Abstract
Purpose of review
This review focuses on recent advances in functional connectivity MRI and renewed interest in knowing the large-scale functional network assemblies in the brain. We also consider some methodological aspects of graph theoretical analysis.
Recent findings
Network science applied to neuroscience is quickly growing in recent years. The characterization of the functional connectomes in normal and pathological brain conditions is now a priority for researchers in the neuropsychiatric field and current findings have provided new insights regarding the pivotal role of network epicenters and specific configurations of the functional networks in the brain.
Summary
Functional connectivity and its analytical tools are providing organization of the functional brain that will be key for the understanding of pathologies in neurology.
Keywords: functional connectivity, neuroimaging, brain network, graph theory
Introduction
During the last decade, we have witnessed the discovery and characterization of large-scale functional networks in the living human brain. Using analytical techniques in functional MRI such as independent component analysis [1], clustering [2, 3] and graph theory [4–7], neuroscientists have been able to disentangle key coherent systems of the brain chronoarchitecture. For instance, a paradigmatic finding is the functional dominance of the Default Mode Network (DMN) associated with internal mentation states [11] -uncovered by Raichle and others [8] and whose anatomical connections were previously pointed out in non-human primate studies [9, 10]-. Even though neuroimaging has traditionally focused on task-based studies, the analysis of resting-state MRI has expanded our ability to complement anatomical and structural findings by revealing the functional delimitations of the brain systems. In this sense, the analysis of the synchrony of spontaneous low-frequency (<0.1 Hz) blood oxygen level-dependent fluctuations [12–14] has demonstrated to be especially useful in the field of human brain connectomics [15–18].
The enormous effort of describing distinct segregated functional systems through parcelation schemes is now associated with a renewed interest in knowing the merging structure of functional networks of the human brain. For instance, it is still a matter of controversy how the articulations and transitions from modular sensory regions to parallel-organized heteromodal or limbic processing systems take place or how the high-cognitive functional networks of the brain integrate between each other. This review aims to examine recent representative examples of studies that have focused on the analysis of connectional fingerprints for the assemblies of brain networks and to expose some methodological concerns in functional connectivity MRI.
Network Science and Graph Theory
The working idea of the brain organized into large-scale functional networks has a long history. Classical anatomo-pathological studies that correlated clinical and neuropsychological data with brain injuries brought the idea of “disconnection syndromes” (see reviews in [16, 19]); an idea that has reemerged in the field of structural connectomics in neuroimaging ([20]). On the other hand, seminal works using invasive techniques, such as anatomic tract tracing and ablation methods in non-human primates, pointed to large networks that presumably subserve behaviors and integrative functions in their cytoarchitectonically equivalent and expanded regions in humans [9, 10, 21, 22]. A recent addition to this line of research is the development of network analytical tools applied to functional neuroimaging in an attempt to capture the complex interactions displayed by brain regions. For instance, graph theory has attracted researchers mostly due to its ability to reduce the dimensionality of the brain and to convert complex problems in tractable analytical frameworks by defining nodes (brain regions) and edges (coupling between brain regions). Unlike other methods mostly based on the aim of segregating brain networks, such as conventional ICA or K-means [3, 23], graph theory is especially appropriate to visualize and characterize the network interactions and topological merging between systems [23–25]. In fact, the ability to portray the brain as a whole network and our ability to visualize the network interactions is what many neuroscientists have envisioned for a long time. The brain can be only fully explained by itself and by doing cerebral graphs we are building “mediocre brains”-mimicking Turing’s famous statement-. However, functional connectivity MRI (fcMRI) graph theory approaches are probably one the most complete methods so far that we can use to study the large-scale functional connectome and to reveal the network integration secrecies.
