Abstract
Maintenance of the corneal epithelium is essential for vision and is a dynamic process incorporating constant cell production, movement and loss. Although cell based therapies involving the transplantation of putative stem cells are well advanced for the treatment of human corneal defects, the scientific understanding of these interventions is poor. No definitive marker that discriminates stem cells that maintain the corneal epithelium from the surrounding tissue has been discovered and the identity of these elusive cells is, therefore, hotly debated. The key elements of corneal epithelial maintenance have long been recognised but it is still not known how this dynamic balance is coordinated during normal homeostasis to ensure the corneal epithelium is maintained at a uniform thickness. Most indirect experimental evidence supports the limbal epithelial stem cell (LESC) hypothesis, which proposes that the adult corneal epithelium is maintained by stem cells located in the limbus at the corneal periphery. However, this has been challenged recently by the corneal epithelial stem cell (CESC) hypothesis, which proposes that during normal homeostasis the mouse corneal epithelium is maintained by stem cells located throughout the basal corneal epithelium with LESCs only contributing during wound healing. In this chapter we review experimental studies, mostly based on animal work, that provide insights into how stem cells maintain the normal corneal epithelium and consider the merits of the alternative LESC and CESC hypotheses. Finally, we highlight some recent research on other stem cell systems and consider how this could influence future research directions for identifying the stem cells that maintain the corneal epithelium.
19.1 Introduction
19.1.1 Introduction to the cornea
The transparent adult cornea has rightly been called our window on the world. Its unique properties allow it to maintain transparency, refract light and form a protective, impermeable barrier. The cornea comprises an outer squamous, non-keratinised epithelium of keratinocytes, which is about 5- 6 cells thick, a thick stroma of flattened keratocytes embedded in collagen and the corneal endothelium, comprising a single inner cell layer (Fig 19.1). In addition, an acellular, collagenous basement membrane (Descemet’s membrane) separates the corneal stroma and endothelium, and in humans and other primates there is also a distinct acellular Bowman’s layer (anterior limiting lamina) between the stroma and corneal epithelium. This is rudimentary and indistinct in mice but visible by electron microscopy (Haustein 1983). The cornea is avascular and absorbs oxygen and nutrients from the tear film and aqueous humour but it is innervated and the nerves provide additional trophic support. Mouse corneal anatomy is described in detail in Smith et al. (2002).
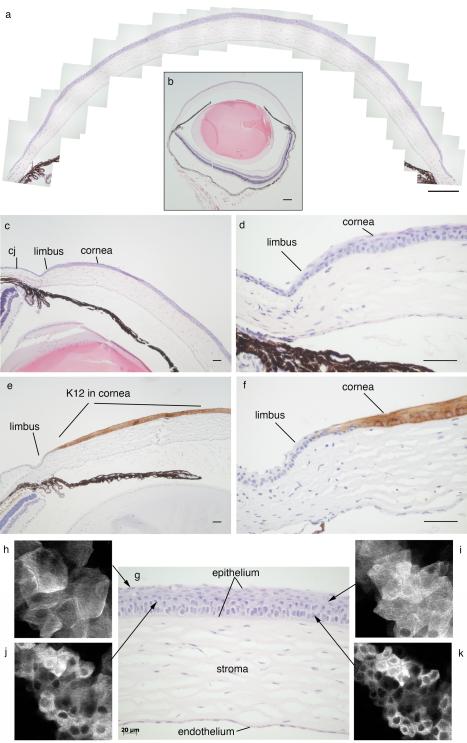
Fig. 19.1. Mouse cornea and limbus.
(a) Montage of mouse cornea. (b) Whole eye used to produce montage shown in (a). (c) Peripheral cornea, limbus and part of the conjunctiva. (d) Higher magnification of the limbal region shown in (c). (e) Peripheral cornea, limbus and part of the conjunctiva immunostained for keratin 12 (K12; dark staining) to show the border between the corneal epithelium (K12 positive) and limbal epithelium (K12 negative). (f) Higher magnification of dark K12 immunostaining shown in (e) to demonstrate border between corneal and limbal epithelia. (g) Central cornea showing corneal epithelium, stroma and endothelium. (h-k) Confocal images of corneal epithelium from tau-GFP transgenic mouse (TgTP6.3; Pratt et al. 2000) showing optical sections of different epithelial layers, as indicated in (g), from flattened squamous cells in the superficial layer at the corneal surface (h) to intermediate “wing cells” in the suprabasal layers and compact basal epithelial cells (k). Scale bars: (a, b) 200μm; (c-f), 50μm; (g), 20μm. Abbreviation in (c): cj, conjunctiva.
The corneal epithelium develops from the head surface ectoderm and both the stromal keratocytes and corneal endothelium are produced by mesenchyme (Haustein 1983), which in mice is derived predominantly from neural crest cells with an additional contribution from cranial mesoderm (Gage et al. 2005). During development, nerves grow into the stroma from the limbus and form a nerve plexus beneath the epithelium which projects fine nerves through the epithelium to the ocular surface (McKenna and Lwigale 2011).
The corneal epithelium has more cell layers than the neighbouring conjunctival epithelium, which is distinguished by the presence of goblet cells and blood vessels, both of which are incompatible with transparency and absent from the corneal epithelium (Smith et al. 2002). Mitosis is restricted to the basal layer in both the corneal and conjunctival epithelia. The basal corneal epithelial cells are cuboidal while the suprabasal cells are progressively more flattened towards the anterior. These comprise 2-3 layers of polyhedral ‘wing cells’ and 1-3 layers of superficial squamous cells with flattened nuclei (Fig. 19.1), which are held together by tight junctions to form an effective barrier. Corneal epithelial cells are continuously being shed (desquamated) from the superficial layer and replenished, yet the tissue maintains a uniform structure and thickness, so transparency is not compromised.
In the adult, neither the corneal stromal nor endothelial cells divide unless injured; endothelial cells are arrested in G1 and show contact inhibition (Joyce 2003) whereas stromal keratocytes exit the cell cycle around the time the eyes open in mice, at postnatal days (P) 12-14, and remain quiescent in G0 (Zieske 2004; Zieske et al. 2004). The corneal endothelium consists of a single layer of cells that is critical for maintaining correct hydration of the corneal stroma via metabolic pumps that actively transport fluid out of the stroma and into the anterior chamber. The corneal stroma is less hydrated than the neighbouring sclera and if the cornea becomes too hydrated it swells and becomes opaque.
Laterally, the corneal stroma merges with the sclera and forms a region known as the limbus at the corneoscleral junction. The limbus is less pronounced in mouse than humans but it forms a morphological ‘dent’ in the mouse ocular surface that is not always apparent in histological sections. The epithelial layer of the limbus forms a transition zone between the corneal epithelium and the conjunctival epithelium. The mouse corneo-limbal epithelial boundary can be identified by immunostaining for keratin 15 (K15) or K19, which are both present in the conjunctiva and limbus but not the cornea, or immunostaining for K12, which is specific for the corneal epithelium (Fig. 19.1e,f). The corneal epithelium is thinner at the periphery and, unlike the human, the mouse limbal epithelium is also thin. According to the conventional limbal epithelial stem cell (LESC) hypothesis (Schermer et al. 1986; Cotsarelis et al. 1989), some basal limbal epithelial cells are stem cells that maintain the corneal epithelium (see section 19.3). There is some evidence that there may also be separate populations of stem cells for the stromal keratocytes (Du et al. 2005; Funderburgh et al. 2005) and endothelium (McGowan et al. 2007) that reside in or close to the limbal region (but below the epithelial layer).
19.1.2 Scope of this review
The focus of this review is how adult stem cells maintain a healthy cornea. Maintenance involves a balance of cell production, movement and loss, orchestrated by stem cells. The conventional LESC hypothesis has recently been challenged. The alternative view proposes that stem cells scattered throughout the mouse corneal epithelium are responsible for homeostatic maintenance of the tissue and that LESCs are only active during wound healing (Majo et al. 2008). Although human limbal epithelium is used successfully as a source of stem cells for treating human corneal epithelial conditions that are thought to involve a stem cell deficiency (Rama et al. 2010), this does not prove that limbal epithelial cells maintain the cornea during normal homeostasis. Such controversies highlight the difficulty in defining a stem cell niche, however this is a matter crucial to understanding the basis of corneal stem cell biology. The availability of genetic and transgenic resources makes the mouse the experimental animal of choice for many investigations of the cornea. Mouse models of human corneal abnormalities provide insights into many aspects of normal and abnormal corneal epithelial maintenance. The aim of this review, therefore, is to discuss our present understanding of the basic cell biology of stem cells that maintain the corneal epithelium, to focus on the mouse to examine its contribution and to discuss its relevance as an effective model for human corneal epithelial maintenance.
19.2 Maintenance of stratified squamous epithelia by adult stem cells
Adult stratified squamous epithelia are composed of layers of flattened epithelial cells lying on top of a basement membrane and are often found in tissues that are subjected to constant abrasive forces such as the skin, the oral mucosa and the corneal epithelium. These tissues are highly dynamic; cells are constantly lost from the tissue surface and therefore must be replaced in order to maintain a constant cellular mass. Their inherent self-renewing capacity is driven by an adult stem cell (SC) population that resides in the basal layer of the epithelium. SCs divide to produce transient (or transit) amplifying cells (TACs) that proliferate in the basal layer before differentiating, moving through the suprabasal layers to the outer stratified layers and finally being shed from the tissue surface (Kruse 1994; Ren and Wilson 1996). Although, overall, net asymmetric division is required to maintain the SC pool and produce TACs, in some systems SCs need not always divide asymmetrically but may also divide symmetrically to produce two TACs or two SCs, providing a stochastic mechanism whereby some SC lineages expand and others are lost (Nakagawa et al. 2007; Snippert et al. 2010; Lopez-Garcia et al. 2010; Klein and Simons 2011). The adult stem cells that maintain these epithelia divide relatively infrequently, however they have the potential to divide indefinitely (Braun and Watt 2004). Identification of the stem cells has, therefore, exploited these features using incorporation of tritiated thymidine (3H-TdR) or the thymidine analogue bromodeoxyuridine (BrdU) into the DNA of dividing cells during S-phase of the cell cycle (Potten and Loeffler 1990). Although the stem cells divide infrequently they can be labelled by prolonged exposure and the label is retained over time but lost from the more rapidly dividing daughter cells. However, these traditional label-retaining methods may preferentially identify a subset of functional stem cells. For example, SCs may alternate between active and quiescent periods or separate populations of active and quiescent SC populations may exist (see section 19.8). The more quiescent SCs, though less likely to incorporate label during the period of exposure, are more likely to retain that label during the chase period (Li and Clevers 2010). The use of this approach to identify stem cells that maintain the corneal epithelium is discussed in section 19.3.2. In addition to cell production by stem cells and proliferation of TACs, the mechanism of corneal epithelial maintenance involves centripetal migration of cells from the periphery (as discussed in section 19.4.2).
Label-retaining stem cells in the mouse epidermis have also been identified by an elegant transgenic system whereby chromatin is labelled with GFP when a transgene producing a histone-2B (H2B)-GFP fusion protein is expressed early in development and then switched off for the chase period via a Tet-Off switch, activated by continuous doxycycline treatment (Tumbar et al. 2004). This GFP label-retaining cell approach is more powerful and easier to interpret than the conventional BrdU label-retaining cell method because (i) all the potential stem cells can be labelled with H2B-GFP before the chase period, (ii) live H2B-GFP-positive cells can be FACS-sorted and used for transcriptional profiling, (iii) H2B-GFP labelling can be restricted to specific tissues, so simplifying analysis of label-retaining cells. It is likely that this and similar transgenic approaches will be used to identify putative stem cells as GFP label-retaining cells in other tissues, including the ocular surface.
19.3 Stem cells that maintain the corneal epithelium
19.3.1 The limbal epithelial stem cell hypothesis
The burden of evidence (discussed in section 19.3.2) suggests that during adult corneal epithelial homeostasis cell production depends on a population of adult stem cells at the periphery of the cornea. These are known as limbal epithelial stem cells (LESCs) because they are thought to reside in the basal layer of the limbal epithelium (limbus) at the corneoscleral junction (Schermer et al. 1986; Cotsarelis et al. 1989; Lehrer et al. 1998; Li et al. 2007); Fig. 19.1. LESCs cycle slowly unless stimulated to proliferate by corneal insult. Daughter TACs move into the corneal epithelium, where they undergo several further rounds of division to maintain the epithelium, move centripetally and leave the basal layer of the epithelium at variable times as pairs of cells (Beebe and Masters 1996). TAC populations near the periphery have a greater replicative potential than their more centrally located counterparts (Lehrer et al. 1998). Once in the suprabasal layers, the cells differentiate, become post-mitotic, move vertically to the superficial layer and are subsequently lost by desquamation at the corneal surface. In order to maintain a uniform corneal epithelial thickness, some TACs must leave the basal layer at the periphery of the cornea and others must move on towards the centre, but it is not known how this process is regulated.
19.3.2 Indirect evidence that stem cells in the limbus maintain the corneal epithelium
The hypothesis that the corneal epithelium is maintained by SCs in the limbus was proposed by Schermer et al. (1986), based on the conclusion that the basal limbal epithelium was less differentiated than the suprabasal limbal epithelium and both basal and suprabasal corneal epithelia in the rabbit. More recent studies also indicate that basal limbal epithelial cells are morphologically distinct from basal corneal epithelial cells, being smaller and euchromatin-rich with a high nucleus to cytoplasm ratio (Romano et al. 2003; Chen et al. 2004). These are properties that are believed to be typical of stem cells in a variety of tissues (Barrandon and Green 1985; Tani et al. 2000; Gaspar-Maia et al. 2009). Evidence that cells move centripetally from the limbal region was also emerging at the time that the LESC hypothesis was proposed (Kinoshita et al. 1981; Buck 1985) and this was confirmed later (section 19.4.2).
