Abstract
Interferon (IFN-α) is effective therapy for polycythemia vera (PV) patients, but it is frequently interrupted because of adverse events. To permit the long-term use of IFN, we propose combining low doses of IFN with Nutlin-3, an antagonist of MDM2, which is also capable of promoting PV CD34+ cell apoptosis. Combination treatment with subtherapeutic doses of Peg IFN-α 2a and Nutlin-3 inhibited PV CD34+ cell proliferation by 50% while inhibiting normal CD34+ cells by 30%. Combination treatment with Nutlin-3 and Peg IFN-α 2a inhibited PV colony formation by 55%-90% while inhibiting normal colony formation by 22%-30%. The combination of these agents also decreased the proportion of JAK2V617F-positive hematopoietic progenitor cells in 6 PV patients studied. Treatment with low doses of Peg IFN-α 2a combined with Nutlin-3 increased phospho-p53 and p21 protein levels in PV CD34+ cells and increased the degree of apoptosis. These 2 reagents affect the tumor suppressor p53 through different pathways with Peg IFN-α 2a activating p38 MAP kinase and STAT1, leading to increased p53 transcription, whereas Nutlin-3 prevents the degradation of p53. These data suggest that treatment with low doses of both Nutlin-3 combined with Peg IFN-α 2a can target PV hematopoietic progenitor cells, eliminating the numbers of malignant hematopoietic progenitor cells.
Introduction
The chronic myeloproliferative neoplasms (MPNs) originate at the level of hematopoietic stem cells (HSCs) and/or progenitor cells.1–4 The identification of an acquired somatic mutation on Janus kinase 2 (JAK2V617F) in the myeloid cells of MPN patients has led to the development of small-molecule inhibitors of JAK2.5–9 Clinical trials of these agents revealed their ability to decrease the degree of splenomegaly and to improve systemic symptoms in MPN patients, but unfortunately, the progeny of the malignant clone have not been substantially affected.10–13 IFN-α therapy has been effective in controlling the excess production of red cells and platelets, reducing the burden of malignant cells (granulocyte JAK2V617F allele burden), occasionally inducing cytogenetic remissions, correcting marrow morphologic abnormalities, and reestablishing polyclonal hematopoiesis in a substantial number of polycythemia vera (PV) patients.14–17 However, prolonged therapy of PV patients with IFN-α is not in frequently limited by a variety of adverse events, necessitating its discontinuation.18 Because many of these adverse events are dose dependent, the identification of drugs that can be combined with lower doses of IFN-α potentially would provide a means of treating greater numbers of PV patients with IFN for longer periods of time.
IFN-α binds to the type I IFN receptor and activates the JAK/TYK/STAT pathway, leading to multiple downstream events.19 Furthermore, IFN-α also activates a p38 mitogen-activated protein kinase (MAP kinase) resulting in apoptosis of PV hematopoietic progenitor cells (HPCs).20 Both of these pathways have been shown to act through the p53 tumor suppressor protein.21–23
p53 plays an important role in the control of DNA repair, cell cycling, and apoptosis, and is frequently inactivated in human cancers.23 In chronic phases of the MPN, including PV wild-type forms of p53, are universally present, whereas p53 mutations have been identified in only a limited number of patients undergoing transformation to acute leukemia.24–27 The murine double minute (MDM2) plays a major role in keeping p53 cellular levels low.28 MDM2 not only facilitates p53 degradation, but also binds p53 and inhibits its transcriptional activity. A small-molecule antagonist of MDM2, Nutlin-3, specifically inhibits the p53-MDM2 interactions in the cellular context, activates the p53 pathway in cancer cells with wild-type p53, resulting in cellular blockade in G1 and G2, and increased apoptosis.29 MDM2 and p53 play a role in normal HSC homeostasis in mice. Unrestrained p53 activity depletes HSCs and HPCs via induction of cell cycle arrest, senescence, and ultimately cell death. Recently, Nakatake et al demonstrated that, in cell lines, JAK2V617F alters p53 responses to DNA damage through up-regulation of La antigen that increases MDM2 protein translation.30 These experiments were repeated with erythroblasts derived from CD34+ cells isolated from peripheral blood of healthy donors or JAK2V617F-positive PV and primary myelofibrosis patients. They observed that expression of p53 was significantly reduced in PV patients compared with control cells. This reduced expression was associated with a significant decrease in Bcl-2 associated X protein (BAX) and the p53 up-regulated modulator of apoptosis (PUMA) mRNA expression in primary myelofibrosis compared with control cells. Molecules with nutlin-like activities are currently being evaluated in clinical trials for a variety of human malignancies.31 We hypothesized that Nutlin-3 might be an effective agent to combine with IFN α to treat MPN patients. Targeting alternative pathways that can up-regulate p53 might serve as a novel therapeutic strategy for treating PV patients. In this study, the potential therapeutic utility of IFN-α combined with Nutlin-3 targeting of Mdm2/p53 proteins in PV patients was investigated.
Methods
Cell preparation
Peripheral blood was obtained from 19 PV patients being treated with phlebotomy alone and/or aspirin after informed consent was obtained according to guidelines established by the Institutional Review Board of the Mount Sinai School of Medicine, New York, NY. Approval was obtained from the Institutional Review Board of the Mount Sinai School of Medicine, New York, NY for these studies. Informed consent was provided according to the Declaration of Helsinki. All patients met the World Health Organization diagnostic criteria for PV. The peripheral blood samples were layered onto Ficoll-Hypaque (1.077 g/mL; GE Healthcare) and low-density mononuclear cells separated after centrifugation. The CD34+ cell population was isolated using a human CD34+ cell selection kit (StemCell Technologies) according to the manufacturer's instructions. The purity of the CD34+ cell population was analyzed using a FACSCalibur flow cytometer (BD Biosciences); the purity of CD34+ cells used in all experiments was ≥ 85%. Fresh normal human bone marrow CD34+ cells were purchased from ALLCELLS. The characteristics of the PV patients studied are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
HPC assays
CD34+ cells were cultured in serum free medium (StemCell Technologies)32,33 containing 50 ng/mL stem cell factor (SCF), 50 ng/mL thrombopoietin (TPO), 50 ng/mL fms-like tyrosine kinase 3 (Flt-3) ligand, and 50 ng/mL IL-3, and were treated with a low dose of Peg IFN-α 2a (200 U/mL; Roche Diagnostics), or a low dose of Nutlin-3 (200nM; Cayman; 48108), or in combination for 4 days. After 4 days of treatment, CD34+ cells were assayed in semisolid media as described previously.34 Briefly, 5 × 102 CD34+ cells were plated per dish in duplicate cultures containing 1 mL IMDM with 1.1% methylcellulose and 20% FBS, to which SCF, TPO, Flt-3 ligand, IL-3, and GM-CSF at each 50 ng/mL, and 2 U/mL erythropoietin (EPO) were added. Colonies were enumerated after 14 days of incubation as described previously, and individual colonies were plucked and genotyped for JAK2V617F.
