Abstract
Tumor cells can induce certain cytokines and soluble receptors that have a suppressive effect on the immune system. In this study, we showed that an extracellular portion of a membrane-bound ligand of CD40 (soluble CD40 ligand; sCD40L) was significantly elevated in the serum of cancer patients compared with healthy donors. In addition, PBMCs from cancer patients had a relatively larger population of myeloid-derived suppressor cells (MDSCs), defined as CD33+HLA-DR− cells, and these cells expressed higher levels of CD40. T-cell proliferation and IFN-γ production decreased when stimulated T cells were cocultured with an increased amount of autologous MDSCs. The addition of recombinant monomeric sCD40L enriched MDSCs and had an additive inhibitory effect on T-cell proliferation. PBMCs cultured in vitro with sCD40L also showed an expansion of regulatory T cells (CD4+CD25highFoxp3+), as well as induction of cytokines, such as IL-10 and IL-6. Moreover, sCD40L-induced enrichment of programmed death-1–expressing T cells was greater in cancer patients than in healthy donors. Preexisting sCD40L also inhibited IL-12 production from monocytes on activation. These data suggest that the higher levels of sCD40L seen in cancer patients may have an immunosuppressive effect. These studies were registered at www.clinicaltrials.gov as NCT00060528, NCT00019695, NCT00179309, NCT00514072, NCT00081848, and NCT00436956.
Introduction
Cancer cells can induce a variety of soluble factors, which have an immunosuppressive effect that helps tumor cells evade host immune responses. Emerging evidence suggests that myeloid-derived suppressor cells (MDSCs) and T regulatory cells (Tregs) play a critical role in generating these tumor-derived soluble factors.1 In humans, MDSCs are commonly defined as cells that express the myeloid marker CD33 but lack expression of HLA-DR.2 An endocrine loop between tumor and suppressor cells, bridged by tumor-derived soluble factors, such as TGF-β, IL-10, GM-CSF, and VEGF, can generate a potent immunoinhibitory effect on antitumor responses and promote survival and proliferation of cancer cells.3,4 Thus, studying new tumor-derived soluble factors necessary for the generation of suppressor-cell populations, and then targeting these factors, could be an additional strategy for treating cancer patients with immunotherapies.
CD40-CD40 ligand (CD40L) is a member of the TNF superfamily and is expressed at various levels on antigen-presenting cells, epithelial cells, and hematopoietic progenitor cells.5,6 The CD40-CD40L costimulatory pathway has been shown to play a crucial role in humoral responses in humans and in the production of cytokines, such as IL-10 and IL-12, by monocytes and macrophages. These cytokines modulate the function of T lymphocytes in antitumor responses.7 A recent murine study suggested that CD40 is essential not only for MDSC-mediated immune suppression, but also for tumor-specific Treg expansion. Specifically, blockade of CD40-CD40L interaction by anti-CD40 antibody inhibited the development of Tregs and enhanced the efficacy of an established immunomodulatory therapy in an advanced tumor model.8 In addition to its role in immune regulation of this pathway, evidence suggests that ligation of CD40-CD40L can directly promote either tumor-cell apoptosis or tumor growth because many tumor cells express CD40. This contradictory effect depends on the level of CD40L signaling: higher CD40L signaling induces tumor cell death, whereas lower signaling promotes tumor growth.9 CD40L's indirect role in promoting tumor growth is a result of angiogenesis, which is mediated primarily by VEGF, TGF-β, and other chemokines. Murine studies have suggested that CD40-CD40L promotes angiogenesis by inducing VEGF production from endothelial cells and by activating platelets.8,10
sCD40L is an 18-kDa functional trimer that is shed from activated T lymphocytes and platelets.8,11 The pathophysiologic function of sCD40L has been investigated mainly in cardiovascular diseases and certain autoimmune disorders.12,13 Patients with unstable angina have elevated plasma levels of sCD40L. An increased level of this protein is thus considered a very important factor in the evaluation of cardiovascular disease.14 In cancer studies, investigation has mainly focused on the role of the membrane-bound CD40L in anticancer responses. To date, 15 clinical trials in the United States aimed at modulating this pathway to enhance immunity in cancer patients have been completed or are ongoing (www.clinicaltrials.gov). However, 2 reported studies showed that sCD40L is elevated in patients with metastasized lung cancer and undifferentiated nasopharyngeal carcinoma.15,16 In cancer patients, sCD40L is more likely derived from activated platelets than from T cells, a notion supported by evidence that cancer patients have significant platelet activation, as well as inadequate T-cell activation.17–20 These findings raise the question of whether the CD40-CD40L pathway functions as a double-edged sword, turning CD40-induced antitumor immunity into CD40-mediated angiogenesis and immune suppression. A better understanding of the underlying mechanisms of the CD40-CD40L pathway could thus lay the foundation for the development of new strategies for cancer immunotherapy.
In this study, we focused on the potential role of monomeric sCD40L, probably the predominant form in human serum, on phenotypic and functional changes in immune cells and hypothesized that elevated sCD40L in cancer patients may have an immunosuppressive effect.
Methods
Sera and cells
Serum samples from healthy donors were purchased from Innovative Research and Valley Biomedical. Cancer patients' sera were obtained from clinical trials conducted at the National Cancer Institute (www.clinicaltrials.gov): (1) a second-generation poxviral vaccine (PSA-TRICOM) targeting prostate-specific antigen (PSA) in metastatic castration-resistant prostate cancer (NCT00060528)21; (2) a prostate cancer phase 2 trial using a combination of ketoconazole with and without alendronate (NCT00019695)22; (3) a breast cancer phase 2 trial using docetaxel alone or in combination with vaccine (NCT00179309); (4) a prostate cancer vaccine trial targeting patients with no radiographic evidence of disease (NCT00514072); and (5) vaccine therapy and radiation to liver metastasis in patients with CEA-positive solid tumors (NCT00081848). All healthy donors' PBMC samples were isolated from the buffy coat by density gradient centrifugation (MP Biomedicals). PBMC samples were obtained from prostate cancer patients enrolled in clinical trials conducted at the National Cancer Institute: a prostate cancer trial using cediranib (NCT00436956) and the breast cancer phase 2 trial (NCT00179309). This study was conducted in accordance with the Declaration of Helsinki.
