Abstract
FLT3 is frequently mutated in acute myeloid leukemia (AML), but resistance has limited the benefit of tyrosine kinase inhibitors (TKI). We demonstrate that statins can impair FLT3 glycosylation, thus leading to loss of surface expression and induction of cell death, as well as mitigation of TKI resistance. Immunofluorescence microscopy confirms a reduction in surface localization and an increase in intracellular FLT3/internal tandem duplication (ITD) accumulation. This aberrant localization was associated with increased STAT5 activation but inhibition of both MAPK and AKT phosphorylation. Growth inhibition studies indicate that FLT3/ITD-expressing cells were killed with an IC50 within a range of 0.2-2μM fluvastatin. Several mechanisms of resistance could be circumvented by fluvastatin treatment. An increase in the IC50 for inhibition of phosphorylated FLT3/ITD by lestaurtinib caused by exogenous FLT3 ligand, resistance to sorafenib caused by the D835Y or FLT3/ITD N676K mutations, and activation of the IL-3 compensatory pathway were all negated by fluvastatin treatment. Finally, fluvastatin treatment in vivo reduced engraftment of BaF3 FLT3/ITD cells in Balb/c mice. These results demonstrate that statins, a class of drugs already approved by the US Food and Drug Administration, might be repurposed for the management of FLT3 mutant acute myeloid leukemia cases either alone or in conjunction with FLT3 TKI.
Introduction
FLT3 is a class III tyrosine kinase receptor that is composed of an extracellular domain that binds FLT3 ligand (FL), a single-pass transmembrane domain, a short juxtamembrane domain, and an interrupted kinase domain that contains a typical activation loop.1–3 FLT3 is expressed on hematopoietic stem and progenitor cells where it functions in cell differentiation, proliferation, and survival. After translation, FLT3 undergoes glycosylation in the endoplasmic reticulum to form an immature receptor and progresses to the Golgi complex where final glycosylation produces a mature receptor before it translocates to the surface. Once at the surface, FLT3 binding to FL leads to receptor dimerization, autophosphorylation, and activation.4 The transient activation of FLT3 by FL activates several downstream pathways, including Ras/MAPK, PI3K/AKT, and JAK/STAT.4–9 Besides FL binding, FLT3 can also be constitutively activated by mutation, either internal tandem duplications (ITD) of the juxtamembrane domain or point mutations of the tyrosine kinase domain (TKD), that produce altered signaling.10–12 The ITD mutations result in in-frame repeats of varying length. Most TKD mutations result in missense mutations of the activation loop, most frequently the D835 residue. The activating mutations of FLT3 are found in approximately 30% of patients with acute myeloid leukemia (AML).13,14 When cytokine-dependent cell lines are engineered to express FLT3 mutations they are transformed to factor independence in vitro. FLT3/ITD knock-in mice and mice whose bone marrow is retrovirally transduced with mutant FLT3 develop a lethal myeloproliferative disease.15–18 When combined with other mutations such as MLL-AF9, AML1/ETO, NUP-98/HOXD13, or NPM known to occur in human AML, FLT3/ITD mutations cooperate to cause acute leukemia in the mice.19–22
This evidence indicates a cooperative role for FLT3 in leukemia and has led to the development of drugs that target FLT3 kinase activity. Numerous tyrosine kinase inhibitors (TKIs) have been identified that all inhibit FLT3/ITD phosphorylation and are cytotoxic to FLT3/ITD-dependent cells.23–25 Wild-type FLT3 is often inhibited to a lesser extent by many of the FLT3 TKI. Some FLT3 TKI have very little activity against certain FLT3 kinase domain-activating mutations, particularly D835Y, rendering the cells functionally resistant.26–28 In addition, there are several mutations within or outside the drug-binding cleft that have been selected for in vitro or in vivo that impart varying levels of resistance to TKI.28–32 Thus, newer TKIs or a different class of drugs that can inhibit FLT3 are merited for management of leukemias that express FLT3 as a significant transformative component of malignancy.
Statins have been developed to lower cholesterol and total triglyceride levels in patients who are considered to be at risk for heart attack based in part on serum cholesterol levels and are considered to be very safe drugs.33 They act by blocking 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG CoA reductase), the rate-limiting step in the mevalonate pathway.34 This pathway generates not only cholesterol but also produces many isoprenoids that are critical for multiple cellular processes. Because ras GTPase activity is dependent on isoprenylation derived from the mevalonate pathway, studies have been conducted to determine whether statins could inhibit proliferation of cancer cells in vitro and in vivo.35–37 Based on subsequent positive findings, several statins have been tried clinically against various tumors but have provided only modest benefit.38–40 However, the mevalonate pathway also produces dolichol, which is responsible for the cotranslational transfer of oligosaccharides to nascent polypeptides that undergo N-linked glycosylation.41 Proper protein folding and localization depend on efficient glycosylation, without which cells may respond to the presence of unfolded protein by pathways that lead to apoptosis. Thus, transformation of cells that are highly dependent on a cell-surface signaling protein such as mutant FLT3 that requires glycosylation might be disrupted by exposure to statins. We demonstrate for the first time that statins can attenuate mutant FLT3 kinase activity by preventing complex glycosylation of the receptor; this in turn promotes altered localization and signaling and ultimately leads to induction of apoptosis, regardless of their resistance to TKIs.
Methods
Reagents and antibodies
Fluvastatin was purchased from Tocris Bioscience. Lovastatin and pravastatin were from Sigma-Aldrich. Atorvastatin, lestaurtinib, and sorafenib were purchased from LC Laboratories. Recombinant human interleukin-3 (IL-3) and human FL were purchased from PeproTech Inc. The FLT3 S-18 and STAT5 antibodies were from Santa Cruz Biotechnology, 4G10 phosphotyrosine mouse monoclonal antibody and recombinant protein A-agarose were from Upstate Biotechnology, and CD135-phycoerythrin (PE) conjugate and annexin V–PE antibodies were from BD Pharmingen. Phospho-MAPK, phospho-STAT5, phospho-AKT, MAPK, and AKT antibodies were from Cell Signaling Technologies Inc. Goat anti–mouse and goat anti–rabbit horseradish peroxidase antibodies and the enhanced chemiluminescence kit were from Amersham Biosciences. The C-19 antibody (Santa Cruz Biotechnology) was used for immunoprecipitating and detecting c-Kit. For immunofluorescence microscopy, FLT3 was detected using mouse anti–human FLT3 antibody MAB812 from R&D Systems and a secondary Cy3-conjugated antibody from BioLegend.
DNA constructs and cells
All cell lines were routinely cultured at 37°C in 5% CO2 in RPMI medium (Invitrogen Corporation) with 10% fetal bovine serum (FBS; Gemini Bio-Products) containing penicillin/streptomycin (Invitrogen Corporation). BaF3 cells were supplemented with 1 ng/mL recombinant human IL-3. BaF3 FLT3/ITD cells were obtained as previously described.42 FLT3 point mutations expressed in BaF3 cells were generated by site-directed mutagenesis in the pBabe Neo vector using the QuickChange Site-Directed Mutagenesis kit following the manufacturer's recommendations (Stratagene). Transfection was performed using the Nucleofector II from Amaxa Biosystems, after which cells were selected in 1 mg/mL G418 in the presence of IL-3 and sorted for surface FLT3 expression using CD135 PE-conjugated antibody on a FACSCalibur flow cytometer (BD Biosciences) and analyzed using CellQuest Version 3.3 software. Total FLT3 levels were measured by permeabilizing and fixing cells with Cytofix/Cytoperm buffer (BD Biosciences) for 30 minutes.
Growth inhibition and apoptosis
Cells were seeded in the presence or absence of inhibitor for the indicated times with or without 1 ng/mL IL-3 or 100 ng/mL FL. For day 0 measurements, viability was measured 1 hour after addition of fluvastatin. Cell viability was measured using the MTT assay according to the manufacturer's instructions (Roche Applied Science). Apoptosis was measured by incubating treated cells with annexin V and 7-AAD for 15 minutes according to the manufacturer's instructions (BD Biosciences) and analyzing on a FACSCalibur cytometer.