Nested allele-specific PCR for JAK2V617F-positive colonies
Genomic DNA was isolated from randomized plucked colonies using the Extract-N-Amp Blood PCR Kits (Sigma-Aldrich). JAK2V617F was detected by using a nested allele-specific PCR as described previously.34 The final PCR products were analyzed on 2.0% agarose gels. The nested PCR product had a size of 453 bp. A 279-bp product indicated allele-specific JAK2V617F-positive, whereas a 229-bp product denoted allele-specific wild-type product. Colonies were classified as homozygous for JAK2V617F if they contained only the 279-bp band, whereas heterozygous colonies were identified based on the presence of both the 279-bp and 229-bp bands.
Apoptosis assay
Treated cells were collected and washed with PBS for staining with annexin-V (BD Biosciences); the staining procedures were performed according to the protocols provided by the manufacturer. Data were acquired on a FACSCalibur flow cytometer (BD Biosciences), and at least 10 000 live cells were acquired for each analysis (BD FACS Diva software; BD Biosciences).
Western blot analysis
CD34+ cells were purified from the peripheral blood of patients with PV and cultured in serum-free medium contained with SCF, FL-3 ligand, IL-3, and TPO. The cells were treated with a low dose of Peg IFN-α 2a, or a low dose of Nutlin-3 or in combination for 4 hours. Cells were harvested and the whole cells protein extracts were prepared with RIPA lysis buffer (Boston BioProducts) containing protease inhibitors cocktail (Thermo Scientific) for Western blotting.
To prepare the cytoplasmic and nuclear protein fractions of cells from patients with PV, CD34+ were expanded in serum-free media containing SCF, FL-3 ligand, and IL-3 for 10 days. CD34+ cells were then repurified and treated with a low dose of Peg IFN-α 2a, or Nutlin-3 alone or in combination for 48 hours in the presence of SCF, FL-3 ligand, IL-3, and TPO. The protein extracts were prepared using the NE-PER nuclear and cytoplasmic extraction reagent (Thermo Scientific) according to the manufacturer's instructions.
Before Western blotting, all the samples were denatured with Laemmli SDS-sample buffer (Boston BioProducts) by heating at 95°C for 5 minutes; each sample was separated on SDS-PAGE gels and transferred to polyvinyldifluoridine membranes (Bio-Rad). Phospho-p53, p53, MDM2, p21, p-STAT1, PUMA, and Bak were visualized using the antibodies (Cell Signaling Technologies) and ECL Western blotting reagents (Denville Scientific).
Statistical analysis
Results were reported as the mean ± SD of individual data points obtained from the various number of experiments. Statistical significance was determined using Student t tests or paired-samples t test.
Results
PV CD34+ cells contained higher levels of MDM2 protein
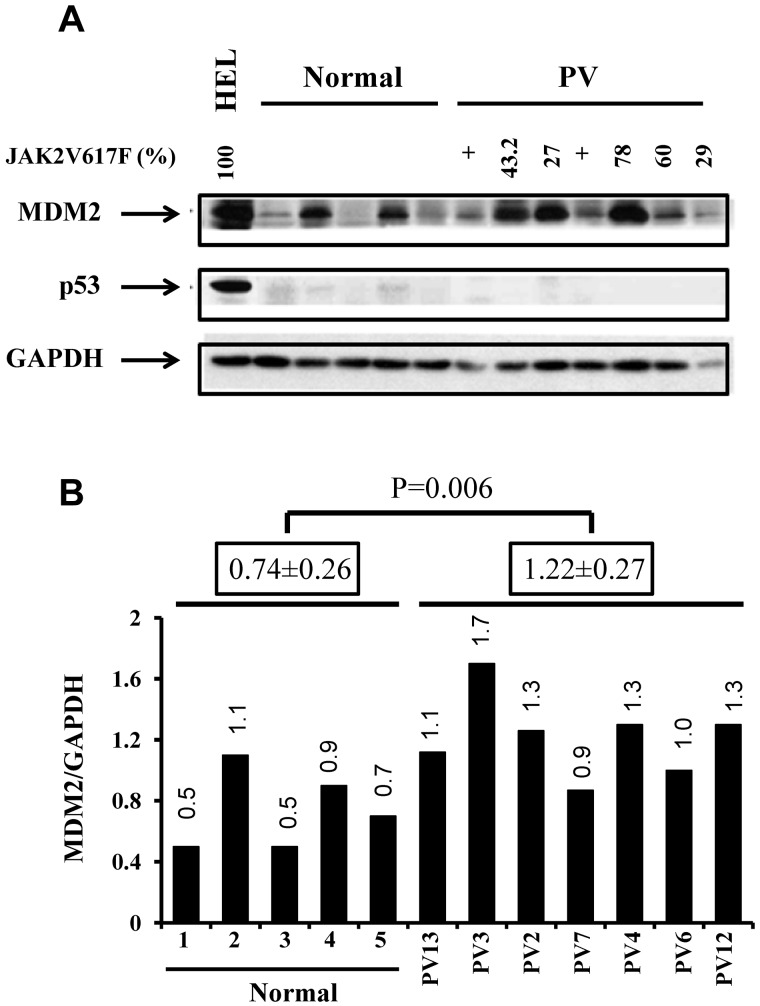
To evaluate the potential therapeutic effects of IFN-α and Nutlin-3 alone or in combination, we first tested the basal level of MDM2 protein in CD34+ cells from 7 PV patients and 5 normal bone marrow samples by Western blot analysis. Although p53 protein level was too low to be calculated in both normal and PV CD34+ cells, we documented by real-time PCR that p53 mRNA levels were much lower in CD34+ cells from PV than that observed in normal CD34+ cells (supplemental Figure 1). The expression of MDM2 protein was significantly higher in PV CD34+ cells compared with normal controls as determined by densitometric quantitation of Western blots (Figure 1). These data are consistent with the report of Nakatake et al.30
Figure 1.
PV CD34+ cells contained higher levels of MDM2 protein. (A) Western blotting demonstrated the increased expression of MDM2 and lower levels of p53 in PV CD34+ cells (7 PVs and 5 normal BMs). (B) The quantification of protein levels was performed densitometrically and normalized to GAPDH levels.
