Abstract
The reduction in levels of the potentially toxic amyloid-β peptide (Aβ) has emerged as one of the most important therapeutic goals in Alzheimer's disease. Key targets for this goal are factors that affect the expression and processing of the Aβ precursor protein (βAPP). Earlier reports from our laboratory have shown that a novel cholinesterase inhibitor, phenserine, reduces βAPP levels in vivo. Herein, we studied the mechanism of phenserine's actions to define the regulatory elements in βAPP processing. Phenserine treatment resulted in decreased secretion of soluble βAPP and Aβ into the conditioned media of human neuroblastoma cells without cellular toxicity. The regulation of βAPP protein expression by phenserine was posttranscriptional as it suppressed βAPP protein expression without altering βAPP mRNA levels. However, phenserine's action was neither mediated through classical receptor signaling pathways, involving extracellular signal-regulated kinase or phosphatidylinositol 3-kinase activation, nor was it associated with the anticholinesterase activity of the drug. Furthermore, phenserine reduced expression of a chloramphenicol acetyltransferase reporter fused to the 5′-mRNA leader sequence of βAPP without altering expression of a control chloramphenicol acetyltransferase reporter. These studies suggest that phenserine reduces Aβ levels by regulating βAPP translation via the recently described iron regulatory element in the 5′-untranslated region of βAPP mRNA, which has been shown previously to be up-regulated in the presence of interleukin-1. This study identifies an approach for the regulation of βAPP expression that can result in a substantial reduction in the level of Aβ.
The major pathological hallmarks of Alzheimer's disease (AD), a progressive neurodegenerative condition leading to loss of memory, are characterized by the appearance of senile plaques that are primarily composed of Aβ and neurofibrillary tangle aggregates (1, 2). Aβ, a 40- to 42-residue peptide, is derived from a larger protein, βAPP (695–770 residues) whose biological functions remain to be fully determined but whose pathological role may be separated on the basis of its final proteolyzed form (1, 3). βAPP derivatives are generated by three enzymatic activities termed α-, β-, and γ-secretases to produce different protein fragments that are either neuroprotective or amyloidogenic. Recently, four groups have identified an aspartyl protease with β-secretase-like properties (4–7) that may serve as a therapeutic marker. However, its value as a target for drug development is complicated by its location within two (plasma and Golgi) membranes. Furthermore, the role of alternative compensatory activities remains unclear. Indeed, a second enzyme, Thimet oligopeptidase, was found capable of β-secretase activity in transfected COS cells (8). A major pharmaceutical industry focus has been to look for agents that reduce amyloidogenic processing using compounds that can manipulate βAPP to produce nonamyloidogenic by-products. However, it is important to note that the role of alternative βAPP fragments in AD is unclear.
Regarding regulatory mechanisms involved in βAPP processing, environmental agents have been demonstrated to accelerate βAPP turnover into its pathological Aβ form (1). Furthermore, the cellular surroundings of neurons, particularly astrocytes and microglia, are additional and nonneuronal sources of βAPP (9, 10). Thus, amyloid plaque occurrence is often associated with enlarged microglia that produce IL-1, a potent mediator of astroglial proliferation and βAPP production (10). The fact that IL-1 can influence this process suggests that signaling pathways induced by cytokines are interconnected with βAPP metabolism. Another example of receptor–signaling association and βAPP homeostasis is demonstrated through the activation of muscarinic m1 and m3 receptors, which modify βAPP synthesis and processing through mitogen-activated protein (MAP) kinase-dependent and independent pathways (11–13). Reductions in muscarinic receptors, as in AD, may alter βAPP metabolism and result in subsequent Aβ deposition. Cholinergic system impairment has been reversed with moderate success by the use of cholinesterase inhibitors (ChEIs) (14, 15), the only approved drugs for AD treatment.
We have synthesized a family of ChEIs, phenserine, and analogues. Phenserine dramatically improves cognitive performance in rodents (14, 16) and is in clinical trials. Studies of rats with forebrain cholinergic lesions, known to increase βAPP in cholinergic projection areas, have shown that phenserine can protect against this and, additionally, reduce βAPP production in naive animals (17). As both βAPP processing and cholinesterase activity are affected in the AD brain (18), and as the ChEI, tacrine, has been shown to decrease βAPP and Aβ in neuronal cells in vitro (19), our current studies have focused on the molecular changes induced by phenserine. We report herein the mechanism through which phenserine interacts with the cellular processing of βAPP and reduces Aβ. With this mechanism, we have identified an approach to lower βAPP synthesis rates, an unfortunately neglected route for treatment of AD, which will not overproduce alternative fragments whose physiological effects are currently unpredictable.
Materials and Methods
Phenserine.
Phenserine is a member of a family of compounds that are phenylcarbamates of hexahydropyrrol indoles and is selective for acetylcholinesterase (14, 15). The compound was synthesized in its optically (>99.9%) and chemically (>99.9%) pure (−) and (+) enantiomeric forms as a tartrate salt (14, 20). The concentration of compound required to inhibit 50% acetylcholinesterase activity was 22 nM for (−)-phenserine, whereas >25,000 nM was inactive for (+)-phenserine.
Cell Lines.
Human neuroblastoma (SK-N-SH and SH-5Y-5Y) and human astrocytoma (U373) cell lines were obtained from American Type Culture Collection.
Drug Treatment.