Isolation of CD33+DR− cells and coculture with autologous T cells
To isolate CD33+HLA-DR− cells, fresh leukapheresis samples from healthy donors were first depleted of HLA-DR+ cells. Samples were stained with anti–HLA-DR-FITC antibody and anti-FITC beads, followed by positive selection of CD33+ cells using anti-CD33 beads (Miltenyi Biotec). The purity of the isolation was tested by FACS analysis. Autologous T cells were also isolated from these samples. The T cells were labeled with CFSE and cocultured with the autologous CD33+HLA-DR− cells at different ratios, in the presence or absence of different concentrations of sCD40L (Enzo Life Sciences) or human serum albumin (Sigma-Aldrich) as an irrelevant protein. In some experiments, anti-CD40 blocking antibody (5 μg/mL) was added to cultures (BioLegend). Supernatants were collected after 18 hours and IFN-γ release was analyzed by ELISA. T-cell proliferation was assayed by CFSE dilution after 3-4 days of coculture.
Isolation of CD14+ cells and monocyte stimulation
CD14+ cells were isolated from fresh PBMCs by positive selection using anti-CD14–conjugated magnetic beads (MACS MicroBeads, Miltenyi Biotec). The cells (106/mL) were then incubated with 2 μg/mL of sCD40L for 20 hours, after which 300 U/mL of IFN-γ and 1 μL/mL of Golgi Stop, a protein transporter inhibitor maintaining the cytokine intracellularly (BD Biosciences), were added. Two hours later, a second signal consisting of 50 ng/mL lipopolysaccharide (LPS) was added to the culture. Cells were harvested 6 hours after the initial IFN-γ stimulation, and intracellular staining of IL-12 was performed.
Measurement of sCD40L in serum samples
The serum level of sCD40L was determined by human sCD40L Platinum ELISA kit, according to the manufacturer's instructions (eBioscience). The standard range of this kit is 0-10 ng/mL.
Antibodies and flow cytometry analysis
Percentages of phenotypic markers on the surface of PBMCs were determined by flow cytometry. Direct staining by monoclonal antibodies was used to detect each of the cell-surface antigens. Intracellular staining with Foxp3 and IL-12 was performed according to the manufacturer's protocol (eBioscience). Programmed death-1 (PD-1), CD33, CD25, HLA-DR, CD11b, CD40, CD70, and CD14 were purchased from BD Biosciences. Flow cytometry analysis was measured by FACSCalibur (BD Biosciences), and the resulting data were analyzed with FlowJo Version 7.6 software (TreeStar).
T-cell proliferation assay
T-cell proliferation was determined by CFSE dilution analysis. CD3+ cells were labeled with CFSE (Invitrogen) according to the manufacturer's instructions. The labeled CD3+ T cells were then cultured in conditions according to the experimental designs. The level of CFSE in CD3+ cells was analyzed by flow cytometry after 4 days of culture.
Results
sCD40L is elevated in serum of cancer patients
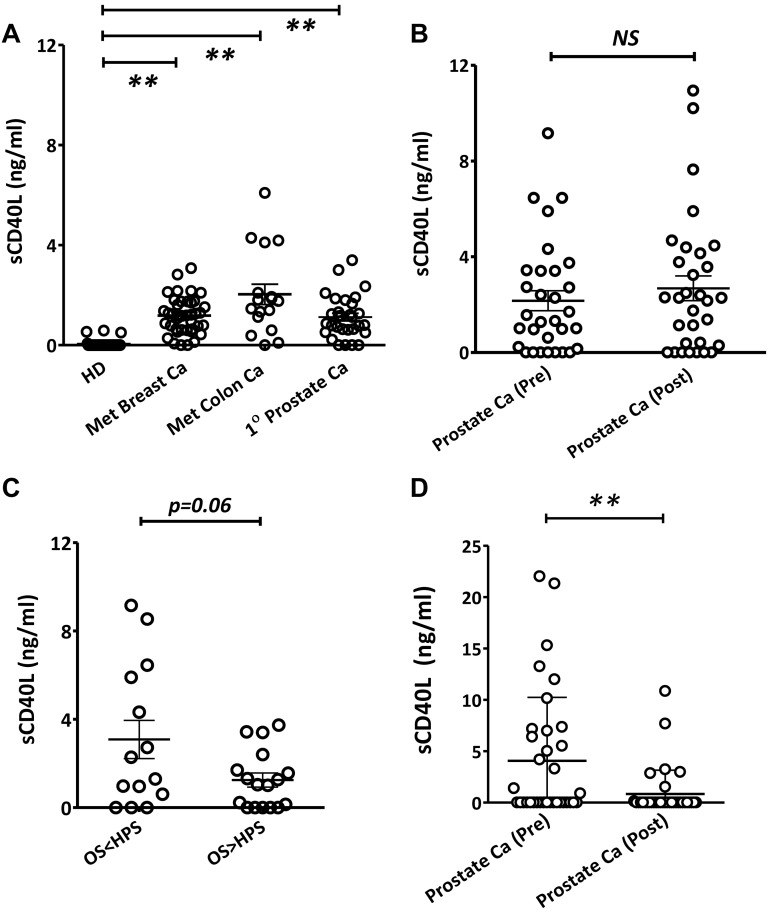
Before the comparison of sCD40L in serum samples between healthy donors and cancer patients (pretreatment), serum samples were evaluated from 2 different age groups: younger healthy donors (both male and female, average age 23 years) and older healthy donors (both male and female, average age 54 years). There were no age-related differences in sCD40L serum levels among the healthy donors, indicating that age is not a factor (data not shown). Next, the comparison of serum sCD40L levels between age-matched healthy donors and pretreatment cancer patients was carried out. Results showed significantly higher levels of serum sCD40L in pretreatment cancer patients compared with healthy donors (Figure 1A). We also found no differences between pre- and posttreatment levels of serum sCD40L in prostate cancer patients participating in a clinical trial of a second-generation poxviral vaccine (PSA-TRICOM) targeting PSA21 (Figure 1B). The Halabi nomogram23 uses 7 baseline parameters to predict overall survival in prostate cancer patients treated with chemotherapy or second-line hormonal agents. Patients in the vaccine study whose survival was shorter than predicted by the Halabi nomogram showed a trend (P = .06) toward higher pretreatment levels of sCD40L in serum than patients who outlived their Halabi-predicted survival (Figure 1C). In addition, serum samples from a previously described clinical trial22 that used a combination of ketoconazole and alendronate to treat patients with castration-resistant prostate cancer were also analyzed and showed that the treatments lowered the levels of sCD40L in these patients (Figure 1D). Treatment with ketoconazole, an adrenal steroid inhibitor, was associated with sustained ≥ 50% declines in PSA in about half of the patients in this trial, a proportion of responding patients similar to that seen with docetaxel-based therapy,24 and abiraterone, a recently approved adrenal steroid inhibitor.25 This observed difference was not the result of the quality of the serum samples because the samples were also tested for another soluble protein, sCD27, which appeared in reverse proportions to sCD40L in healthy donors and cancer patients (J.H., C.J., A.A., T.T., A.J., R.A.M., J. W. Hodge, K.Y.T., D. J. Liewehr, S. M. Steinberg, J.L.G., J.S., Soluble CD27-pool in humans may contribute to T cell activation and tumor immunity, manuscript in preparation). These results suggested that there was a difference in serum levels of sCD40L between healthy persons and cancer patients before treatment, and cancer chemotherapy could lower its levels.