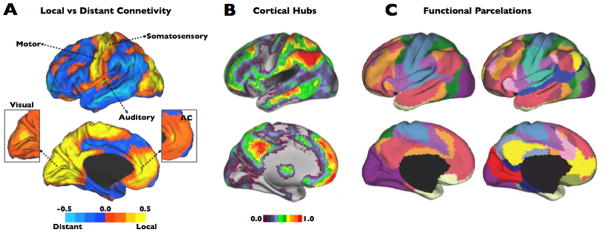
Graph theory in fcMRI is not absent of methodological problems [23, 26–30]. For instance, although used in the past in the pioneer network theory studies, now we know that anatomical definition of the nodes provides worse estimates of intrinsic functional connectivity than parcelations obtained from functional modules of the cortex [15, 23, 31]. Nodes based on anatomic parcelations seem to be poor proxies of the functional borders and they produce aberrant interpretations of the cerebral functional properties. An alternative strategy to avoid the limitations of parcelation schemes and the use of ROIs is to create the graphs at the voxel-level or at least the closest computationally efficient fraction to the image acquisition unit [32, 33] (see example at Figure 1–A and 1–B). Voxel-level graphs bring the possibility to capture the full richness of the functional connectivity variability in between and within modules and networks, especially covering the transitions between functional modules. For instance, there is evidence that even local modules, such as the visual cortex, have unique degree and functional connectivity profiles in a neighboring voxel-to-voxel basis [3, 5] that presumably supports distinctive unimodal functions. Moreover, it is well-known that functional modules overlap between them [34] and parcelation schemes change with different parameter set-ups and threshold constraints (see for instance Figure 1–C) creating difficult scenarios for a gold-standard definition of the functional borders. Some groups have argued that the voxel-level graphs are suboptimal representations of the brain regions because the functional brain areas vary in size relative to one another and therefore it is more accurate to select specific ROIs based on functional modularity criteria [23]. Although functional defined ROIs are also valid approaches, it is important to notice that voxel-level graphs introduce the size information in the graphs by representing each functional region with the corresponding number of voxels. Moreover, they have the advantage of incorporating meaningful within modular heterogeneity information of the brain regions and avoid the simplification and homogenizing of the network functional modules. In a related issue, it is important to remark that the inclusion of ROIs, sometimes rather big ROIs, could cause misinterpretations about the local processing of the brain. Local network properties, at least at the level of neuroimaging resolution acquisition, can only be inferred if small units, such as voxels of mm size, are included in the graph.
Figure 1.
The human brain is organized in regions of predominant local and distant functional connectivity (A). Local hubs are located in primary and secondary information processing regions and also in an ACC region previously associated with self and interceptive information processing (adapted from [5]). The cortical hubs of the human brain associate widespread brain regions most likely supporting the integration of cognitive functions (B) (adapted from [32]). Example of a recent study separating distinct set of functional connectivity networks at different levels of clustering (C; partition in 7 networks in left side; partition in 17 networks in right side) (adapted from [3]).
Finally, another methodological concern that has been elegantly remarked in recent times is movement correction. It has been consistently proven by independent groups that conventional correction of subjects’ motion may not be sufficient to avoid important bias in fcMRI studies when comparing different study samples such as young adults, elderly adults, children or neuropsychiatric samples [26, 29]. More research is needed to fully understand the consequences of movement in functional connectivity metrics but we certainly need to handle very carefully the correction of movement to avoid potential confounds in the results of the studies.
Assembly of Brain Networks
Currently, we are facing the challenge of understanding the fine assembly of the large-scale functional networks in the brain. Anatomical and functional findings suggest the existence of modal areas with dense local connectivity [5, 21, 35–37] (see an example in Figure 1–A), as well as cortical hubs that associate widespread brain regions [5, 10, 22, 32, 38–40] (Figure 1–B), most likely supporting the integration of high cognitive functions [6, 32, 41–43]. However, there is increased interest in knowing how these two organizational extremes merge together to integrate the internal and external world information and also the interdigitations of cognitive networks in the human brain [34, 39, 44, 45].