Immunoprecipitation and Western blotting
FLT3 was analyzed by performing immunoprecipitation, SDS-PAGE, and Western blotting. Proteins were transferred to a polyvinyl diflouride (PVDF) membrane (Millipore) and probed for FLT3 using S-18 and 4G10 phosphotyrosine antibodies. FLT3 was then detected on a Licor imager using Odyssey Version 3.0 software. c-Kit was detected in the same manner using C-19 and 4G10. Other proteins were detected in whole-cell lysates using the indicated antibodies and a horseradish peroxidase–conjugated goat anti–rabbit secondary antibody followed by enhanced chemiluminescence (ECL).
Immunofluorescence microscopy
Cells were processed for immunofluorescence microscopy using standard procedures. Briefly, BaF3 FLT3/ITD cells were washed and resuspended in ice-cold PBS containing WGA-AF488 for 20 minutes at 4°C to stain the cell membrane. For intracellular staining, cells were washed and transferred onto poly-L-lysine–coated slides on ice. Cells were then fixed using 4% paraformaldehyde and permeabilized using 0.2% Triton X-100. FLT3 antibody in FBS was added for 1 hour at room temperature followed by washing and incubation in secondary antibody conjugated to Cy3. Slides were mounted in ProLong antifade mounting medium (Invitrogen) and visualized on a Nikon TE2000 Eclipse microscope using a Plan Apo 60 × 1.4 oil-immersion objective using EZ-C1 for software analysis.
Patient samples
Leukemic samples were collected under an institutionally approved protocol with informed patient consent in accordance with the Declaration of Helsinki. Blasts were purified by centrifugation in Ficoll and frozen until use in liquid nitrogen.
Engraftment in Balb/c mice
BaF3 FLT3/ITD cells were transfected with the L3GFP plasmid (a gift from Dr Linzhao Cheng, Johns Hopkins University) containing genes for luciferase and green fluorescent protein (GFP). Cells were sorted for GFP and CD135 expression on a FACSAria II cytometer using FACSDiva Version 6.1 software. For engraftment, 2 × 105 cells were injected by tail vein into Balb/c mice (day 0; The Jackson Laboratory). Starting 3 days later, mice were treated with 20 mg/kg fluvastatin dissolved in PBS twice daily by oral gavage. Mice were imaged by injecting luciferin and visualizing on an IVIS Spectrum imager (Caliper Life Sciences) using Living Image software for analysis on day 3 after inoculation to monitor engraftment and on day 7 to assess drug effect. All animal procedures were conducted in accordance with the policy of the Johns Hopkins University School of Medicine Animal Care and Use Committee.
Results
Fluvastatin impairs glycosylation of the FLT3 receptor
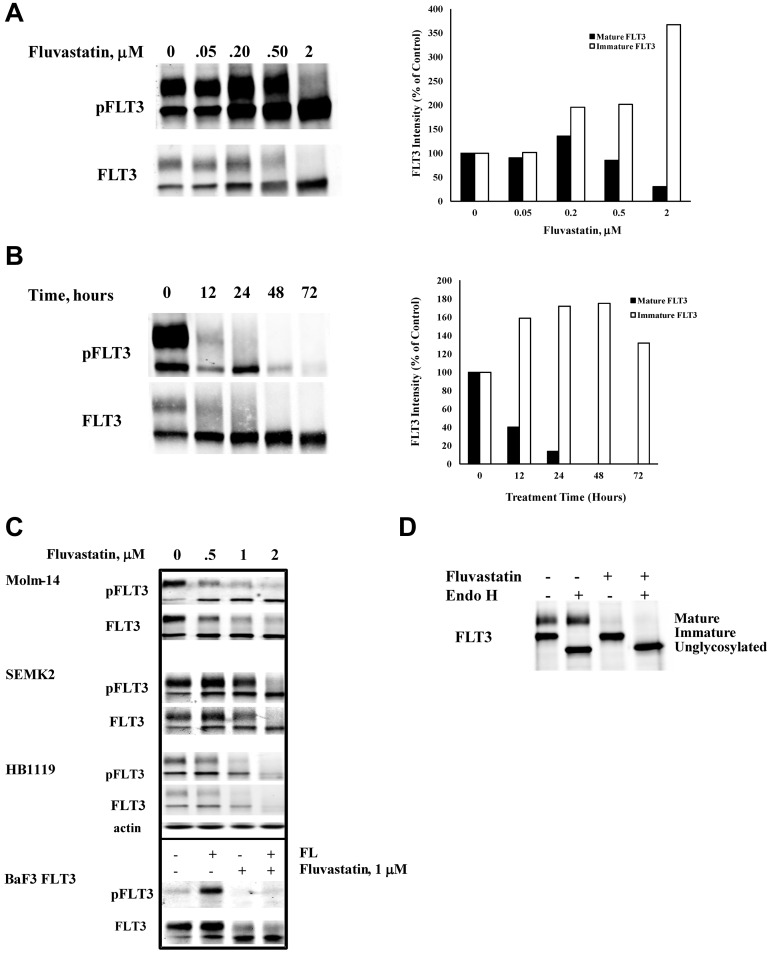
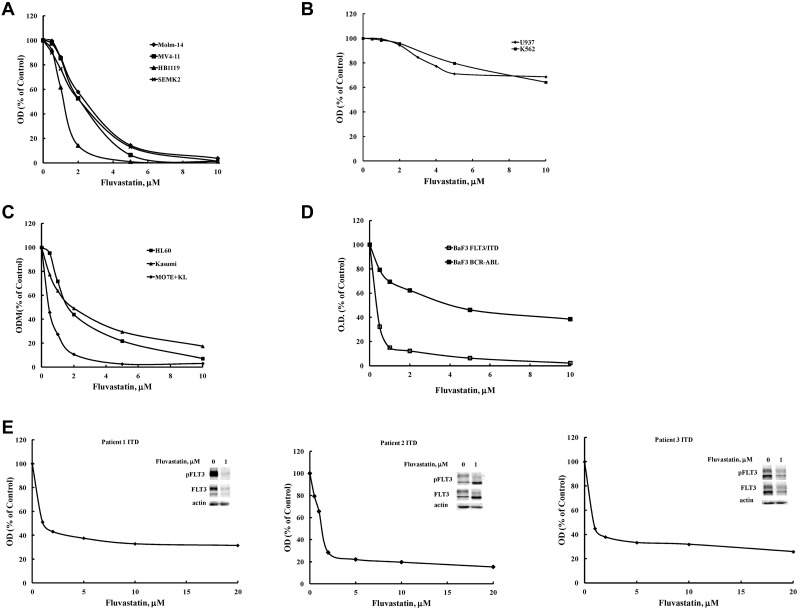
Tunicamycin directly disrupts the first step involved in N-linked protein glycosylation, and it was recently demonstrated to inhibit FLT3 glycosylation as well.43 Our results demonstrated that FLT3 ligand cannot bind and activate FLT3 after cells were treated with tunicamycin (data not shown). Thus, it was postulated that drugs acting through a different pathway of inhibiting glycosylation that can be safely tolerated in humans might produce similar results. Statins have a well-defined safety profile and they also reduce cellular levels of dolichol, which is necessary for N-linked protein glycosylation. Previous reports showed that lovastatin blocked mature glycosylation of the erythropoietin and insulin-like growth factor-1 (IGF) receptors through inhibition of mevalonate synthesis.44,45 Because N-linked glycosylation functions both in targeting proteins to the cell surface and increasing protein stability, inhibition of mevalonate biosynthesis by fluvastatin treatment might be expected to alter FLT3 localization and/or lead to its degradation. Figure 1A shows that BaF3 FLT3/ITD cells treated with fluvastatin for 24 hours showed a concentration-dependent inhibition of the glycosylated, mature FLT3/ITD (and of its accompanying autophosphorylation) with a concomitant increase in levels of the immature FLT3/ITD (and of its autophosphorylation activity). Significant inhibition of FLT3/ITD glycosylation was observed by 12 hours of fluvastatin treatment, was maximal by 24 hours, and was maintained throughout the 72-hour testing period (Figure 1B). Next, we investigated whether fluvastatin could inhibit glycosylation in other cell lines expressing FLT3. Molm-14, SEMK-2, and HB1119 cells are human-derived cell lines that harbor constitutively activated endogenous FLT3 through ITD mutation, amplification of wild-type FLT3 with autocrine/intracrine activation, and kinase domain mutation, respectively. Western blots show that fluvastatin inhibited complex glycosylation of FLT3 (and its associated autophosphorylation) in Molm-14, SEMK-2, and HB1119 cells, similar to the effects seen in BaF3 FLT3/ITD cells (Figure 1C).