Subsequent label retaining experiments in the mouse identified a population of putative stem cells in the basal layer of the limbal epithelium (Cotsarelis et al. 1989; Lehrer et al. 1998). The early label retaining studies relied on wounding of the central cornea in order to stimulate LESCs to divide during an initial pulse of tritiated thymidine (3H-TdR) label. Such methods may not recapitulate the unwounded homeostatic mechanisms accurately (Cotsarelis et al. 1989). However, the results were confirmed using BrdU perfusion by osmotic minipump, which avoided the need for wounding because of the much-increased length of the pulse period (Lehrer et al. 1998).
SCs may divide infrequently in vivo but their proliferative potential can be unmasked by culturing them in vitro. Barrandon and Green classified human epidermal cultures as holoclones, paraclones or meroclones, depending on their proliferation characteristics and the morphology of the colonies produced (Barrandon and Green 1987). Holoclones have the greatest proliferative potential and are thought to be produced from SCs. Paraclones form abortive colonies and are believed to consist mainly of terminally differentiated cells. Meroclones constitute a transitional class and are cultures formed by cells with limited growth potential, now thought to be TACs. Pellegrini et al. (1999) showed that cells cultured from human limbal tissue, but not central or paracentral corneal tissue, were able to produce holoclones, suggesting that stem cells are found in the human limbus but not in the cornea itself. As discussed in section 19.8, care must be taken in the interpretation of ex-vivo culture experiments, as it is possible to unmask proliferative potential that is not relevant to normal homeostasis.
Clinical data also suggest that the limbus contains a population of stem cells. If the corneal epithelium is completely destroyed along with the limbal zone, a situation that occurs in patients with chemical burns, standard cornea replacement is ineffective but transplantation of small pieces of healthy, human limbal epithelial tissue can reconstitute the entire corneal epithelium (Kenyon and Tseng 1989; Tseng 1989). However, grafts that include both limbal and corneal tissue seem to be more successful than limbal grafts alone and treatment with cultured limbal cells is also proving effective (Rama et al. 2010; Shortt et al. 2011).
19.3.3 The quest for markers of LESCs
Table 19.1 summarises a selection of the most promising positive and negative markers of the various human limbal/corneal epithelial compartments. Although a number of markers are specifically enriched in the basal limbal epithelium no single marker has been unambiguously confirmed as LESC specific. The most promising combination of markers currently appears to be a C/EBPδ-positive, Bmi1-positive, ΔNp63α-positive population identified in the human peripheral limbus (Barbaro et al. 2007).
Table 19.1.
Markers of human corneal and limbal epithelial compartments
| Marker | LESCs* | TACs | Differentiated | Function | Reference | |
|---|---|---|---|---|---|---|
|
|
||||||
| bLE | bCE | sLE | sCE | |||
| Positive limbal epithelial markers | ||||||
| Bmi1 | Yes (rLESC) |
SC self-renewal | Barbaro et al. 2007 | |||
| C/EBPδ | Yes (rLESC) |
Cell-cycle arrest | Barbaro et al. 2007 | |||
| ΔNp63 | Yes | ** | Proliferation | Pellegrini et al. 2001 | ||
| ΔNp63α | Yes | Proliferation | Di Iorio et al. 2005 | |||
| ABCG2 | Yes | Cell-surface transport | Chen et al. 2004 | |||
| Integrin α9 | Yes | ECM binding | Chen et al. 2004 | |||
| N-cadherin | Yes | Cell adhesion | Hayashi et al. 2007 | |||
| Non-specific markers | ||||||
| GDNF | Yes | Yes | Cell survival | Qi et al. 2008 | ||
| GFR α1 | Yes | Yes | Cell survival | Qi et al. 2008 | ||
| Keratin 15 | Yes | Yes | Structural | Yoshida et al. 2006 | ||
| Keratin 5/Keratin 14 | Yes | Yes | Structural | Schlotzer-Schrehardt and Kruse 2005 | ||
| Nestin | Yes | Yes | Intermediate filaments | Chen et al. 2004 | ||
| α-enolase | Yes | Yes | Yes | Yes | Metabolic enzyme | Zieske et al. 1992 |
| Negative limbal epithelial markers | ||||||
| NGF receptor (p75NTR) |
Yes | Yes | Yes | Survival/differentiation | Chen et al. 2004 | |
| Involucrin | Yes | Yes | Yes | Structural | Chen et al. 2004 | |
| Keratin 3/Keratin 12 | Yes | Yes | Yes | Structural | Chen et al. 2004 | |
| Connexin 43 | Yes | Yes | Yes | Structural | Chen et al. 2004 | |
| E-cadherin | Yes | Yes | Yes | Cell adhesion | Chen et al. 2004 | |
LESCs are located in the basal limbal epithelium. Some markers identify a sub-set of LESCs in the peripheral basal limbal epithelium that are thought to be resting LESCs (shown here as rLESC). Other LESC markers may be expressed in both active and resting LESCs or this information may be unknown.
ΔNp63 is not expressed in the human basal corneal epithelium (Pellegrini et al. 2001) but it is expressed in the mouse (Collinson et al. 2002; Moore et al. 2002; Ramaesh et al. 2005) and rat (Moore et al. 2002).
Abbreviations: bCE, basal corneal epithelium; bLE, basal limbal epithelium; sLE, suprabasal limbal epithelium; sCE, suprabasal corneal epithelium. aLESC, active limbal epithelial stem cell; rLESC, resting limbal epithelial stem cell.
The markers outlined in Table 19.1 are indicative of a tissue hierarchy that is initiated in the basal limbus and terminated in the suprabasal cornea, further supporting the LESC hypothesis. Markers of basal limbus are associated with SC-like properties including quiescence (C/EBPδ), self-renewal and proliferation (Bmi1 and ΔNp63α) and cell adhesion (N-cadherin and integrin α9) whereas markers of suprabasal cornea appear more associated with terminal differentiation (keratin 3/12, connexin 43). Five of the most promising and widely cited markers of LESCs/TACs are discussed in further detail below.
ΔNp63α
The p63 transcription factor has been identified as a marker of the most proliferative basal cells in the skin (Yang et al. 1998) and is required for the development of all stratified epithelia. Homozygous p63 null mouse embryos die postnatally and have severe limb defects and lack development of epidermal tissues (Mills et al. 1999). Six isoforms are generated from the p63 gene. Three isoforms (p63α, β and γ), having transactivating activity, are transcribed from the upstream promoter and have distinct C termini, whilst the three ΔN isoforms which lack the N-terminal coding exons are transcribed from a downstream intronic promoter (Parsa et al. 1999; Yang and McKeon 2000). Di Iorio et al. (2005) showed that ΔNp63α is present in the human basal limbal epithelium and that ΔNp63γ may be expressed at low levels in superficial cornea and limbus. Neither ΔNp63α nor ΔNp63β is present in resting (non-wounded) human corneas but all three ΔNp63 isoforms are expressed widely in the corneal epithelium after wounding. ΔNp63α has, therefore, been proposed as a marker of both quiescent and active LESCs (Pellegrini et al. 2001; Barbaro et al. 2007) but its expression pattern suggests it is also likely to be expressed in early TACs. In the mouse, unspecified ΔNp63 isoforms are detectable in the basal layer of the corneal epithelium at all times (Collinson et al. 2002; Moore et al. 2002; Ramaesh et al. 2005). In agreement with a role for ΔNp63α in LESCs, a recent report shows that individuals who are heterozygous for some p63 mutations have LESC deficiency (Di Iorio et al. 2012).
ABCG2
The multi-drug resistance gene ATP-binding cassette sub-family G member 2 (ABCG2) was identified in a subpopulation of bone marrow cells (known as the side-population) that efluxed Hoechst 33342 nuclear dye and exhibited SC-like properties (Goodell et al. 1996). ABCG2 has been proposed as a stem cell marker in other systems. It may protect stem cells from exogenous damage by exporting toxic compounds from the cell. It is detectable in the basal limbal epithelium by immunohistochemistry or RT-PCR of different species, including mice (Krulova et al. 2008). Cells from limbal explants that express ABCG2 show high clonogenic potential, however its broad expression pattern in basal limbus suggests that it is a marker of early TACs as well as LESCs (Budak et al. 2005; Chen et al. 2004; de Paiva et al. 2005; Krulova et al. 2008).
C/EBPδ and Bmi1
Bmi1 is a proposed oncogene that has been shown to promote neural stem cell self-renewal by repressing the cell cycle inhibitors p16Ink4a and p19Arf (Molofsky et al. 2005). It is essential for the maintenance of neural stem cells (distinguishing their self renewal from progenitor cell proliferation) and is responsible for the maintenance of adult self-renewing haematopoietic cells (Molofsky et al. 2003; Park et al. 2003). Knockout mice have defects in haematopoiesis, skeletal patterning and neurological development (Van der Lugt et al. 1994; Park et al. 2003). Bmi1 is expressed in limbal epithelium side-population cells (see above) but is expressed at extremely low levels if at all in the central cornea (Umemoto et al. 2006).
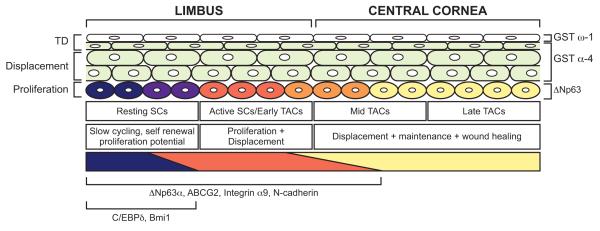
The CCAAT enhancer-binding protein δ (C/EBPδ) is a transcription factor that has been shown to regulate the cell cycle in an epithelial specific manner by inducing a G0/G1 arrest (O’Rourke et al. 1999; Hutt et al. 2000). Barbaro et al. (2007) demonstrated colocalisation of C/EBPδ and Bmi1 in human peripheral basal limbal epithelial cells constituting ~10% of the total population. They show that this expression overlaps with ΔNp63α expression but that after wounding there is a distinct population of cells that are ΔNp63α-positive but Bmi1 and C/EBPδ-negative. They suggest that ΔNp63α marks quiescent LESCs, activated SCs and early TACs but C/EBPδ and Bmi1 only mark a subpopulation of LESCs that are quiescent during normal corneal maintenance (Fig. 19.2) but may be activated during wounding (also see section 19.5). The balance of proliferative proteins (Bmi1 and ΔNp63α) and quiescence-associated factors (C/EBPδ) may allow slow cycling LESCs to exist in an intermediate state between quiescence and proliferation, poised to proliferate more rapidly if stimulated to do so by corneal injury.
Fig. 19.2. A model of mammalian corneal regeneration and maintenance.
Quiescent LESCs that express Bmi1, C/EBPδ (which sustain their quiescence and self renewal) and ΔNp63α (which primes them for proliferation) reside at the periphery of the basal limbal epithelium (dark shading; Barbaro et al. 2007). They divide in response to injury or periodically during tissue maintenance to produce active LESCs and/or early TACs that down-regulate Bmi1 and C/EBPδ but continue to express ΔNp63α as they proliferate to produce TACs. Basal limbal cells also express integrin α9, N-cadherin and ABCG2 (Chen et al. 2004; Hayashi et al. 2007; Budak et al. 2005). In humans ΔNp63α is restricted to the basal limbal epithelium and although ΔNp63β and ΔNp63γ are expressed in the basal corneal epithelium during wound healing (not shown) no ΔNp63 isoforms are detectable in the resting basal corneal epithelium (Pellegrini et al. 2001; Di Iorio et al. 2005). However, in mice unspecified ΔNp63 isoforms are detectable in the resting basal corneal epithelium (Collinson et al 2002, Moore et al 2002, Ramaesh et al 2005). During normal maintenance TACs differentiate and migrate to the suprabasal layers. Here they become wing cells, down regulate all p63 isoforms and begin to express GST-α4 in the intermediate layers (Norman et al. 2004). In the most superficial layer of the corneal epithelium they down-regulate GST-α4 and up-regulate GST-ω1 (Norman et al. 2004). It is not known whether GST-α4 and GST-ω1 are expressed in the limbal epithelium. The diagram is adapted from one published by Barbaro et al. (2007) but includes information from both human and mouse studies. Bmi1 and C/EBPδ have been identified in the human ocular surface but not yet in mouse whereas GST-α4 and GST-ω1 have been identified in mouse but not yet in human.
Integrin α9
Integrins are membrane-bound glycoproteins important in cell-cell and cell-matrix adhesion. The corneo-limbal distribution of integrin α9 changes postnatally. At P7 in the mouse (a time when the cornea epithelium is still growing in size) integrin α9 positive cells are distributed throughout the epithelium but by P42 expression is restricted to the basal limbal epithelium (Pajoohesh-Ganji et al. 2004). The expression of integrin α9 is up-regulated during wound healing (section 19.5). This suggests that integrin α9 is a marker of LESCs and the early TAC populations (Stepp et al. 1995; Pal-Ghosh et al. 2004).
Identification of markers through mRNA and proteomic expression studies
Table 19.2 summarises recent attempts to identify genes specific to corneo-limbal compartments using mRNA and proteomic expression techniques. A selection of the markers that have been independently validated from these studies is outlined in Table 19.3. There is a surprising lack of overlap with the markers described in Table 19.1 and also a lack of concordance between different studies. Crucially, the methods used to collect the tissue vary from laser capture dissection (Zhou et al. 2006) to selection of cells by their adhesive properties (Bian et al. 2010). Zhou et al. (2006) identified around 50 genes that are differentially expressed between basal limbal and basal corneal epithelium in the mouse. Further characterisation of randomly picked genes from the initial screen used semi-quantitative RT-PCR and showed that Ereg (epiregulin), Dach2 (dachshund 2), and Sry (sex determining region of chromosome Y) transcripts were found exclusively in limbal basal cells and not in corneal basal cells. Furthermore, diablo, cyclin M2 and multiple PDZ domain protein were expressed at significantly higher levels in corneal basal cells compared to limbal basal cells. Epiregulin-LacZ mice showed β-galactosidase-positive staining restricted to the basal limbal epithelium (Zhou et al. 2006). Because the basal limbal epithelium contains early TACs as well as LESCs it is not clear if these markers will be useful and further studies are required to determine if any are expressed specifically in LESCs.
Table 19.2.