Figure 1.
Differential levels of serum sCD40L in cancer patients and healthy donors (HD). (A) Elevated serum sCD40L in cancer patients. Serum sCD40L levels in HDs (n = 16) were compared with levels in patients with metastatic breast cancer (Breast Ca, n = 40), metastatic colon cancer (Colon Ca, n = 17), or first-degree (1°) prostate cancer (Prostate Ca, n = 30) before treatment of any kind. (B) sCD40L levels did not change after vaccination with PSA-TRICOM. Levels of sCD40L in 31 metastatic prostate cancer patients' sera before and after treatment with PSA-TRICOM vaccine were analyzed. (C) Association of pretreatment serum sCD40L levels with overall survival (OS) and Halabi-predicted survival (HPS) in prostate cancer patients vaccinated with PSA-TRICOM. Pretreatment serum samples were evaluated and compared for sCD40L between patients who had longer or shorter survival than their HPS. (D) Decrease in sCD40L in serum of prostate cancer patients after a combination of ketoconazole and alendronate treatments. Levels of serum sCD40L were analyzed by ELISA, and statistical analysis was performed using an unpaired t test. **P < .01. NS indicates not significant.
PBMCs from cancer patients had more MDSCs and CD40-expressing MDSCs compared with healthy donors
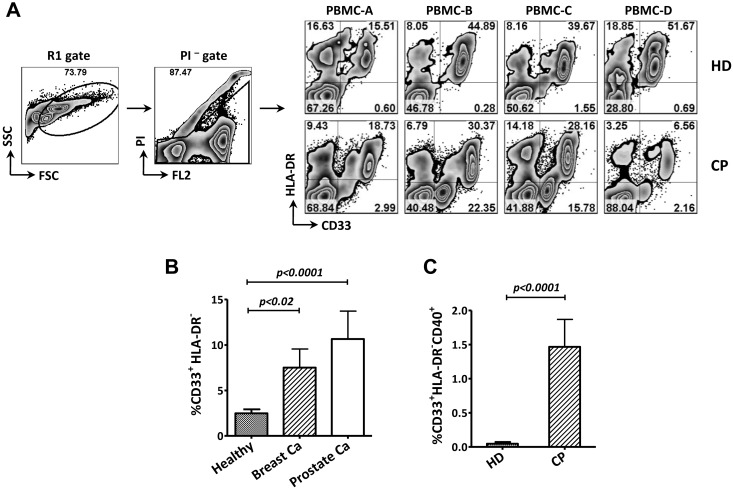
To test the hypothesis that sCD40L plays an immunosuppressive role in cancer patients, we analyzed the expression of CD40 on CD33+HLA-DR− cells (MDSCs) in PBMC samples collected from healthy donors and pretreatment cancer patients. The results showed more MDSCs in samples from patients with either metastatic breast or prostate cancer than in samples from healthy donors (Figure 2A-B). Further analysis revealed significantly higher numbers of CD40-expressing MDSCs in total PBMC in cancer patients than in healthy donors (Figure 2C), and the frequency of CD40+ MDSC in cancer patients was 20%, compared with only 2% in healthy donors. These results suggest that the MDSCs in cancer patients are more likely to respond to sCD40L signaling.
Figure 2.
Differential levels of MDSCs and CD40-expressing MDSCs in PBMCs from cancer patients (CP) and healthy donors (HD). (A) Representative FACS data from 4 HDs and 4 CPs. Age-matched PBMCs from HDs and CPs were stained with antibodies, and propidium iodide (PI) was added 10 minutes before FACS analysis. R1 and PI− cell populations were gated, and the frequency of MDSCs (CD33+HLA-DR− cells) is shown in the right lower quadrant. (B) CPs had significantly higher frequencies of MDSCs in PBMCs than HDs. PBMCs from HDs (n = 30) and untreated patients with metastatic breast cancer (Breast Ca, n = 9) or metastatic prostate cancer (Prostate Ca, n = 11) were evaluated. Statistical analysis was performed using an unpaired t test. (C) Higher frequency of CD40-expressing MDSCs in CPs than in HDs. Samples used in panel B were evaluated for CD40 expression on the MDSC population. The data shown in CPs were from all CPs (9 breast Ca and 11 prostate Ca). The results shown are representative of 2 experiments. Statistical analysis was performed using an unpaired t test. FSC indicates forward scatter; and SSC, side scatter.
sCD40L may induce MDSCs and enhance their suppression of activated T cells
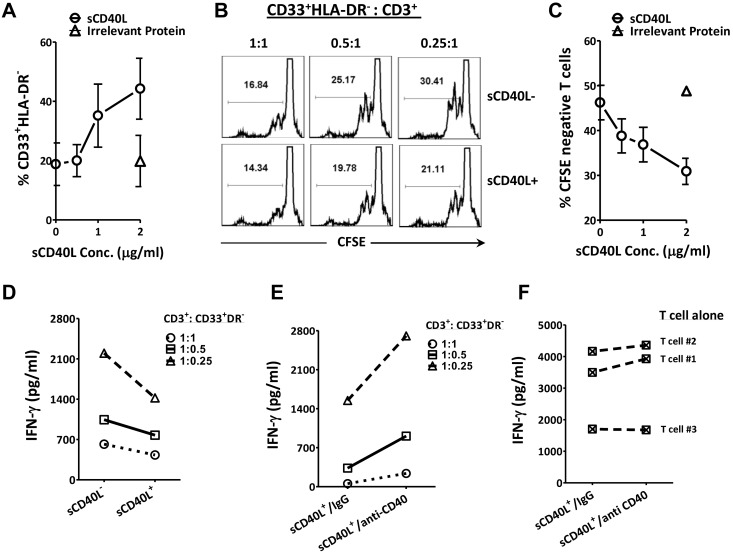
We next investigated the influence of sCD40L on MDSCs in regard to autologous T-cell activation. It would have been ideal to use cancer patient PBMCs to test our hypothesis because more MDSCs and CD40+ MDSCs were seen in cancer patients; however, only a small amount of cells was available in each blood draw from cancer patients. Hence, for the MDSCs/T separation and coculture experiments, we used fresh PBMCs from healthy donors, which were easier to obtain in large numbers from the blood bank. First, MDSCs were enriched from PBMCs, and different amounts of sCD40L or a single dose of irrelevant protein (human serum albumin) were added. The results indicated that, with an increasing amount of sCD40L in the culture, there was an expansion of MDSCs (Figure 3A). Next, we determined whether these sCD40L-treated MDSCs can inhibit T-cell activation. CFSE-labeled T cells were cocultured with autologous MDSCs at different ratios (1:1, 0.5:1, and 0.25:1) in the presence or absence of 2 μg/mL of sCD40L. Results showed that T-cell proliferation and IFN-γ release increased when fewer MDSCs were added to the culture and that the addition of sCD40L inhibited T-cell proliferation and IFN-γ production (Figure 3B,D); this inhibition was sCD40L dose-dependent (Figure 3C). To test whether the inhibitory effect was specific to sCD40L, MDSCs were incubated with anti-CD40 blocking antibody (5 μg/mL) for 4 hours before T cells and sCD40L were added. This resulted in the inhibitory effect of sCD40L on IFN-γ release being reversed (Figure 3E). There was no difference between anti-CD40 antibody and control IgG on T cells alone (Figure 3F). This suggest that the anti-CD40 blocking antibody acted directly on the MDSCs in the MDSCs and T-cell coculture, rather than an action of the antibody on the T cells. These data suggest that sCD40L might expand and enhance the suppressive function of MDSCs, resulting in further suppression of T-cell activation.