SK-N-SH neuroblastoma cells were cultured on 60-mm dishes at a concentration of 3 × 106 cells, and SH-SY-5Y neuroblastoma and U373 astrocytoma cell lines were plated in 100-mm dishes at a concentration of 3 × 105 cells. The cells were allowed to grow in complete media (10% FBS/2 mM glutamine in DMEM) for 3–4 days until they reached 70% confluence. To start the experiment, spent media were removed and replaced with fresh media (SK-N-SH: 4 ml of DMEM/0.5% FBS; U373: 5 ml of DMEM/2.5% FBS) containing 0, 5, or 50 μM phenserine. The cells were incubated at 37°C, 5% CO2 for the specific times indicated. Different media and sera were purchased from Life Technologies (Rockville, MD)
Inhibitor Treatment.
A day before drug treatment, confluent cultures of U373 cells were pretreated with 25 nM extracellular signal-regulated kinase (ERK)-specific inhibitor, PD98059 (Calbiochem), in 4.5 ml of 2.5% FBS/2 mM glutamine/DMEM for 16 h. Phenserine was added to each assay plate in a final volume of 5 ml. To examine for phosphatidylinositol 3-kinase (PI3-kinase) involvement, an active 2 μM concentration of the PI3-kinase inhibitor, LY294002 (Calbiochem), in 4.5 ml of 2.5% FBS/2 mM glutamine/DMEM was added to each assay plate and incubated for 30 min before the addition of phenserine. Appropriate vehicle controls were run alongside treated samples.
Lysate Preparation.
At each time point, the spent medium was collected and stored at −70°C for later analysis of secretory βAPP levels. The cells were washed twice with PBS, pH 7.4 and incubated on ice for 15 min for lysis with 100 μl of lysis buffer (20 mM Hepes/2 mM EGTA/50 mM β-glycophosphate/1 mM sodium orthovanadate/1% Triton X-100/10% glycerol) containing appropriate protease inhibitors (2 mM PMSF/100 μg/ml aprotinin/25 μM leupeptin/20 μg/ml soybean trypsin inhibitor). Each lysate was microcentrifuged for 15 min at 14,000 rpm. Protein levels of the supernatant were analyzed by the Bradford protein assay (Bio-Rad).
Western Blot.
Fifteen micrograms of protein from each sample was mixed with the appropriate volume of 5 × Laemmli buffer and boiled for 5 min at 100°C. The samples were loaded onto a 10% NuPAGE Bis-Tris gel in 1 × NuPAGE Mops SDS running buffer (NOVEX, San Diego), and the proteins were separated at 200 V for 45 min. The gels then were transferred onto nitrocellulose at 25 V for 1.5 h. The blots were blocked with 5% nonfat dry milk in 10 mM Tris, pH 8.0, containing 150 mM NaCl for 1 h and washed twice for 15 min in large volumes of TBST (10 mM Tris, pH 8.0/150 mM NaCl/0.05% Tween 20). Each blot was probed for 2 h with either 22C11 anti-βAPP N-terminal antibody (Roche Molecular Biochemicals) diluted to 2.5 μg/ml or anti-activated ERK antibody (Promega) diluted to 25 ng/ml. The blots were washed twice for 15 min in TBST and placed in secondary antibody, anti-mouse IgG, or anti-rabbit IgG conjugated to horseradish peroxidase (Sigma) for 30 min. Three final TBST washes of 20 min in duration each were performed before the samples were detected by chemiluminescence and exposed to film (Amersham Pharmacia). Additionally, all blots were stained with Ponceau S (Sigma) to determine equivalent loading of samples. Densitometric quantification of blots was undertaken by using a CD camera and NIH-IMAGE (version 4.1).
Lactate Dehydrogenase (LDH) Assay.
Measurement of released LDH in conditioned medium was undertaken as a marker of cell membrane integrity, cellular toxicity, and, indirectly, its viability, as described (19, 21).
Total Aβ Assay.
Total Aβ levels released in SH-SY-5Y and SK-N-SH cultured samples were assayed by ELISA (22). For total Aβ levels in conditioned medium, the rabbit polyclonal antibody no. 3160 (1–40 residues of Aβ) was used as a capture antibody for all species of Aβ (Aβ1–40 and Aβ1–42), whereas mAb 4G8 (17–25 residues of Aβ) was used to detect Aβ levels, and the values were expressed as the mean of six independent assays.
Transfection.
One day before transfection, U373 cells were plated onto 100-mm dishes (3 × 105 cells). On the day of transfection, the cells were given 5 ml of fresh media containing 10% FBS/2 mM glutamine in DMEM. They were transfected using a calcium phosphate precipitation method (23). Briefly, for each plate, 3 μg of DNA [5′ untranslated region (UTR) APP-PSV2-CAT or PSV2-CAT vector] were placed in a final volume of 500 μl of 250 mM CaCl2. The CAT gene was used as a reporter gene. The DNA solution was slowly pipetted into an aerated equivalent volume of 2 × HeBS, pH 7.05. The resulting precipitate was allowed to stand 10–20 min at room temperature before its addition to the cells. After 18 h, the media were changed, and the transfected cells were left for 2 days before drug treatment.
CAT Assay.
The cell lysates from transfected U373 cells treated with phenserine were analyzed for their CAT activity using a colorimetric enzyme immunoassay (Roche Molecular Biochemicals). Briefly, 50 μg of protein (an amount within the linear range of the assay) was placed onto anti-CAT coated microtiter plate modules and allowed to bind for 1 h at 37°C. The plates were washed thoroughly after each step. Next, a digoxigenin-labeled anti-CAT antibody was added to the samples and incubated for 1 h at 37°C. A subsequent antibody, anti-digoxigenin conjugated to peroxidase, was placed in the wells for another hour under similar conditions. Finally, peroxidase substrate, ABTS, was added, and the absorbance of each sample was measured at 405 nM wavelength.