PV CD34+ cells responded to the treatment of Nutlin-3 in a dose-dependent fashion
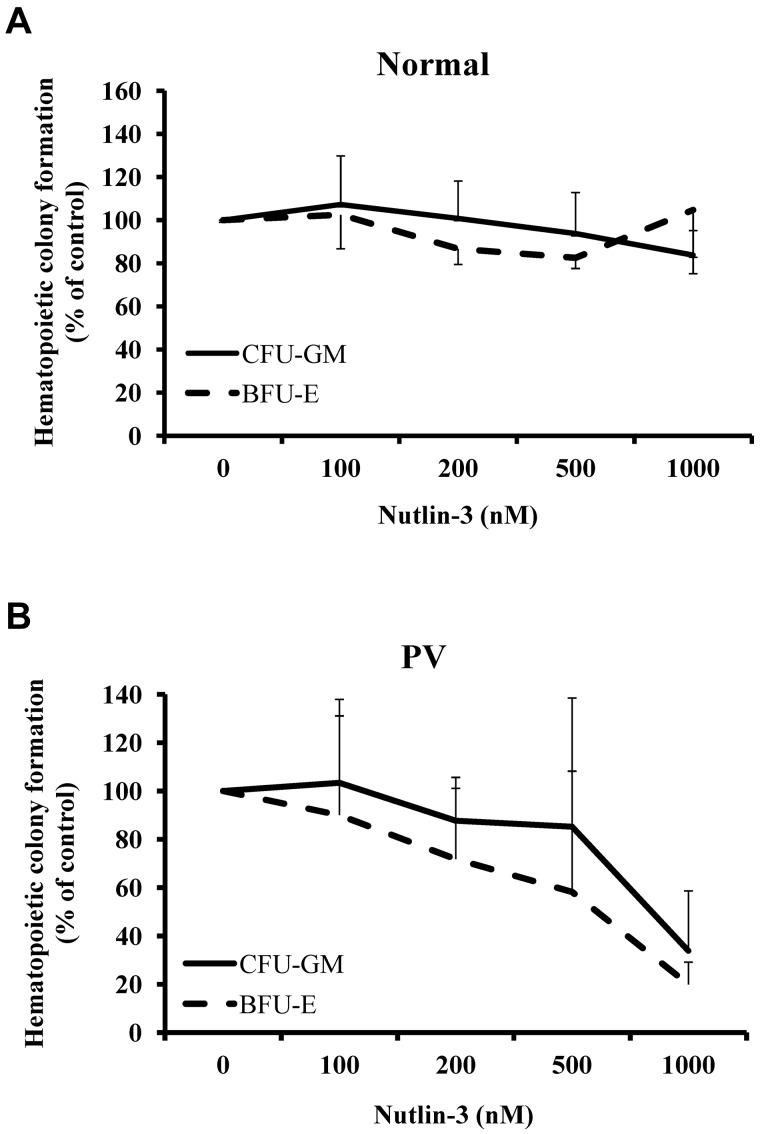
The effect of increasing concentrations of Nutlin-3 on the ability of PV CD34+ cells to generate CFU-GM– and BFU-E–derived colonies was assessed with. CD34+ cells were isolated from 5 patients with PV and cultured in serum-free medium with SCF, Flt-3 ligand, IL-3, and TPO, cells treated with Nutlin-3 at doses from 100nM to 1000nM for 4 days. After treatment, the same numbers of CD34+ cells were assayed for colony formation. Nutlin-3 was capable of suppressing BFU-E– and CFU-GM–derived colony formation by PV CD34+ cells in dose-dependent fashion. The IC50 of Nutlin-3 was 800nM for CFU-GM and 600nM for BFU-E (Figure 2). By contrast, normal CD34+ cells were less responsive to the effects of Nutlin-3. Doses of Nutlin-3 up to 1000nM did not affect colony formation by normal marrow CD34+ cells.
Figure 2.
PV CD34+ cells responded to the treatment of Nutlin-3. Effects of increasing concentrations of Nutlin-3 on CFU-GM– and BFU-E–derived colony formation by normal bone marrow (A) and PV (B) CD34+ cells.
Treatment with a low dose of Peg IFN-α 2a combined with low doses of Nutlin-3 significantly inhibited the proliferation of PV CD34+ cells
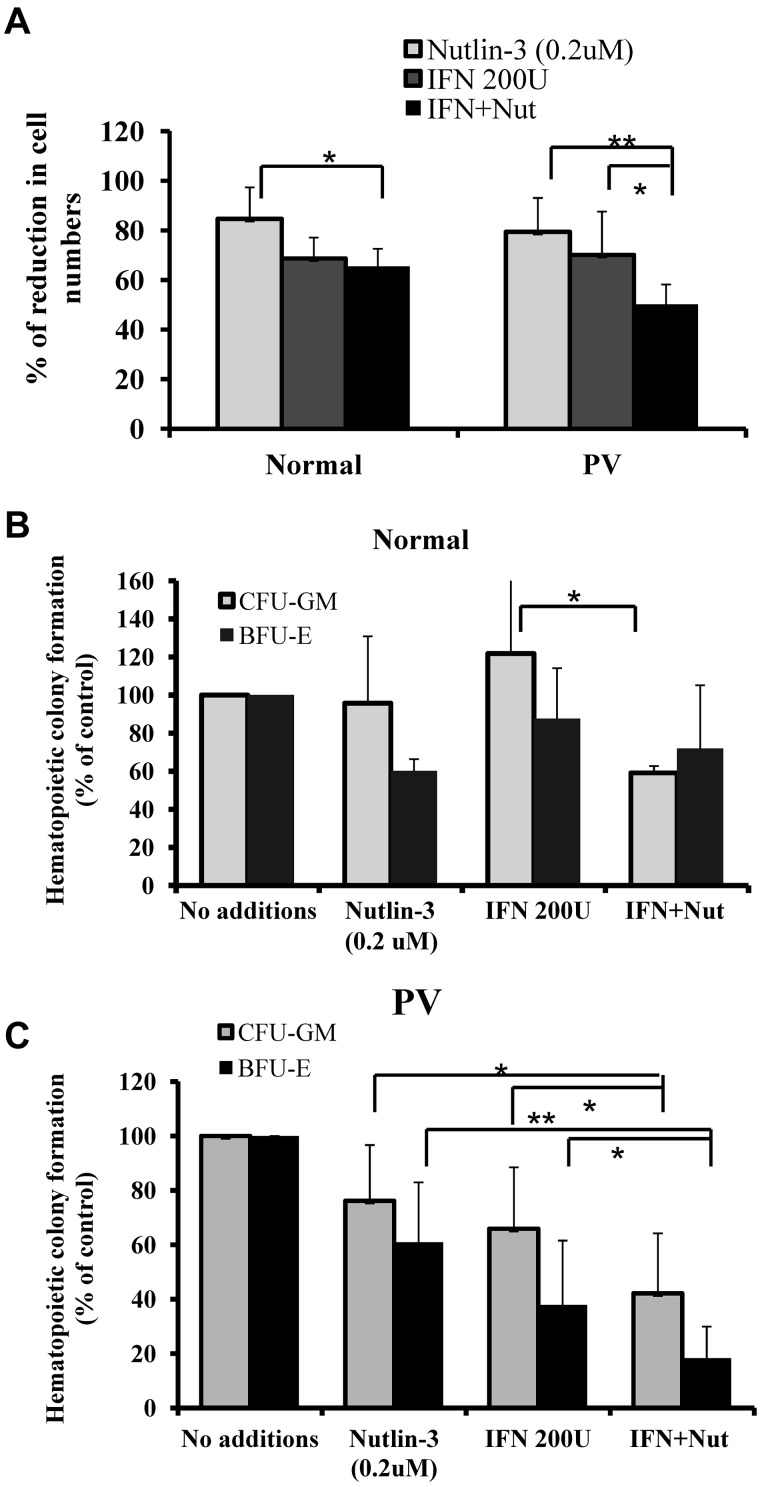
We investigated the antiproliferative effect of low doses of Peg IFN-α 2a and Nutlin-3 on HPCs. The doses chosen for these studies (200 U/mL of Peg IFN-α 2a and 200nM of Nutlin-3) each had suboptimal inhibitory effects on CD34+ cell proliferation based on data presented in Figure 2B and previous study reported from our laboratory.20 Treatment with Peg IFN-α 2a or Nutlin-3 alone or in combination inhibited the PV CD34+ cell numbers of CD34+ cells after 4 days of culture to a greater extent than normal CD34+ cells (Figure 3A). We then investigated the effect of low doses of Peg IFN-α 2a and Nutlin-3 alone or in combination on hematopoietic colony formation by PV and normal CD34+ cells. As shown in Figure 3B-C, treatment with 200nM of Nutlin-3 alone decreased PV CFU-GM– and BFU-E–derived colony formation by 24% and 40%, respectively, whereas treatment with 200 U/mL of Peg IFN-α 2a alone decreased PV CFU-GM– and BFU-E–derived colony formation by 34% and 62%, respectively. Combination treatment with low doses of Peg IFN-α 2a and Nutlin-3, however, resulted in dramatic suppression of PV CFU-GM– and BFU-E–derived colony formation by 62% and 82%, respectively. Meanwhile, treatment with the same doses of Peg IFN-α 2a and Nutlin-3 alone did not affect the ability of normal human marrow CD34+ cells to generate CFU-GM–derived colony formation and exposure to 200nM of Nutlin-3 alone decreased BFU-E–derived colony formation by only 40%. Combination treatment with low doses of Peg IFN-α 2a and Nutlin-3 decreased normal CFU-GM– and BFU-E–derived colony formation by 40% and 30%, respectively, which was dramatically less than the effect observed with PV HPCs. These results suggested that treatment with a low dose of Peg IFN-α 2a combined with a low dose of Nutlin-3 preferentially targets PV CD34+ cells.