Figure 3.
sCD40L may enhance MDSC suppression of activated T cells. (A) sCD40L expanded MDSCs in vitro. Fresh PBMCs from healthy donors (HD) were enriched for the cell population of CD33+HLA-DR− using the Miltenyi isolation kit, and 0, 0.5, 1, or 2 μg/mL of sCD40L or a single dose of irrelevant protein human serum albumin (2 μg/mL) was added to the culture (2.5 × 105/mL), respectively. Three days later, FACS analysis was performed; the frequency of CD33+HLA-DR− cells is shown. (B) sCD40L further decreased T-cell proliferation in the presence of autologous MDSCs. A uniform number of isolated CD3+ cells (106/mL) from 3 HDs were labeled with CFSE and cocultured with various numbers of autologous MDSCs (106/mL, 5 × 105/mL, and 2.5 × 105/mL, respectively). A total of 2 × 105/mL anti-CD3/CD28 beads were added to the cultures, and 2 μg/mL of sCD40L was present or absent in the cultures. FACS assay was carried out by analyzing CFSE dilution of CD3+ T cells 3 days later. The figure shows representative FACS data from 1 sample. (C) sCD40L inhibited T-cell proliferation in MDSC/T-cell coculture in a dose-dependent manner. Autologous MDSCs and CFSE-labeled T cells were isolated from 4 fresh PBMC samples derived from HDs and cocultured. The MDSC to T-cell ratio was 1:4, and anti-CD3/CD28 beads and the indicated concentration of sCD40L or irrelevant protein were also added to the culture. Three days later, FACS analysis was carried out by looking at CFSE dilution of CD3+ T cells. (D) sCD40L further decreased IFN-γ release in the supernatant of the autologous T-cell/MDSC coculture. The supernatant from panel B was collected 18 hours after coculture, and IFN-γ was tested by ELISA. Each data point represents the mean values of the 3 samples. (E) CD40 blockade reversed the sCD40L inhibitory effect on IFN-γ production. MDSCs and autologous T cells were isolated, and 5 μg/mL of anti-IgG or CD40 blocking antibody was preincubated with the isolated MDSCs for 2-4 hours. The autologous T cells stimulated with CD3/CD28 and 2 μg/mL of sCD40L were then added to the cultures. IFN-γ release in cell culture supernatant was tested by ELISA 18 hours after the coculture. Each data point represents the mean values of 3 samples. (F) CD40 blocking antibody did not alter the IFN-γ release from purified T cells in the presence of sCD40L. T cells (106/mL) from these 3 PBMC samples were isolated and cultured in medium containing 2 × 105/mL anti-CD3/CD28 beads in the presence of either 5 μg/mL IgG or CD40 blocking antibodies and 2 μg/mL of sCD40L. The supernatant was collected 18 hours after treatment and IFN-γ was tested by ELISA. Each data point represents the mean values of 3 T-cell cultures. All results shown are representative of 2-4 individual experiments.
sCD40L may increase Tregs in vitro
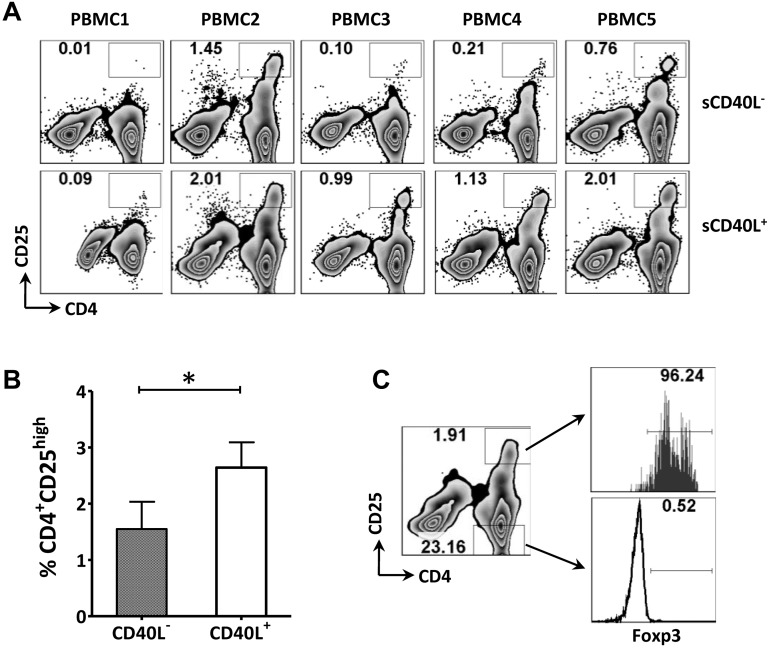
To assess the impact of sCD40L on the expansion of Tregs in vitro, 5 samples of PMBCs from healthy donors were cultured in medium containing low-dose IL-2 with or without 2 μg/mL of sCD40L. PBMCs cultured in the presence of sCD40L showed a significant increase (2.5-fold on average, for 5 different healthy donors being tested individually) in CD4+CD25high cells (Figure 4A-B). Further analysis demonstrated that the CD4+CD25high cells were Foxp3+ (Figure 4C). Taken together, these data suggest that sCD40L may stimulate/expand Tregs in vitro.
