissue and isolated cells from vascular smooth muscle. J Biol Chem. 1980 Apr 25;255(8):3770–3776. [PubMed] [Google Scholar]
- Corbin J. D., Lincoln T. M. Comparison of cAMP and cGMP-dependent protein kinases. Adv Cyclic Nucleotide Res. 1978;9:159–170. [PubMed] [Google Scholar]
- Garrison J. C. The effects of glucagon, catecholamines, and the calcium ionophore A23187 on the phosphorylation of rat hepatocyte cytosolic proteins. J Biol Chem. 1978 Oct 10;253(19):7091–7100. [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Haeusler G. Differential effect of verapamil on excitation-contraction coupling in smooth muscle and on excitation-secretion coupling in adrenergic nerve terminals. J Pharmacol Exp Ther. 1972 Mar;180(3):672–682. [PubMed] [Google Scholar]
- Ives H. E., Casnellie J. E., Greengard P., Jamieson J. D. Subcellular localization of cyclic GMP-dependent protein kinase and its substrates in vascular smooth muscle. J Biol Chem. 1980 Apr 25;255(8):3777–3785. [PubMed] [Google Scholar]
- Janis R. A., Diamond J. Relationship between cyclic nucleotide levels and drug-induced relaxation of smooth muscle. J Pharmacol Exp Ther. 1979 Dec;211(3):480–484. [PubMed] [Google Scholar]
- Katsuki S., Arnold W. P., Murad F. Effects of sodium nitroprusside, nitroglycerin, and sodium azide on levels of cyclic nucleotides and mechanical activity of various tissues. J Cyclic Nucleotide Res. 1977 Aug;3(4):239–247. [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Katsuki S., Murad F. Regulation of adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol Pharmacol. 1977 Mar;13(2):330–341. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mariash C. N., Seelig S., Oppenheimer J. H. A rapid, inexpensive, quantitative technique for the analysis of two-dimensional electrophoretograms. Anal Biochem. 1982 Apr;121(2):388–394. doi: 10.1016/0003-2697(82)90498-5. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Schlichter D. J., Casnellie J. E., Greengard P. An endogenous substrate for cGMP-dependent protein kinase in mammalian cerebellum. Nature. 1978 May 4;273(5657):61–62. doi: 10.1038/273061a0. [DOI] [PubMed] [Google Scholar]
- Schlichter D. J., Detre J. A., Aswad D. W., Chehrazi B., Greengard P. Localization of cyclic GMP-dependent protein kinase and substrate in mammalian cerebellum. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5537–5541. doi: 10.1073/pnas.77.9.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz K., Schultz K., Schultz G. Sodium nitroprusside and other smooth muscle-relaxants increase cyclic GMP levels in rat ductus deferens. Nature. 1977 Feb 24;265(5596):750–751. doi: 10.1038/265750a0. [DOI] [PubMed] [Google Scholar]