Summary of differential expression studies
| Method | Species | Differentially regulated transcripts |
Reference | |||
|---|---|---|---|---|---|---|
| Group 1 (n) | Group 2 (n) | Group 3 (n) | ||||
| 1 | PCR array | Human | LE only (21) | NA | CE only (24) | Nieto-Miguel et al. 2011 |
| 2 | Microarray | Human | LEC (95) | LE (169) | CE (1,237) | Kulkarni et al. 2010 |
| 3 | Microarray | Human | CE (93) | NA | CJE (211) | Turner et al. 2007 |
| 4 | Microarray | Human | FL (33) | LXE (22) | CE (11) | Figueira et al. 2007 |
| 5 | Microarray | Human | RA LE (499) | NA | N/SA LE (277) | Bian et al. 2010 |
| 6 | Microarray | Mouse | Basal LE (32*) | NA | Basal CE (17*) | Zhou et al. 2006 |
| 7 | SAGE | Mouse | P9 TC only (3,052) |
Both (3,483) | Adult TC only (2,394) |
Norman et al. 2004 |
| 8 | SAGE | Rat | LE only (759) | Both (2,292) | C only CE (844) | Adachi et al. 2006 |
| 9 | Microarray | Monkey | CJE (506) | LE (186) | CE (644) | Ding et al. 2008 |
| 10 | Microarray | Pig | LE SP (382) | NA | LE SP (296) | Akinci et al. 2009 |
| 11 | 2D-Page/MSpec | Human | LE (70) | NA | C CE (32) | Lyngholm et al. 2008b |
n = number of markers identified in each group
Of the top 49 differentially expressed genes listed
Abbreviations: LE, limbal epithelium; CE, corneal epithelium; P9, postnatal day 9; TC, total cornea; C, central; TL, total limbus; LEC, limbal epithelial crypt; SP, side population; CJE, conjunctival epithelium; FL, fetal limbus; FC, fetal cornea; LXE, limbal explant epithelium; LEP, limbal epithelial progenitors; RA, rapidly adherent; N/SA, none or slowly adherent. 2D-Page, 2-dimensional polyacrylamide gel electrophoresis; Mspec, mass spectrometry.
Table 19.3.
Top confirmed potential LESC markers from differential expression studies
| Molecule | Species | Confirmed by | Reference |
|---|---|---|---|
| Epiregulin | Human | Microarray + IHC | Zhou et al. 2006 |
| Wnt-4 | Human | Microarray + IHC | Figueira et al. 2007 |
| Keratin 14 | Human | Microarray + IHC | Figueira et al. 2007 |
| P-Cadherin | Human | Microarray + IHC | Figueira et al. 2007 |
| SOD2 | Human | Microarray +IHC +Mass Spec | Lyngholm et al. 2008a; Kulkarni et al. 2010 |
| Keratin 15 | Human/Rat | Microarray +IHC + Mass Spec |
Figueira et al. 2007; Akinci et al. 2009; Lyngholm et al. 2008a |
| ID4 | Human | 2 independent expression studies | Takacs et al. 2009; Wolosin et al. 2000 |
| Spondin-1 | Human | 2 independent expression studies | Takacs et al. 2009; Zhou et al. 2006 |
| S100A8 | Human | 2 independent expression studies | Takacs et al. 2009; Lyngholm et al. 2008a |
| Catenin-α2 | Human | 2 independent expression studies | Figueira et al. 2007; Zhou et al. 2006 |
| GPX2 | Monkey/Pig | 2 independent expression studies | Ding et al. 2008; Akinci et al. 2009 |
| Keratin 13 | Rat/Monkey | 2 independent expression studies | Adachi et al. 2006; Ding et al. 2008 |
Abbreviations: IHC, immunohistochemistry; Mass Spec, mass spectrometry.
19.3.4 The limbal niche
The microenvironment surrounding adult SCs plays a vital role in supporting, protecting and regulating the function of somatic and germ stem cells (Moore and Lemischka 2006). Evidence suggests that, in humans, LESCs reside in a specific anatomical location within the limbus known as the palisades of Vogt (Davanger and Evensen 1971; Townsend 1991; Dua and Azuara-Blanco 2000). These are subepithelial, vascularised papillae between which the epithelium projects downwards (Goldberg and Bron 1982). The limbus is a good candidate for a stem cell niche because it has blood vessels, (which supply growth factors and nutrients), immune cells (such as Langerhans cells and T-lymphocytes which protect against pathogens) and, in some species, melanocytes which may protect SCs from ultraviolet light and potential DNA damage (Baum 1970) (Vantrappen et al. 1985; Boulton and Albon 2004; Li et al. 2007).
In humans three anatomical sites, associated with the palisades of Vogt, have been identified as probable LESC locations. These are known as the limbal epithelial crypts (LECs), limbal crypts (LCs) and focal stromal projections (FSPs). LECs were the first to be described (Dua et al. 2005) and are anatomical projections from the peripheral aspect of the limbal palisades that extend either radially into the conjunctival stroma or circumferentially along the limbus and contain epithelial cells that express K14 and the proposed LESC marker ABCG2 (see section 19.3.3). Despite their similar names, LECs and LCs are anatomically distinct, with only the more peripheral LECs extending from the limbus into the conjunctival stroma. (It may be helpful to rename LECs and/or LCs because the similarity in their names is confusing and the original terminology has not always been used consistently.) Shortt et al. (2007b) describe LCs as downward focal projections of the basal limbal epithelium into the corneal limbal stroma and the FSPs as finger-like projections of stroma, containing a central blood vessel, extending upwards into the corneal limbal epithelium. LCs and FSPs are asymmetrically distributed, with the majority concentrated in the superior and inferior limbal quadrants (Shortt et al. 2007b). Other evidence also suggests the distribution of the limbal niche is asymmetrical in humans (Lauweryns et al. 1993; Wiley et al. 1991) and mice (Pajoohesh-Ganji et al. 2004). Epithelial cells in the LC/FSP regions are generally smaller, have a higher nuclear to cytoplasm ratio and express high levels of p63 and ABCG2. In addition, cells cultured from LC/FSP-rich regions of the limbus have a higher colony forming potential than those from non LC/FSP-containing tissue (Shortt et al. 2007b). The palisades of Vogt are not present in most species and LCs have not been identified in mouse, rat or rabbit but have been identified in pig (Notara et al. 2011).
19.3.5 The corneal epithelial stem cell hypothesis
Barrandon and colleagues propose that during normal homeostasis the corneal epithelium is maintained by stem cells residing throughout the basal layer of the corneal epithelium (Majo et al. 2008). Central to their argument was the observation that limbal and central corneal tissue, transplanted into immunocompromised mice, produced clones of centripetally migrating donor tissue only when the host corneal epithelium was removed. In these cases, both the corneal and limbal tissues behaved similarly. The authors proposed that LESCs were active during wound healing but played no role in normal maintenance of the unwounded corneal epithelium. Although Majo et al. (2008) do not address the question experimentally, they also suggest that, in contrast to the conventional LESC hypothesis, any movement in the corneal epithelium was more likely to be centrifugal than centripetal (Fig. 19.3). Using in vitro colony-forming assays, they also demonstrated that the replicative potential of the limbus and central cornea in culture was similar for many species (Majo et al. 2008). However, consistent with an earlier observation (Pellegrini et al. 1999), there was no evidence that the human central and intermediate cornea produced holoclones. For technical reasons, the holoclone assay currently does not work for the mouse cornea, so they were unable to investigate whether the mouse cornea produced holoclones.
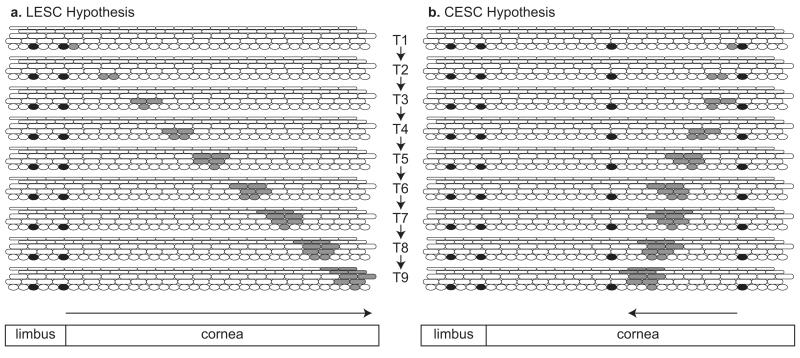
Fig 19.3. Alternative hypotheses for maintaining the corneal epithelium.
(a) Conventional Limbal Epithelial Stem Cell Hypothesis. Diagram representing the limbal epithelium and peripheral corneal epithelium with LESCs shown as black basal cells in the limbus. At time T1, one LESC produces a TAC (shaded grey). At later times (T2-T9) the daughter cells of this TAC are shown moving centripetally, dividing and, in some cases, leaving the basal layer, becoming non-mitotic and more differentiated and moving to the surface. For simplicity, only one clone of labelled cells is illustrated, only one TAC is shown dividing at each time point, basal and suprabasal cells move centripetally at the same rate and cells move vertically through the suprabasal layers to the surface relatively slowly. At T2, T4, T6 and T8 both daughter cells remain in the basal layer but at T3, T5, T7 and T9 both daughter cells move to the first suprabasal layer. (b) Alternative Corneal Epithelial Stem Cell Hypothesis, (see Majo et al. 2008). Diagram representing the limbal epithelium and peripheral corneal epithelium with CESCs shown as black basal cells in the corneal epithelium and quiescent LESCs shown as black basal cells in the limbal epithelium. At time T1, one CESC produces a TAC (shaded grey). At times T2-T9 the daughter cells of this TAC are shown moving slowly centrifugally, dividing and, in some cases, leaving the basal layer. (TACs produced by CESCs in the corneal epithelium could either maintain the local area of corneal epithelium or move centrifugally (towards the limbus). For simplicity the clone of cells is shown moving centrifugally but relatively slowly.)
The results reported by Majo et al. (2008) for the mouse are controversial and open to several interpretations but importantly they prompt re-evaluation of the conventional LESC hypothesis. Several authors have reported results that they consider support the new corneal epithelial stem cell (CESC) hypothesis for humans as well as mice but none is conclusive. A 12-hour, ex-vivo study of human corneal wound healing showed that the initial stage of wound closure was independent of the limbus (Chang et al. 2008). This experiment has been interpreted as supporting the CESC hypothesis (Sherwin 2009), but a 12-hour study would be too short to evaluate the role of the limbus adequately. However, p63-positive, cultured clonogenic spheres can be isolated from the human central cornea as well as the limbus, albeit less efficiently, indicating that the human central cornea may contain some cells with progenitor potential (Chang et al. 2011). Other evidence suggests that the central cornea of both humans and rabbits has some ability to self-maintain. Thus, some patients with total LESC deficiency (with conjunctivalisation) retain central islands of normal corneal epithelium for several years (Dua et al. 2009). Similarly, the central cornea survives for months after surgical removal or isolation of the limbus in rabbits (Huang and Tseng 1991; Kawakita et al. 2011), but corneal integrity slowly degenerates and it is incapable of responding adequately when challenged by corneal wounding. These studies do not disprove the LESC hypothesis for humans or rabbits because although they imply that the central corneal epithelium has cells able to act as progenitors, either in culture or if LESCs are unable to maintain the corneal epithelium, they do not show these cells act as progenitors during normal homeostasis. Furthermore, autografts of ex vivo-labelled rabbit keratolimbal explants have shown that transplanted limbal tissue can colonise the corneal epithelium without wounding of the host tissue (Bradshaw et al. 1999). Thus, there is no reason to reject the LESC hypothesis for rabbits. If some progenitor TACs are able to assume the stem cell role in situations where LESCs are unable to maintain the corneal epithelium this would also explain the transplantation results obtained by Majo et al. (2008) with mice. This latent potential of some central corneal epithelial cells to proliferate in vitro and in transplantation experiments does not mean that they act as stem cells during normal tissue homeostasis in vivo. In younger individuals such cells could be equivalent to stem cells that persist from fetal stages (Tanifuji-Terai et al. 2006) and in older individuals these could be early TACs derived from LESCs.
The evidence for the existence of a LESC population in the mouse cornea is strong. In interpreting their results to formulate the new CESC hypothesis (Majo et al. 2008), the authors disregard convincing evidence that during normal maintenance of the unwounded cornea, corneal epithelial cells move centripetally not centrifugally (Kinoshita et al. 1981; Buck 1985; Nagasaki and Zhao 2003; Collinson et al. 2002; Endo et al. 2007; Mort et al. 2009), whereas conjunctival epithelial cells do not move significantly in either direction (Nagasaki and Zhao 2005). The distribution of individual β-galactosidase-positive radial stripes in the corneal epithelium of KRT5LacZ/− mosaic transgenic mice suggests the stripes extended centripetally from the limbus, so favouring the LESC hypothesis over the CESC hypothesis (Douvaras et al. 2012), but this experiment should be repeated with an inducible lineage marker to provide more conclusive evidence (section 19.8). Thus, while the transplantation experiment reported by (Majo et al. 2008) has focused attention on the progenitor potential of the central corneal epithelium, current evidence favours the conventional LESC hypothesis over the new CESC hypothesis for the mouse as well as other species.
19.4 Maintaining corneal epithelial homeostasis
19.4.1 Evolution of ideas about corneal epithelial maintenance (XYZ to LESC)
The corneal epithelium undergoes continuous cell renewal, which can replace the whole tissue every 2 weeks (Cenedella and Fleschner 1990) and corneal homeostasis must be carefully regulated to ensure a uniform epithelial thickness. Before the role of adult stem cells was understood, the corneal epithelium was thought to be a self-renewing tissue. Studies with 3H-TdR labelling had shown that the basal corneal epithelial cells divided and moved vertically and were sloughed off within 3.5-7 days in mice and rats (Hanna and O’Brien 1960), so the corneal epithelium was thought to be maintained by proliferation of the basal epithelial cells. By 1982 evidence was beginning to emerge that corneal epithelial cells moved centripetally (Kinoshita et al. 1981; Buck 1982) and in 1983 Thoft and Friend proposed the ‘X, Y, Z hypothesis’ (Thoft and Friend 1983). This described the balance of proliferation, migration and cell loss by the simple formula X + Y = Z, where X was defined as “the proliferation of basal epithelial cells”, Y as “the contribution to the cell mass by centripetal movement of peripheral cells” and Z as “the epithelial cell loss from the surface”. It was not known whether the centripetal movement included movement of conjunctival cells across the limbus to the cornea as well as movement of cells from the peripheral cornea. It had been thought that conjunctival epithelium could transdifferentiate into corneal epithelium and was a source of new corneal epithelial cells during corneal wound healing (Friedenwald 1951). However, by 1986 it was becoming clear that this was not the case (Buck 1986) and the concept of centripetal movement was more firmly established (Buck 1985). Davanger and Evensen (1971) had already shown that the limbus produced cells that could colonise the corneal epithelium but the idea that the corneal epithelium was maintained by stem cells located in the limbus did not gain acceptance until later (Schermer et al. 1986; Cotsarelis et al. 1989).