Figure 4.
sCD40L increased Tregs and CD4+CD25int cells in vitro. (A) FACS analysis of the frequency of the CD4+CD25high population in CD3+ T cells after adding sCD40L. PBMCs from 5 healthy donors (HD) were incubated in medium with or without 2 μg/mL of sCD40L. Both sCD40L− and sCD40L+ cultures contained 25 U/mL of IL-2. Four days later, cells were analyzed by multicolor FACS for CD3, CD4, CD25, and intracellular Foxp3. (B) The cells were analyzed by gating on the CD4+CD25high population. (C) CD4+CD25high cells were mainly Foxp3+. Intracellular staining of Foxp3 was performed, and a comparison of Foxp3 expression between CD4+CD25high and CD4+CD25− cells is shown for 1 sample that is representative of 5 PBMCs that were tested individually. The data were repeated 3 times. Statistical analysis was performed using a paired t test.
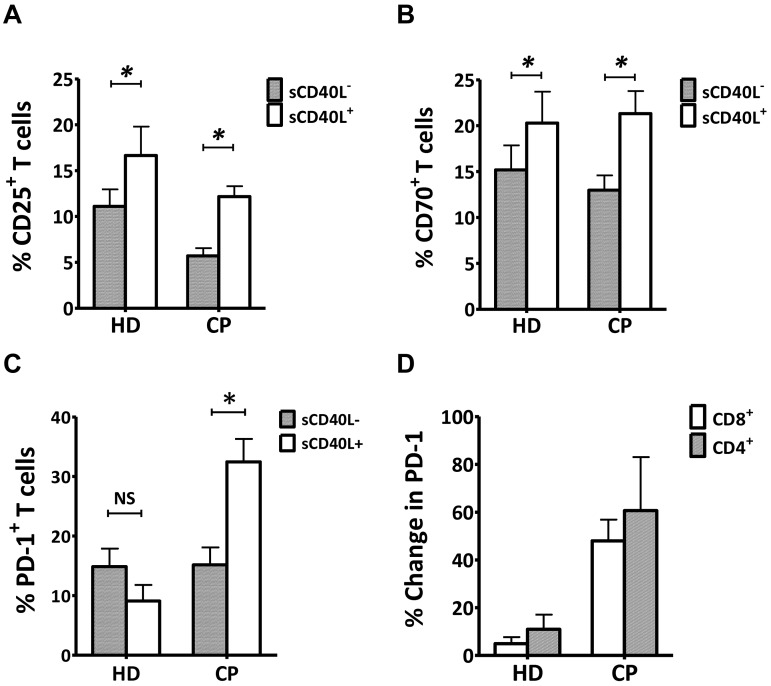
sCD40L induced greater PD-1 expression in vitro on activated T cells from cancer patients compared with healthy persons
Next, we evaluated the effect of sCD40L on surface expression of T-cell activation markers from PBMC samples from pretreatment metastatic prostate cancer patients and healthy donors and found that sCD40L enhanced CD3/CD28-stimulated T-cell activation markers, such as CD25 and CD70 after 4 days in culture (Figure 5A-B). Interestingly, there was an enrichment of the PD-1+ T-cell population in stimulated PBMCs only from the prostate cancer patients but no effect on samples from healthy donors (Figure 5C). When we divided the population of T cells into CD4+ and CD8+ T cells, there was on average a 5.5- and 9.7-fold increase in PD-1 expression for CD4+ and CD8+ T cells, respectively, in cancer patients compared with healthy donors (Figure 5D).
Figure 5.
sCD40L induced greater PD-1 expression in vitro on T cells from cancer patients (CP) than T cells from healthy donors (HD). (A-B) Induction of CD25 and CD70 on T cells. PBMCs from 6 age-matched HD and 5 metastatic prostate CP were incubated in medium containing 25 U/mL of IL-2 with or without 2 μg/mL of sCD40L for 4 days. Cells were then analyzed by FACS. (C) Comparison of PD-1–expressing T cells in PBMCs between HD and CP after the addition of sCD40L to the culture. The samples were also evaluated for PD-1 expression on CD3+ T cells. (D) Differential enhancement of PD-1 expression on CD4+ and CD8+ T cells after the addition of sCD40L to cell cultures in HD and CP. PD-1 expression on CD4+ and CD8+ T cells was evaluated. All results are representative of 2 individual repeats. Statistical analysis was performed using a paired t test. *P < .05. NS indicates not significant.
sCD40L significantly increased IL-10 and IL-6 production in PBMCs from cancer patients on stimulation
The previous results led us to evaluate how sCD40L affects the cytokine profile of stimulated PBMCs from cancer patients. Pretreatment PBMC samples from 6 prostate cancer patients were cultured in 4 treatment groups, and the supernatants were tested by ELISA (Table 1). The results demonstrated that, among the tested cytokines, IL-10 and IL-6 were significantly elevated on the addition of sCD40L after anti-CD3/CD28 stimulation. In addition, for IL-2 and GM-CSF, there was a trend of increase, but it was not statistically significant.
Table 1.
Cytokine production after the addition of sCD40L
| Treatment | Cytokines, pg/mL |
||||||
|---|---|---|---|---|---|---|---|
| IL-10 | IL-6 | IL-8 | TGF-β | IL-2 | GM-CSF | TNF-α | |
| 1. sCD40L− | 25 ± 15 | 466 ± 323 | 1852 ± 683 | 2013 ± 551 | 653 ± 37 | 62 ± 10 | 120 ± 80 |
| 2. sCD40L+ | 24 ± 7 | 445 ± 252 | 1063 ± 188 | 2426 ± 552 | 633 ± 56 | 61 ± 22 | 125 ± 63 |
| 3. sCD40L− + anti-CD3/28 | 530 ± 123 | 494 ± 123 | 506 ± 85 | 2624 ± 717 | 15 000 ± 3134 | 1298 ± 209 | 12 236 ± 1192 |
| 4. sCD40L+ + anti-CD3/28 | 792 ± 143*† | 651 ± 149*‡ | 427 ± 52 | 2430 ± 672 | 20 004 ± 3879 | 1955 ± 375 | 17 031 ± 1738 |
Six PBMC samples (106/mL) from metastatic prostate cancer patients were incubated in X-VIVO15 medium with (2 μg/mL) or without sCD40L, as well as ± anti-CD3/28 beads. Three days later, the supernatant was harvested for cytokine analysis. Results represent a mean of 6 samples ± SE. Parametric t test was analyzed either between treatments 1 and 2 or between treatments 3 and 4.
Statistical difference.
P < .01.
P < .05.