Figure 3.
Low doses of Peg IFN-α 2a combined with Nutlin-3 significantly inhibited the proliferation of PV CD34+ cells. (A) After treatment with a low dose of Peg IFN-α 2a combined with Nutlin-3, the percentage of reduction in CD34+ cell numbers from PV and normal BM compared with cytokines alone. *P < .05. **P < .01. (B) Effects of 200 U/mL of Peg IFN-α 2a combined with 200nM of Nutlin-3 on CFU-GM- and BFU-E-derived colony formation by normal BM CD34+ cells. *P < .05. n = 7. (C) Effects of 200 U/mL of Peg IFN-α 2a combined with 200nM of Nutlin-3 on CFU-GM- and BFU-E-derived colony formation by PV CD34+ cells. *P < .05. **P < .01. n = 17.
Low dose of Peg IFN-α 2a combined with low doses of Nutlin-3 reduces the numbers of JAK2V617F-positive HPCs
To further explore the effects of low doses of Peg IFN-α 2a and Nutlin-3 on PV HPC, individual hematopoietic colonies were randomly plucked from cultures receiving treatment with either this combination or cytokines alone, genomic DNA was isolated, and the JAK2V617F allele status was assessed. The combined treatment with Peg IFN-α 2a and Nutlin-3 decreased the total number of JAK2V617F-positive HPCs (P = .001; Table 1) from 6 PV cases with JAK2V617F granulocyte allele burdens ranging from 28% to 75%, leading to an increased number of colonies with wild-type JAK2. As can be seen in Table 1, combined treatment with a low dose of Peg IFN-α 2a and Nutlin-3 for 4 days was most effective in decreasing the numbers of JAK2V617F heterozygous HPCs. These results indicated that short-term exposure to a low dose of Peg IFN-α 2a and Nutlin-3 can preferentially eliminate malignant PV HPCs with a low burden of JAK2V617F.
Table 1.
Effect of combination treatment with Peg-IFNα 2a and Nutlin-3 on the JAK2 genotype of PV hematopoietic colonies
| Patient no. | Granulocyte JAK2 V617F allele burden, % | Additions to culture |
|||||
|---|---|---|---|---|---|---|---|
| None |
Peg IFNα 2a 200 U/mL + Nutlin-3 200nM |
||||||
| Homo, % | Hetero, % | WT, % | Homo, % | Hetero, % | WT, % | ||
| PV 1 | 53 | 29.2 | 33.3 | 37.5 | 42 | 8 | 50 |
| PV 2 | 27 | 0 | 25 | 75 | 0 | 9 | 91 |
| PV 3 | 43.2 | 4.1 | 16.7 | 79.2 | 2.1 | 4.2 | 93.7 |
| PV 4 | 78 | 54.2 | 25 | 20.8 | 30 | 20 | 50 |
| PV 5 | 20 | 38.6 | 29.6 | 31.8 | 40.5 | 14.3 | 45.2 |
| PV 6 | 60 | 0 | 43 | 57 | 0 | 32.4 | 67.6 |
At least 50% of colonies were examined if the number of colonies exceeded 50 in an individual dish. If the number of colonies in an individual dish were < 40, 90% of colonies were plucked and examined.