Figure 1.
Fluvastatin prevents glycosylation of FLT3 and impedes receptor maturation. (A) BaF3 FLT3/ITD cells were treated with increasing concentrations of fluvastatin for 24 hours. Lysates were immunoprecipitated with anti-FLT3 antibody and resolved by SDS-PAGE and then probed with FLT3 or 4G10 antiphosphotyrosine antibodies. Quantitation of the upper and lower FLT3 bands is shown in the graph. (B) Cells were treated with 1μM fluvastatin for the indicated time points and analyzed for FLT3 expression as described. Quantitation of the upper and lower FLT3 bands is shown in the graph. (C) The effect of increasing fluvastatin concentration treatment for up to 24 hours on the FLT3-expressing cell lines HB1119, Molm-14, and SEMK-2 cells. In the bottom panel, wild-type FLT3-expressing BaF3 cells were treated ± 1μM fluvastatin for 24 hours followed by ± stimulation with 20 ng/mL FLT3 ligand for 5 minutes. (D) BaF3 FLT3/ITD cells were treated with 1μM fluvastatin for 24 hours after which immunoprecipitated FLT3/ITD was digested with endoglycosidase H for 1 hour. Results are typical of at least 3 independent experiments.
While these mutant and amplified forms of FLT3 show constitutive activation, wild-type FLT3 is dependent on binding of its ligand to the extracellular domain of the mature receptor to activate its kinase activity. Therefore, we examined whether fluvastatin would inhibit the activation of mature wild-type FLT3 on exogenous stimulation with FL. In BaF3 FLT3 cells, fluvastatin prevented FL-induced phosphorylation of FLT3, likely by blocking receptor maturation and translocation to the cell surface, preventing it from interacting with ligand (Figure 1C). Digestion of FLT3/ITD molecules with endoglycosidase H confirmed the single band visible on a Western blot after treatment with fluvastatin as the high-mannose immature form of FLT3 (Figure 1D).
Fluvastatin inhibits cell proliferation and induces apoptosis
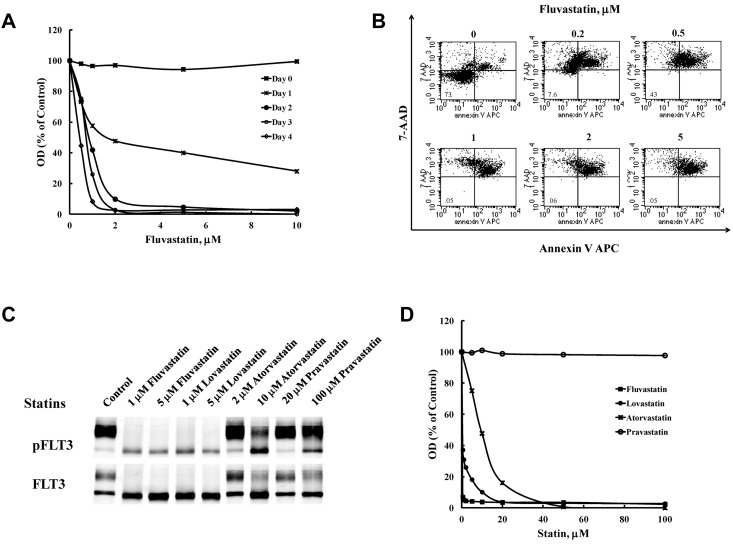
Constitutive FLT3 activation provides a growth advantage to cells and some are dependent on its kinase activity for survival. FLT3 TKIs inhibit proliferation in cell lines that are dependent on FLT3/ITD activity by blocking cell-cycle progression and inducing apoptosis. We thus tested the effect of fluvastatin treatment on proliferation and survival. Fluvastatin inhibited proliferation of BaF3 FLT3/ITD cells in a concentration-dependent fashion (Figure 2A). Even a 24-hour exposure resulted in significant inhibition of growth that became more pronounced on further time of exposure. Annexin V/7-AAD binding shows that inhibition of BaF3 FLT3/ITD cell growth was because of induction of apoptosis by fluvastatin (Figure 2B). The percentage of cells staining positive for annexin V and/or 7-AAD increased to > 92% after 48 hours of treatment with 0.2μM fluvastatin or higher concentrations. Because the pharmacokinetics of statins vary widely, other statins were tested for their ability to inhibit glycosylation of FLT3/ITD and proliferation in BaF3 cells. Figure 2C and D show that, in addition to fluvastatin, lovastatin and atorvastatin impair glycosylation of FLT3/ITD and inhibit cell proliferation in BaF3 FLT3/ITD cells while pravastatin failed to show activity in either assay at the concentrations tested. To test the possibility that fluvastatin cytotoxicity in these cells was because of depletion of cholesterol, we cotreated BaF3 FLT3/ITD cells with fluvastatin and 5μM cholesterol for 48 hours. The results indicate that cholesterol replenishment could not reverse the effect of fluvastatin on cell survival (data not shown).
Figure 2.
Treatment with statins inhibits proliferation in BaF3 FLT3/ITD cells. (A) BaF3 FLT3/ITD cells were treated with fluvastatin for the indicated times and processed using the MTT assay or (B) by measuring binding of annexin V and 7-AAD after 48 hours to assess induction of apoptosis. (C) Western blotting and (D) the MTT assay were also used to measure the effect of fluvastatin, lovastatin, atorvastatin, or pravastatin on FLT3 glycosylation and growth inhibition. Results are representative of 3 independent experiments.
Fluvastatin inhibits FLT3/ITD translocation to the cell surface
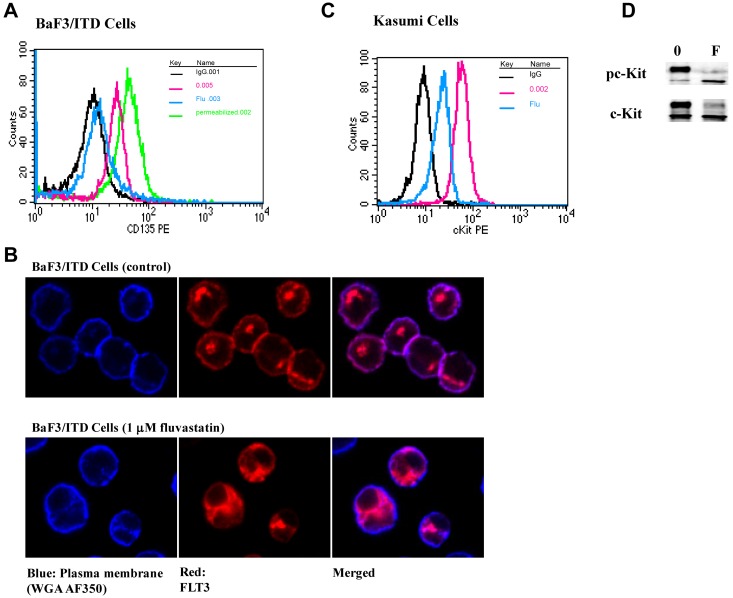
Fully glycosylated FLT3 has been shown to localize to the plasma membrane while the immature and unglycosylated FLT3 forms are restricted to the cell interior.46 To determine whether loss of the fully glycosylated receptor on the surface correlated with the patterns observed by Western blots, FLT3/ITD surface expression was analyzed by flow cytometry for CD135. Compared with untreated BaF3 FLT3/ITD cells, fluvastatin caused a dramatic reduction in surface FLT3 expression (Figure 3A) though intracellular FLT3/ITD was still detectable. The mean fluorescence intensity for surface FLT3/ITD decreased after treatment with 1μM fluvastatin for 24 hours, similar to what has been previously demonstrated by inhibition of receptor glycosylation using tunicamycin by other groups.47 Longer treatment (48 hours) with fluvastatin resulted in greater loss of detectable surface FLT3/ITD comparable with expression levels seen in cells stained with the IgG control (data not shown). Furthermore, immunofluorescence microscopy confirmed that fluvastatin treatment led to intracellular retention of FLT3/ITD in BaF3 cells as little surface FLT3 was detected compared with untreated cells (Figure 3B). To confirm that inhibition of receptor glycosylation is not unique to FLT3, Kasumi cells expressing the constitutively activated c-Kit tyrosine kinase receptor were treated with fluvastatin. Figure 3C shows that fluvastatin resulted in down-regulation of surface c-Kit expression by FACS analysis, which correlated with inhibition of formation of fully glycosylated mature c-Kit (Figure 3D).