Northern Blotting.
Total RNA (10 μg) was extracted and prepared from treated astrocytoma cells using an RNA-STAT kit (Tel-Test, Friendswood, TX). Samples were denatured in formamide, Mops buffer, formaldehyde, dye mix, and ethidium bromide at 65°C for 10 min, placed on ice for 5 min, and electrophoresed on a 1.0% agarose-formaldehyde gel. The gel was blotted onto Hybond nitrocellulose filters and immobilized by UV crosslinking and heating filters for 2 h. Each filter was prehybridized in hybridization buffer (1% BSA/7% SDS/0.5 M phosphate buffer, pH 7/1 mM EDTA) for at least 2 h. The filter was hybridized overnight with probe. After hybridization, the filters were washed twice with wash solution containing 0.5% BSA/5% SDS/40 mM phosphate buffer, pH 7/1 mM EDTA for 30 min at 65°C. The βAPP cDNA probe corresponded to a unique internal BglII/SpeI fragment generated from human βAPP cDNA (provided by John Kusiak, National Institutes of Health). Equal loading of samples was verified by rehybridizing the filter with a human actin gene by using an actin β-cDNA probe (CLONTECH).
Plasmid Constructs.
The pSV2(APP)CAT was provided by J.R. (23). Subcloning of all constructs is described in ref. 23. Briefly, the pSV2(APP)CAT construct was generated by inserting a 90-bp fragment of the βAPP gene 5′ UTR immediately upstream of the CAT gene into the pSV2 vector.
Statistics.
A two-tailed Student's t test was carried out to compare two means. When more than two means were compared, one-way ANOVA, together with a Bartlett's test for homogeneity of variances and a Dunnett's multiple comparison test, were used. The level of significance was defined as P < 0.05.
Results
Phenserine Decreases βAPP and Aβ Levels in Neuroblastoma Cells.
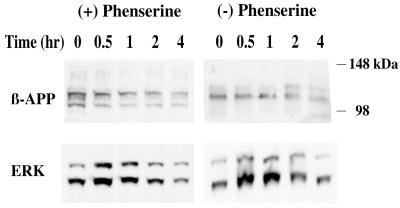
βAPP protein levels were measured after treatment of the SH-SY-5Y cells with both 5 μM (+)-phenserine and (−)-phenserine for 0.5, 1, 2, and 4 h (Fig. 1). These stereoisomeric forms of the drug have opposite affects on ChEI activity; (+)-phenserine exhibits no anti-cholinesterase activity, whereas (−)-phenserine has potent enzymatic activity (14, 15). In both experiments, the βAPP levels in the cell lysates slowly decreased at each time point, with the most dramatic decline observed after 4 h. During this period, the cells were also examined for their ability to induce signal transduction pathways. Mitogen-stimulated kinase, ERK1/2, was detected in the treated samples at all times and peaked at the 30-min to 1-h period. Stress-activated transcription kinases, p38 and JNK, were not detected in the samples (data not shown). Furthermore, media samples were analyzed for levels of Aβ at 4, 8, and 16 h to assess whether or not a decline in βAPP translated into a decline in total Aβ levels. Unfortunately, levels of Aβ were below detectable levels in both control and phenserine-treated cells. Hence, studies were repeated with SK-N-SH cells with (−)-phenserine, which was used in all subsequent studies unless otherwise indicated. These human neuroblastoma cells have a higher basal level of Aβ secretion compared with SH-SY-5Y cells.
Figure 1.
Phenserine treatment of SH-SH-5Y neuroblastoma cells decreased βAPP protein levels and affected ERK transcription factor levels. SH-SY-5Y cells were incubated with 5 μM (+)- or (−)-phenserine for 0, 0.5, 1, 2, and 4 h to determine the effect of the drug on βAPP protein levels. Western blots of cell lysates containing 15 μg of total protein per lane were analyzed. The blot was sectioned into two halves, and the top portion was probed with an N-terminal directed anti-βAPP antibody; the remaining portion was probed with an antibody directed to phosphorylated ERK. In accord with prior reports (19, 21, 39), two high molecular mass bands corresponded to alternate forms of βAPP (100–125 kDa) and ERK 1/2 (42–46 kDa).
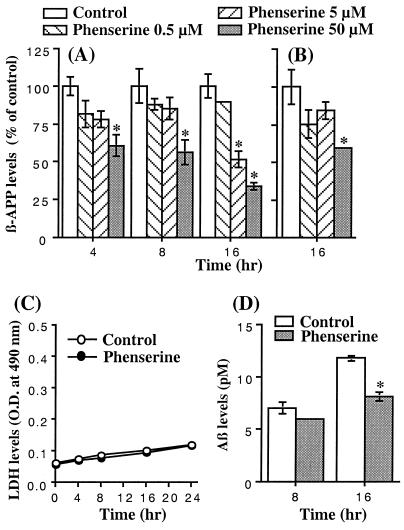
After phenserine treatment of SK-N-SH cells for 16 h, βAPP levels were reduced in a time- and concentration-dependent manner in both conditioned media (Fig. 2A) and cell lysates (Fig. 2B). This was not associated with cellular dysfunction, as determined by measurement of LDH levels versus untreated controls (Fig. 2C). Quantification of levels of total Aβ was undertaken at 8 and 16 h, and results in Fig. 2D demonstrate a phenserine-induced reduction of 14% and 31% (P < 0.002), respectively, versus untreated controls. (+)-Phenserine possessed a similar concentration- and time-dependent action on βAPP levels (data not shown).