Homo indicates JAK2V617F homozygous; Hetero, JAK2V617F heterozygous; and WT, contains only wild-type JAK2.
Low dose of Nutlin-3 and low dose of Peg IFN-α 2a in combination promote apoptosis of PV HPCs
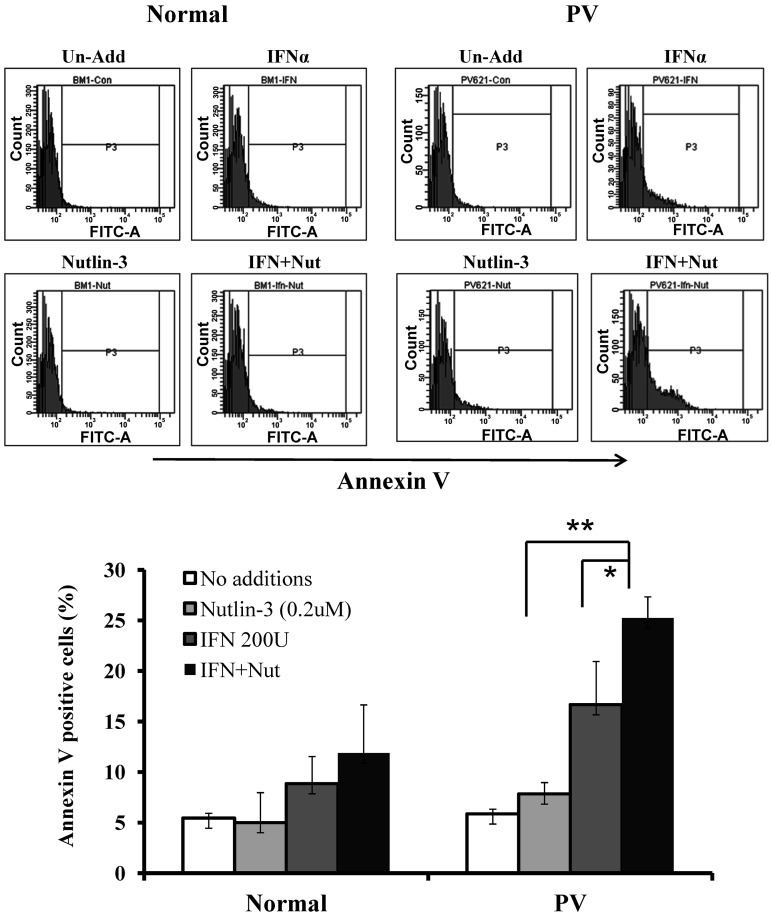
To test whether a low dose of Peg IFN-α 2a and/or Nutlin-3 preferentially induces apoptosis of PV HPCs, CD34+ cells from patients with PV and normal bone marrow were cultured in serum-free medium containing cytokines and treated with 200 U/mL of Peg IFN-α 2a and 200nM of Nutlin-3 alone and in combination In Figure 4, one can see that neither Peg IFN-α 2a nor Nutlin-3 alone or in combination induced apoptosis of normal CD34+ cells, whereas Peg IFN-α 2a alone induced apoptosis of PV CD34+ cells to a limited degree and combination treatment induced apoptosis to a far greater extent.
Figure 4.
Low doses of Nutlin-3 enhance the effects of a low dose of Peg IFN-α 2a on the apoptosis of PV hematopoietic progenitor cells. Flow cytometric analysis of normal and PV CD34+ cells after 4 days of treatment with 200nM of Nutlin-3 alone or 200 U/mL of Peg IFN-α 2a alone or in combination. None of the treatments increased the number of apoptotic cells in cultures of normal CD34+ cells (n = 4). By contrast, Peg IFN-α 2a and Nutlin-3 alone increased to a limited degree apoptosis of PV CD34+ cells, whereas Nutlin-3 combined with Peg IFN-α 2a induced apoptosis in PV CD34+ cells to a far greater extent. *P < .05. **P < .01. n = 4.
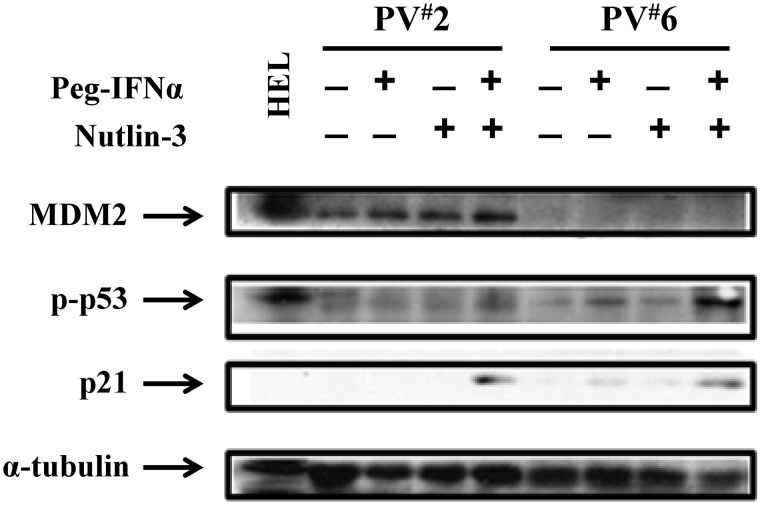
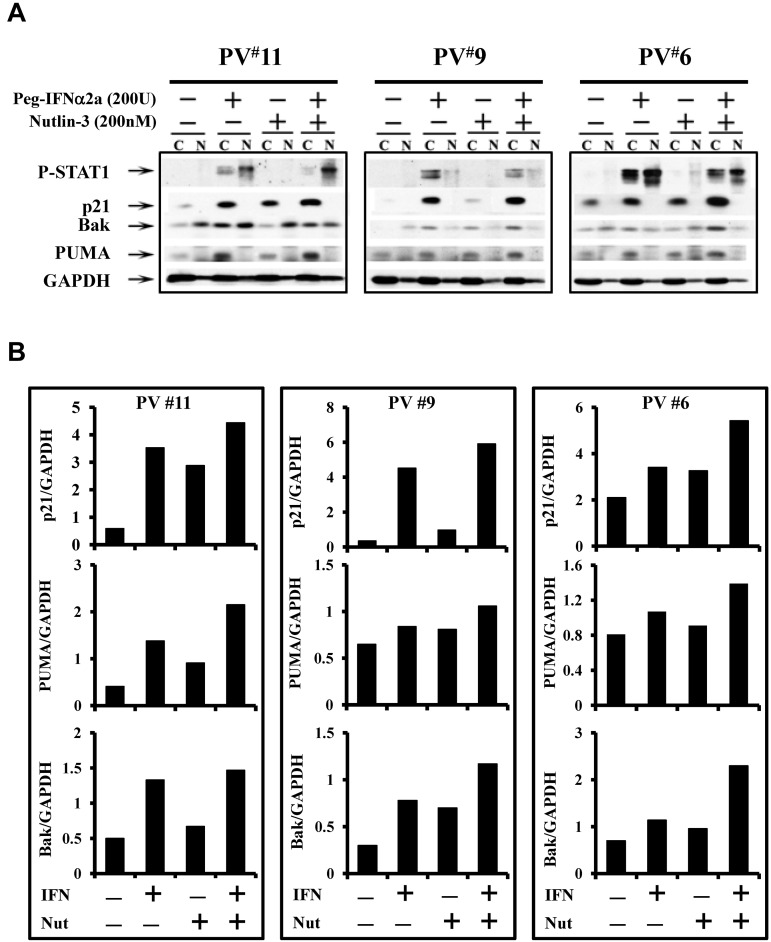
Combination treatment with a low dose of Peg IFN-α 2a and Nutlin-3 increases p53 activity and stabilization through different mechanisms
To further understand the mechanisms by which Peg IFN-α 2a combined with Nutlin-3 affects PV HPCs, phosphor-p53 as well as p53 directed p21 levels were evaluated after treatment of PV CD34+ cells with Peg IFN-α 2a, or Nutlin-3, alone or a combination. Figure 5 shows that combination treatment for 4 hours induced phosphor-p53 in both of the PV cases studied. In 1 PV case, treatment with even the low dose of 200 U/mL of Peg IFN-α 2a alone induced phosphor-p53 to a limited degree. p21 protein levels were also increased in cells treated with the drug combination. Furthermore, CD34+ cells of 3 individual PV patients were treated with a low dose of Peg IFN-α 2a or Nutlin-3 alone or in combination for 48 hours to examine the effects of these drugs on downstream regulators associated with cell cycle arrest and apoptosis. In the presence of cytokines alone, the levels of p21, PUMA, and Bak were limited (Figure 6). Treatment with a low dose of Peg IFN-α 2a, but not Nutlin-3, activated STAT1, as well as increased the nuclear translocation of phosphor-STAT1 in CD34+ cells. In addition, both drugs alone increased p21, PUMA, and Bak protein levels in the cytosol of PV CD34+ cells after 48 hours of treatment, and a combination of these agents increased p21, PUMA, and Bak protein levels in cytosol to an even greater degree. Several studies have suggested that STAT1 is a key molecular mediator of cell death and apoptosis by interacting with p53.35–37 IFN-α also is known to induce apoptosis of PV CD34+ cells by activation of the p38 MAPK pathway. Nutlin-3 was incapable of increasing phosphor-STAT1 levels; it specifically blocked the MDM2-p53 interaction, leading to p53 stabilization and accumulation. These data indicate that combination treatment with low doses of Peg IFN-α 2a and Nutlin-3 inhibit the proliferation and induce apoptosis of JAK2V617F cells by affecting the p53 pathway through distinct different pathways, including JAK/STAT1, p38MAPK, and the blocking of the interaction between MDM2 and p53.
Figure 5.