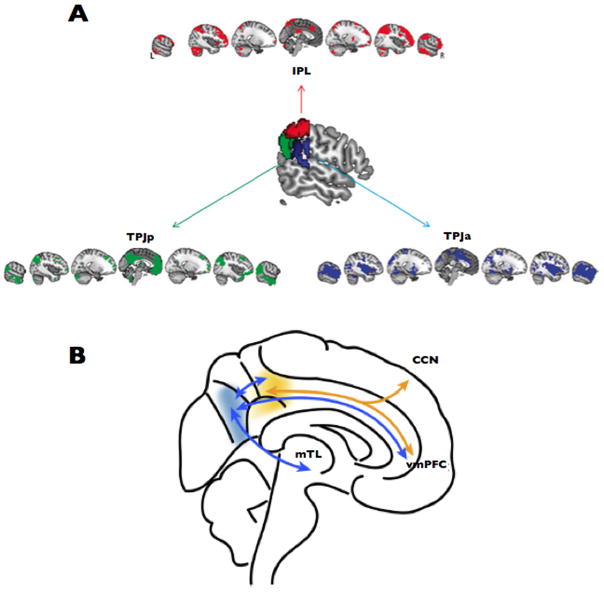
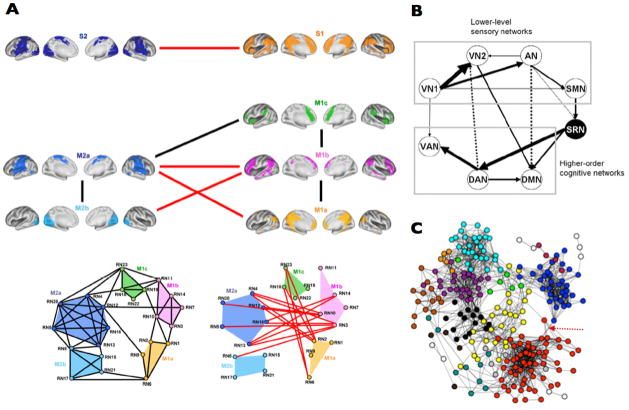
In the last two years, several notable papers have been published that directly focused on the study of the network articulation features of the brain. They used two main research strategies. First, several groups analyzed pivotal hubs or epicenters [39] of the brain in order to describe the confluence of distinct networks in strategic cortical points. For instance, Mars RB et al. described that the temporo-parietal joint subserve as critical articulator of the control, default mode and ventral attention networks [46] (Figure 2–A). On the other hand, Leech et al used ICA and fcMRI during rest and task to show that PCC/precuneus area is a functional heterogeneous region that converge internal thoughts and cognitive control systems [47] (Figure 2–B). The authors show that the dorsal region is strongly connected to “task-positive” areas within the cognitive control network, and the ventral region is more related to medial temporal lobe structures including the hippocampus. Second, another strategy used in recent investigations is to study the block assembly between brain functional networks. For instance, Doucet et al. have described hierarchical bridges between brain modules [48] (Figure 3–A). They found that primary cortices and dorsal attentional networks (or “extrinsic” systems) are located in one extreme of the hierarchy, while three networks, the generation of spontaneous thoughts (DMN), inner maintenance and manipulation of information, and cognitive control and switching activity networks (or the “intrinsic” systems) are placed in the other hierarchical extreme. Moreover, the cognitive control system seems to mediate exchange of information between the intrinsic and extrinsic systems. On the other hand, Li et al used ICA and a Bayesian network learning approach to describe that a ventral attention/salience network interrelates, in a great extend, the communications between primary cortices and high order cognitive networks such as the DMN [49] (Figure 3–B), supporting a sensory-fugal interpretation of the overall brain organization. A similar finding can be also visualized in a study that uses graph theory analysis [50] (Figure 3–C). Primary cortex and DMN nodes occupy peripheral parts of the graph and other networks, such as attentional and control networks, inter-locates in-between them. Of note, it is interesting to see that primary cortex and DMN modules would be in opposite network extremes if only one link is released in the network structure (arrow in Figure 3–C). This connectivity is probably due to the physical proximity between visual and PCC/precuneus cortices (same issue is noticeable in Figure 3–A). Overall the data these studies support a hierarchical organization from the modal to high order cognitive hubs and the in-between modulation of multimodal and ventral attention networks.
Figure 2.
The temporo-parietal joint is a pivotal hub for the control, default mode and ventral attention networks (A) (adapted from [46]). PCC/precuneus is pivotal hub for the convergence of internal thoughts and cognitive control systems (B) (adapted from [47]).
Figure 3.
Recent findings in the literature have described in more detail the hierarchical organization of the human brain, from modal cortices to attentional networks and high order cognitive networks. Three examples of the functional connectivity structure between networks are shown in (A; adapted from [48]), (B; adapted from [49]) and (C; adapted from [50]).
