Abstract
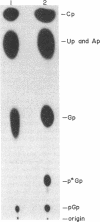
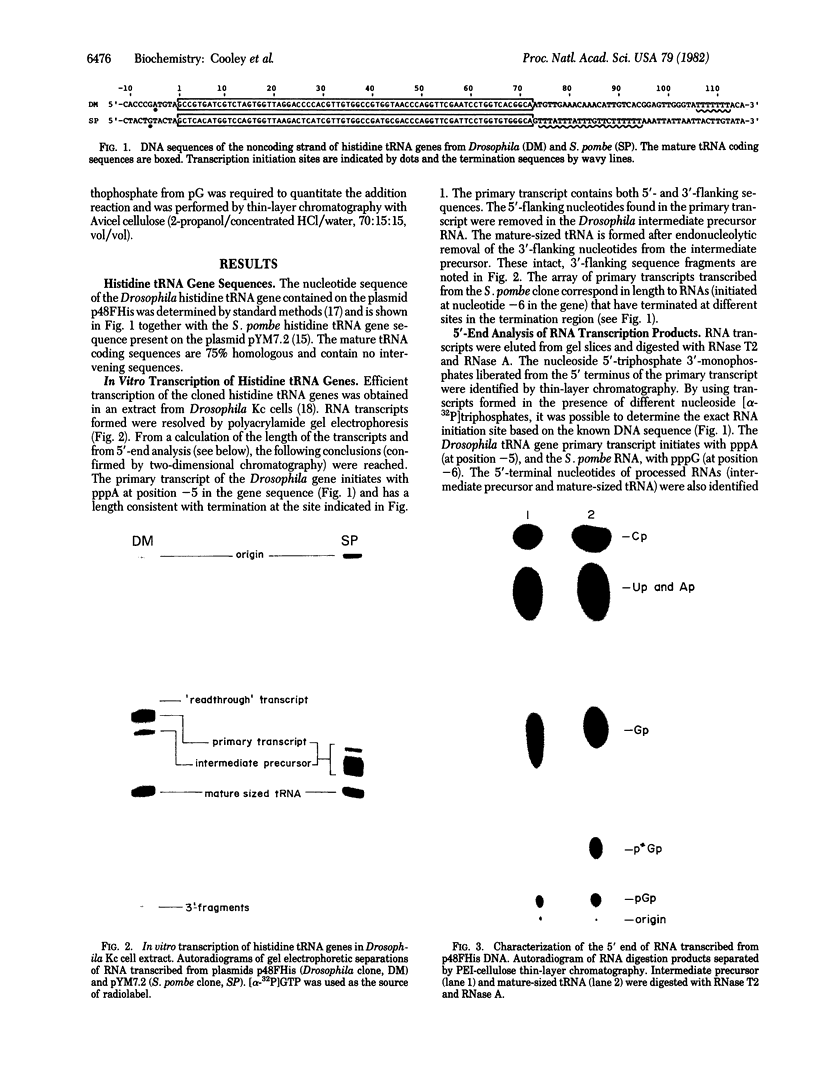
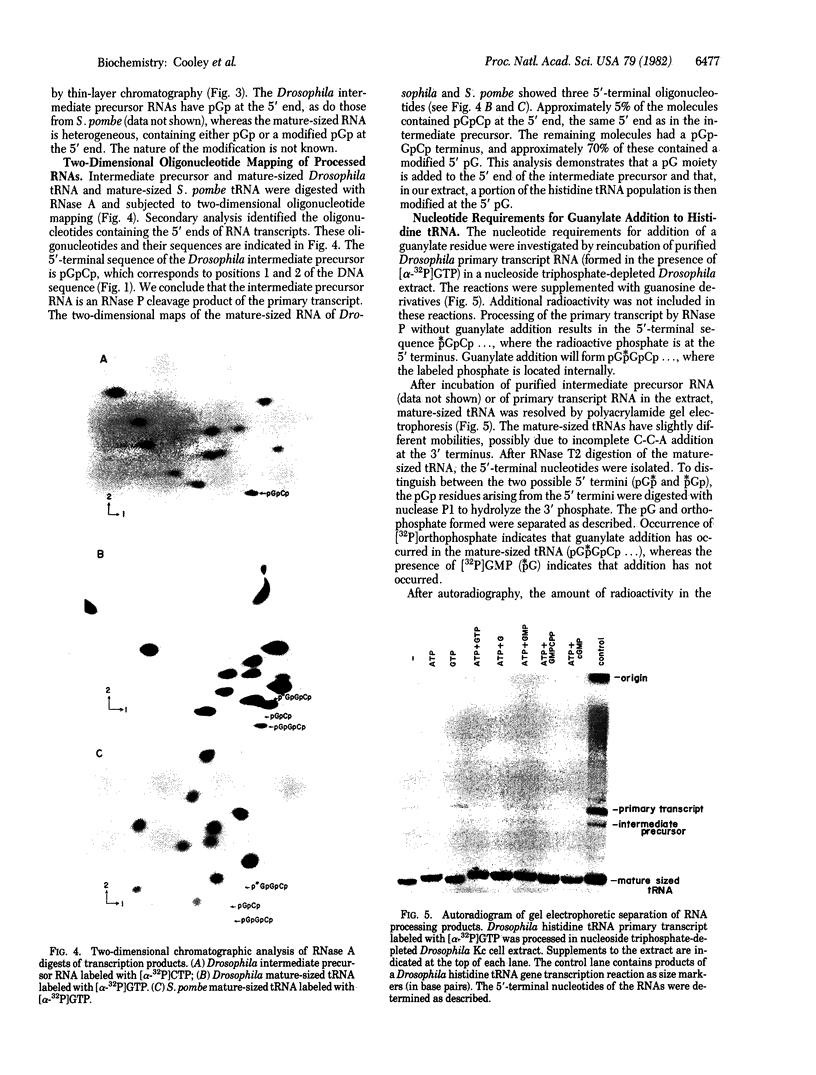
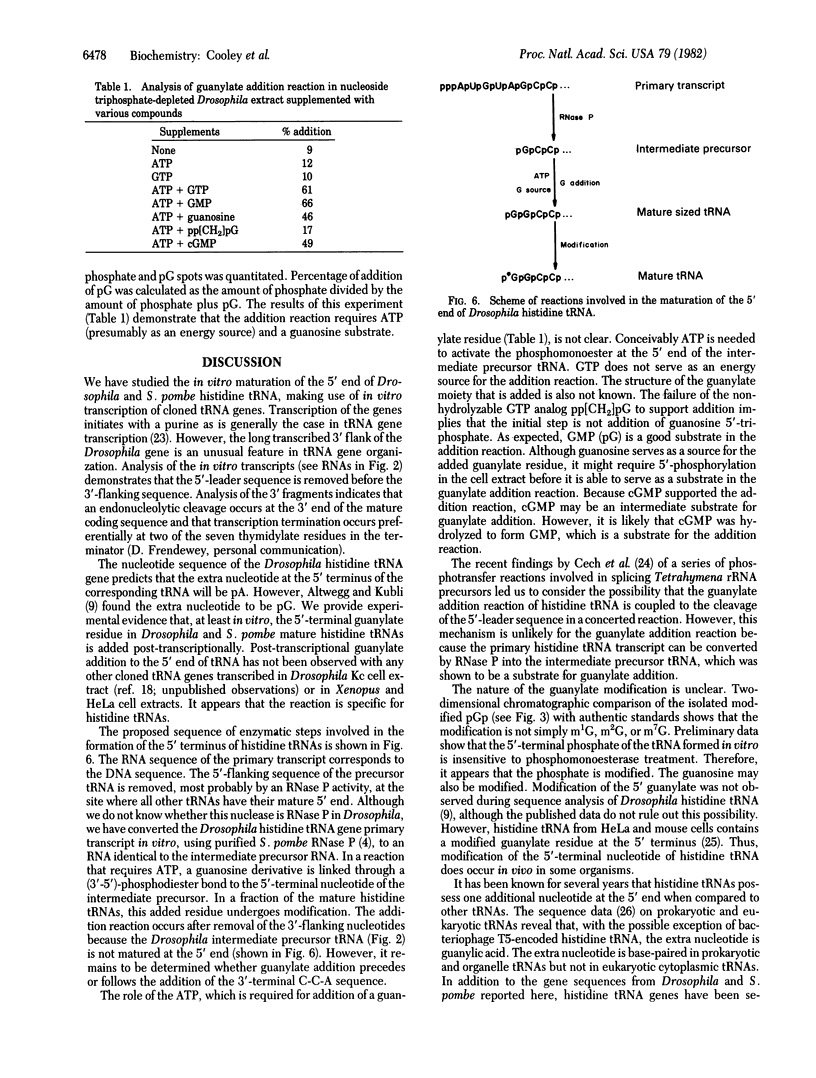
All sequenced histidine tRNAs have one additional nucleotide at the 5' end when compared to other tRNA species. Sequence analysis of histidine tRNA genes from Drosophila melanogaster and Schizosaccharomyces pombe showed that the terminal guanylate residue of the mature tRNAs is not encoded by the genes. Analysis of the products from in vitro transcription of these genes in extracts from Drosophila Kc cells demonstrated that the 5'-terminal nucleotide present in the mature tRNA is added post-transcriptionally. The addition reaction requires ATP. A portion of the mature tRNAs are then modified at the 5'-terminal pG. Analysis of the RNA species formed during the in vitro maturation of the Drosophila histidine tRNA primary transcript uncovered the following maturation scheme: (i) the primary transcript is processed by RNase P at the 5' end to form an intermediate precursor; (ii) the 3'-flanking sequence is endonucleolytically removed, and a guanylate moiety is added to the 5' end to form mature-sized histidine tRNA; and (iii) a fraction of the 5'-terminal guanylate residues then undergoes modification. In contrast to the capping of eukaryotic mRNA, the guanylate addition to histidine tRNA results in the formation of a (3'-5')-phosphodiester bond. There are no precedents for the post-transcriptional addition of nucleotides (in phosphodiester linkage) to the 5' end of RNA precursors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J. RNA processing and the intervening sequence problem. Annu Rev Biochem. 1979;48:1035–1069. doi: 10.1146/annurev.bi.48.070179.005131. [DOI] [PubMed] [Google Scholar]
- Altman S. Transfer RNA processing enzymes. Cell. 1981 Jan;23(1):3–4. doi: 10.1016/0092-8674(81)90262-2. [DOI] [PubMed] [Google Scholar]