Although the ’X, Y, Z’ hypothesis of corneal epithelial maintenance has provided the framework for much of the subsequent research into corneal epithelial homeostasis leading to the LESC hypothesis, in its original form it does not take account of the role of stem cells in corneal maintenance. Nowadays the “contribution to the cell mass by centripetal movement of peripheral cells” (Y) would be interpreted as production of basal corneal epithelial cells (TACs) by stem cells in the limbus (see section 19.3.1). Production of cells by stem cells must precede proliferation of TACS, so if the X, Y, Z hypothesis was updated to allow for stem cells it could be redefined as: YSC + XTAC = ZL, where YSC is production of basal corneal epithelial cells by limbal epithelial stem cells (LESCs), XTAC is proliferation of basal corneal epithelial TACs, as originally proposed (Thoft and Friend 1983), and ZL is epithelial cell loss from the surface (Fig. 19.1). By including the qualification that the stem cells are located in the limbus, this revised formulation is equivalent to the LESC hypothesis. Although the X, Y, Z hypothesis is still much cited it is now obsolete and has long been superseded by the LESC hypothesis, which currently provides the most likely explanation of how the corneal epithelium is maintained (as discussed in section 19.3).
19.4.2 Centripetal migration, differentiation and desquamation of corneal epithelial cells
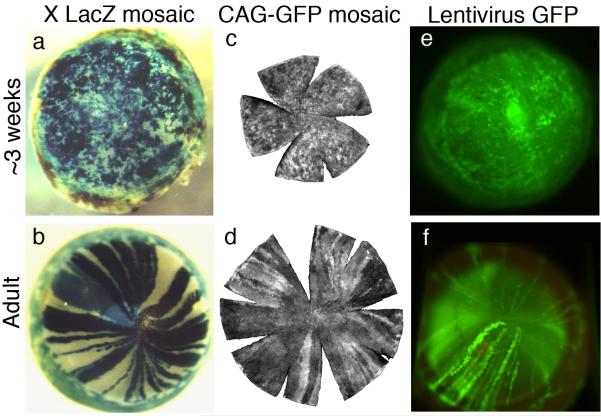
Centripetal migration in the mouse corneal epithelium has been demonstrated robustly both directly and indirectly by a number of experimental systems. Directly labelling superficial and wing cells with India ink allowed Buck (1985) to estimate a rate of centripetal migration of 17 μm/day. More recently Nagasaki and Zhao (2003) directly visualised centripetal migration using time-lapse imaging of GFP-bright clusters of corneal epithelial cells in ubiquitous-GFP reporter mice and estimated the rate of migration to be around 26 μm/day. Indirect methods using mouse genetic mosaics have also been very informative. When cells are randomly labelled with an X-linked LacZ transgene or a GFP-tagged lentiviral vector, a postnatal switch is observed from a randomly orientated mosaic pattern to a pattern of radial stripes in the corneal epithelium (Fig. 19.4). These patterns extend from the limbal region towards the central cornea and are evident from about 5 weeks postnatally. The stripes are thought to represent clones of centripetally migrating epithelial cells produced by LESCs that become active in the postnatal period prior to stripe formation (Collinson et al. 2002; Collinson et al. 2004b; Mort et al. 2009; Endo et al. 2007). In most mosaic corneas the stripes meet at a central clockwise or anticlockwise spiral (Collinson et al. 2002). The frequency of spirals increases with age, suggesting that they form stochastically but are stable once formed (Mort et al. 2009). Corneal stripes and spirals are also visualised in some human conditions (Bron 1973) and are unexplained. They may reflect failure of centripetally migrating cells to meet precisely at the centre of the tissue, or could arise from small stochastic variations in movement of the epithelial sheet.
Fig. 19.4. Corneal epithelia of different types of mosaic mice show a transition from patches to radial stripes.
(a, b) β-galactosidase staining in XLacZ X-inactivation mosaics (Collinson et al. 2002). (c, d) GFP fluorescence in CAG-GFP transgenic mosaics (Zhang et al. 2008). (e, f) GFP fluorescence in corneal epithelium after transfecting conceptuses with lentiviral vectors encoding green fluorescent protein (GFP) at embryonic day 9 or 10 (Endo et al. 2007). Images (c & d) are reproduced with permission of the authors and Molecular Vision. Images (e & f) are reproduced with permission of the authors and Molecular Therapy.
The causes of centripetal movement are still unknown and possibilities include (1) population pressure from the periphery due to production of new TACs by LESCs (Bron 1973; Sharma and Coles 1989; Wolosin et al. 2000); (2) preferential desquamation of epithelial cells from the central cornea drawing peripheral cells toward the centre (Lemp and Mathers 1989; Lavker et al. 1991); (3) chemotaxis involving a gradient resulting in either attraction to a central signal or repulsion from the periphery (e.g. limbal blood vessels) (Buck 1985); (4) stimulation by corneal nerves (Jones and Marfurt 1996); (5) response to endogenous electric currents due to ion flow in wounded and unwounded corneas (McCaig et al. 2005). Unlike the cornea, the conjunctival epithelium does not show any cell movement (Nagasaki and Zhao 2005) and mosaic patterns appear as patches rather than stripes (Collinson et al. 2002; Mort et al. 2009). This is consistent with the idea that stem cells renewing this epithelium are distributed throughout the tissue (Nagasaki and Zhao 2005).
Differentiation of the corneal epithelium continues after birth. Evidence from expression of an ocular surface marker in the rat suggests that during development ‘stem-like’ cells reside throughout the basal layer of the corneal epithelium but become restricted to the limbus postnatally (Chung et al. 1992). This postnatal loss of stem cells from the central cornea is supported by analysis of mosaic mouse corneas which shows that transition to LESC-maintained corneal epithelium occurs between postnatal weeks 5 and 8 and the pattern is not fully mature until at least 10 weeks (Collinson et al. 2002; Mort et al. 2009). Changing postnatal expression of the keratins K12 and K14 suggests that the mouse corneal epithelium may not be fully mature until 3-6 months after birth (Tanifuji-Terai et al. 2006). Expression of integrin α9β1 suggests that the limbus also matures progressively during the first 8 weeks after birth (Pajoohesh-Ganji et al. 2004). Care must be taken when extrapolating results from animal models to human because there are some notable species differences in corneal differentiation as well as more obvious differences of tissue size and lifespan. For example, mice have no Krt3 (keratin 3) gene but keratin 5 is present in the mouse cornea (Byrne and Fuchs 1993; Lu et al. 2006; Ou et al. 2008). It has been suggested that K5 may pair with K12 in the cornea of mice and some other species with low K3 levels, including humans and dogs (Chaloin-Dufau et al. 1993; Hesse et al. 2004).
When TACs undergo their final division both daughter cells leave the basal layer together and begin the next stage of differentiation synchronously (Beebe and Masters 1996) and then move to the superficial layer within a few days (Hanna and O’Brien 1960). Few studies have been undertaken to measure the rate of epithelial shedding in mouse or human corneal epithelium. However, Ren and Wilson (1996), using both in vivo and in vitro measurements of corneal epithelial shedding rates in the rabbit, calculated that on average cells are shed at a rate of 5-15 cells/min from each cornea.
19.5 Corneal epithelial wound healing and homeostasis
Wound healing has been used as an experimental tool to challenge corneal homeostasis and, thus, has already been mentioned several times in this review. The nature of the corneal wound healing response depends on the type of injury and is regulated by molecules produced by the epithelium, stroma and lacrimal glands (Imanishi et al. 2000; Schultz et al. 1994; Wilson et al. 2001; Wilson et al. 2003). Briefly, healing proceeds through three stages; an initial migratory stage to cover the wound, a proliferative stage to restore the epithelial thickness and a period of differentiation to restore the epithelium’s complex structure (Suzuki et al., 2003). In essence this regenerative process mirrors normal corneal maintenance and has therefore been used as a tool to investigate this process. There are obvious caveats in the interpretation of results from wound healing studies, already alluded to elsewhere in this review (e.g. sections 19.3.3 and 19.3.5).
Several studies have shed light on the tissue hierarchy in the corneal epithelium by examining re-epithelialisation and have helped to identify compartments of putative resting SCs, activated SCs, TACs and differentiated cells. Cell-cycle double-labelling techniques have shown that the cornea uses three strategies to generate new epithelial cells in response to injury: (1) the activation of SCs to replenish the TAC population; (2) additional rounds of TAC proliferation and (3) the shortening of TAC cell cycle time to produce new tissue more quickly (Lehrer et al. 1998).
These cell cycle changes are accompanied by changes in the distribution of cells expressing proposed markers of LESCs and/or early TACs. Upon corneal wounding, early TACs (expressing ΔNp63α) migrate into the basal layer of the corneal epithelium from the limbus (Di Iorio et al. 2005). As outlined in section 19.3.3, Barbaro and co-workers identified a subpopulation of basal limbal epithelial cells that are positive for C/EBPδ, Bmi1 and ΔNp63α. These are present during normal homeostasis and are thought to represent relatively quiescent LESCs. After wounding, some of these cells lose expression of C/EBPδ and Bmi1 but continue to express ΔNp63α as they proliferate, suggesting that some quiescent LESCs become active LESCs and early TACs, which move from the limbus to the cornea (Barbaro et al. 2007).
During healing of very large wounds, involving most of the cornea, integrin α9-expressing TACs move to repair the wound and this is accompanied by a depletion of integrin α9-expressing basal limbal epithelial cells (Pal-Ghosh et al. 2004). The loss of integrin α9 expression at the limbus after formation of large wounds seemed to correlate with the presence of goblet cells in the central cornea suggesting ingression of cells from the surrounding bulba-conjunctiva. Overall, this suggests that these very large corneal epithelial wounds place high demands on the stem cell reserve in the limbus, which may cause a depletion of stem cells and early TACs in the limbus and ingression of conjunctival cells.
19.6 Effects of ageing on corneal epithelial maintenance
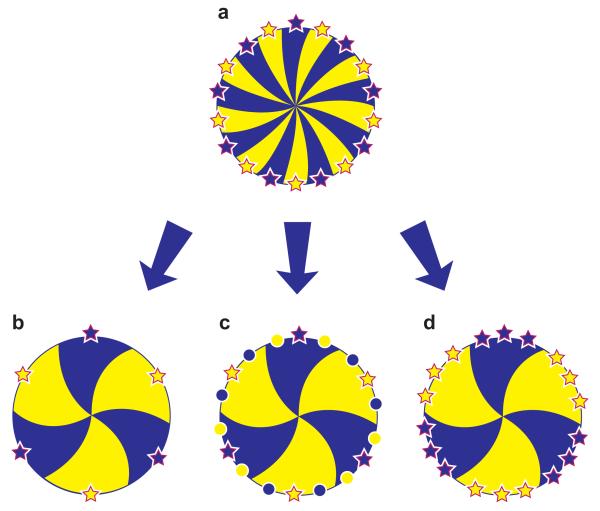
Numerical analysis of striping patterns in corneas of adult X-inactivation mosaic mice (Fig. 19.4b) can give an indirect estimate of the number of coherent clones of LESCs maintaining the corneal epithelium (Collinson et al. 2002; Collinson et al. 2004b; Mort et al. 2009). Although this is not equal to the number of active stem cells, it is useful for comparing dynamic patterns of SC maintenance in different experimental groups. The corrected number of radial stripes in the corneal epithelium declines from ~100 at 10-weeks postnatally to ~50 at 39-weeks, with no further decline up to 52-weeks (Collinson et al. 2002; Mort et al. 2009). This suggests that the number of coherent clones of LESCs maintaining the corneal epithelium decreases with age (Fig. 19.5), which could reflect either a loss of LESCs (Fig. 19.5b) or an increase in the proportion of quiescent LESCs (Fig. 19.5c). Alternatively, stochastic neutral drift in the distribution of LESCs may reduce the number of LESC clones but not the number of active LESCs (Fig. 19.5d). This possibility is suggested by lineage tracing studies which have identified similar coarsening of mosaic patterns derived from SC clones in the mouse testis (Nakagawa et al. 2007) and mouse intestine (Snippert et al. 2010; Lopez-Garcia et al. 2010; Klein and Simons 2011). Stochastic replacement of a SC clone by a neighbouring one could occur if SCs did not always divide asymmetrically to produce one SC and one TAC at each division, but sometimes divided symmetrically to produce two SCs or two TACs (Fig. 19.6). Similarly, the age-related decline in frequency of rare corneal epithelial stripes in mosaic KRT5LacZ/− transgenic mice (Douvaras et al. 2012) could reflect a decline in SC function with age, or neutral drift in stem cell clones without an overall reduction in SC number. Other methods are required to investigate whether SC numbers decline with age.
Fig. 19.5. Alternative explanations of age-related reduction in corrected stripe number in corneal epithelia of mouse X-inactivation mosaics.