Figure 2.
Phenserine treatment of SK-N-SH neuroblastoma cells decreased βAPP protein and total Aβ peptide levels without cellular dysfunction. SK-N-SH cells were incubated with (−)-phenserine for up to 16 h to determine the effect of the drug on βAPP protein (A and B), LDH (C), and total Aβ levels (D). (A and B) βAPP levels as a percent of controls after pretreatment with 0.5, 5, and 50 μM (−)-phenserine for 4, 8, and 16 h (*, significantly different from control, P < 0.05). Western blots of conditioned media (A) and cell lysates (B) were probed with an N-terminal directed anti-βAPP antibody. (C) LDH levels in media from cells treated with and without 50 μM (−)-phenserine for up to 16 h. There was no significant difference between treated and untreated levels up to 16 h (P > 0.05). (D) Concentration of total Aβ peptide in media from SK-N-SH cells incubated with 50 μM (−)-phenserine for up to 16 h. Levels fell from 6.95 to 5.95 pM (14%) at 8 h and from 11.75 to 8.1 pM (31%, P < 0.02) at 16 h in control and phenserine-treated cells, respectively.
Phenserine-Associated Decrease of βAPP Levels in Astrocytoma Cell Line U373 Does Not Depend on ERK Activation.
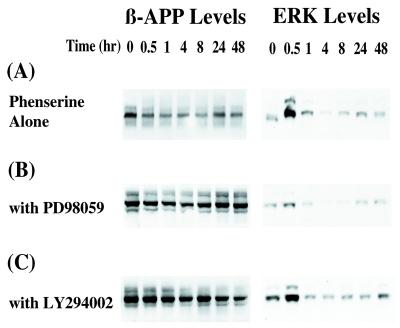
After an extended period of phenserine treatment, U373 cells exhibited a similar pattern of decreased βAPP protein synthesis. Fig. 3 is a representative of four experiments that shows that βAPP levels gradually decreased between 1 and 8 h of treatment. After 8 h, a slow recovery of βAPP was detectable (Fig. 3A), but its level was still lower than in untreated cells. Activation of ERK1/2 peaked at 30 min and remained elevated at a low level for the remainder of the assay. To determine whether or not ERK involvement was directly related to phenserine treatment, the cells were pretreated with PD98059, a specific inhibitor of MAP kinase (Fig. 3B). Although ERK levels decreased significantly, the pattern of βAPP levels induced by phenserine remained largely similar to U373 cells treated with drug without PD98059. In all cases, βAPP levels decreased in excess of 25%, as determined by densitometry.
Figure 3.
Phenserine treatment of U373 MG astrocytoma cells decreased βAPP protein levels, yet cotreatment with ERK and PI3-kinase inhibitors did not affect this process. U373 MG astrocytoma cells were treated with (−)-phenserine to determine their effect on βAPP protein levels. ERK inhibitor, PD98059, and PI3-kinase inhibitor, LY294002, were added and used to ascertain whether or not phenserine action on βAPP was directed via these signaling pathways. (A) Western blots of lysates (15 μg/lane) of U373 cells incubated with 50 μM (−)-phenserine for 0, 0.5, 1, 4, 8, 24, and 48 h were analyzed. The blot was divided into two sections. The blot was probed with anti-βAPP antibody (Left) or with anti-phosphorylated ERK antibody (Right). (B) U373 MG cells were pretreated with 25 nM PD98059 for 16 h before (−)-phenserine treatment. Lysates were analyzed by Western blots, described above. (C) U373 MG cells were pretreated with 200 μM LY294002 for 1.5 h before addition of (−)-phenserine. The cell lysate of each sample was analyzed as described above. βAPP protein levels were reduced by in excess of 25% (P < 0.05) with phenserine treatment in A, B, and C, as determined by densitometric quantification.
Phenserine action on βAPP through ERK independent, PI3-kinase stimulation was also assessed. Treatment of astrocytoma cells with phenserine and LY294002, a specific inhibitor of PI3-kinase, showed a similar pattern of βAPP levels when compared with phenserine alone treated cells (Fig. 3C).
Phenserine Decreases βAPP Protein Levels Through the Action of a Translational Enhancer in the APP–mRNA 5′ UTR.
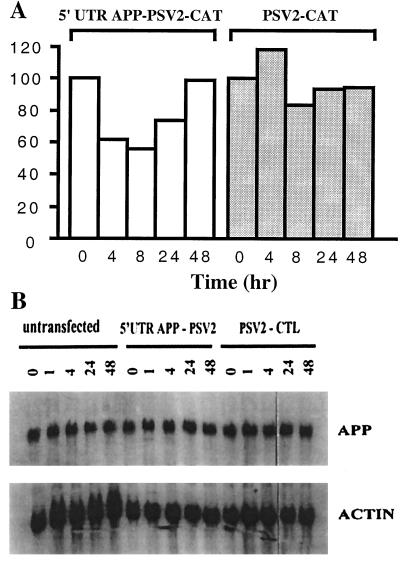
A recent report identified a 90-nt element from the 146-nt 5′ UTR of the βAPP mRNA that is able to confer a 3-fold IL-1 responsive gene expression to CAT reporter mRNA in astrocytoma cells (23). IL-1 was able to induce βAPP protein levels in the absence of increased βAPP mRNA synthesis. Parallel experiments with phenserine were examined for its ability to regulate βAPP protein levels in an identical manner. Fig. 4 is a representative CAT assay that shows that phenserine is able to decrease the level of APP–mRNA 5′ UTR enhancement to a CAT reporter mRNA in pSV2 (APP) CAT-transfected astrocytoma cells. A 2-fold decrease after phenserine treatment was sustained between 4 and 8 h in cells transfected with the 5′ UTR of βAPP mRNA. This mirrored the reduction in βAPP protein levels, illustrated in Fig. 3A. Similar to βAPP protein levels in Fig. 3, CAT activity returned to pretreatment levels by 48 h. In control samples, pSV2 CAT-transfected cells exhibited no significant inhibition by phenserine at any time, indicating that the parental vector was unresponsive to drug treatment. The expression level of CAT in the control vector versus the 5′ UTR containing vector before phenserine treatment was similar.