Figure 3.
Fluvastatin prevents translocation of FLT3/ITD to the cell surface leading to intracellular accumulation of the immature form. (A) BaF3 FLT3/ITD cells were treated with 1μM fluvastatin for 24 hours. Afterward, surface or total FLT3/ITD was measured by incubating with CD135-PE for 30 minutes and analyzed by flow cytometry. The untreated cells are shown in red, fluvastatin-treated cells in blue, and total FLT3 from cells treated with fluvastatin and then permeabilized is shown in green. Cell-surface staining with IgG is shown in black for both the permeabilized and nonpermeabilized cells. (B) Cells treated with or without fluvastatin were adhered to poly-L-lysine slides and stained for plasma membrane (WGA-A350, blue) or FLT3 (Cy3, red). Slides were visualized by immunofluorescence microscopy using a Nikon TE2000 Eclipse microscope and 60 × 1.4 oil-immersion lens. (C) Kasumi cells were treated with 1μM fluvastatin for 24 hours and assessed for surface c-Kit expression. Cells stained with IgG are shown in black, untreated cells in red, and fluvastatin-treated cells in blue. (D) Western blot results show fluvastatin treatment led to a reduction in the amount of mature c-Kit expression in Kasumi-1 cells. Results are representative of at least 3 independent experiments.
FLT3/ITD signaling is altered by fluvastatin
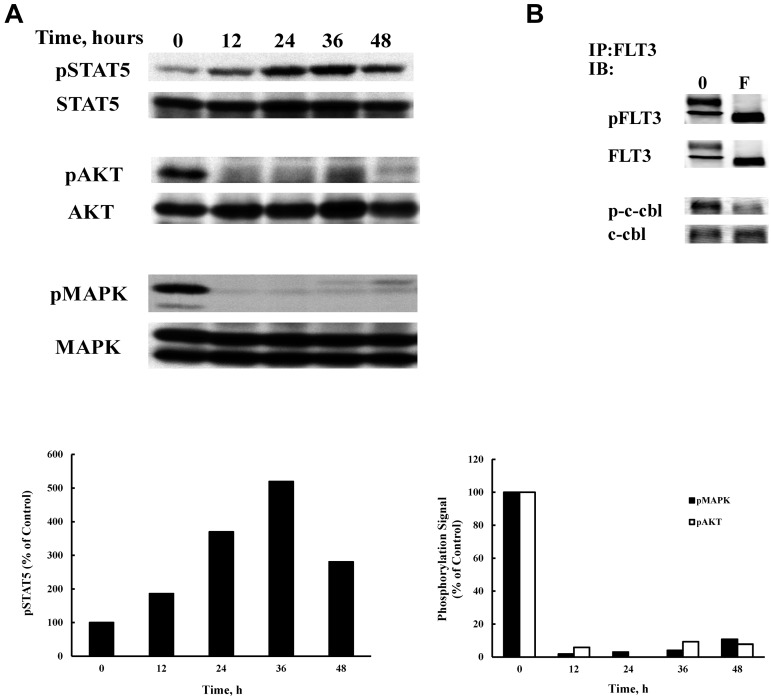
The transformative capacity of FLT3/ITD is mediated by its constitutive activation of STAT5, AKT, and MAPK pathways. FLT3 signaling may be compartment specific in that MAPK and AKT are activated only when FLT3/ITD is present in the plasma membrane.43 In contrast, STAT5 phosphorylation is enhanced when FLT3/ITD was prevented from reaching the surface.43 Because statin treatment produces altered localization of FLT3, we examined the ensuing effects on activation of these signaling pathways. Treatment of BaF3 FLT3/ITD cells with 1μM fluvastatin produced a time-dependent increase in STAT5 activation that was detectable by 12 hours and peaked at 36 hours of treatment (Figure 4A). In contrast, AKT and MAPK activity were inhibited by fluvastatin by 12 hours of incubation and this was maintained over the treatment course. Although inhibition of ras isoprenylation may explain the cytotoxicity for statin therapy in some cells, clearly the effect seen on localization of FLT3/ITD, which eliminates both MAPK and AKT activity, is a direct contributor to cytotoxicity. Analysis of c-cbl phosphorylation provides further evidence that fluvastatin alters signaling from surface expression of FLT3/ITD. Activated c-cbl is a common mechanism for regulating receptor tyrosine kinase signaling and causes internalization of the activated receptor. Because fluvastatin eliminates activated surface FLT3/ITD expression, treatment also led to a reduction in phosphorylated c-cbl that was associated with immunoprecipitated FLT3/ITD (Figure 4B).
Figure 4.
Fluvastatin alters FLT3/ITD signaling. (A) BaF3 FLT3/ITD cells were treated with 1μM fluvastatin for the indicated times and 50 μg of lysate from each time point was analyzed by SDS-PAGE and Western blotting. (B) Cells were treated with 1μM fluvastatin for 24 hours and lysates were immunoprecipitated with a FLT3 antibody (S-18). Blots were then probed for FLT3, phosphotyrosine, c-cbl, or phospho c-cbl. Typical results of 3 experiments are shown.
Fluvastatin inhibits proliferation driven by multiple glycosylated transmembrane receptors
Statins inhibit processes that are crucial to the survival of many different cell types, so we wanted to ascertain whether growth in cells other than BaF3 cell lines was affected. Any cell line expressing a transmembrane glycoprotein that is essential to its survival should be susceptible to fluvastatin-induced cytotoxicity, whereas cells that use other mechanisms (eg, nonreceptor tyrosine kinases) responsible for their survival should be able to tolerate higher concentrations of fluvastatin with less cytotoxicity seen. Molm-14, MV4-11, SEMK2, and HB1119 are human cell lines that all express endogenous FLT3 at the cell surface and are dependent on activated FLT3 for their growth and survival. All FLT3-dependent cell lines tested responded to fluvastatin with similar IC50 values of < 2μM (Figure 5A).
Figure 5.
Fluvastatin inhibits proliferation that is driven by glycosylated transmembrane receptors. Inhibition of growth was assessed after a 48-hour incubation period using the MTT assay in cell lines driven by either: (A) activated FLT3 including FLT3/ITD (MV4-11, Molm-14 cells), FLT3 (SEMK2 cells), or a D835H kinase domain mutant (HB1119 cells); (B) the nonglycosylated oncogenic intracellular kinases BCR-ABL (K562 cells) or Fes kinase (U937 cells); or (C) other glycosylated transmembrane receptors, such as insulin (HL60 cells), c-Kit (MO7E cells), or mutant c-Kit (Kasumi cells). (D) The effect of fluvastatin was assessed on proliferation of BaF3 cells expressing either FLT3/ITD or BCR-ABL after treatment for 48 hours by MTT. (E) Primary patient AML samples harboring a FLT3/ITD mutation were treated with the indicated concentrations of fluvastatin for 72 hours and analyzed by MTT assay or Western blotting. Typical results are shown for at least 2 separate experiments.