- Altwegg M., Kubli E. The nucleotide sequence of histidine tRNA gamma of Drosophila melanogaster. Nucleic Acids Res. 1980 Aug 11;8(15):3259–3262. doi: 10.1093/nar/8.15.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlani R. E., Pentella C., Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: isolation of mitochondrial transfer ribonucleic acid mutants and characterization of transfer ribonucleic acid genes of Saccharomyces cerevisiae. J Bacteriol. 1980 Mar;141(3):1086–1097. doi: 10.1128/jb.141.3.1086-1097.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisnard M., Petrissant G. The nucleotide sequence of sheep liver histidine-tRNA (anticodon Q-U-G). FEBS Lett. 1981 Jun 29;129(1):180–184. doi: 10.1016/0014-5793(81)80785-5. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Osinga K. A., Van der Horst G., Borst P. Nucleotide sequence of the mitochondrial structural genes for cysteine-tRNA and histidine-tRNA of yeast. Nucleic Acids Res. 1979 Jul 25;6(10):3255–3266. doi: 10.1093/nar/6.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Zaug A. J., Grabowski P. J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell. 1981 Dec;27(3 Pt 2):487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingermann T., Sharp S., Appel B., DeFranco D., Mount S., Heiermann R., Pongs O., Söll D. Transcription of cloned tRNA and 5S RNA genes in a Drosophila cell free extract. Nucleic Acids Res. 1981 Aug 25;9(16):3907–3918. doi: 10.1093/nar/9.16.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudler R., Egg A. H., Kubli E., Artavanis-Tsakonas S., Gehring W. J., Steward R., Schedl P. Transfer RNA genes of Drosophila melanogaster. Nucleic Acids Res. 1980 Jul 11;8(13):2921–2937. doi: 10.1093/nar/8.13.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber R. L., Gage L. P. Transcription of a cloned Bombyx mori tRNA2Ala gene: nucleotide sequence of the tRNA precursor and its processing in vitro. Cell. 1979 Nov;18(3):817–828. doi: 10.1016/0092-8674(79)90134-x. [DOI] [PubMed] [Google Scholar]