The mosaic corneal epithelium is shown as a disk with radial dark and light stripes produced by LESCs, represented by dark and light stars, at the edge of the cornea. (For purposes of illustration dark and light LESCs alternate around the circumference so all dark and light stripes are the same width.) Dark and light circles represent quiescent LESCs. (a) Full complement of active LESCs in young eye. (b) Reduced number of LESCs in older eye. (c) Increased proportion of quiescent LESCs in older eye. (d) Number of LESC clones declines (without reducing the number of LESCs) by stochastic neutral drift in the distribution of LESCs (also see Fig. 19.6). The corrected stripe number produced by quantitative analysis of X-inactivation mosaic patterns indicates the relative number of active LESC coherent clones rather than the actual or relative number of active LESCs (Collinson et al 2002; Mort et al 2009). Nevertheless, the possibilities illustrated in the figure still apply – i.e. stripe numbers can be reduced by stem cell loss, inactivation or redistribution. Adapted from Mort et al. (2009).
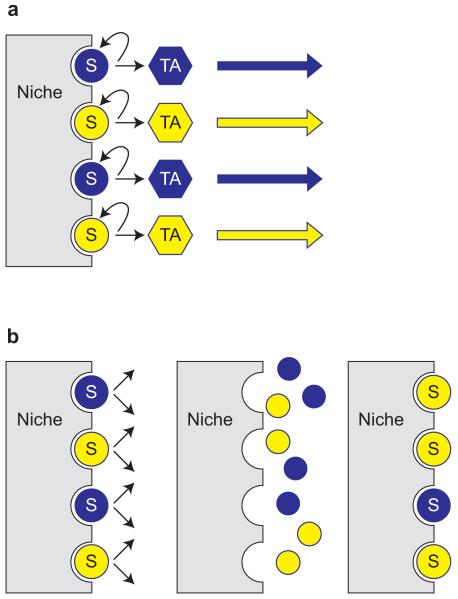
Fig. 19.6. Asymmetric and symmetric stem cell divisions.
Stem cells (SCs) are represented by dark and light circles marked with the letter ‘S’ associated with a niche; transient (or transit) amplifying cells (TACs) are shown as dark and light hexagons, marked with the letters ‘TA’. (a) Asymmetric division of stem cells always produces one stem cell and one TAC, which will divide a limited number of times to produce more differentiated cells. (b) Stem cells might also divide symmetrically to produce two equivalent daughter cells, which compete with neighbours to occupy limited space on the niche. Daughter cells securing contact with the niche would become stem cells and others would become TACs. This would provide scope for stochastic replacement of a SC by a daughter cell of a neighbouring SC. In the diagram, the top dark SC is lost and both daughter cells produced by the neighbouring light SC occupy the niche so the light SC clone expands.
Some observations on the human cornea and limbus support the notion that ageing affects stem cell numbers. The frequency of holoclones generated from skin of an infant was ten times greater than from an individual aged 64 (Barrandon and Green 1987) and a recent study showed that the efficiency of holoclone production from the human corneal limbus declined with age (Notara et al. 2012). Cells derived from younger donors (0-30 years old) had a significantly higher colony forming efficiency than cells from middle aged (30-60 years) and older donors (60-90 years). Other age-related changes noted for the human limbus include increased frequency of eyes without detectable palisades of Vogt (Zheng and Xu 2008), reduction in limbal crypts and focal stromal projections (see section 19.3.4) in people over 60 years old (Notara et al. 2012) and an increase in average size of cells in the basal limbal epithelium (Zheng and Xu 2008). It has also been reported recently that the frequency of side-population cells (see discussion of ABCG2 marker in section 19.3.3) declines with age and this could indicate an age-related decline in the number of SCs and early TACs (Chang et al. 2011).
Despite experimental evidence suggesting that stem cell function may decline with age, corneal epithelial maintenance appears to be remarkably robust and there is no evidence that ageing significantly compromises the integrity of the normal, healthy corneal epithelium. However, older mice of some strains are prone to corneal stromal mineralisation and some types of mouse cage bedding may trigger neovascularisation and keratitis in older mice (Smith et al. 2002).
19.7 Genetic defects of corneal epithelial maintenance
Corneal maintenance is impaired when LESCs are depleted by injury or disease (Daniels et al. 2001; Shortt et al. 2007a). When LESC deficiency only affects one eye, it can be treated with autografts of limbal tissue from the unaffected eye (Kenyon and Tseng 1989) or by transplanting cultured limbal epithelial cells (enriched for putative LESCs and early TACs) derived from the contralateral eye (Rama et al. 2010). LESC deficiency is also thought to underlie the corneal deterioration that occurs in people who are heterozygous for PAX6 mutations that cause aniridia (discussed below) and those who are heterozygous for some p63 mutations (Di Iorio et al. 2012).
19.7.1 Aniridia-related keratopathy (ARK)
Aniridia is an inherited eye disease caused by heterozygosity for a PAX6 mutation (PAX6+/−), which results in low levels of the PAX6 transcription factor. PAX6 expression is reduced in eyes of PAX6+/− aniridia patients and also in the abnormal corneal pannus tissue of patients with Stevens-Johnson syndrome (Li et al. 2008). Eyes of PAX6+/− aniridia patients develop abnormally and the cornea usually deteriorates progressively causing corneal opacity that sometimes leads to blindness (Mackman et al. 1979; Nelson et al. 1984; Nishida et al. 1995). This corneal deterioration is known as aniridic keratopathy or aniridia-related keratopathy (ARK) and features include irregular thickening of the peripheral corneal epithelium, in-growth of blood vessels from the limbus, associated with connective tissue (pannus), stromal scarring and accumulation of goblet cells within the corneal epithelium. Goblet cells are normally only found in the conjunctival epithelium (beyond the limbus) and their presence in the corneal epithelium has been interpreted as evidence for encroachment of conjunctival cells to compensate for poor LESC activity (Nishida et al. 1995). Limbal morphology is abnormal in PAX6+/− aniridia patients and the palisades of Vogt (putative LESC niche) are absent (Nishida et al. 1995). The conclusion that ARK is at least partly caused by LESC-deficiency is supported by the clinical observation that transplantation treatment has much better success when transplanted tissue is healthy limbal and peripheral corneal tissue (kerato-limbal allograft) rather than central corneal tissue (penetrating keratoplasty) (Holland et al. 2003).
19.7.2 Pax6+/− mouse aniridia model
Heterozygous Pax6+/− (e.g. Pax6+/Sey-Neu) mice provide an excellent model of both the developmental eye defects seen in aniridia and the progressive corneal changes characteristic of ARK, although, unlike humans with aniridia, Pax6+/− mice also have small eyes (microphthalmia) (Ramaesh et al. 2003; Davis et al. 2003; Sivak et al. 2004; Ramaesh et al. 2005; Ramaesh et al. 2006; Leiper et al. 2006). It is clear that low levels of Pax6 expression cause abnormal maintenance of the adult cornea in both mice and humans, but this may involve multiple mechanisms. The presence of goblet cells is consistent with LESC deficiency (Nishida et al. 1995) but this remains circumstantial because other explanations, such as abnormal differentiation, are possible (Ramaesh et al. 2005). Proliferation of TACs in the basal layer of the corneal epithelium is higher in Pax6+/− mice than in wild-type mice (Davis et al. 2003; Ramaesh et al. 2005) and this could be a secondary response to greater cell loss from a fragile epithelium (Davis et al. 2003) and/or reduced LESC function, rather than a direct effect of reduced Pax6 levels on cell proliferation.
Patterns visualised in the corneal epithelia of mosaic and chimeric mice provide clues about corneal epithelial maintenance. Wild-type (WT) XLacZ, X-inactivation mosaic mice exhibit radial striping patterns in the corneal epithelium, with stripes converging at a central spiral or midline and only very rarely do abnormal patterns occur (Fig. 19.7a-c). The pattern in Pax6+/− XLacZ mosaics is usually disorganised, implying that corneal epithelial cell movement is abnormal (Collinson et al. 2004a; Mort et al. 2011; Fig. 19.7d-f). However, it is unclear whether this is caused by some intrinsic movement defect or a response to chronic wounding of a thin and fragile corneal epithelium that diverts cells from their normal centripetal migration. Quantitative analysis implies that Pax6+/− mice have fewer active clones of LESCs maintaining the corneal epithelium than normal (Collinson et al. 2004a; Mort et al. 2011). However, it is unclear whether there are fewer active LESCs or if LESCs are simply clustered into larger clones in Pax6+/− eyes. Quantitative analysis of Pax6+/− ↔ WT mouse chimeras showed that Pax6+/− cells were under-represented in the corneal epithelium of adults (Collinson et al. 2004a) but not in embryonic day (E) 16.5 fetuses (Collinson et al. 2001). This implies that Pax6+/− LESCs contribute less well to the adult corneal epithelium than WT cells, which indicates either that in these chimeras Pax6+/− LESCs are under-represented relative to WT LESCs or that they produce fewer TACs. Overall, the mosaic and chimera analyses are consistent with a mild LESC abnormality in the Pax6+/− mouse model of aniridia.
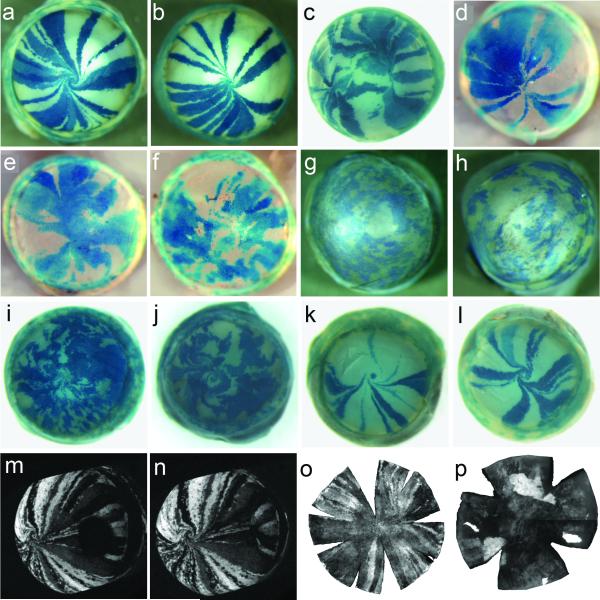
Fig. 19.7. Normal and abnormal mosaic patterns in the mouse corneal epithelium.
(a-l) β- galactosidase staining in XLacZ X-inactivation mosaics; (m-p) GFP fluorescence in GFP transgenic mosaics. (a, b) Mosaic patterns in corneal epithelia of normal, wild-type XLacZ X-inactivation mosaic mice show radial stripes that meet at either a central spiral (a) or at the midline (b). (c) A rare wild-type XLacZ X-inactivation mosaic cornea with two points of stripe convergence. (d-f) Pax6+/Sey-Neu XLacZ mosaics may have normal striped patterns (d) but in most cases stripes are disrupted (e, f). (g-j) Pax6+/Leca4 XLacZ mosaics (g, h) and double heterozygous Pax6+/Sey-Neu Gli3+/XtJ XLacZ mosaics (i, j) have a pattern of patches rather than stripes. Cornea (j) has a double spiral pattern. (k, l) PAX77Tg/− XLacZ mosaics over-express Pax6 but usually show a normal pattern of radial stripes. (m, n) Images from time-lapse confocal microscopy of healing of a 1 mm peripheral wound in a Y001deltaDRR (PAX6-GFP) mosaic corneal epithelium shows closure of the wound between 5.25 h. after wounding (m) and 18.75 h. (n) to form a second point of stripe convergence (Mort et al. 2009). (o, p) Flat whole mount CAG-EGFP corneas show radial stripes of GFP fluorescence (o) but adult homozygous GFP-Dstncorn1/corn1 corneas (p) show globular and diffuse GFP patterns (Zhang et al. 2008). Images (o) and (p) are reproduced from Zhang et al. (2008) with permission of the authors and publishers of Molecular Vision. Others are from our own studies: a, b, c, m, n (Mort et al. 2009); d (Mort 2007); e,f (Collinson et al. 2004a); i, j (Kucerova et al. 2012); g, h, k, l unpublished.
19.7.3 Other examples of abnormal corneal epithelial maintenance in mice
Although Pax6+/− XLacZ mosaic corneal patterns were mildly disrupted, other Pax6 defects are associated with more abnormal patterns. Pax6 +/Leca4 XLacZ mosaics (Mort et al. 2011) and Pax6+/Sey-Neu Gli3+/XtJ XLacZ double heterozygotes (Kucerova et al. 2012) both produced randomly-orientated patterns of patches in the adults (Fig. 19.7g-j). This suggests that movement of cells from the limbus is severely reduced or absent and that the corneal epithelium may be maintaining itself, either because of LESC deficiency or a primary failure of centripetal cell movement, as reported for Dstncorn1/corn1 mosaics (Zhang et al. 2008), discussed below. Wild-type mice carrying the PAX77 transgene (Schedl et al. 1996) over-express Pax6 and show some corneal epithelial abnormalities characteristic of PAX6+/− heterozygotes (Dorà et al. 2008) but PAX77Tg/− XLacZ mosaics have normal radial stripes (Fig. 19.7k,l), implying centripetal movement is normal (Mort et al. 2011). Abnormal striped patterns also occur occasionally in wild-type XLacZ mosaics (Fig. 19.7c) and may be caused by healing of lateral wounds to generate a second point of stripe convergence during wound closure. Studies with GFP mosaics confirmed that lateral wounding can create a new convergence point and cell migration can reorientate after wounding (Fig. 19.7m,n; Mort et al. 2009).
In Dstncorn1/corn1, CAG-EGFP mosaics, GFP radial stripes begin to emerge but their centripetal extension ceases at around 6 weeks of age and mosaic patterns become more globular (Zhang et al. 2008) (Fig. 19.7o,p). Cells in the corneal epithelium are stationary but proliferative (with regions of epithelial hyperplasia) and comprise a mixture of corneal epithelial and conjunctival epithelial phenotypes (including goblet cells in older mice), with BrdU label-retaining cells being distributed throughout the corneal epithelium instead of being confined to the limbus. The absence of cell movement suggests that the goblet cells arise by abnormal differentiation within the corneal epithelium, rather than by immigration from the conjunctiva. The authors proposed that the Dstncorn1/corn1 corneal epithelium maintains itself with one or more populations of stem cells within the corneal epithelium that can produce K12-positive corneal epithelial cells, K8-positive conjunctiva-like epithelial cells and goblet cells (Zhang et al. 2008).