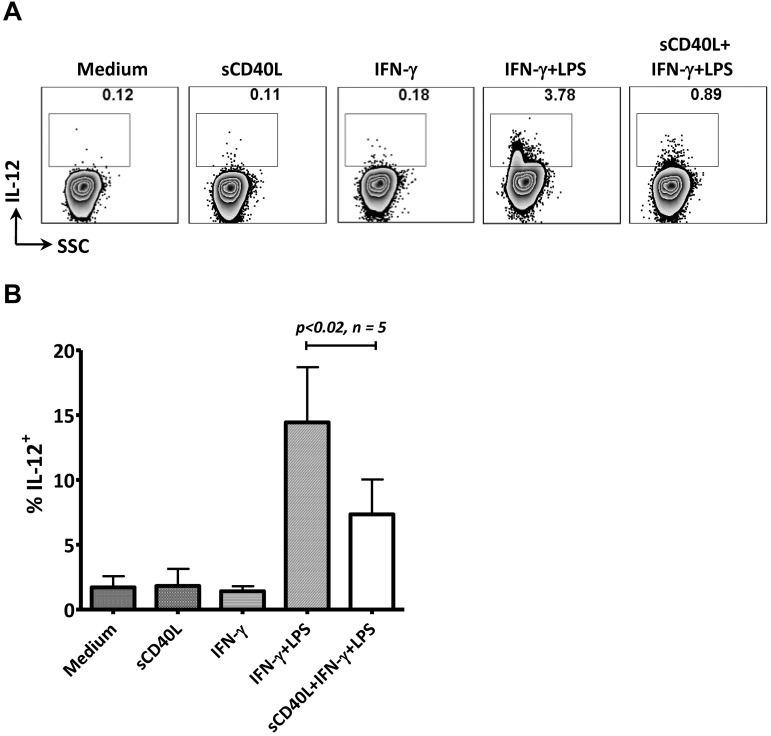
Preexisting sCD40L decreased IL-12 production in monocytes stimulated in vitro with IFN-γ and LPS
As described in this study, sCD40L may inhibit T-cell activation via MDSCs, expand the Treg population in vitro, enhance PD-1 expression on T cells, and elevate IL-10 and IL-6 production from anti-CD3/CD28–stimulated PBMCs from cancer patients. We further hypothesized that preexisting sCD40L in serum from cancer patients could potentially influence monocyte function by altering IL-12 production after activation signals were engaged. The addition of sCD40L alone to the isolated monocytes produced no change in IL-12 production. However, the cells pretreated with sCD40L for 24 hours produced significantly less IL-12 when activated with IFN-γ and LPS (Figure 6A-B). These data suggest that preexisting sCD40L potentially blocks monocyte activation, and thus the production of IL-12.
Figure 6.
Preexisting sCD40L decreased IL-12 production from monocytes after in vitro stimulation. (A) Monocytes were isolated from PBMCs of a healthy donor using CD14 beads. The cells (106/mL) were incubated with 2 μg/mL of sCD40L for 20 hours, after which IFN-γ (300 U/mL) and Golgi Stop (0.8 μL/mL) were added. Two hours later, a second signal (50 ng/mL LPS) was added to the culture. Cells were harvested 6 hours after the initial IFN-γ stimulation, and intracellular staining of IL-12 was performed. (B) Summary of data from 5 individual assays described in panel A of monocytes derived from 5 PBMC samples. The data were repeated 4 times. Statistical analysis was performed using a paired t test. SSC indicates side scatter.
Discussion
The immune system's innate and adaptive responses are carefully orchestrated by soluble and membrane-bound regulators that establish the most efficient effectors for preventing cancer development. Tumor cells, however, are also able to induce various soluble factors that favor the recruitment and stimulation of immune suppressor rather than immune effector cell types. In some cases, 1 soluble factor may play a dual role: one for cancer immunity and another for carcinogenesis.9 The switch between the 2 actions may be controlled by the strength of the soluble factor-induced signaling or by conditions in the tumor microenvironment that favor one action over another. Details of how such countercurrent processes can be regulated remain unclear.
CD40 was initially identified as a B-cell receptor that is key to inducing an effective adaptive immune response. Evidence suggested that immunity against tumors was crucially dependent on CD40-CD40L interactions. Paradoxically, CD40 was also shown to be expressed by endothelial cells and to promote angiogenesis.5,8–10 Ligation of CD40 and CD40L can cause sCD40L to be shed from the cell surface and released into body fluids. Compared with the membrane-bound CD40L, sCD40L is a functional trimer, meaning it can perform functions similar to membrane-bound CD40L but with less signaling strength.6 Early studies identified increased levels of serum sCD40L in autoimmune diseases, such as systemic lupus erythematosus,26 as well as a range of vascular diseases, including diabetes, hypertension, peripheral vascular disease, and coronary artery disease, in which angiogenesis is actively involved in disease development.12,13 Although a similar sCD40L elevation in sera was seen in these categories of disease, sCD40L was thought to be derived from 2 different cell types, T cells or platelets. In tumor-prone transgenic mice, CD40-mediated neovascularization is essential for early-stage tumorigenicity.27 In humans, 2 studies found elevated sCD40L in the serum of patients with lung cancer and undifferentiated nasopharyngeal carcinoma.15,16 In cancer patients, sCD40L is more likely derived from activated platelets than from T cells, a notion supported by evidence that cancer patients have significant platelet activation, as well as inadequate T-cell activation.17–20 Several other studies have focused on the role of membrane-bound CD40/CD40L in angiogenesis, carcinogenesis, and immune activation, but limited data are available on sCD40L and its possible contribution to the negative regulation of immune responses in cancer. In this study, we found levels of serum sCD40L in patients with breast, prostate, and colon cancer that were significantly higher than levels in healthy persons. More importantly, we provide evidence potentially linking elevated serum sCD40L to immunosuppression.
The dramatic difference between levels of serum sCD40L in cancer patients (≤ 10 ng/mL) and healthy donors (∼ 0 ng/mL) led us to hypothesize that serum sCD40L may promote cancer development by suppressing immune activation. MDSCs are key players in immunosuppression. They compose a heterogeneous cell population with potent immune suppressive function and are seen in large numbers in tumor-bearing mice and human cancer patients.3,4,28 We confirmed this observation in patients with metastatic breast and prostate cancer. Our data demonstrated that, before treatment, these patients had a relatively higher frequency of CD33+HLA-DR− cells in PBMCs compared with healthy donors. In addition, more of these cells expressed CD40 on the surface than the same cells in healthy donors (P < .001). These results suggest that the function of MDSCs may be modulated through the CD40 signaling pathway.