- Han J. H., Harding J. D. Isolation and nucleotide sequence of a mouse histidine tRNA gene. Nucleic Acids Res. 1982 Aug 25;10(16):4891–4900. doi: 10.1093/nar/10.16.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline L., Nishikawa S., Söll D. Partial purification of RNase P from Schizosaccharomyces pombe. J Biol Chem. 1981 May 25;256(10):5058–5063. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., De Robertis E. M., Cortese R. Order and intracellular location of the events involved in the maturation of a spliced tRNA. Nature. 1980 Mar 13;284(5752):143–148. doi: 10.1038/284143a0. [DOI] [PubMed] [Google Scholar]
- Randerath K., Gupta R. C., Randerath E. 3H and 32P derivative methods for base composition and sequence analysis of RNA. Methods Enzymol. 1980;65(1):638–680. doi: 10.1016/s0076-6879(80)65065-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Schwarz Z., Jolly S. O., Steinmetz A. A., Bogorad L. Overlapping divergent genes in the maize chloroplast chromosome and in vitro transcription of the gene for tRNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3423–3427. doi: 10.1073/pnas.78.6.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibler A. P., Martin R. P., Dirheimer G. The nucleotide sequence of yeast mitochondrial histidine-tRNA. FEBS Lett. 1979 Nov 1;107(1):182–186. doi: 10.1016/0014-5793(79)80491-3. [DOI] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R. Histidine regulation in Salmonella typhimurium. 13. Nucleotide sequence of histidine transfer ribonucleic acid. J Biol Chem. 1972 May 25;247(10):2989–3000. [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r1–55. [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Jou W. M., Fiers W. Analysis of 32P-labeled bacteriophage MS2 RNA by a mini-fingerprinting procedure. Anal Biochem. 1976 May 7;72:433–446. doi: 10.1016/0003-2697(76)90551-0. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]