Low doses of Peg IFN-α 2a and Nutlin-3 increase p53 activity. Western blotting demonstrated that the phospho-p53 level in PV CD34+ cells from 2 individual patients (PV1 and PV2) was increased after treatment with low doses of Nutlin-3 combined with Peg IFN-α 2a. The p21 protein level was also increased by combination drug therapy.
Figure 6.
Low doses of Peg IFN-α 2a and Nutlin-3 activate p53 pathway through different mechanisms. (A) Peg IFN-α 2a, but not Nutlin-3, increased p-STAT1 in CD34+ cells. Treatment with Nutlin-3 or Peg IFN-α 2a alone for 48 hours led to increased cytoplasmic p21, PUMA, and Bak protein levels; treatment with the 2 drugs in combination led to an even greater increase in p21, PUMA and Bak levels. (B) The quantification of cytoplasmic levels of p21, PUMA, and Bak was determined densitometrically and normalized to GAPDH.
Discussion
The Philadelphia chromosome-negative MPN are hematologic malignancies originating at the level of the HSCs and/or HPCs. JAK2V617F has been found in > 90% of patients with PV. JAK2 has served as a target for drug development, leading to the development of several novel small-molecule inhibitors directed against this intracellular kinase. Treatment with these JAK2 inhibitors has resulted in a reduction of the degree of splenomegaly and improvement of systemic symptoms but did not lead to correction of the underlying hematologic parameters, marrow histopathologic abnormalities, or reduction of the JAK2V617F allele burden.5–13 By contrast, the recent clinical experience with IFN-α and Peg IFN-α 2a in patients with high-risk PV and ET indicates that IFN-α provides a potential means of eliminating malignant PV hematopoietic cells.14–17 Treatment with IFN-α results in reduction of JAK2V617F allele burden, elimination of cytogenetic abnormalities, and reestablishment of polyclonal hematopoiesis.18 Unfortunately, this therapy is interrupted, not infrequently, by adverse events that prevent persons for prolonged periods during which the drug can be administered without compromising their quality of life. Because many of the adverse consequences of IFN-α are dose related, the use of lower doses of this drug, if equally effective, might make such a long-term treatment strategy possible. Furthermore, the successful treatment of most hematologic malignancies requires the use of combinations of drugs affecting different targets that are associated with nonoverlapping toxicities, rather than the use of a single drug. The effect of additional drugs, such as hydroxyurea, which is currently used to treat PV, may also possibly be enhanced by the addition of nutlin.
In the present study, we showed that treatment with a low dose of Peg IFN-α 2a combined with Nutlin-3, a specific small-molecule antagonist of MDM2, significantly inhibited the proliferation by PV CD34+ cells as well as the ability of PV HPCs to form hematopoietic colonies in vitro to a greater extent than that observed with normal CD34+ cells. More importantly, combination treatment decreased the numbers of JAK2V617F-positive hematopoietic colonies by PV CD34+ cells. In 1 patient with JAK2V617F-negative primary myelofibrosis, but with a deletion of the long arm of chromosomal 20, treatment with a low dose of IFN-α combined with Nutlin-3 was observed to reduce the number of the chromosomally abnormal cells from 12% to 1% using FISH analysis (supplemental Figure 2). These results indicate that the combined drug treatment can preferentially eliminate the malignant MPN HPCs. A brief exposure (4 days) to combination therapy also decreased the number of JAK2V617F heterozygous HPCs resulting in increased number of assayable HPCs with wild-type JAK2. We previously reported that in vitro treatment with higher doses of Peg IFN-α 2a for 14 days, however, decreased the numbers of both JAK2V617F homozygous and heterozygous HPCs.20 More prolonged exposure to combination therapy or higher doses of IFN-α might be needed to eliminate JAK2V617F homozygous HPCs.