19.8 Research directions for identification of stem cells
As discussed in sections 19.2 and 19.3, the conventional view is that most adult tissues, including the corneal epithelium, are maintained by populations of long-lived, predominantly quiescent, SCs, residing in a specialised niche. These SCs show the hallmarks of being able to ‘self-renew’ (duplicate without losing developmental potential) and give rise to all differentiated cell types of the tissue or organ in which they reside (Potten and Loeffler 1990; He et al. 2009). In the case of the corneal epithelium this SC population is unipotent generating only differentiated keratinocytes (and undifferentiated SCs). As discussed in the preceding sections the most plausible hypothesis to explain corneal epithelial maintenance at present is the LESC hypothesis.
A number of recent observations have forced stem cell biologists to re-appraise their interpretation of tissue stem cell hierarchies and may inform the current debate. For example, fast cycling populations of cells with stem cell or progenitor cell characteristics have been identified in mouse tail epidermis, the hair follicle epithelium and the small intestine. This evidence suggests either that quiescence is not a prerequisite for an adult SC population or that, in some tissues, TACs/progenitors are responsible for much of the normal maintenance (Clayton et al. 2007; Barker et al. 2007; Jaks et al. 2008). Furthermore, evidence from the small intestine, spermatogonia and interfollicular epidermis suggest that some tissues may be maintained in a stochastic manner with competition between daughter cells to occupy the SC niche, rather than by rigid asymmetrical stem cell division (Lopez-Garcia et al. 2010; Klein et al. 2010; Li and Clevers 2010; Klein and Simons 2011); also see Fig. 19.6. In addition, SC like potential can be ‘unmasked’ in hair follicle bulge cells that contribute to repair of the interfollicular epidermis despite the fact that they appear not to be involved during normal homoeostasis (Ito et al. 2005; Levy et al. 2007; Yu et al. 2008).
These studies suggest that mechanisms of stem cell maintenance may differ between tissues and that the interaction between the tissue ‘environment’ and the ‘plasticity’ of a particular cell population is key. Experimental approaches that modify the local environment (by wounding, transplantation or ex vivo expansion) may therefore induce SC like behaviour in cells that do not normally contribute to homeostasis (Potten and Loeffler 1990). One current model suggests that in some tissues there may exist a rapidly cycling ‘committed progenitor’ (CP) responsible for the bulk of tissue maintenance as well as a population of quiescent or slow cycling SCs. These proposed CPs maintain the tissue and retain a degree of ‘stemness’ which enables them to act as an alternative source of stem cells in response to injury or physiological stress (Li and Clevers 2010; Klein and Simons 2011; Kaur and Potten 2011); however CPs have a lower proliferative potential than SCs. In tissues where this model fits the experimental data, identification of the relevant tissue-maintaining SC will require additional approaches. It is not yet known whether the corneal epithelium is maintained by such a combination of quiescent SCs and more active CPs. However it is intriguing that, for human cornea, Barbaro et al. (2007) identified a Bmi1-positive, C/EBPδ-positive and ΔNp63α-positive population (possibly a quiescent SC) that responds to injury and a separate Bmi1- negative, C/EBPδ-negative and ΔNp63α-positive population (possibly a CP) in uninjured limbus. It remains to be seen however, whether this potential quiescent SC population contributes to normal tissue maintenance or whether the potential CPs fulfil this role alone. Lineage tracing studies (see below) should resolve this.
What is clear is that, in order to identify the SC that is responsible for corneal epithelial maintenance, experimental approaches are required that seek to unpick the tissue hierarchy without altering the tissue ‘environment’ or unmasking latent ‘plasticity’. To date the most useful and transparent techniques (in their execution if not in their interpretation) have been those that use genetic markers driven by the promoters of specific genes to demonstrate lineage, coupled with conditional diphtheria toxin-mediated cell ablation of those populations. For example, in the case of the small intestine, two potential SC populations have been identified. A slow cycling population that expresses Bmi1 is located at the +4 position immediately above the base of the crypt (Sangiorgi and Capecchi 2008). The faster cycling crypt base columnar cells (CBCs) are interspersed with the Paneth cells at the base of the crypt and expresses Lgr5 (Barker et al. 2007). Lineage tracing has shown that both populations give rise to all mature intestinal epithelial cell lineages. However whilst ablation of CBCs either genetically or by irradiation leads to expansion of the +4 cells to restore tissue maintenance (Tian et al. 2011; Yan et al. 2012), genetic ablation of the Bmi1-positive, +4 cell population results in widespread cell death and is not compatible with crypt maintenance (Sangiorgi and Capecchi 2008). The evidence suggests, therefore, that CBCs are responsible for the bulk of tissue maintenance, but that +4 cells sit at the top of the lineage as quiescent SCs able to divide in response to injury.
This demonstrates how the combination of cell lineage tracing and genetic cell ablation can be used as powerful tools to tease apart potential SC populations within a tissue. Combined with the identification of additional cell-specific markers and theoretical modelling of stem cell dynamics (Lopez-Garcia et al. 2010; Klein and Simons 2011), similar approaches are likely to be crucial in defining (or rejecting the existence of) SC/CP cell hierarchies in the corneal epithelium. The expression pattern of Bmi1 in the human cornea (Barbaro et al. 2007) and the availability of Bmi1 transgenic mouse lines (Sangiorgi and Capecchi 2008) make the gene an obvious starting point for such an analysis. To unambiguously identify the corneal epithelium-maintaining stem cell population the following key points should be demonstrated. (1) The prospective SC population can be shown to populate the entire corneal epithelium though lineage analysis. (2) Ablation of the prospective SC population should result in corneal deterioration. Thus, although C/EBPδ and Bmi1 appear to be good candidates as markers of a subpopulation of quiescent LESCs in the human limbus, lineage tracing and ablation studies are required to determine whether these are the ultimate stem cells that maintain the corneal epithelium.
19.9 Conclusions
Ocular surface damage and disease are major causes of blindness and so are of great clinical significance. This has motivated the development of new clinical procedures, including transplantation of cells cultured from the corneal limbus to treat limbal epithelial stem cell deficiency (Rama et al. 2010; Shortt et al. 2011). In some respects clinical science is running ahead of the biological understanding and many fundamental basic research questions remain unresolved, not least the isolation and characterisation of the proposed LESC population.
The evidence reviewed here favours a model by which the corneal epithelium is maintained by LESCs. Evidence that describes a stem cell population within the corneal epithelium itself (CESCs) may represent the unmasking of latent ’plasticity’ within the resident TAC population and does not support a fundamental difference between humans and the mouse. The balance of experimental evidence supports the conventional LESC hypothesis over the new CESC hypothesis for the mouse as well as other species. Nevertheless, there are significant differences among species, which need to be taken into account when extrapolating results from animal models to humans. Despite these differences the mouse has many advantages as an experimental animal and the availability of mouse genetic mutants, mosaics and various transgenic models provide powerful experimental resources with which to unravel the secrets of corneal epithelial maintenance and to model corneal epithelial defects.
There are currently some promising gene expression patterns that that may open the door to reliable LESC biomarkers. Careful cell lineage and ablation studies combined with expression profiling will be required to validate these potential markers and unpick the tissue hierarchy responsible for corneal epithelial maintenance. With a better understanding of the basic biology, clinicians will be able to refine their existing therapies and achieve better clinical outcomes. Furthermore, they will gain better insight and a clearer understanding of the long-term efficacies of these still relatively new therapies.
Acknowledgements
We thank Ronnie Grant for assistance with illustrations, Dr Alan W. Flake and Molecular Therapy for permission to reproduce images shown in Figure 19.4e,f and Dr Takayuki Nagasaki and Molecular Vision for permission to reproduce images shown in Figures 19.4c,d and 19.7o,p. We also thank Developmental Dynamics, BMC Developmental Biology, Investigative Ophthalmology & Vision Science and Molecular Vision for permission to reproduce our own previously published images included in Figs 19.4 and 19.7. We are grateful to the Wellcome Trust, Medical Research Council, Biotechnology and Biological Sciences Research Council, Fight for Sight (UK), the RS MacDonald Charitable Trust and the Royal College of Surgeons of Edinburgh for support for our own research.
References
- Adachi W, Ulanovsky H, Li Y, Norman B, Davis J, Piatigorsky J. Serial analysis of gene expression (SAGE) in the rat limbal and central corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47(9):3801–3810. doi: 10.1167/iovs.06-0216. doi:10.1167/iovs.06-0216. [DOI] [PubMed] [Google Scholar]
- Akinci MAM, Turner H, Taveras M, Wolosin JM. Differential gene expression in the pig limbal side population: Implications for stem cell cycling, replication, and survival. Invest Ophthalmol Vis Sci. 2009;50(12):5630–5638. doi: 10.1167/iovs.09-3791. doi:10.1167/iovs.09-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaro V, Testa A, Di Iorio E, Mavilio F, Pellegrini G, De Luca M. C/EBP delta regulates cell cycle and self-renewal of human limbal stem cells. J Cell Biol. 2007;177(6):1037–1049. doi: 10.1083/jcb.200703003. doi:10.1083/jcb.200703003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barrandon Y, Green H. Cell size as a determinant of the clone forming ability of human keratinocytes. Proce Natl Acad Sci USA. 1985;82(16):5390–5394. doi: 10.1073/pnas.82.16.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84(8):2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum JL. Melanocyte and Langerhans cell population of cornea and limbus in albino animal. Am J Ophthalmol. 1970;69(4):669–676. doi: 10.1016/0002-9394(70)91637-5. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Masters BR. Cell lineage and the differentiation of corneal epithelial cells. Invest Ophthalmol Vis Sci. 1996;37(9):1815–1825. [PubMed] [Google Scholar]
- Bian F, Liu WB, Yoon KC, Lu R, Zhou N, Ma P, Pflugfelder SC, Li DQ. Molecular signatures and biological pathway profiles of human corneal epithelial progenitor cells. Int J Biochem Cell Biol. 2010;42(7):1142–1153. doi: 10.1016/j.biocel.2010.03.022. doi:10.1016/j.biocel.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Albon J. Stem cells in the eye. Int J Biochem Cell Biol. 2004;36(4):643–657. doi: 10.1016/j.biocel.2003.10.013. doi:10.1016/j.biocel.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Bradshaw JJ, Obritsch WF, Cho BJ, Gregerson DS, Holland EJ. Ex vivo transduction of corneal epithelial progenitor cells using a retroviral vector. Invest Ophthalmol Vis Sci. 1999;40(1):230–235. [PubMed] [Google Scholar]
- Braun KM, Watt FM. Epidermal label-retaining cells: Background and recent applications. J Invest Derm Symp Proc. 2004;9(3):196–201. doi: 10.1111/j.1087-0024.2004.09313.x. [DOI] [PubMed] [Google Scholar]
- Bron AJ. Vortex patterns of corneal epithelium. Trans Ophthalmol Soc UK. 1973;93:455–472. [PubMed] [Google Scholar]
- Buck RC. Hemidesmosomes of normal and regenerating mouse corneal epithelium. Virchows Archiv B. 1982;41(1-2):1–16. doi: 10.1007/BF02890267. [DOI] [PubMed] [Google Scholar]
- Buck RC. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Invest Ophthalmol Vis Sci. 1985;26(9):1296–1299. [PubMed] [Google Scholar]
- Buck RC. Ultrastructure of conjunctival epithelium replacing corneal epithelium. Curr Eye Res. 1986;5(2):149–159. doi: 10.3109/02713688609015103. doi:10.3109/02713688609015103. [DOI] [PubMed] [Google Scholar]
- Budak MT, Alpdogan OS, Zhou MY, Lavker RM, Akinci MAM, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005;118(8):1715–1724. doi: 10.1242/jcs.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C, Fuchs E. Probing keratinocyte and differentiation specificity of the human K5 promoter in vitro and in transgenic mice. Mol Cell Biol. 1993;13(6):3176–3190. doi: 10.1128/mcb.13.6.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenedella RJ, Fleschner CR. Kinetics of corneal epithelium turnover in vivo - Studies of Lovastatin. Invest Ophthalmol Vis Sci. 1990;31(10):1957–1962. [PubMed] [Google Scholar]
- Chaloin-Dufau C, Pavitt I, Delorme P, Dhouailly D. Identification of keratin-3 and keratin-12 in corneal epithelium of vertebrates. Epithelial Cell Biol. 1993;2(3):120–125. [PubMed] [Google Scholar]
- Chang CY, Green CR, McGhee CNJ, Sherwin T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci. 2008;49(12):5279–5286. doi: 10.1167/iovs.07-1260. doi:10.1167/iovs.07-1260. [DOI] [PubMed] [Google Scholar]
- Chang CYA, McGhee JJ, Green CR, Sherwin T. Comparison of stem cell properties in cell populations isolated from human central and limbal corneal epithelium. Cornea. 2011;30(10):1155–1162. doi: 10.1097/ICO.0b013e318213796b. doi:10.1097/ICO.0b013e318213796b. [DOI] [PubMed] [Google Scholar]