To test this hypothesis, we next isolated MDSCs (CD33+HLA-DR− cells) from fresh PBMCs of healthy donors. Different amounts of MDSCs were cocultured with a uniform amount of autologous T cells stimulated with anti-CD3/CD28, in the presence or absence of recombinant monomeric sCD40L in the culture medium, which was the dominant form in serum of systemic lupus erythematosus patients.26 The results indicated an inverse relationship between the number of MDSCs added to the coculture and the level of T-cell proliferation and IFN-γ production. The addition of sCD40L modestly enhanced this inhibitory effect, resulting in up to 44% decrease in T-cell proliferation and 54% decrease in IFN-γ production in the coculture. In prolonged culture, the suppressive effect diminished (data not shown); this could be the result of the strong stimulation of anti-CD3/CD28, which do not exist in vivo, directly working on the T cells and overpowering the effect of sCD40L signaling. Blocking the interaction between CD40 and CD40L with antagonistic anti-CD40 antibody decreased the effect of sCD40L. There was no significant expansion of MDSCs by sCD40L when whole PBMCs were used for the test (data not shown). However, when PBMCs were enriched for the CD33+HLA-DR− cell population, enhancement of the MDSC population by sCD40L was observed. In addition, our data also showed that there was a trend of increased IL-2 production from activated PBMCs when sCD40L was added. However, the significantly increased production of suppressive cytokines, such as IL-6 and IL-10, may counteract the stimulatory action of IL-2. In accordance with this hypothesis, sCD40L was found to decrease T-cell proliferation in the MDSC and T-cell coculture. Furthermore, GM-CSF, a driver of MDSCs, was also enhanced (P = .054) by sCD40L. Studies conducted during the past decade have identified several factors involved in the proliferation of MDSCs and have documented varied mechanisms used by MDSCs to block antitumor immunity.3,28 Our results demonstrate that the elevated sCD40L found in the serum of cancer patients may be one of the factors that enhance the suppressive function of MDSCs.
To investigate sCD40L's involvement in the modulation of T-cell function via MDSCs, we added sCD40L to a culture of whole PBMCs, in which MDSCs and T cells were both present. Murine studies have suggested that MDSCs promote the generation of Tregs, a process that requires the interaction of CD40, which is expressed by MDSCs, with CD40L.29,30 Our results demonstrated that adding sCD40L in vitro did expand the MDSCs, as well as the CD4+CD25highFoxp3+ (Treg) population, indicating that enhanced T-cell suppression may be the result of sCD40L promoting the expansion of MDSCs and Tregs, 2 potent immunosuppressive cell populations. The molecular mechanisms behind sCD40L's enhancement of MDSCs' suppressive activity need to be further studied.
Phenotypic analysis of T cells in PBMCs from healthy donors and cancer patients, cultured with sCD40L, revealed significantly enhanced markers for T-cell activation, such as CD25 and CD70. Compared with healthy donors, cancer patients showed a more dramatic increase in the inhibitory receptor PD-1 on T cells after incubation with sCD40L. Both murine and human cancer studies have suggested that PD-1 signaling blockade has a therapeutic benefit,31,32 decreasing the inhibitory effect of PD-1 on immune responses to cancer. T-cell activation and the generation of protective immunity are usually the result of a balance between positive and negative signals. Although sCD40L has been shown to promote some level of T-cell activation, significant up-regulation of negative signaling molecules in cancer patients could have a stronger inhibitory effect that overcomes the positive effects. Furthermore, several previous studies have indicated that Tregs that express PD-1 have a strong suppressive function. We found more sCD40L-induced PD-1–expressing CD4+ T cells (including Tregs) in cancer patients, implying that sCD40L may enhance the suppressive function of Tregs by up-regulating PD-1 expression on their surface. It is still not clear how sCD40L upregulates PD-1 expression on T cells in cancer patients. Our data provide evidence that CD40L may enhance the suppressive function of MDSCs on T cells because MDSCs have been shown to modulate Treg function. sCD40L could also directly alter PD-1 expression because some T-cell subsets express the receptor CD40. We hypothesize that lowering the levels of sCD40L could enhance the efficacy of immunotherapy, such as PD-1 blockade. Taken together, these findings provide potential evidence that, in cancer patients, higher levels of serum sCD40L may inhibit the antitumor immune response.
In laboratory research, sCD40L is widely used to mature monocyte-derived dendritic cells in vitro. sCD40L added to a monocyte culture after differentiation agents, such as GM-CSF, IL-4, TNF-α, or other stimuli, induces dendritic cell maturation and the production of IL-12.33,34 However, preexisting serum sCD40L in cancer patients may lead to a very different outcome in terms of monocyte activation. A previous study reported significant inhibition of IL-12 production when monocytes were preincubated with sCD40L before adding the activation agents IFN-γ and LPS.34 We reproduced this experiment with monocytes derived from PBMCs of healthy donors and observed a significant inhibitory effect on IL-12 production when sCD40L was added to cultures 24 hours before monocyte stimulation by IFN-γ and LPS. This result suggests that preexisting sCD40L in cancer patients may block production of IL-12 by monocytes when activation signals are present and further suppress T-cell function by a different pathway. Taken together, these data provide further insight into the process of immunosuppression in cancer patients.
Previous studies have suggested that the majority of soluble receptors compete with their membrane-bound counterparts for ligands and thus act antagonistically; very few have been found to be agonistic.35 It remains unclear how sCD40L acts after shedding from the cell surface in cancer patients, but data from previous studies and from this study suggest 3 potential roles for sCD40L: (1) sCD40L directly promotes tumor-cell growth by engaging CD40 on tumor cells. The strong ligation signal produced by membrane-bound CD40L causes tumor-cell apoptosis, whereas the low ligation signal of sCD40L increases tumor-cell survival and proliferation. Although trimer is the original form of CD40L shed from the cell surface, 2 additional forms, monomer and dimer, were detected in serum of patients with autoimmune disease, and the monomeric sCD40L apparently was the dominant form,26 suggesting that much weaker ligand signals may occur. (2) sCD40L induces angiogenesis. (3) sCD40L orchestrates an immunosuppressive response in cancer patients by enhancing the suppressive activity of MDSCs, expanding the Treg population, up-regulating PD-1 expression on CD4+ T cells, increasing suppressive cytokine production, and inhibiting IL-12 production by monocytes. All of these potential functions suggest that blocking the production of sCD40L could enhance antitumor responses. Our results demonstrate that, for patients in the PSA-TRICOM phase 2 trial, vaccine therapy did not alter the serum levels of sCD40L. However, patients who had relatively low levels of serum sCD40L before treatment outlived their Halabi-predicted survival, suggesting that a reduction of sCD40L could help enhance the efficacy of subsequent immunotherapy (Figure 1C). In addition, there was a significant decrease (P < .01) in serum sCD40L in castration-resistant prostate cancer patients treated with ketoconazole with or without alendronate22 (Figure 1D). Treatment with ketoconazole, an adrenal steroid inhibitor, was associated with sustained ≥ 50% declines in PSA in about half of the patients in this trial, a proportion of responding patients similar to that seen with docetaxel-based therapy,24 and abiraterone, a recently approved adrenal steroid inhibitor.25 Analysis of sCD40L levels was performed before and after therapy. Figure 1D shows that, indeed, the treatment resulted in a significant reduction of the sCD40L levels. Anticlotting agents, such as aspirin and clopidogrel bisulfate (Plavix), have been shown to significantly lower sCD40L production in patients with type II diabetes and stable coronary artery disease, conditions in which platelet activation is a factor in pathogenesis.36,37 A retrospective analysis of 682 prostate cancer patients showed significant benefit with the combination of anticlotting agents and radiation therapy,38 adding clinical support to the hypotheses and findings of this study.