U937 are human-derived myelomonocytic cells that do not express FLT3. Instead, these cells are transformed by the intracellular tyrosine kinase activity of Fes kinase that is responsible for providing proliferative signals. Likewise, K562 cells are a human myeloid leukemia line devoid of FLT3 activity, but growth is driven by expression of the nonreceptor BCR-ABL tyrosine kinase fusion. In contrast, neither U937 nor K562 cells were sensitive, as their IC50 was not reached in the concentration range studied (Figure 5B). These results bolster the argument that statins are more effective at blocking cell growth in which a glycosylated transmembrane receptor drives proliferation. We further tested this hypothesis by treating HL60, MO7E, and Kasumi cells with fluvastatin. HL60 cells are human-derived promyelocytic cells that are completely dependent on insulin and transferrin receptors for growth, both of which are glycosylated transmembrane receptors. MO7E is a human myeloid leukemia cell line that requires expression of the wild-type c-Kit receptor for its survival and proliferation. Kasumi cells are a human myeloid line that is transformed by expression of the glycosylated constitutively activated mutant c-Kit receptor tyrosine kinase. As predicted, fluvastatin proved to be cytotoxic to HL60, MO7E, and Kasumi cells (Figure 5C). To eliminate the possibility that BaF3 FLT3/ITD cells are exquisitely sensitive to fluvastatin because of traits particular to the BaF3 cells rather than expression of a glycosylated transmembrane oncogene, we subsequently tested fluvastatin against BaF3 cells transformed with the intracellular oncogene BCR-ABL. Although fluvastatin produced inhibition in the BaF3 BCR-ABL cells (which could be because of effects on a variety of cell-surface receptors normally stimulated by factors in serum), the BaF3 FLT3/ITD cells were 10-fold more sensitive (Figure 5D).
Effect of fluvastatin on human AML blasts expressing FLT3/ITD
Statins have been reported to induce apoptosis in some multiple myeloma (MM) cell lines and patient MM samples.48 Our data suggest AML patient samples that rely on constitutively activated FLT3/ITD mutants would also be sensitive to statins. Therefore, we tested whether fluvastatin could affect survival/proliferation in 3 FLT3/ITD AML patient samples. FLT3/ITD-positive AML blasts from 3 different patients were susceptible to fluvastatin-induced growth inhibition at concentrations similar to those observed in mutant FLT3 cell lines (Figure 5E). Western blotting shows that fluvastatin also reduces expression levels of immature and mature FLT3 in primary patient samples similar to the results seen in some cell lines at high fluvastatin concentrations.
Fluvastatin overcomes resistance to tyrosine kinase inhibitors
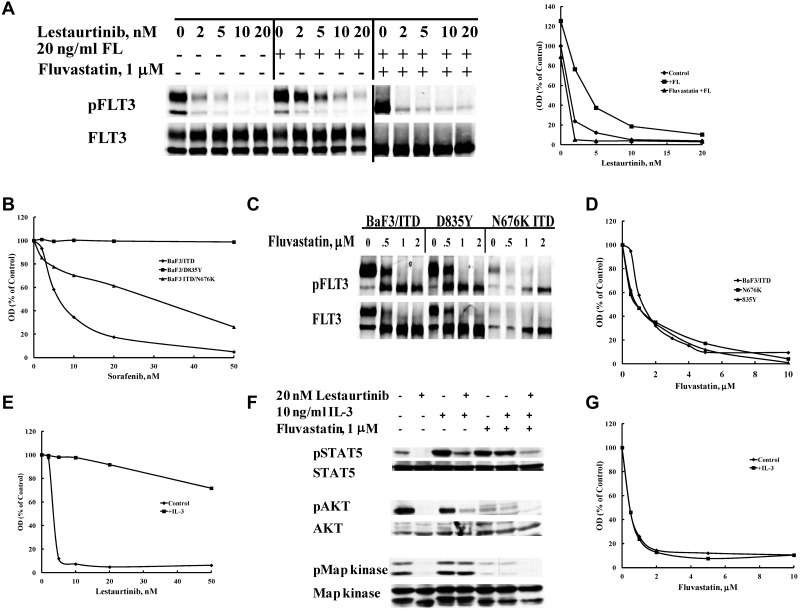
In light of the data showing the inhibitory effect of fluvastatin on FLT3/ITD glycosylation and localization, we were prompted to evaluate statins for their potential to overcome several mechanisms of TKI resistance. Because fluvastatin inhibits activation of FLT3 by FL by reducing the level of cell-surface receptor available to ligand, we hypothesized it would inhibit the enhancement of FLT3/ITD phosphorylation that was recently documented as well.49 This is important because recent findings implicate elevated serum FL levels in chemotherapy treated FLT3/ITD AML patients as a putative mechanism of increasing FLT3 TKI resistance several fold.50 In patients, this may translate into a need for increased drug levels that may be difficult to achieve or maintain because of associated greater toxicity. As shown in Figure 6A, 24-hour pretreatment with fluvastatin reverses the partial resistance to lestaurtinib mediated by FL stimulation. Treatment of cells with lestaurtinib for 1 hour caused a concentration-dependent decrease in FLT3/ITD phosphorylation with an IC50 of <1nM. However, stimulation with 20 ng/mL FL shifted the lestaurtinib IC50 to 3nM, and phosphorylation could still be observed even at concentrations as high as 20nM. Pretreatment with fluvastatin blocked surface localization of FLT3/ITD and thus restored the IC50 of lestaurtinib to < 1nM despite the presence of high levels of exogenous FL.
Figure 6.
Fluvastatin reverses resistance to FLT3 tyrosine kinase inhibitors mediated by multiple mechanisms. (A) Resistance to lestaurtinib induced by stimulation with FLT3 ligand (FL) is overcome by prior treatment with fluvastatin. BaF3 FLT3/ITD cells were treated with or without 1μM fluvastatin for 24 hours followed by incubation in lestaurtinib for 1 hour and then stimulated with 20 ng/mL FL for 5 minutes. Densitometry of the pFLT3 bands plotted in response to treatment is shown. (B) BaF3 cells expressing a FLT3/ITD, D835Y, or FLT3/ITD N676K resistance mutation were treated with sorafenib for 48 hours and viability was assessed by MTT assay. (C) The same cells were treated for 24 hours with increasing concentrations of fluvastatin to study FLT3 glycosylation or (D) for 48-96 hours and analyzed by MTT assay. Cells were simultaneously treated with either (E) lestaurtinib or (G) fluvastatin and 10 ng/mL interleukin-3 for 48 hours after which cell growth was evaluated by MTT. (F) Activation of IL-3 receptor signaling pathways was assessed by treating cells with or without 1μM fluvastatin for 24 hours followed by incubation with or without lestaurtinib for 1 hour and a 10-minute stimulation with or without 10 ng/mL IL-3 followed by SDS-PAGE Western blotting analysis of STAT5, AKT, and MAPK activation states. Results are representative of at least 3 experiments.
While FL stimulation has been shown to reduce the potency of TKI inhibition of phosphorylation of FLT3/ITD by > 2-fold, some mutations within FLT3 can lead to resistance that greatly decreases TKI activity even at very high concentrations for many inhibitors. The FLT3 D835Y-activating mutation has long been known to be resistant to many TKI and occurs in 7%-10% of AML cases. Other FLT3-resistance mutations that occur on the backbone of a FLT3/ITD mutation have also been shown to confer TKI resistance by altering the ATP-binding cleft. For example, 1 AML patient with a FLT3/ITD mutation developed an N676K resistance mutation while on midostaurin treatment. Sorafenib is currently in clinical trials for mutant FLT3, but it is less effective against both the D835Y and N676K mutants (Figure 6B). All FLT3 mutants undergo N-linked glycosylation and are thus susceptible to the effects fluvastatin has on its maturation and thus signal transduction ability. Figure 6C shows that glycosylation of mature FLT3 in both the D835Y and FLT3/ITD N676K mutants was inhibited by fluvastatin similar to the FLT3/ITD cells. In response, proliferation was inhibited in both mutants by treatment with fluvastatin (Figure 6D). Thus, specific FLT3 mutations that are resistant to TKI remain sensitive to statin-mediated glycosylation inhibition.