IFN-α is a pleiotropic biologic response modifier; it binds to the cell surface receptor, the type I IFN receptor, activates JAK/TYK/STATs pathway, leading to the activation of multiple signal pathways to eliminate malignant cells, including suppressing proliferation, inducing apoptosis of HPC, and promoting the cycling of HSC. In our previous study, we found that IFN-α induced PV HPC apoptosis by activating the p38 MAP kinase pathway, whereas others have reported that p38 MAP kinase pathway can activate p53.20–23 IFN-α also can activate STAT1 through its action on the JAK-STAT pathway.36,38 Our results indicate that treatment with Peg IFN-α 2a not only significantly increased phosphor-STAT1 level but also increased its nuclear translocation. Several studies have suggested that STAT1 is a regulator of cell death.36–41 Townsend et al demonstrated that STAT1 is required for DNA damage-induced apoptosis through its direct interaction with p53.39 They showed that STAT1 is a negative transcriptional regulator of Mdm2 and might play an important role in mediating apoptosis resulting from IFN-α. On the other hand, our data showed that Nutlin-3 does not induce phosphor-STAT1 but still increases p53 levels; these data indicate that IFN-α and Nutlin-3 result in p53 accumulation by affecting different pathways. In chronic MPN, wild-type p53 is present almost exclusively; p53 mutations have, however, been identified exclusively in MPN patients undergoing transformation to acute leukemia. Nakatake et al reported that, in MPN, expression of JAK2V617F affects the p53 response to DNA damage30 and that JAK2V617F negatively regulates p53 stabilization by enhancing MDM2 levels. The role of inactivation of wild-type p53 resulting from up-regulation of MDM2 (and/or MDM4) has recently been explored.28–31,42,43 Our data confirmed that MDM2 protein levels are higher to various degrees in PV CD34+ cells than that in normal CD34+ cells. Additional studies will be required to determine whether MDM2 levels can be used to predict in vitro or in vivo responses to IFN-α and nutlin treatment.
In conclusion, our data indicate that IFN-α plus the MDM2 antagonist Nutlin-3 specifically target malignant MPN HPCs by acting through different pathways resulting ultimately in p53 accumulation in vitro, which we propose can be exploited therapeutically to eliminate JAK2V617F-positive HPCs in PV patients. These results strongly suggest that combinations of low doses of IFN-α and Nutlin-3 might serve as a novel therapeutic strategy for the long-term treatment of PV patients.
Acknowledgments
This study was supported by the National Cancer Institute (grant 1P01CA108671, R.H.).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.L. designed and performed the research, collected and analyzed data, and wrote the manuscript; X.W., Y.L., J.T., G.M., and J.M. assisted in the performance of the experiments; M.K. assisted in the compilation of the patient characteristics and the creation of the manuscript; V.N. performed the cytogenetic analyses and assisted in the creation of the manuscript; and R.H. designed the research, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Hoffman, Division of Hematology/Oncology, Tisch Cancer Institute, Department of Medicine, Mount Sinai School of Medicine, One Gustave L. Levy Pl, Box 1079, New York, NY 10029; e-mail: ronald.hoffman@mssm.edu.
References
- 1.Hoffman R, Xu M, Finazzi G, Barbui T. The polycythemias. In: Hoffman R, Benz EJ Jr, Shattil SJ, et al., editors. Hematology: Basic Principles and Practice. Philadelphia, PA: Churchill Livingstone; 2008. pp. 1073–1108. [Google Scholar]
- 2.Kralovics R, Skoda RC. Molecular pathogenesis of Philadelphia chromosome negative myeloproliferative disorders. Blood Rev. 2005;19(1):1–13. doi: 10.1016/j.blre.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Mesa RA. Clinical and scientific advances in the Philadelphia-chromosome negative chronic myeloproliferative disorders. Int J Hematol. 2002;76(Suppl 2):193–203. doi: 10.1007/BF03165117. [DOI] [PubMed] [Google Scholar]
- 4.Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100(13):4272–4290. doi: 10.1182/blood-2001-12-0349. [DOI] [PubMed] [Google Scholar]
- 5.Lucet IS, Fantino E, Styles M, et al. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood. 2006;107(1):176–183. doi: 10.1182/blood-2005-06-2413. [DOI] [PubMed] [Google Scholar]
- 6.Williams NK, Bamert RS, Patel O, et al. Dissecting specificity in the Janus kinases: the structures of JAK-specific inhibitors complexed to the JAK1 and JAK2 protein tyrosine kinase domains. J Mol Biol. 2009;387(1):219–232. doi: 10.1016/j.jmb.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Hedvat M, Huszar D, Herrmann A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16(6):487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009;23(8):1441–1445. doi: 10.1038/leu.2009.50. [DOI] [PubMed] [Google Scholar]
- 9.Tyner JW, Bumm TG, Deininger J, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010;115(25):5232–5240. doi: 10.1182/blood-2009-05-223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor in myelofibrosis. N. Engl J Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardanani A, Gotlib JR, Jamieson C, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29(7):789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tefferi A, Pardanani A. JAK inhibitors in myeloproliferative neoplasms: rationale, current data and perspective. Blood Rev. 2011;25(5):229–237. doi: 10.1016/j.blre.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Atallah E, Verstovsek S. Prospect of JAK2 inhibitor therapy in myeloproliferative neoplasms [review]. Expert Rev Anticancer Ther. 2009;9(5):663–670. doi: 10.1586/era.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messora C, Bensi L, Vecchi A, et al. Cytogenetic conversion in a case of polycythaemia vera treated with interferon-alpha. Br J Haematol. 1994;86(2):402–404. doi: 10.1111/j.1365-2141.1994.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 15.Kiladjian JJ, Cassinat B, Turlure P, et al. High molecular response rate of polycythemia vera patients treated with pegylated interferon alpha-2a. Blood. 2006;108(6):2037–2040. doi: 10.1182/blood-2006-03-009860. [DOI] [PubMed] [Google Scholar]