- Chen Z, De Paiva CS, Luo LH, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22(3):355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EH, Bukusoglu G, Zieske JD. Localization of corneal epithelial stem-cells in the developing rat. Invest Ophthalmol Vis Sci. 1992;33(7):2199–2206. [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446(7132):185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Chanas SA, Hill RE, West JD. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the PAX6+/− mouse. Invest Ophthalmol Vis Sci. 2004a;45(4):1101–1108. doi: 10.1167/iovs.03-1118. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Hill RE, West JD. Analysis of mouse eye development with chimeras and mosaics. Int J Dev Biol. 2004b;48(8-9):793–804. doi: 10.1387/ijdb.041885jc. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Morris L, Reid AI, Ramaesh T, Keighren MA, Flockhart JH, Hill RE, Tan SS, Ramaesh K, Dhillon B, West JD. Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev Dyn. 2002;224(4):432–440. doi: 10.1002/dvdy.10124. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Quinn JC, Buchanan MA, Kaufman MH, Wedden SE, West JD, Hill RE. Primary defects in the lens underlie complex anterior segment abnormalities of the Pax6 heterozygous eye. Proc Natl Acad Sci USA. 2001;98(17):9688–9693. doi: 10.1073/pnas.161144098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57(2):201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Daniels JT, Dart JKG, Tuft SJ, Khaw PT. Corneal stem cells in review. Wound Repair Regen. 2001;9(6):483–494. doi: 10.1046/j.1524-475x.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- Davis J, Duncan MK, Robison WG, Piatigorsky J. Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci. 2003;116(11):2157–2167. doi: 10.1242/jcs.00441. [DOI] [PubMed] [Google Scholar]
- de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23(1):63–73. doi: 10.1634/stemcells.2004-0093. doi:10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of Delta Np63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102(27):9523–9528. doi: 10.1073/pnas.0503437102. doi:10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio E, Kaye SB, Ponzin D, Barbaro V, Ferrari S, Böhm E, Nardiello P, Castaldo G, McGrath JA, Willoughby CE. Limbal stem cell deficiency and ocular phenotype in ectrodactyly-ectodermal dysplasia-clefting syndrome caused by p63 mutations. Ophthalmology. 2012;119(1):74–83. doi: 10.1016/j.ophtha.2011.06.044. doi:10.1016/j.ophtha.2011.06.044. [DOI] [PubMed] [Google Scholar]
- Ding ZH, Dong J, Liu J, Deng SX. Preferential gene expression in the limbus of the vervet monkey. Mol Vis. 2008;14(240):2031–2041. [PMC free article] [PubMed] [Google Scholar]
- Dorà N, Ou J, Kucerova R, Parisi I, West JD, Collinson JM. PAX6 dosage effects on corneal development, growth and wound healing. Dev Dyn. 2008;237(5):1295–1306. doi: 10.1002/dvdy.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvaras P, Webb S, Whitaker DA, Dorà N, Hill RE, Dorin JR, West JD. Rare corneal clones in mice suggest an age-related decrease of stem cell activity and support the limbal epithelial stem cell hypothesis. Stem Cell Res. 2012;8:109–119. doi: 10.1016/j.scr.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Du YD, Funderburgh ML, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23(9):1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44(5):415–425. doi: 10.1016/s0039-6257(00)00109-0. doi:10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- Dua HS, Miri A, Alomar T, Yeung AM, Said DG. The role of limbal stem cells in corneal epithelial maintenance testing the dogma. Ophthalmology. 2009;116(5):856–863. doi: 10.1016/j.ophtha.2008.12.017. doi:10.1016/j.ophtha.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89(5):529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Zoltick PW, Chung DC, Bennett J, Radu A, Muvarak N, Flake AW. Gene transfer to ocular stem cells by early gestational intraamniotic injection of lentiviral vector. Mol Therap. 2007;15(3):579–587. doi: 10.1038/sj.mt.6300092. [DOI] [PubMed] [Google Scholar]
- Figueira EC, Di Girolamo N, Coroneo MT, Wakefield D. The phenotype of limbal epithelial stem cells. Invest Ophthalmol Vis Sci. 2007;48(1):144–156. doi: 10.1167/iovs.06-0346. [DOI] [PubMed] [Google Scholar]
- Friedenwald JS. Growth pressure and metaplasia of conjunctival and corneal epithelium. Doc Ophthalmol. 1951;5-6:184–192. doi: 10.1007/BF00143661. doi:10.1007/bf00143661. [DOI] [PubMed] [Google Scholar]
- Funderburgh ML, Du YQ, Mann MM, SundarRaj N, Funderburgh JL. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005;19(7):1371–1373. doi: 10.1096/fj.04-2770fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46(11):4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Gaspar-Maia A, Alajem A, Polesso F, Sridharan R, Mason MJ, Heidersbach A, Ramalho-Santos J, McManus MT, Plath K, Meshorer E, Ramalho-Santos M. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature. 2009;460(7257):863–868. doi: 10.1038/nature08212. doi:10.1038/nature08212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MF, Bron AJ. Limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1982;80:155–171. [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. doi:10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna C, O’Brien JE. Cell production and migration in the epithelial layer of the cornea. Arch Ophthal. 1960;64(4):536–539. doi: 10.1001/archopht.1960.01840010538009. [DOI] [PubMed] [Google Scholar]
- Haustein J. On the ultrastructure of the developing and adult mouse corneal stroma. Anat Embryol. 1983;168(2):291–305. doi: 10.1007/BF00315823. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Yamato M, Sugiyama H, Sumide T, Yang J, Okano T, Tano Y, Nishida K. N-cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells. 2007;25(2):289–296. doi: 10.1634/stemcells.2006-0167. doi:10.1634/stemcells.2006-0167. [DOI] [PubMed] [Google Scholar]
- He SH, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. In: Ann Rev Cell Dev Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. doi:10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- Hesse M, Zimek A, Weber K, Magin TM. Comprehensive analysis of keratin gene clusters in humans and rodents. Eur J Cell Biol. 2004;83(1):19–26. doi: 10.1078/0171-9335-00354. [DOI] [PubMed] [Google Scholar]
- Holland EJ, Djalilian AR, Schwartz GS. Management of aniridic keratopathy with keratolimbal allograft: A limbal stem cell transplantation technique. Ophthalmology. 2003;110(1):125–130. doi: 10.1016/s0161-6420(02)01451-3. [DOI] [PubMed] [Google Scholar]
- Huang AJW, Tseng SCG. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32(1):96–105. [PubMed] [Google Scholar]
- Hutt JA, O’Rourke JP, DeWille J. Signal transducer and activator of transcription 3 activates CCAAT enhancer-binding protein delta gene transcription in G(0) growth-arrested mouse mammary epithelial cells and in involuting mouse mammary gland. J Biol Chem. 2000;275(37):29123–29131. doi: 10.1074/jbc.M004476200. doi:10.1074/jbc.M004476200. [DOI] [PubMed] [Google Scholar]
- Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19(1):113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu YP, Yang ZX, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11(12):1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, Van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40(11):1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jones MA, Marfurt CF. Sympathetic stimulation of corneal epithelial proliferation in wounded and nonwounded rat eyes. Invest Ophthalmol VisSci. 1996;37(13):2535–2547. [PubMed] [Google Scholar]
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22(3):359–389. doi: 10.1016/s1350-9462(02)00065-4. doi:10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Kaur P, Potten CS. The interfollicular epidermal stem cell saga: sensationalism versus reality check. Exp Dermatol. 2011;20(9):697–702. doi: 10.1111/j.1600-0625.2011.01338.x. doi:10.1111/j.1600-0625.2011.01338.x. [DOI] [PubMed] [Google Scholar]
- Kawakita T, Higa K, Shimmura S, Tomita M, Tsubota K, Shimazaki J. Fate of corneal epithelial cells separated from limbus in vivo. Invest Ophthalmol Vis Sci. 2011;52(11):8132–8137. doi: 10.1167/iovs.11-7984. doi:10.1167/iovs.11-7984. [DOI] [PubMed] [Google Scholar]
- Kenyon KR, Tseng SCG. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96(5):709–723. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Friend J, Thoft RA. Sex chromatin of donor corneal epithelium in rabbits. Invest Ophthalmol Vis Sci. 1981;21(3):434–441. [PubMed] [Google Scholar]
- Klein AM, Nakagawa T, Ichikawa R, Yoshida S, Simons BD. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell. 2010;7(2):214–224. doi: 10.1016/j.stem.2010.05.017. doi:10.1016/j.stem.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Klein AM, Simons BD. Universal patterns of stem cell fate in cycling adult tissues. Development. 2011;138(15):3103–3111. doi: 10.1242/dev.060103. [DOI] [PubMed] [Google Scholar]
- Krulova M, Pokorna K, Lencova A, Fric J, Zajicova A, Filipec M, Forrester JV, Holan V. A rapid separation of two distinct populations of mouse corneal epithelial cells with limbal stem cell characteristics by centrifugation on Percoll gradient. Invest Ophthalmol Vis Sci. 2008;49(9):3903–3908. doi: 10.1167/iovs.08-1987. doi:10.1167/iovs.08-1987. [DOI] [PubMed] [Google Scholar]
- Kruse FE. Stem cells and corneal epithelial regeneration. Eye. 1994;8:170–183. doi: 10.1038/eye.1994.42. [DOI] [PubMed] [Google Scholar]
- Kucerova R, Dorà N, Mort RL, Wallace K, Leiper LJ, Lowes C, Neves C, Walczysko P, Bruce F, Fowler PA, Rajnicek AM, McCaig CD, Zhao M, West JD, Collinson JM. Interaction between hedgehog signalling and PAX6 dosage mediates maintenance and regeneration of the corneal epithelium. Mol Vis. 2012;18:139–150. [PMC free article] [PubMed] [Google Scholar]
- Kulkarni BB, Tighe PJ, Mohammed I, Yeung AM, Powe DG, Hopkinson A, Shanmuganathan VA, Dua HS. Comparative transcriptional profiling of the limbal epithelial crypt demonstrates its putative stem cell niche characteristics. BMC Genomics. 2010;11:526. doi: 10.1186/1471-2164-11-526. doi: 10.1186/1471-2164-11-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauweryns B, Vandenoord JJ, Devos R, Missotten L. A new epithelial-cell type in the human cornea. Invest Ophthalmol Vis Sci. 1993;34(6):1983–1990. [PubMed] [Google Scholar]
- Lavker RM, Dong G, Cheng SZ, Kudoh K, Cotsarelis G, Sun TT. Relative proliferative rates of limbal and corneal epithelia - Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Invest Ophthalmol Vis Sci. 1991;32(6):1864–1875. [PubMed] [Google Scholar]
- Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998;111:2867–2875. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- Leiper LJ, Walczysko P, Kucerova R, Ou JX, Shanley LJ, Lawson D, Forrester JV, McCaig CD, Zhao M, Collinson JM. The roles of calcium signaling and ERK1/2 phosphorylation in a PAX6+/− mouse model of epithelial wound-healing delay. BMC Biol. 2006;4:27. doi: 10.1186/1741-7007-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemp MA, Mathers WD. Corneal epithelial cell movement in humans. Eye. 1989;3:438–445. doi: 10.1038/eye.1989.65. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21(7):1358–1366. doi: 10.1096/fj.06-6926com. doi:10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- Li LH, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–545. doi: 10.1126/science.1180794. doi:10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Chen YT, Hayashida Y, Blanco G, Kheirkah A, He H, Chen SY, Liu CY, Tseng SCG. Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J Pathol. 2008;214(1):114–122. doi: 10.1002/path.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Hayashida Y, Chen YT, Tseng SCG. Niche regulation of corneal epithelial stem cells at the limbus. Cell Research. 2007;17(1):26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330(6005):822–825. doi: 10.1126/science.1196236. doi:10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- Lu H, Zimek A, Chen J, Hesse M, Bussow H, Weber K, Magin TM. Keratin 5 knockout mice reveal plasticity of keratin expression in the corneal epithelium. Eur J Cell Biol. 2006;85(8):803–811. doi: 10.1016/j.ejcb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Lyngholm M, Hoyer PE, Vorum H, Nielsen K, Ehlers N, Mollgard K. Immunohistochemical markers for corneal stem cells in the early developing human eye. Exp Eye Res. 2008a;87(2):115–121. doi: 10.1016/j.exer.2008.05.004. doi:10.1016/j.exer.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Lyngholm M, Vorum H, Nielsen K, Ostergaard M, Honore B, Ehlers N. Differences in the protein expression in limbal versus central human corneal epithelium - a search for stem cell markers. Exp Eye Res. 2008b;87(2):96–105. doi: 10.1016/j.exer.2008.05.001. doi:10.1016/j.exer.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Mackman G, Brightbill F, Optiz J. Corneal changes in aniridia. Am J Ophthalmol. 1979;8:497–502. doi: 10.1016/0002-9394(79)90238-1. [DOI] [PubMed] [Google Scholar]
- Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456(7219):250–255. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: Current views and future potential. Physiol Rev. 2005;85(3):943–978. doi: 10.1152/physrev.00020.2004. doi:10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- McGowan SL, Edelhauser HF, Pfister RR, Whikehart DR. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol Vis. 2007;13(223-27):1984–2000. [PubMed] [Google Scholar]
- McKenna CC, Lwigale PY. Innervation of the mouse cornea during development. Invest Ophthalmol Vis Sci. 2011;52(1):30–35. doi: 10.1167/iovs.10-5902. doi:10.1167/iovs.10-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Zheng BH, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398(6729):708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, He SH, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19(12):1432–1437. doi: 10.1101/gad.1299505. doi:10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425(6961):962–967. doi: 10.1038/nature02060. doi:10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JE, McMullen CBT, Mahon G, Adamis AP. The corneal epithelial stem cell. DNA and Cell Biology. 2002;21(5-6):443–451. doi: 10.1089/10445490260099737. [DOI] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311(5769):1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Mort RA. Stem cells function in the mouse corneal epithelium. University of Edinburgh; 2007. PhD thesis. [Google Scholar]