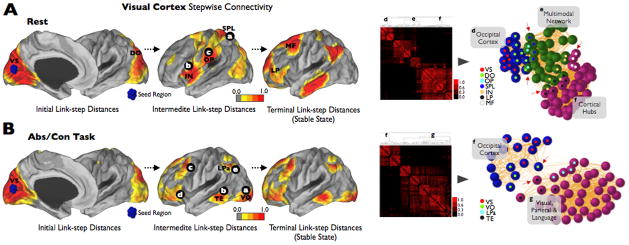
The idea of attentional networks as involved in the dynamical role of allocating brain information flows from primary cortices to the appropriate higher order cognitive network has been proposed before. For instance, the salience-awareness and ventral attentional networks have been specifically postulated in the switching between the DMN and control network [51–53] when relevant intrapersonal or extrapersonal events take place. Furthermore, evidence from the stepwise connectivity profile of primary systems, such as the visual cortex, supports that attentional and multimodal areas of the brain are in deed playing a role in the transfer of primary information to the cognitive cortical hubs of the brain (letters a, b and c in Figure 4–A).
Figure 4.
(A) shows that primary systems, such as the visual cortex, merge the cortical hubs of the human brain through attentional and multimodal areas of the human brain (areas previously reported, for instance, in [52, 62]). (B) shows that task activity change the linkscapes of the brain and produce alternative connectivity pathways specific to task contingencies. In (A) and (B) we use a stepwise functional connectivity (SFC) approach in which the degree of stepwise connectivity (Djl) is computed from the count of all paths that connect voxel j to a seed voxel i (V1 region) in an exactly length of l (where l is the specific link-step distance). To aid visualization, all surface SFC images were displayed using a normalized color scale from 0 to 1, where 0 is the intensity corresponding to the one-sample t-test p-value of 0.0001 and 1 is the maximum intensity of the image corresponding to the smallest one-sample t-test p-value. We used Pajek software [63] and Kamada-Kawai energy layout [64] for the network graphs displays. Nodes were obtained from all main results displayed in the cortical maps. We used an average-linkage hierarchical clustering analysis to highlight the modules of the networks. Red arrows show the main merging points between networks. Only nodes that are key in the transition between networks are labeled in the cortical maps and graphs. VS: visual seed region; DO: dorsal occipital; OP: operculum parietale; SPL: superior parietal lobe; IN: insula; LP: lateral prefrontal; MF: mid Frontal; VO: ventral occipital; LPa: lateral parietal; TE: Temporal.
Another piece of information pointed out by Spreng et al. is that the control network seems to be interfacing the DMN and goal directed attentional network [54] and probably mediating the equilibrium of internal-external goal-directed cognition in a more top-down manner [54, 55]. In summary, if salience network is guiding the active awareness state by modulating the mutually exclusive dominance of the DMN or control networks depending on external stimuli, the control network may drive individuals either to an active goal directed attention state or a resting-state depending on mental internal stimuli.
Finally, it is important to highlight that a major challenge for functional connectivity researchers is to understand the synchrony changes of the whole-brain network structure during task states. Figure 4–B shows an example of the stepwise connectivity of the visual cortex in a verbal task (continuous abstract-concrete task [5]). The network structures at rest (Figure 4–A) and task (Figure 4–B) present different transition and dominant states and show the dynamic adaptability of synchrony patterns due to task environmental contingencies. The connectivity pattern of the verbal task shows, for instance, that not all visual presentation of stimuli have inevitably to flow through ventral or dorsal attentional networks (Figure 4–B). There are alternatives to merge external and internal information and one of them, at least for visual stimuli of verbal content, seems to be through a ventral path from visual to temporal areas (letter a and b in Figure 4–B) that also connects to other language regions in the brain (c, d and e Figure 4–B). These results show that the brain is embedded in uninterrupted dynamical changes of connectivity. Not surprisingly it has been postulated that it is constantly on the edge of chaos [19, 56, 57], moving between different network states in search for dominant “stabilities”. In this sense, task changes may induce perturbations in more stable states such as the DMN, probably induced by local emergence of coupling patterns [5]. However, further investigations are needed to empirically prove, for instance, that DMN act as stable state or main attractor of the brain systems.