Figure 4.
Phenserine regulation of βAPP protein levels in U373 MG astrocytoma cells is conferred through the action of the βAPP–mRNA 5′ UTR. U373 MG astrocytoma cells were transfected with 3 μg of pSV2 (APP) CAT plasmid or the parental vector pSV2 CAT. Each set of transfection plates was left unstimulated or treated with 50 μM of phenserine for the experimental times listed. (A) CAT activity was assessed from lysates of transfected cells treated with (−)-phenserine for 0, 4, and 8 h, and 50 μg from each sample was measured in duplicate for each assay. Quantitation of the fold stimulus of CAT gene activity conferred by the 5′ UTR βAPP–mRNA was measured. ELISA readings of CAT expression were measured at 405 nm. (B) Phenserine does not affect the steady-state levels of βAPP–mRNA levels. Ten micrograms of RNA isolated from untransfected and transfected cells treated with (−)-phenserine for 0, 1, 4, 24, and 48 h was analyzed by Northern blot. Phosphoimager analysis revealed steady-state expression of βAPP–mRNA in each sample. The filter was stripped and rehybridized with a labeled human actin probe to standardize the loading differences in individual samples.
In contrast, phenserine treatment of U373 cells did not affect βAPP mRNA levels. Fig. 4B is a representative Northern blot of U373 treated with phenserine for time points up to 48 h. Standardization of each sample to actin mRNA expression showed consistent levels of βAPP mRNA without any major fluctuations in densitometry readings. Clearly, phenserine's action of βAPP protein is at the level of translation, as Northern blot analyses of untransfected and transfected cells show little differences in levels of mRNA transcription.
Discussion
The consistent association of familial AD mutations with an increase in Aβ42 provides strong evidence for its role in triggering the cascade of events that lead to the neurodegeneration in AD. Thus, reduction of Aβ levels has become an important goal for many drug development programs. However, due to the large cholinergic deficiency in AD, the first drugs developed to correct this deficit and restore cognitive abilities were cholinergic agents. Interestingly, Tacrine, a first-generation ChEI used in AD, also showed a capacity to reduce levels of secreted βAPP and Aβ in tissue culture (21), although the mechanisms involved need to be elucidated. The present study characterizes the regulation of βAPP processing by using a ChEI, phenserine, currently in AD clinical trials and effective in improving learning in rats. Interestingly, phenserine protects rats with forebrain cholinergic lesions from associated elevations in βAPP protein synthesis (17). These studies provided us the impetus to examine the specific mechanisms involved in phenserine's action on βAPP regulation at the cell culture level. In examining the treatment of cells centrally affected in AD with this drug, the findings in this report may provide clues for the identification of regulating elements.
The neuroblastoma cell line, SH-SY-5Y, is an accepted model of neuronal differentiation (24, 25). Undifferentiated, it is an ideal candidate for this study as the cells exhibit muscarinic membrane receptors and neurotransmitter enzymes and display a basal level of βAPP expression (26). Phenserine treatment of SH-SY-5Y cells rapidly reduced levels of endogenous βAPP, under the dose and conditions described. This action was not because of the ChEI activity of phenserine, as the stereoisomeric (+)-phenserine, which lacks ChEI activity (15), reduced the level of βAPP protein to a similar magnitude as its ChEI potent (−)-enantiomer. As a lipophilic compound, phenserine is able to diffuse across membrane barriers with rapid ease. Indeed, pharmacological reports of its ability to cross the blood–brain barrier, reaching brain/plasma ratios of 10:1 (14), substantiate its ability to penetrate and exert its action within neuroblastoma cells. These actions were, additionally, replicated in two other cell lines of nervous system origin: specifically, SK-N-SH neuroblastoma and U373 astrocytoma cells. In contrast, other ChEI drugs (physostigmine and metrifonate) are unable to alter βAPP levels in various neuronal cell lines (21). For phenserine-treated cells, the described reductions in βAPP translated into reduced secreted levels of total Aβ.
There is growing evidence that changes within the brain microenvironment can alter βAPP metabolism and, subsequently, the course of AD development (2, 27, 28). Several studies have reported an increased risk of AD after head trauma and in active microglial and astroglial microenvironments in Down's syndrome and AD patients, as well as an inverse relationship between AD pathology and the use of antiinflammatory drugs (2, 27). Changes in βAPP processing by cytokines are supported by the fact that activated microglial cells are invariably associated with developing lesions, and these cells are major sources of IL-1, a potent stimulator of βAPP in surrounding astrocytes. Cytokines such as IL-1, released into the microenvironment, have the potential to activate nonneuronal cells to produce βAPP, and this action can play a significant role in the progression of AD (28, 29). Activation of classical signaling pathways by mitogen stimulation as well as muscarinic m1 and m3 receptor engagement results in the increased secretion of βAPP and its derivatives (12, 13, 28). Detailed reports describing the increased level of βAPP demonstrate that protein kinase C (PKC) stimulation is involved (30–32) and that it acts through a member of the MAP kinase family of transcription factors, ERK1/2 (33–35). However, there is also evidence that ERK1/2-independent pathways are also involved; m1 and m3 receptor-mediated changes in βAPP levels can be detected in absence of ERK1/2. Hence, the likelihood of alternate signaling pathways using other transcription factors is strong (36, 37).