- Mort RL, Bentley AJ, Martin FL, Collinson JM, Douvaras P, Hill RE, Morley SD, Fullwood NJ, West JD. Effects of aberrant Pax6 gene dosage on mouse corneal pathophysiology and corneal epithelial homeostasis. PLoS ONE. 2011;6(12):e28895. doi: 10.1371/journal.pone.0028895. doi:doi:10.1371/journal.pone.0028895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort RL, Ramaesh T, Kleinjan DA, Morley SD, West JD. Mosaic analysis of stem cell function and wound healing in the mouse corneal epithelium. BMC Dev Biol. 2009;9:4. doi: 10.1186/1471-213X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki T, Zhao J. Centripetal movement of corneal epithelial cells in the normal adult mouse. Invest Ophthalmol Vis Sci. 2003;44(2):558–566. doi: 10.1167/iovs.02-0705. [DOI] [PubMed] [Google Scholar]
- Nagasaki T, Zhao J. Uniform distribution of epithelial stem cells in the bulbar conjunctiva. Invest Ophthalmol Vis Sci. 2005;46(1):126–132. doi: 10.1167/iovs.04-0356. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima YI, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12(2):195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Nelson LB, Spaeth GL, Nowinski TS, Margo CE, Jackson L. Aniridia - a review. Surv Ophthalmol. 1984;28(6):621–642. doi: 10.1016/0039-6257(84)90184-x. doi:10.1016/0039-6257(84)90184-x. [DOI] [PubMed] [Google Scholar]
- Nieto-Miguel T, Calonge M, de la Mata A, Lopez-Paniagua M, Galindo S, de la Paz MF, Corrales RM. A comparison of stem cell-related gene expression in the progenitor-rich limbal epithelium and the differentiating central corneal epithelium. Mol Vis. 2011;17(229-31):2102–2117. [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Kinoshita S, Ohashi Y, Kuwayama Y, Yamamoto S. Ocular surface abnormalities in aniridia. Am J Ophthalmol. 1995;120(3):368–375. doi: 10.1016/s0002-9394(14)72167-1. [DOI] [PubMed] [Google Scholar]
- Norman B, Davis J, Piatigorsky J. Postnatal gene expression in the normal mouse cornea by SAGE. Invest Ophthalmol Vis Sci. 2004;45(2):429–440. doi: 10.1167/iovs.03-0449. doi:10.1167/iovs.03-0449. [DOI] [PubMed] [Google Scholar]
- Notara M, Schrader S, Daniels JT. The porcine limbal epithelial stem cell niche as a new model for the study of transplanted tissue-engineered human limbal epithelial cells. Tissue Eng Pt A. 2011;17(5-6):741–750. doi: 10.1089/ten.TEA.2010.0343. doi:10.1089/ten.TEA.2010.0343. [DOI] [PubMed] [Google Scholar]
- Notara M, Shortt AJ, O’Callaghan AR, Daniels JT. The impact of age on the physical and cellular properties of the human limbal stem cell niche. Age. 2012 doi: 10.1007/s11357-011-9359-5. Published online 18 January 2012. doi:10.1007/s11357-011-9359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke JP, Newbound GC, Hutt JA, DeWille J. CCAAT/enhancer-binding protein delta regulates mammary epithelial cell G(0) growth arrest and apoptosis. J Biol Chem. 1999;274(23):16582–16589. doi: 10.1074/jbc.274.23.16582. doi:10.1074/jbc.274.23.16582. [DOI] [PubMed] [Google Scholar]
- Ou J, Walczysko P, Kucerova R, Rajnicek AM, McCaig CD, Zhao M, Collinson JM. Chronic wound state exacerbated by oxidative stress in PAX6+/− aniridia-related keratopathy. J Pathol. 2008;215(4):421–430. doi: 10.1002/path.2371. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Ghosh SP, Stepp MA. Regional distribution of α9β1 integrin within the limbus of the mouse ocular surface. Dev Dynam. 2004;230(3):518–528. doi: 10.1002/dvdy.20050. [DOI] [PubMed] [Google Scholar]
- Pal-Ghosh S, Pajoohesh-Ganji A, Brown M, Stepp MA. A mouse model for the study of recurrent corneal epithelial erosions: α9β1 integrin implicated in progression of the disease. Invest Ophthalmol Vis Sci. 2004;45(6):1775–1788. doi: 10.1167/iovs.03-1194. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian DL, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423(6937):302–305. doi: 10.1038/nature01587. doi:10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Parsa R, Yang A, McKeon F, Green H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol. 1999;113(6):1099–1105. doi: 10.1046/j.1523-1747.1999.00780.x. doi:10.1046/j.1523-1747.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98(6):3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145(4):769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties - Lessons for and from the crypt. Development. 1990;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Pratt T, Sharp L, Nichols T, Price DJ, Mason JO. Embryonic stem cells and transgenic mice ubiquitously expressing a tau-tagged green fluorescent protein. Dev Biol. 2000;228(1):19–28. doi: 10.1006/dbio.2000.9935. [DOI] [PubMed] [Google Scholar]
- Qi H, Li DQ, Shine HD, Chen Z, Yoon KC, Jones DB, Pflugfelder SC. Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Exp Eye Res. 2008;86(1):34–40. doi: 10.1016/j.exer.2007.09.003. doi:10.1016/j.exer.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. New Eng J Med. 2010;363(2):147–155. doi: 10.1056/NEJMoa0905955. doi:10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- Ramaesh T, Collinson JM, Ramaesh K, Kaufman MH, West JD, Dhillon B. Corneal abnormalities in PAX6+/− small eye mice mimic human aniridia-related keratopathy. Invest Ophthalmol Vis Sci. 2003;44(5):1871–1878. doi: 10.1167/iovs.02-0576. [DOI] [PubMed] [Google Scholar]
- Ramaesh T, Ramaesh K, Collinson JM, Chanas SA, Dhillon B, West JD. Developmental and cellular factors underlying corneal epithelial dysgenesis in the PAX6+/− mouse model of aniridia. Exp Eye Res. 2005;81(2):224–235. doi: 10.1016/j.exer.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ramaesh T, Ramaesh K, Leask R, Springbett A, Riley SC, Dhillon B, West JD. Increased apoptosis and abnormal wound-healing responses in the heterozygous PAX6+/− mouse cornea. Invest Ophthalmol Vis Sci. 2006;47(5):1911–1917. doi: 10.1167/iovs.05-1028. [DOI] [PubMed] [Google Scholar]
- Ren HW, Wilson G. The cell shedding rate of the corneal epithelium - A comparison of collection methods. Curr Eye Res. 1996;15(10):1054–1059. doi: 10.3109/02713689609017655. [DOI] [PubMed] [Google Scholar]
- Romano AC, Espana EM, Yoo SH, Budak MT, Wolosin JM, Tseng SCG. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci. 2003;44(12):5125–5129. doi: 10.1167/iovs.03-0628. doi:10.1167/iovs.03-0628. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40(7):915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl A, Ross A, Lee M, Engelkamp D, Rashbass P, van Heyningen V, Hastie ND. Influence of Pax6 gene dosage on development - overexpression causes severe eye abnormalities. Cell. 1996;86(1):71–82. doi: 10.1016/s0092-8674(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64k corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81(3):247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Schultz G, Khaw PT, Oxford K, Macauley S, Vansetten G, Chegini N. Growth factors and ocular wound healing. Eye. 1994;8:184–187. doi: 10.1038/eye.1994.43. [DOI] [PubMed] [Google Scholar]
- Sharma A, Coles WH. Kinetics of corneal epithelial maintenence and graft loss - A population balance model. Invest Ophthal Vis Sci. 1989;30(9):1962–1971. [PubMed] [Google Scholar]
- Sherwin T. A new niche for the corneal epithelial stem cell. Clin Exp Ophthalmol. 2009;37(7):644–645. doi: 10.1111/j.1442-9071.2009.02138.x. doi:10.1111/j.1442-9071.2009.02138.x. [DOI] [PubMed] [Google Scholar]
- Shortt AJ, Secker GA, Limb GA, Khaw PT, Daniels JT. Transplantation of ex vivo cultured limbal epithelial stem cells: A review of techniques and clinical results. Surv Ophthalmol. 2007a;52(5):483–502. doi: 10.1016/j.survophthal.2007.06.013. doi:10.1016/j.survophthal.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Shortt AJ, Secker GA, Munro PM, Khaw PT, Tuft SJ, Daniels JT. Characterization of the limbal epithelial stem cell niche: Novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007b;25(6):1402–1409. doi: 10.1634/stemcells.2006-0580. doi:10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- Shortt AJ, Tuft SJ, Daniels JT. Coneal stem cells in the eye clinic. Br Med Bull. 2011;100(1):209–225. doi: 10.1093/bmb/ldr041. doi:10.1093/bmb/ldr041. [DOI] [PubMed] [Google Scholar]
- Sivak JM, West-Mays JA, Yee A, Williams T, Fini ME. Transcription factors Pax6 and AP-2 alpha interact to coordinate corneal epithelial repair by controlling expression of matrix metalloproteinase gelatinase B. Mol Cell Biol. 2004;24(1):245–257. doi: 10.1128/MCB.24.1.245-257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Sundberg JP, John SWM. The anterior segment and ocular adnexae. In: Smith RS, editor. Systematic Evaluation of the Mouse Eye. Anatomy, Pathology and Biomethods. CRC Press; Boca Raton, Florida: 2002. pp. 1–23. [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143(1):134–144. doi: 10.1016/j.cell.2010.09.016. doi:10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Zhu L, Sheppard D, Cranfill RL. Localized distribution of α9 integrin in the cornea and changes in expression during corneal epithelial cell differentiation. J Histoch Cytochem. 1995;43(4):353–362. doi: 10.1177/43.4.7534781. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Saito J, Yanai R, Yamada N, Chikama T, Seki K, Nishida T. Cell-matrix, and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003;22(2):113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Takacs L, Toth E, Berta A, Vereb G. Stem cells of the adult cornea: From cytometric markers to therapeutic applications. Cytometry Part A. 2009;75A(1):54–66. doi: 10.1002/cyto.a.20671. [DOI] [PubMed] [Google Scholar]
- Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 2000;97(20):10960–10965. doi: 10.1073/pnas.97.20.10960. doi:10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanifuji-Terai N, Terai K, Hayashi Y, Chikama TI, Kao WWY. Expression of keratin 12 and maturation of corneal epithelium during development and postnatal growth. Invest Ophthalmol Vis Sci. 2006;47(2):545–551. doi: 10.1167/iovs.05-1182. [DOI] [PubMed] [Google Scholar]
- Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24(10):1442–1443. [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong K, Rangell L, Klein O, de Sauvage F. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478(7368):255–259. doi: 10.1038/nature10408. doi:10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend W. The limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1991;89:721–756. [PMC free article] [PubMed] [Google Scholar]
- Tseng SCG. Concept and application of limbal stem cells. Eye. 1989;3:141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner HC, Budak MT, Akinci MAM, Wolosin JM. Comparative analysis of human conjunctival and corneal epithelial gene expression with oligonucleotide microarrays. Invest Ophthalmol Vis Sci. 2007;48(5):2050–2061. doi: 10.1167/iovs.06-0998. doi:10.1167/iovs.06-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem Cells. 2006;24(1):86–94. doi: 10.1634/stemcells.2005-0064. doi:10.1634/stemcells.2005-0064. [DOI] [PubMed] [Google Scholar]
- Van der Lugt NMT, Domen J, Linders K, Vanroon M, Robanusmaandag E, Teriele H, Vandervalk M, Deschamps J, Sofroniew M, Vanlohuizen M, Berns A. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the Bmi-1 protooncogene. Genes Dev. 1994;8(7):757–769. doi: 10.1101/gad.8.7.757. doi:10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- Vantrappen L, Geboes K, Missotten L, Maudgal PC, Desmet V. Lymphocytes and Langerhans cells in the normal human cornea. Invest Ophthalmol Vis Sci. 1985;26(2):220–225. [PubMed] [Google Scholar]
- Wiley L, Sundarraj N, Sun TT, Thoft RA. Regional heterogeneity in human corneal and limbal epithelia - An immunohistochemical evaluation. Invest Ophthalmol Vis Sci. 1991;32(3):594–602. [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Ambrosio R, Hong JW, Lee JS. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20(5):625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Netto M, Ambrosio R. Corneal cells: Chatty in development, homeostasis, wound healing, and disease. Am J Ophthalmol. 2003;136(3):530–536. doi: 10.1016/s0002-9394(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Wolosin JM, Xiong XL, Schutte M, Stegman Z, Tieng A. Stem cells and differentiation stages in the limbo-corneal epithelium. Prog Retin Eye Res. 2000;19(2):223–255. doi: 10.1016/s1350-9462(99)00005-1. doi:10.1016/s1350-9462(99)00005-1. [DOI] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li XN, Ootani A, Su J, Lee JY, Su N, Luo YL, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA. 2012;109(2):466–471. doi: 10.1073/pnas.1118857109. doi:10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, McKeon F. P63 and p73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol. 2000;1(3):199–207. doi: 10.1038/35043127. doi:10.1038/35043127. [DOI] [PubMed] [Google Scholar]
- Yang AN, Kaghad M, Wang YM, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2(3):305–316. doi: 10.1016/s1097-2765(00)80275-0. doi:10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Shimmura S, Kawakita T, Miyashita H, Den S, Shimazaki J, Tsubota K. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47(11):4780–4786. doi: 10.1167/iovs.06-0574. [DOI] [PubMed] [Google Scholar]
- Yu BD, Mukhopadhyay A, Wong C. Skin and hair: models for exploring organ regeneration. Hum Mol Genet. 2008;17:R54–R59. doi: 10.1093/hmg/ddn086. doi:10.1093/hmg/ddn086. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhao J, Chen L, Urbanowicz MM, Nagasaki T. Abnormal epithelial homeostasis in the cornea of mice with a destrin deletion. Mol Vis. 2008;14:1929–1939. [PMC free article] [PubMed] [Google Scholar]
- Zheng TY, Xu JJ. Age-related changes of human limbus on in vivo confocal microscopy. Cornea. 2008;27(7):782–786. doi: 10.1097/ICO.0b013e31816f5ec3. [DOI] [PubMed] [Google Scholar]
- Zhou MY, Li XM, Lavker RM. Transcriptional profiling of enriched populations of stem cells versus transient amplifying cells - A comparison of limbal and corneal epithelial basal cells. J Biol Chem. 2006;281(28):19600–19609. doi: 10.1074/jbc.M600777200. doi:10.1074/jbc.M600777200. [DOI] [PubMed] [Google Scholar]
- Zieske JD. Corneal development associated with eyelid opening. Int J Dev Biol. 2004;48(8-9):903–911. doi: 10.1387/ijdb.041860jz. doi:10.1387/ijdb.041860jz. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Bukusoglu G, Yankauckas MA, Wasson ME, Keutmann HT. Enolase is restricted to basal cells of stratified squamous epithelium. Dev Biol. 1992;151(1):18–26. doi: 10.1016/0012-1606(92)90209-y. doi:10.1016/0012-1606(92)90209-y. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Francesconi CM, Guo XQ. Cell cycle regulators at the ocular surface. Exp Eye Res. 2004;78(3):447–456. doi: 10.1016/s0014-4835(03)00205-7. doi:10.1016/s0014-4835(03)00205-7. [DOI] [PubMed] [Google Scholar]