- 16.Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–3072. doi: 10.1182/blood-2008-03-143537. [DOI] [PubMed] [Google Scholar]
- 17.Kiladjian J-J, Chomienne C, Fenaux P. Interferon-alpha therapy in bcr-abl-negative myeloproliferative neoplasms. Leukemia. 2008;22(11):1990–1998. doi: 10.1038/leu.2008.280. [DOI] [PubMed] [Google Scholar]
- 18.Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107(3):451–458. doi: 10.1002/cncr.22026. [DOI] [PubMed] [Google Scholar]
- 19.de Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Chem. 2007;282(28):20053–20057. doi: 10.1074/jbc.R700006200. [DOI] [PubMed] [Google Scholar]
- 20.Lu M, Zhang W, Berenzon D, et al. Interferon-alpha targets JAK2V617F-positive hematopoietic progenitor cells and acts through the p38 MAPK pathway. Exp Hematol. 2010;38(6):472–480. doi: 10.1016/j.exphem.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Amicis F, Giordano F, Vivacqua A, et al. Resveratrol, through NF-Y/p53/Sin3/HDAC1 complex phosphorylation, inhibits estrogen receptor alpha gene expression via p38MAPK/CK2 signaling in human breast cancer cells. FASEB J. 2011;25(10):3695–3707. doi: 10.1096/fj.10-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamy V, Bousserouel S, Gossé F, Minker C, Lobstein A, Raul F. Lupulone triggers p38 MAPK-controlled activation of p53 and of the TRAIL receptor apoptotic pathway in human colon cancer-derived metastatic cells. Oncol Rep. 2011;26(1):109–114. doi: 10.3892/or.2011.1273. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Matsumori H, Nakayama Y, et al. SIRT2 down-regulation in HeLa can induce p53 accumulation via p38 MAPK activation-dependent p300 decrease, eventually leading to apoptosis. Genes Cells. 2011;16(1):34–45. doi: 10.1111/j.1365-2443.2010.01460.x. [DOI] [PubMed] [Google Scholar]
- 24.Feinstein E, Cimino G, Gale RP, et al. p53 in chronic myelogenous leukemia in acute phase. Proc Natl Acad Sci U S A. 1991;88(14):6293–6297. doi: 10.1073/pnas.88.14.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez-Boussard T, Rodriguez-Tome P, Montesano R. IARC p53 mutation database: a relational database to compile and analyze p53 mutations in human tumors and cell lines. International Agency for Research on Cancer. Hum Mutat. 1999;14(1):1–8. doi: 10.1002/(SICI)1098-1004(1999)14:1<1::AID-HUMU1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.Tsurumi S, Nakamura Y, Maki K, et al. N-ras and p53 gene mutations in Japanese patients with myeloproliferative disorders. Am J Hematol. 2002;71(2):131–133. doi: 10.1002/ajh.10188. [DOI] [PubMed] [Google Scholar]
- 27.Harutyunyan A, Klampfl T, Cazzola M, Kralovics R. p53 lesions in leukemic transformation. N Engl J Med. 2011;364(5):488–490. doi: 10.1056/NEJMc1012718. [DOI] [PubMed] [Google Scholar]
- 28.Vassilev LT. MDM2 inhibitors for cancer therapy. Trends Mol Med. 2007;13(1):23–31. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 30.Nakatake M, Monte-Mor B, Debili N, et al. JAK2(V617F) negatively regulates p53 stabilization by enhancing MDM2 via La expression in myeloproliferative neoplasms. Oncogene. 2012;31(10):1323–1333. doi: 10.1038/onc.2011.313. [DOI] [PubMed] [Google Scholar]
- 31.Andreeff M, Kojima K, Padmanabhan S, et al. A multi-center, open-label, phase I study of single agent RG7112, a first in class p53-MDM2 antagonist, in patients with relapsed/refractory acute myeloid and lymphoid leukemias (AML/ALL) and refractory chronic lymphocytic leukemia/small cell lymphocytic lymphomas (CLL/SCLL). Blood. 2010;116:657. [Google Scholar]
- 32.Shadduck RK, Gilmore GL, Lister J. Role of serum-free medium in the ex vivo expansion of human cord blood hematopoietic stem cells. Stem Cells. 2000;18(2):154–155. doi: 10.1634/stemcells.18-2-154. [DOI] [PubMed] [Google Scholar]
- 33.Ivanovic Z, Duchez P, Chevaleyre J. Clinical-scale cultures of cord blood CD34(+) cells to amplify committed progenitors and maintain stem cell activity. Cell Transplant. 2011;20(9):1453–1463. doi: 10.3727/096368910X552853. [DOI] [PubMed] [Google Scholar]
- 34.Ishii T, Bruno E, Hoffman R, Xu M. Involvement of various hematopoietic-cell lineages by the JAK2V617F mutation in polycythemia vera. Blood. 2006;108(9):3128–3134. doi: 10.1182/blood-2006-04-017392. [DOI] [PubMed] [Google Scholar]
- 35.Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21WAF1/CIP1 mediated by STAT1. Science. 1996;272(5262):719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 36.Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE., Jr Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci U S A. 1996;93(15):7673–7678. doi: 10.1073/pnas.93.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephanou A, Latchman DS. STAT-1: a novel regulator of apoptosis. Int J Exp Pathol. 2003;84(6):239–244. doi: 10.1111/j.0959-9673.2003.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arulampalam V, Kolosenko I, Hjortsberg L, Björklund AC, Grandér D, Tamm KP. Activation of STAT1 is required for interferon-alpha-mediated cell death. Exp Cell Res. 2011;317(1):9–19. doi: 10.1016/j.yexcr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Townsend PA, Scarabelli TM, Davidson SM, et al. STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. J Biol Chem. 2004;279(7):5811–5820. doi: 10.1074/jbc.M302637200. [DOI] [PubMed] [Google Scholar]
- 40.Kim HS, Lee MS. STAT1 as a key modulator of cell death. Cell Signal. 2007;19(3):454–465. doi: 10.1016/j.cellsig.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Youlyouz-Marfak I, Gachard N, Le Clorennec C, et al. Identification of a novel p53-dependent activation pathway of STAT1 by antitumour genotoxic agents. Cell Death Differ. 2008;15(2):376–385. doi: 10.1038/sj.cdd.4402270. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Ma X, Ren S, Buolamwini JK, Yan C. A small-molecule inhibitor of MDMX activates p53 and induces apoptosis. Mol Cancer Ther. 2011;10(1):69–79. doi: 10.1158/1535-7163.MCT-10-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marine JC. MDM2 and MDMX in cancer and development. Curr Top Dev Biol. 2011;94:45–75. doi: 10.1016/B978-0-12-380916-2.00003-6. [DOI] [PubMed] [Google Scholar]