Yet another mechanism responsible for imparting FLT3 TKI resistance is the overexpression and/or activation of alternative signaling pathways, especially those mediated by other receptor tyrosine kinases that compensate for inhibited FLT3. Our laboratory has previously shown that some FLT3/ITD cell lines selected for resistance to lestaurtinib overexpress several receptor tyrosine kinases as a means of resistance when they do not accumulate resistance mutations within FLT3 itself.51 These other pathways are felt to substitute for FLT3 signaling in much the same way that stimulation of the IL-3 pathway can rescue BaF3 FLT3/ITD cells from FLT3 TKI-mediated cytotoxicity (Figure 6E). Examination of the signaling pathways reveals that IL-3 is able to activate STAT5, MAPK, and AKT (to a lesser extent) in BaF3 FLT3/ITD cells treated with lestaurtinib (Figure 6F lanes 1-4). Pretreatment with fluvastatin leads to inhibition of MAPK and AKT regardless of the attempt to activate the IL-3 receptor by the addition of IL-3 (Figure 6F lanes 5 and 6). The addition of fluvastatin to lestaurtinib treatment not only blocks MAPK and AKT but enables lestaurtinib to inhibit STAT5 signaling from FLT3/ITD even in the presence of IL-3 (Figure 6F lane 7). In contrast to the rescue by IL-3 of BaF3 FLT3/ITD cells treated with lestaurtinib, fluvastatin treatment prevents this rescue because of inhibition of glycosylation of the IL-3 receptor (Figure 6G).
Statins reduce engraftment of FLT3/ITD cells in Balb/c mice
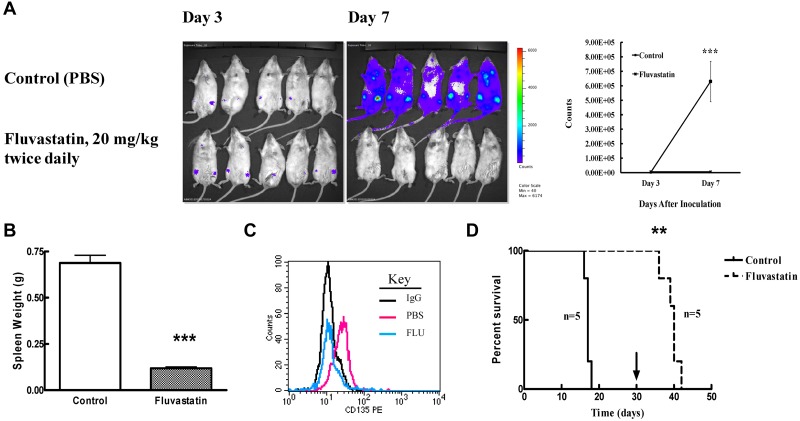
BaF3 FLT3/ITD cells are particularly sensitive in vitro to fluvastatin treatment. We wanted to determine whether this would translate to effective treatment in vivo by studying engraftment of luc+ BaF3 FLT3/ITD cells in Balb/c mice. Transplantation of luc+ BaF3 FLT3/ITD cells via tail vein injection led to engraftment that could be observed by day 3 in all mice. Progression of increased bioluminescence by day 7 in response to fluvastatin treatment was dramatically reduced (P = .0022; Figure 7A). Transplantation of these leukemic cells typically leads to splenomegaly in untreated mice, but fluvastatin treatment caused a significant (P < .0001) size reduction in spleens collected after 2 weeks compared with controls (Figure 7B). To determine whether fluvastatin could inhibit FLT3 glycosylation in vivo, transplanted BaF3 FLT3/ITD cells were allowed to engraft for 10 days before mice were treated for the next 2 days with fluvastatin. FACS analysis of peripheral blood drawn from PBS-treated versus fluvastatin-treated mice showed that fluvastatin did decrease surface FLT3/ITD expression in vivo. We continued to treat some mice further to determine whether fluvastatin could not only reduce engraftment but also prolong survival. Mice in the control group became moribund 2 weeks after transplantation and were euthanized. Mice treated with fluvastatin all survived for the 30-day treatment period. On cessation of fluvastatin treatment, these mice also became moribund in approximately a week. A Kaplan-Meier curve shows that fluvastatin treatment significantly (P = .002) prolonged survival, from a median of 17 days in control mice to 40 days for the treated group (Figure 7D).
Figure 7.
Fluvastatin reduces tumor burden in mice engrafted with FLT3/ITD cells. (A) Balb/c mice were injected via tail vein with 2 × 105 luciferase-positive BaF3 FLT3/ITD cells and treated with PBS (control) or fluvastatin at 20 mg/kg twice daily starting 3 days after injection. Mice were injected with luciferin and analyzed on an IVIS Spectrum imager on days 3 and 7. The mean (+ SEM) bioluminescence for the 5 mice in each group at days 3 and 7 is plotted on the right. (B) Twice-daily treatments were continued as described in panel A until mice became moribund (control group) at which point both groups of mice were euthanized and spleen weights (mean + SEM) were measured. (C) Peripheral blood from transplanted mice treated with or without 20 mg/kg fluvastatin twice daily on days 10 and 11 after transplantation was assayed for surface FLT3 expression. IgG-stained cells are shown in black, cells from PBS-treated mice in red, and cells from fluvastatin-treated mice in blue. (D) Survival of transplanted mice was assessed by twice-daily PBS or fluvastatin treatments from day 3 until day 30 (or until mice became moribund) at which point treatment was stopped (arrow). Five mice were used in each group and the experiment was conducted twice.
Discussion
Targeting FLT3 in AML with TKIs has been hampered by the development of resistance emanating from multiple distinct mechanisms. These include the acquisition of TKI resistance mutations within FLT3, elevation of FLT3 ligand levels with subsequent shift to the right of dose-response curves, activation of alternative signaling pathways bypassing the need for FLT3 signaling, and elevated drug binding to serum proteins reducing the level of free drug available to bind target, among others. Hence, an approach that may circumvent these particular challenges may prove beneficial in treating FLT3/ITD AML. We report here for the first time that statins are able to inhibit FLT3 signaling by preventing complex glycosylation of the receptor, thus leading to aberrant localization, altered signaling and cell death in FLT3-dependent cell lines. In addition, statins may overcome some of the specific challenges outlined above and could potentially be instituted as therapy against leukemias that are driven by activated FLT3.
Statins have been widely used to reduce serum cholesterol levels by targeting HMG CoA reductase that is produced in the mevalonate biosynthetic pathway. Among the many end products and intermediates in this pathway are dolichol and ubiquinone in addition to cholesterol.34 Dolichol serves as a carrier in the transfer of the preassembled 14-sugar core oligosaccharide onto nascent polypeptides at specific asparagine residues. Appropriate glycosylation and processing are important for protein stability and cellular localization. FLT3 is synthesized as a 110-kDa protein that rapidly undergoes dolichol-mediated glycosylation and processing in the endoplasmic reticulum to produce a mannose-rich 130-kDa immature protein. Further processing in the Golgi leads to production of the mature 160-kDa protein that translocates to the cell surface.46 We hypothesized that statins might inhibit HMG CoA reductase sufficiently to alter glycosylation of mutant FLT3 and result in its aberrant localization and activity along with decreased stability. Hence, statin treatment of FLT3-expressing cells was expected to lead to loss of both mature and immature FLT3 because of inhibition of dolichol formation. However, the observation of increased amounts of immature FLT3 means that the role(s) of dolichol in increasing FLT3 stability and in the process of FLT3 glycosylation are not fully understood and will require further investigation.
In this report, we show that statins have an inhibitory effect on glycosylation of mutant FLT3 and, subsequently, on cell survival. Treatment of BaF3 FLT3/ITD cells with fluvastatin resulted in down-regulation of surface FLT3 and concomitant intracellular accumulation of the immature form of FLT3/ITD, indicating a block in the progression of immature to mature receptor. This block leads to an altered pattern of localization characterized by changes in FLT3/ITD signaling. Mutant FLT3 causes persistent activation of multiple oncogenic pathways that contribute to its malignancy. However, on statin treatment and the ensuing altered FLT3 localization, the membrane-associated MAPK and PI3K/AKT components of signaling were eliminated, thus reducing a significant contribution of the transformative capacity of FLT3/ITD. The loss of cell-surface FLT3 and reduced signaling capacity caused by fluvastatin subsequently results in rapid induction of apoptosis.