We additionally examined the effects of phenserine in the astrocytoma cell line U373. As with SH-SY-5Y cells, this cell line displayed constitutive expression of βAPP, and drug treatment induced similar patterns of decreasing βAPP protein levels even at extended times. This observation suggests that identical regulatory elements of βAPP in these cells are likely to be used by phenserine. As several studies have implicated a role for signal transduction in regulating βAPP (38), both cell lines were analyzed for the presence of these proteins after phenserine application. The MAP kinase ERK1/2 was induced by phenserine, maintained in all samples, and peaked at 30 min to 1 h posttreatment. Interestingly, the two MAP kinase relatives, JNK and p38 (39), that are induced by stress (oxidative stress, UV radiation, cyclohexamide treatment) were not detected. Hence, the primary component active in the signal cascade appears to be ERK1/2. To confirm the direct association of this transcription factor with βAPP processing after phenserine treatment, we made use of a specific inhibitor of MAP kinase, PD98059, that prevents the activation of ERK1/2. Interestingly, reduction of βAPP by phenserine under these conditions remained unaffected in the absence of ERK1/2 stimulation. To account for this finding, we examined the possibility that an ERK-independent pathway may also be involved in the regulation of βAPP, as reported (36). Several studies have alluded to the role of PI3-kinase, a membrane-bound activator of phospholipase C that can stimulate phosphoinositol to produce second messengers, diacylglycerol and inositol triphosphate, both of which contribute to subsequent calcium fluxes and mobility (2). Downstream events of these factors can also initiate specific transcription factors and activate changes in protein processing. To correlate the effect of phenserine with possible PI3-kinase activation, a specific inhibitor of the kinase, LY84002, was added to the cells. As with the treatment of the previous inhibitor, similar conclusions were reached with LY84002 that this PI3-kinase inhibitor did not affect the phenserine-associated decrease of βAPP. It is important to note that in all of the former studies characterizing involvement of ERK (13, 36, 40), phorbol esters were used to increase its secretion, and ERK activation during basal βAPP production was not closely investigated. However, we examined the role of signal transduction in the reduction of βAPP from the basal level of protein, and consequently, different activators regulating this process may be involved.
Herein, we report on an action of phenserine: a demonstration of βAPP protein being regulated without βAPP message being changed by any anticholinesterase drug in cultured neuroblastoma cells. Because phenserine treatment did not alter βAPP mRNA levels, the change in protein levels can be attributed to the drug's action on the 5′ UTR, which affects RNA stability, its ribosomal binding, and eventually its translational ability to protein. The role of the UTR in regulating protein synthesis is not unprecedented, and for this reason we examined the structure of the 5′ UTR of βAPP mRNA and observed two important features. First, it contains an element that shows structural and functional homology to the iron regulatory element (IRE) sequence in the 5′ UTR of ferritin mRNA (41). IREs are a family of 28-nt, noncoding elements that control the translation of ferritin mRNA (iron storage), erythroid Δ-aminolevulinic acid synthase mRNA (heme synthesis), and the stability of the transferrin receptor mRNA (iron uptake) (42–44). IREs are localized to either the 5′ or 3′ end of the transcript, and their binding by iron-regulating proteins (IRP) can result in differential mRNA translation (41, 45). IRP binding to 5′-IRE motifs blocks the association of the small ribosomal subunit with mRNA and thus precludes mRNA translation. In contrast, IRP binding to 3′ IRE motifs stabilizes the transcript by preventing mRNA degradation and increases its translational potential. Second, the 5′ UTR of βAPP mRNA contains an important IL-1 responsive element, which conferred translational control of βAPP protein synthesis in U373 MG cells (23). As there was no conclusive identification of a molecule within the signal cascade that was affected by phenserine, and in light of the described report, we decided to assess whether or not phenserine could be down-regulating βAPP levels through this interesting 5′ UTR. Phenserine's action on βAPP 5′ UTR mRNA resulted in a reduced CAT reporter expression. In analogous experiments, phenserine-treated transfected cells showed decreased βAPP protein levels and concurrent maintenance of mRNA steady-state levels, thus reinforcing the likelihood that it exerts its effect through translational modifications.
We hypothesize that phenserine's action on the 5′ UTR βAPP is achieved through similar regulatory mechanisms exhibited in iron regulation. It is possible that phenserine decreases βAPP levels by directly preventing the binding of the ribosomal translational subunit with the mRNA through steric hindrance. Alternatively, phenserine might affect trans-acting proteins, such as IRP, in a similar manner as iron. Iron homeostasis is exquisitely sensitive to its intracellular concentration, and numerous reports have demonstrated that its regulation is also influenced by exposure to cytokines, oxidative stress, and nitric oxide (41), the same factors that coincidentally are involved in regulation of βAPP expression. In addition, there are also similar signaling pathways that are activated in iron metabolism. For example, Eisenstein et al. (46) reported a PKC-dependent increase in IRP binding affinity to IRE, which results in the elevated translational processing of ferritin and transferritin mRNAs. It would be interesting to examine whether protein kinase C can also stimulate like-binding proteins to mediate an increase in translational expression of βAPP. Input from future phenserine studies may reveal and define the connections between these regulatory pathways found in iron metabolism with those involved in βAPP processing.