Acknowledgments
The authors thank Dr Chiara Intrivici for technical assistance, Sandra Doren for help with clinical samples, and Bonnie L. Casey and Debra Weingarten for editorial assistance in the preparation of the manuscript.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.H. designed the study, performed experiments, analyzed data, and wrote the manuscript; C.J. performed experiments, analyzed data, and edited the manuscript; T.T., A.A., and A.J. performed experiments and analyzed data; K.Y.T., R.A.M., J.L.G., and J.S. designed and conducted the preclinical and clinical trial; and J.S. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey Schlom, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 10 Center Dr, Bldg 10, Rm 8B09, Bethesda, MD 20892; e-mail: js141c@nih.gov.
References
- 1.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66(11):5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez PC, Hernandez CP, Quiceno D, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202(7):931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11(7):802–806. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 6.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunda MJ, Luistro L, Warrier RR, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann S, Pandolfi PP. Giving blood: a new role for CD40 in tumorigenesis. J Exp Med. 2006;203(11):2409–2412. doi: 10.1084/jem.20061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murugaiyan G, Martin S, Saha B. CD40-induced countercurrent conduits for tumor escape or elimination? Trends Immunol. 2007;28(11):467–473. doi: 10.1016/j.it.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Melter M, Reinders ME, Sho M, et al. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood. 2000;96(12):3801–3808. [PubMed] [Google Scholar]
- 11.Pietravalle F, Lecoanet-Henchoz S, Blasey H, et al. Human native soluble CD40L is a biologically active trimer, processed inside microsomes. J Biol Chem. 1996;271(11):5965–5967. doi: 10.1074/jbc.271.11.5965. [DOI] [PubMed] [Google Scholar]
- 12.Vakkalanka RK, Woo C, Kirou KA, Koshy M, Berger D, Crow MK. Elevated levels and functional capacity of soluble CD40 ligand in systemic lupus erythematosus sera. Arthritis Rheum. 1999;42(5):871–881. doi: 10.1002/1529-0131(199905)42:5<871::AID-ANR5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Tamura N, Kobayashi S, Kato K, et al. Soluble CD154 in rheumatoid arthritis: elevated plasma levels in cases with vasculitis. J Rheum. 2001;28(12):2583–2590. [PubMed] [Google Scholar]
- 14.Schonbeck U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104(19):2266–2268. doi: 10.1161/hc4401.099447. [DOI] [PubMed] [Google Scholar]
- 15.Roselli M, Mineo TC, Basili S, et al. Soluble CD40 ligand plasma levels in lung cancer. Clin Cancer Res. 2004;10(2):610–614. doi: 10.1158/1078-0432.ccr-0348-03. [DOI] [PubMed] [Google Scholar]
- 16.Caggiari L, Guidoboni M, Vaccher E, et al. High serum levels of soluble CD40-L in patients with undifferentiated nasopharyngeal carcinoma: pathogenic and clinical relevance. Infect Agent Cancer. 2007;2:5. doi: 10.1186/1750-9378-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 18.Sciulli MG, Filabozzi P, Tacconelli S, et al. Platelet activation in patients with colorectal cancer. Prostaglandins Leukot Essen Fatty Acids. 2005;72(2):79–83. doi: 10.1016/j.plefa.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Osada J, Rusak M, Kamocki Z, Dabrowska MI, Kedra B. Platelet activation in patients with advanced gastric cancer. Neoplasma. 2010;57(2):145–150. doi: 10.4149/neo_2010_02_145. [DOI] [PubMed] [Google Scholar]
- 20.Mizoguchi H, O'Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258(5089):1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 21.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59(5):663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figg WD, Liu Y, Arlen P, et al. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. J Urol. 2005;173(3):790–796. doi: 10.1097/01.ju.0000147013.09157.8e. [DOI] [PubMed] [Google Scholar]
- 23.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21(7):1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 24.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 25.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato K, Santana-Sahagun E, Rassenti LZ, et al. The soluble CD40 ligand sCD154 in systemic lupus erythematosus. J Clin Invest. 1999;104(7):947–955. doi: 10.1172/JCI7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiodoni C, Iezzi M, Guiducci C, et al. Triggering CD40 on endothelial cells contributes to tumor growth. J Exp Med. 2006;203(11):2441–2450. doi: 10.1084/jem.20060844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166(9):5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 29.Elmetwali T, Young LS, Palmer DH. CD40 ligand-induced carcinoma cell death: a balance between activation of TNFR-associated factor (TRAF) 3-dependent death signals and suppression of TRAF6-dependent survival signals. J Immunol. 2010;184(2):1111–1120. doi: 10.4049/jimmunol.0900528. [DOI] [PubMed] [Google Scholar]
- 30.Pan P-Y, Ma G, Weber KJ, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70(1):99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalady MF, Onaitis MW, Emani S, Abdel-Wahab Z, Tyler DS, Pruitt SK. Sequential delivery of maturation stimuli increases human dendritic cell IL-12 production and enhances tumor antigen-specific immunogenicity. J Surg Res. 2004;116(1):24–31. doi: 10.1016/j.jss.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Wittmann M, Kienlin P, Mommert S, Kapp A, Werfel T. Suppression of IL-12 production by soluble CD40 ligand: evidence for involvement of the p44/42 mitogen-activated protein kinase pathway. J Immunol. 2002;168(8):3793–3800. doi: 10.4049/jimmunol.168.8.3793. [DOI] [PubMed] [Google Scholar]
- 35.Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol. 1998;64(2):135–146. doi: 10.1002/jlb.64.2.135. [DOI] [PubMed] [Google Scholar]
- 36.Santilli F, Davi G, Consoli A, et al. Thromboxane-dependent CD40 ligand release in type 2 diabetes mellitus. J Am Coll Cardiol. 2006;47(2):391–397. doi: 10.1016/j.jacc.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 37.Azar RR, Kassab R, Zoghbi A, et al. Effects of clopidogrel on soluble CD40 ligand and on high-sensitivity C-reactive protein in patients with stable coronary artery disease. Am Heart J. 2006;151(2):521. doi: 10.1016/j.ahj.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Choe KS, Correa D, Jani AB, Liauw SL. The use of anticoagulants improves biochemical control of localized prostate cancer treated with radiotherapy. Cancer. 2010;116(7):1820–1826. doi: 10.1002/cncr.24890. [DOI] [PubMed] [Google Scholar]