These results suggest that statins may be able to overcome several obstacles encountered when patients are treated with FLT3 TKI. First, the increase in the IC50 for a TKI to inhibit FLT3 because of elevation in FLT3 ligand levels in AML patients treated with chemotherapy was completely abolished by statin pretreatment. Because FLT3 can respond to exogenous FL only while at the cell surface, inhibition of glycosylation and translocation of mature FLT3/ITD by fluvastatin eliminated the resistance to TKI caused by elevated FL binding, thereby restoring inhibitor potency. Second, FLT3 mutants, both activating TKD mutations and resistance mutations selected for within ITD forms, that confer resistance to a particular TKI, remain sensitive to statins. The selection for TKD mutations has begun to emerge as a frequent occurrence for AML patients treated on TKI for a FLT3/ITD mutation and impart resistance to treatment.27,32 Notably, these tend to be mutations at or near D835. In vitro, these mutations produce a high level of resistance to many TKI including, sunitinib, sorafenib, AC220, and KW2449. However, TKD mutants still require glycosylation for localization to the plasma membrane and full signaling and thus should be susceptible to inhibition by statins. In fact, our results showed that proliferation in BaF3 cells expressing a FLT3 TKD mutant, D835Y or D835H, as well as the FLT3/ITD N676K mutation that was isolated from 1 patient who became resistant to PKC412 (midostaurin), was disrupted by fluvastatin treatment. A third mechanism seen to reduce the clinical efficacy of TKI is the development of resistance because of selection of cells with activation of alternative signaling pathways that bypass FLT3 dependence. In one study, quantitative PCR analysis revealed that TKI resistance in the absence of FLT3 mutations was associated with overexpression of IGF1-R, the insulin receptor, PDGF receptor, or other tyrosine kinase receptors.51 These receptors all undergo N-linked glycosylation and would likely all be susceptible to modification by statins. In analogy to FLT3/ITD, the resulting change in localization would be expected to alter signaling qualities from each tyrosine kinase receptor and reduce their contribution to a resistance phenotype. Our results demonstrated that cells that require the glycosylated growth factor receptors IL-3, c-Kit, or insulin were indeed more sensitive to fluvastatin than were cells that are not dependent on cell-surface receptor growth promoting pathways. Thus, statins hold promise as a means to help prevent or circumvent resistance stemming from FLT3 TKI treatment.
Before this study, there were no published findings showing inhibitory effects of statins on FLT3 activity, but there are indications that sufficiently high statin doses can be tolerated and plasma levels achieved to inhibit FLT3 glycosylation. For example, 2 studies of higher dose lovastatin achieved bioactive plasma levels of 3.9-12.3μM.39,40 One report showed that fluvastatin at standard doses for hypercholesterolemia (40 mg/d) resulted in plasma levels up to 1μM.52 In our studies, we showed that fluvastatin at concentrations of 0.2-2μM inhibited proliferation in multiple FLT3 and other receptor tyrosine kinase–expressing cell lines and primary patient mutant FLT3 AML blasts. Dosing of mice transplanted with BaF3 FLT3/ITD cells at 20 mg/kg/d of fluvastatin twice daily showed a significant reduction in engraftment and prolonged survival. Considering that patients have been treated with statins at higher doses and up to 4 times daily, it is possible that similar dosing with fluvastatin could be efficacious and well tolerated.
In summary, we have demonstrated that statins can induce cytotoxicity in mutant FLT3 leukemia cell lines by disrupting FLT3 glycosylation resulting in a disruption of signal transduction, thus reducing tumor burden in vivo. The results suggest that tumor cell proliferation driven by a glycosylated transmembrane receptor such as FLT3/ITD can be inhibited by a combination of statins and TKI at statin doses likely to be achieved in patients. Statins and FLT3 TKI have opposing effects on FLT3 maturation. Thus, preclinical and clinical studies will be necessary to determine how best to combine statins with FLT3 TKI to optimize potential synergistic potential. Thus, repurposing statins merits further consideration for their potential benefit in helping to manage AML in which mutant FLT3 is considered a desirable target.
Acknowledgments
The authors are grateful to Dr Linzhao Cheng (Johns Hopkins University) for providing us with the L3GFP vector used to visualize FLT3/ITD engraftment, Trivikram Rajkhowa (Johns Hopkins University) for isolating leukemic blasts, and members of the laboratory for numerous thoughtful discussions.
This work was supported by grants from the National Cancer Institute (CA90770 and CA90668), the Leukemia & Lymphoma Society, and the Giant Food Pediatric Cancer Research Fund. D.S. is also supported by the Kyle Haydock Professorship.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.B.W. designed experiments, performed research, analyzed data, and wrote the manuscript; L.L. and B.N. performed research; P.B. and M.L. analyzed data and contributed patient samples; and D.S. designed experiments, supervised the project, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donald Small, MD, PhD, Departments of Oncology and Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD 21231; e-mail: donsmall@jhmi.edu.
References
- 1.Small D, Levenstein M, Kim E, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci U S A. 1994;91(2):459–463. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reilly JT. FLT3 and its role in the pathogenesis of acute myeloid leukaemia. Leuk Lymphoma. 2003;44(1):1–7. doi: 10.1080/1042819021000040233. [DOI] [PubMed] [Google Scholar]
- 3.Chan PM. Differential signaling of Flt3 activating mutations in acute myeloid leukemia: a working model. Protein Cell. 2011;2(2):108–115. doi: 10.1007/s13238-011-1020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyman SD, James L, Vanden Bos T, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75(6):1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- 5.Lavagna-Sevenier C, Marchetto S, Birnbaum D, Rosnet O. FLT3 signaling in hematopoietic cells involves CBL, SHC and an unknown P115 as prominent tyrosine-phosphorylated substrates. Leukemia. 1998;12(3):301–310. doi: 10.1038/sj.leu.2400921. [DOI] [PubMed] [Google Scholar]
- 6.Rosnet O, Buhring HJ, deLapeyriere O, et al. Expression and signal transduction of the FLT3 tyrosine kinase receptor. Acta Haematol. 1996;95(3-4):218–223. doi: 10.1159/000203881. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Broxmeyer HE. Flt3 ligand induces tyrosine phosphorylation of gab1 and gab2 and their association with shp-2, grb2, and PI3 kinase. Biochem Biophys Res Commun. 2000;277(1):195–199. doi: 10.1006/bbrc.2000.3662. [DOI] [PubMed] [Google Scholar]
- 8.Kim KT, Baird K, Ahn JY, et al. Pim-1 is up-regulated by constitutively activated FLT3 and plays a role in FLT3-mediated cell survival. Blood. 2005;105(4):1759–1767. doi: 10.1182/blood-2004-05-2006. [DOI] [PubMed] [Google Scholar]
- 9.Tse KF, Mukherjee G, Small D. Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia. 2000;14(10):1766–1776. doi: 10.1038/sj.leu.2401905. [DOI] [PubMed] [Google Scholar]
- 10.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–1918. [PubMed] [Google Scholar]
- 11.Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113(4):983–988. doi: 10.1046/j.1365-2141.2001.02850.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 13.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 14.Yokota S, Kiyoi H, Nakao M, et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia. 1997;11(10):1605–1609. doi: 10.1038/sj.leu.2400812. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Piloto O, Nguyen HB, et al. Knock-in of an internal tandem duplication mutation into murine FLT3 confers myeloproliferative disease in a mouse model. Blood. 2008;111(7):3849–3858. doi: 10.1182/blood-2007-08-109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 2002;99(1):310–318. doi: 10.1182/blood.v99.1.310. [DOI] [PubMed] [Google Scholar]