We have also considered another mechanism to account for phenserine's action. Apart from its role at the 5′ UTR, IL-1 can up-regulate βAPP promoter activity in HUVEC and PC12 cells and thus modulate βAPP synthesis at the transcriptional level (29, 47, 48). Whether phenserine can exert its effect in the IL-1 acting region of the βAPP promoter in other cell lines remains to be studied.
In conclusion, our cell culture studies support the ability of phenserine to reduce βAPP, as demonstrated in vivo (17), and lower secreted levels of Aβ. Phenserine action on βAPP likely occurs via a translational mechanism. This mechanism appears to be independent of cholinergic activity and may involve direct interactions with other proteins. In light of this, phenserine action on the 5′ UTR of βAPP mRNA, identifying a drug target for AD therapy, is a phenomenon that warrants further study.
Acknowledgments
We are indebted to Drs. Catherine Cahill, Martin Farlow, and Harold Holloway. This work was supported by the Intramural National Institute on Aging (to K.T.Y.S., T.U., Q.S.Y., and N.H.G.), National Institutes of Health Grant NIH-NIAR01, and grants from the Alzheimer's Association (to D.K.L.) and The Institute for the Study on Aging (to J.R.).
Abbreviations
- AD
Alzheimer's disease
- Aβ
amyloid-β peptide
- βAPP
β-amyloid precursor protein
- ChEI
cholinesterase inhibitor
- UTR
untranslated region
- ERK
extracellular signal-regulated kinase
- CAT
chloramphenicol acetyltransferase
- IRE
iron regulatory element
- LDH
lactate dehydrogenase
- MAP
mitogen-activated protein
- IRP
iron-regulating proteins
- PI3-kinase
phosphatidylinositol 3-kinase
References
- 1.Selkoe D J. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- 2.Roberson M, Harrell L. Brain Res Rev. 1997;25:50–69. doi: 10.1016/s0165-0173(97)00016-7. [DOI] [PubMed] [Google Scholar]
- 3.Checler F. J Neurochem. 1995;65:1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- 4.Hussain I, Powell D, Howlett D, Tew D, Meek T, Chapman C, Gloger I, Murphy K, Southan C, Ryan D, et al. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 5.Sinha S, Anderson J P, Barbour R, Basi G, Caccavello R, Davis D, Dovey H, Frigon N, Hong J, et al. Nature (London) 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 6.Vassar R, Bennett B D, Babu-Khan S, Kahn S, Mendiaz E A, Denis P, Teplow D B, Ross S, Amarante P, Loeloff R. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 7.Yan R, Bienkowski M, Shuck M, Miao H, Tory M, Pauley A, Brashler J, Stratman N, Mathews W R, Buhl A, et al. Nature (London) 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 8.Koike H, Seki H, Kouchi Z, Ito M, Kinouchi T, Tomioka S, Sorimachi H, Saido T C, Maruyama K, Suzuki K. J Biochem (Tokyo) 1999;126:235–242. doi: 10.1093/oxfordjournals.jbchem.a022428. [DOI] [PubMed] [Google Scholar]
- 9.Funato H, Yoshimura M, Yamazaki T, Sato T C, Ito Y, Yokofujita J, Okeda R, Ihara Y. Am J Pathol. 1998;152:983–992. [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole G M, Cooper N, Eikelenboom P, Emmerling M, Fiebich B L, et al. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felder C, Ma A, Briley E, Axelrod J. Ann NY Acad Sci. 1993;695:15–18. doi: 10.1111/j.1749-6632.1993.tb23020.x. [DOI] [PubMed] [Google Scholar]
- 12.Nitsch R, Slack B, Wurtman R, Growdon J. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 13.Nitsch R, Growdon J, Farber S, Deng M, Wurtman R. In: Alzheimer Disease: Therapeutic Strategies. Giacobini E, Becker R, editors. Boston: Birkhauser; 1994. pp. 54–61. [Google Scholar]
- 14.Greig N H, Pei X, Soncrant T, Ingram D, Brossim A. Med Chem Rev. 1995;15:3–31. doi: 10.1002/med.2610150103. [DOI] [PubMed] [Google Scholar]
- 15.Brossi A, Pei X, Greig N H. Aust J Chem. 1996;49:171–190. [Google Scholar]
- 16.Patel N, Spangler E, Greig N H, Yu Q, Ingram D, Meyer R. NeuroReport. 1998;9:171–176. doi: 10.1097/00001756-199801050-00035. [DOI] [PubMed] [Google Scholar]
- 17.Haroutunian V, Greig N H, Pei X, Utsuki T, Gluck R, Acevedo L, Davis K, Wallace W. Mol Brain Res. 1997;46:161–168. doi: 10.1016/s0169-328x(96)00297-5. [DOI] [PubMed] [Google Scholar]
- 18.Bronfman F, Fernandez H, Inestrosa N. Exp Cell Res. 1996;229:93–99. doi: 10.1006/excr.1996.0347. [DOI] [PubMed] [Google Scholar]
- 19.Lahiri D, Farlow M, Sambamurti K. Mol Brain Res. 1998;62:131–140. doi: 10.1016/s0169-328x(98)00236-8. [DOI] [PubMed] [Google Scholar]
- 20.Yu Q S, Brossi A. Heterocycles. 1988;27:745–751. [Google Scholar]
- 21.Lahiri D, Farlow M, Nurnberger J, Jr, Greig N H. Ann NY Acad Sci. 1997;826:416–421. doi: 10.1111/j.1749-6632.1997.tb48495.x. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki N, Cheung T, Cai X, Odaka A, Eckman C, Golde T, Younkin S. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 23.Rogers J, Leiter L, McPhee J, Cahill C, Zhan S, Potter H, Nilsson L. J Biol Chem. 1999;274:6421–6431. doi: 10.1074/jbc.274.10.6421. [DOI] [PubMed] [Google Scholar]
- 24.Adem A, Mattsson M, Nordberg A, Pahlman S. Dev Brain Res. 1987;33:235–242. doi: 10.1016/0165-3806(87)90156-8. [DOI] [PubMed] [Google Scholar]
- 25.Leli U, Cataldo A, Shea T B, Nixon R A, Hauser G. J Neurochem. 1992;58:1191–1198. doi: 10.1111/j.1471-4159.1992.tb11328.x. [DOI] [PubMed] [Google Scholar]
- 26.Dyrks T, Monning U, Beyreuther K, Turner J. FEBS Lett. 1994;349:210–214. doi: 10.1016/0014-5793(94)00671-7. [DOI] [PubMed] [Google Scholar]
- 27.Breitner J C. Neurobiol Aging. 1997;17:789–794. doi: 10.1016/0197-4580(96)00109-1. [DOI] [PubMed] [Google Scholar]
- 28.Buxbaum J, Oishi M, Chen H, Pinkas-Kramarski R, Jaffe E, Gandy S, Greengard P. Proc Natl Acad Sci USA. 1992;89:10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldgaber D, Harris H, Hla T, Maciag T, Donnelly R, Jacobsen J, Vitek M, Gajdusek D. Proc Natl Acad Sci USA. 1989;86:7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buxbaum J D, Gandy S, Cicchetti P, Ehrlich M, Czernik A, Fracasso R, Ramabhadran T, Unterbeck A, Greengard P. Proc Natl Acad Sci USA. 1990;87:6003–6006. doi: 10.1073/pnas.87.15.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung A, Selkoe D J. EMBO J. 1994;13:534–542. doi: 10.1002/j.1460-2075.1994.tb06291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobsen J, Spruyt M, Brown A, Sahasrabudhe S, Blume A, Vitek M, Muenkel H, Sonnenberg-Reines J. J Biol Chem. 1994;269:8376–8382. [PubMed] [Google Scholar]
- 33.Caputi A, Barindelli S, Pastorino L, Cimino M, Buxbaum J D, Cattabeni F, Di Luca M. J Neurochem. 1997;68:2523–2529. doi: 10.1046/j.1471-4159.1997.68062523.x. [DOI] [PubMed] [Google Scholar]
- 34.Desdouits F, Buxbaum J D, Desdouits-Magnen J, Nairn A C, Greengard P. J Biol Chem. 1996;271:24670–24674. doi: 10.1074/jbc.271.40.24670. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Greengard P, Gandy S. J Biol Chem. 1995;270:23243–23245. doi: 10.1074/jbc.270.40.23243. [DOI] [PubMed] [Google Scholar]
- 36.Buxbaum J D, Ruefli A A, Parker C A, Cypess A M, Greengard P. Proc Natl Acad Sci USA. 1994;91:4489–4493. doi: 10.1073/pnas.91.10.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leblanc A, Koutroumanis M, Goodyer C. J Neurosci. 1998;18:2907–2913. doi: 10.1523/JNEUROSCI.18-08-02907.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desdouits-Magnen J, Desdouits F, Takeda S, Syu L, Saltiel A, Buxbaum J, Czernik A, Nairn A, Greengard P. J Neurochem. 1998;70:524–530. doi: 10.1046/j.1471-4159.1998.70020524.x. [DOI] [PubMed] [Google Scholar]
- 39.Waskiewics A, Cooper J. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 40.Savage M, Trusko S, Howland D, Pinsker L, Mistretta S, Reaume A, Greenberg B, Siman R, Scott R. J Neurosci. 1998;18:1743–1752. doi: 10.1523/JNEUROSCI.18-05-01743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hentze M, Kuhn L. Proc Natl Acad Sci USA. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhasker C, Burgiel G, Neupert B, Emery-Goodman A, Kuhn L, May B. J Biol Chem. 1993;268:12699–12700. [PubMed] [Google Scholar]
- 43.Melefors O, Goossen B, Johansson H, Stripecke R, Gray N, Hentze M. J Biol Chem. 1993;268:5974–5978. [PubMed] [Google Scholar]
- 44.Schalinske K, Chen O, Eisenstein R. J Biol Chem. 1998;273:3740–3746. doi: 10.1074/jbc.273.6.3740. [DOI] [PubMed] [Google Scholar]
- 45.Kim H Y, LaVaute T, Iwai K, Klausner R, Rounault T. J Biol Chem. 1996;271:24226–24230. doi: 10.1074/jbc.271.39.24226. [DOI] [PubMed] [Google Scholar]
- 46.Eisenstein R, Tuazon P, Schalinske K, Anderson S, Traugh J. J Biol Chem. 1993;268:27363–27370. [PubMed] [Google Scholar]
- 47.Donnelly R, Friedhoff A J, Beer B, Blume A J, Vitek M P. Cell Mol Neurobiol. 1990;10:485–495. doi: 10.1007/BF00712843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lahiri D K, Nall C. Mol Brain Res. 1995;32:233–240. doi: 10.1016/0169-328x(95)00078-7. [DOI] [PubMed] [Google Scholar]