- 17.Grundler R, Miething C, Thiede C, Peschel C, Duyster J. FLT3-ITD and tyrosine kinase domain mutants induce 2 distinct phenotypes in a murine bone marrow transplantation model. Blood. 2005;105(12):4792–4799. doi: 10.1182/blood-2004-11-4430. [DOI] [PubMed] [Google Scholar]
- 18.Lee BH, Tothova Z, Levine RL, et al. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell. 2007;12(4):367–380. doi: 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stubbs MC, Kim YM, Krivtsov AV, et al. MLL-AF9 and FLT3 cooperation in acute myelogenous leukemia: development of a model for rapid therapeutic assessment. Leukemia. 2008;22(1):66–77. doi: 10.1038/sj.leu.2404951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenblatt S, Li L, Slape S, et al. Knock-in of a FLT3/ITD mutation cooperates with a NUP98-HOXD13 fusion to generate acute myeloid leukemia in a mouse model. Blood. 2012;119(12):2883–2894. doi: 10.1182/blood-2011-10-382283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rau RE, Magoon D, McIntyre E, et al. Cytoplasmic nucleophosmin (NPMc+) mutations and FMS-like tyrosine kinase 3 (Flt3) internal tandem duplication (ITD) mutations cooperate to cause leukemia in a mouse model. Blood. 2010;116(21):145. [Google Scholar]
- 22.Gilliland DG, Griffin JD. Role of FLT3 in leukemia. Curr Opin Hematol. 2002;9(4):274–281. doi: 10.1097/00062752-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Pemmaraju N, Kantarjian H, Ravandi F, Cortes J. FLT3 inhibitors in the treatment of acute myeloid leukemia: the start of an era? Cancer. 2011;117(15):3293–3304. doi: 10.1002/cncr.25908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.el-Shami K, Stone RM, Smith BD. FLT3 inhibitors in acute myeloid leukemia. Expert Rev Hematol. 2008;1(2):153–160. doi: 10.1586/17474086.1.2.153. [DOI] [PubMed] [Google Scholar]
- 25.Knapper S. FLT3 inhibition in acute myeloid leukaemia. Br J Haematol. 2007;138(6):687–699. doi: 10.1111/j.1365-2141.2007.06700.x. [DOI] [PubMed] [Google Scholar]
- 26.Clark JJ, Cools J, Curley DP, et al. Variable sensitivity of FLT3 activation loop mutations to the small molecule tyrosine kinase inhibitor MLN518. Blood. 2004;104(9):2867–2872. doi: 10.1182/blood-2003-12-4446. [DOI] [PubMed] [Google Scholar]
- 27.Alvarado Y, Kantarjian H, Ravandi F, et al. FLT3 inhibitor treatment in FLT3-mutated AML is associated with development of secondary FLT3-TKD mutations. Blood. 2011;118(21):1493. [Google Scholar]
- 28.Smith CC, Chin J, Wang Q, et al. Validation of FLT3-ITD as a therapeutic target in human acute myeloid leukemia. Blood. 2011;118(21):937. [Google Scholar]
- 29.Cools J, Mentens N, Furet P, et al. Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Res. 2004;64(18):6385–6389. doi: 10.1158/0008-5472.CAN-04-2148. [DOI] [PubMed] [Google Scholar]
- 30.Heidel F, Solem FK, Breitenbuecher F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107(1):293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 31.Breitenbuecher F, Markova B, Kasper S, et al. A novel molecular mechanism of primary resistance to FLT3-kinase inhibitors in AML. Blood. 2009;113(17):4063–4073. doi: 10.1182/blood-2007-11-126664. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Konopleva M, Jacamo RO, et al. Acquired point mutations of TKD are responsible for sorafenib resistance in FLT3-ITD mutant AML [abstract]. Blood (ASH Annual Meeting Abstracts) 2011;118 Abstract 3505. [Google Scholar]
- 33.Eisenberg DA. Cholesterol lowering in the management of coronary artery disease: the clinical implications of recent trials. Am J Med. 1998;104(2A):2S–5S. doi: 10.1016/s0002-9343(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 34.Corsini A, Maggi FM, Catapano AL. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol Res. 1995;31(1):9–27. doi: 10.1016/1043-6618(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 35.Stirewalt DL, Appelbaum FR, Willman CL, Zager RA, Banker DE. Mevastatin can increase toxicity in primary AMLs exposed to standard therapeutic agents, but statin efficacy is not simply associated with ras hotspot mutations or overexpression. Leuk Res. 2003;27(2):133–145. doi: 10.1016/s0145-2126(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 36.Clutterbuck RD, Millar BC, Powles RL, et al. Inhibitory effect of simvastatin on the proliferation of human myeloid leukaemia cells in severe combined immunodeficient (SCID) mice. Br J Haematol. 1998;102(2):522–527. doi: 10.1046/j.1365-2141.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Lee I, Han B, et al. Effect of simvastatin on cetuximab resistance in human colorectal cancer with KRAS mutations. J Natl Cancer Inst. 2011;103(8):674–688. doi: 10.1093/jnci/djr070. [DOI] [PubMed] [Google Scholar]
- 38.Martirosyan A, Clendening JW, Goard CA, Penn LZ. Lovastatin induces apoptosis of ovarian cancer cells and synergizes with doxorubicin: potential therapeutic relevance. BMC Cancer. 2010;10:103. doi: 10.1186/1471-2407-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thibault A, Samid D, Tompkins AC, et al. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin Cancer Res. 1996;2(3):483–491. [PubMed] [Google Scholar]
- 40.Holstein SA, Knapp HR, Clamon GH, Murry DJ, Hohl RJ. Pharmacodynamic effects of high dose lovastatin in subjects with advanced malignancies. Cancer Chemother Pharmacol. 2006;57(2):155–164. doi: 10.1007/s00280-005-0013-8. [DOI] [PubMed] [Google Scholar]
- 41.Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med (Maywood) 2004;229(7):567–585. doi: 10.1177/153537020422900701. [DOI] [PubMed] [Google Scholar]
- 42.Tse KF, Allebach J, Levis M, Smith BD, Bohmer FD, Small D. Inhibition of the transforming activity of FLT3 internal tandem duplication mutants from AML patients by a tyrosine kinase inhibitor. Leukemia. 2002;16(10):2027–2036. doi: 10.1038/sj.leu.2402674. [DOI] [PubMed] [Google Scholar]
- 43.Choudhary C, Olsen JV, Brandts C, et al. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol Cell. 2009;36(2):326–339. doi: 10.1016/j.molcel.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 44.Hamadmad SN, Hohl RJ. Lovastatin suppresses erythropoietin receptor surface expression through dual inhibition of glycosylation and geranylgeranylation. Biochem Pharmacol. 2007;74(4):590–600. doi: 10.1016/j.bcp.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 45.Siddals KW, Marshman E, Westwood M, Gibson JM. Abrogation of insulin-like growth factor-I (IGF-I) and insulin action by mevalonic acid depletion: synergy between protein prenylation and receptor glycosylation pathways. J Biol Chem. 2004;279(37):38353–38359. doi: 10.1074/jbc.M404838200. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt-Arras DE, Bohmer A, Markova B, Choudhary C, Serve H, Bohmer FD. Tyrosine phosphorylation regulates maturation of receptor tyrosine kinases. Mol Cell Biol. 2005;(9):25:3690–3703. doi: 10.1128/MCB.25.9.3690-3703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ling YH, Li T, Perez-Soler R, Haigentz M., Jr Activation of ER stress and inhibition of EGFR N-glycosylation by tunicamycin enhances susceptibility of human non-small cell lung cancer cells to erlotinib. Cancer Chemother Pharmacol. 2009;64(3):539–548. doi: 10.1007/s00280-008-0902-8. [DOI] [PubMed] [Google Scholar]
- 48.Clendening JW, Pandyra A, Li Z, et al. Exploiting the mevalonate pathway to distinguish statin-sensitive multiple myeloma. Blood. 2010;115(23):4787–4797. doi: 10.1182/blood-2009-07-230508. [DOI] [PubMed] [Google Scholar]
- 49.Zheng R, Bailey E, Nguyen B, et al. Further activation of FLT3 mutants by FLT3 ligand. Oncogene. 2011;30(38):4004–4014. doi: 10.1038/onc.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato T, Yang X, Knapper S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117(12):3286–3293. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piloto O, Wright M, Brown P, Kim KT, Levis M, Small D. Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood. 2007;109(4):1643–1652. doi: 10.1182/blood-2006-05-023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tse FL, Jaffe JM, Troendle A. Pharmacokinetics of fluvastatin after single and multiple doses in normal volunteers. J Clin Pharmacol. 1992;32(7):630–638. doi: 10.1002/j.1552-4604.1992.tb05773.x. [DOI] [PubMed] [Google Scholar]