Conclusions
Many neuropsychiatric disorders are seen today more than ever as brain network pathologies. To properly study the network changes in disease conditions it is imperative to know the normal assemblies of the large-scale network structure in the normal conditions of the human brain. The emerging tools of functional connectivity and graph theory in neuroimaging offer a good scenario for uncovering complex mechanisms underlying disorders [19, 32, 58–60]. Currently, there is an axiom that is crystallizing in the researcher’s mind, not only the neurons that “fire together, wire together” (as Donald Hebb popularized) but also it seems that the neurons that “wire together, die together”. We anticipate that this framework, and in particular the understandings of networks assemblies, is going to provide important insights in different fields of neurology in the near future. However to produce upcoming moments of clarity, network science in neuroimaging needs to evolve from its infantile age to a more mature landscape by integrating functional, structural-anatomical and experimental data [61].
Acknowledgments
We thank Randy L. Buckner and Fenna Krienen for generously providing the raw data used in Figure 4. This work was supported by the Alzheimer’s Association (NIRG-11-205690 to JS; ZEN-10-174210 to KAJ) and the NIH (K25EB013649-01 to MRS; R01AG037497 and R01AG036694 to KAJ).
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–52. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 2.Bellec P, et al. Multi-level bootstrap analysis of stable clusters in resting-state fMRI. Neuroimage. 2010;51(3):1126–39. doi: 10.1016/j.neuroimage.2010.02.082. [DOI] [PubMed] [Google Scholar]
- 3.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–40. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- 5*.Sepulcre J, et al. The organization of local and distant functional connectivity in the human brain. PLoS Comput Biol. 2010;6(6):e1000808. doi: 10.1371/journal.pcbi.1000808. This paper describes the organization of local and distant functional connectivity in the human brain and suggests that the brain has evolved a physical connectivity balance that optimizes information-processing efficiency across different classes of specialized areas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sporns O, et al. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8(9):418–25. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Sporns O, Honey CJ. Small worlds inside big brains. Proc Natl Acad Sci U S A. 2006;103(51):19219–20. doi: 10.1073/pnas.0609523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–56. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 10.Jones EG, Powell TP. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93(4):793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- 11.Gusnard DA, et al. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswal B, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 13**.Biswal BB, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107(10):4734–9. doi: 10.1073/pnas.0911855107. The paper presents the 1000 Functional Connectomes Project dataset. This repository, freely accessible dataset at www.nitrc.org/projects/fcon_1000/, is already shaping the field of fcMRI by creating a common research space for many groups around the world. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 15**.Behrens TE, Sporns O. Human connectomics. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.08.005. This paper presents a recent thorough review of the basic principles of connectomics in neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–90. doi: 10.1016/j.tics.2010.04.004. This paper provides an expert review of how cognitive functions emerge from structural and functional networks of the brain. [DOI] [PubMed] [Google Scholar]
- 17.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 18.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 19.Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol. 2009;22(4):340–7. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128(Pt 10):2224–39. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- 21.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 22.Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- 23*.Wig GS, Schlaggar BL, Petersen SE. Concepts and principles in the analysis of brain networks. Ann N Y Acad Sci. 2011;1224:126–46. doi: 10.1111/j.1749-6632.2010.05947.x. This review focuses on understanding some principles in graph theory applied to functional connectivity data. [DOI] [PubMed] [Google Scholar]
- 24.Sporns O, Honey CJ, Kotter R. Identification and classification of hubs in brain networks. PLoS ONE. 2007;2(10):e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 2007;1(1):3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Power JD, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubinov M, Sporns O. Weight-conserving characterization of complex functional brain networks. Neuroimage. 2011;56(4):2068–79. doi: 10.1016/j.neuroimage.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 28*.Van Dijk KR, et al. Intrinsic Functional Connectivity As a Tool For Human Connectomics: Theory, Properties, and Optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. This paper provides essential tips for the optimization of functional connectivity parameters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zalesky A, Fornito A, Bullmore E. On the use of correlation as a measure of network connectivity. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 31**.Smith SM, et al. Network modelling methods for FMRI. Neuroimage. 2011;54(2):875–91. doi: 10.1016/j.neuroimage.2010.08.063. Leaded by authorized experts in the field, this paper provides an excellent comparison between different network functional connectivity methods in neuroimaging. [DOI] [PubMed] [Google Scholar]
- 32.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuo XN, et al. Network Centrality in the Human Functional Connectome. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]
- 34.Ferrarini L, et al. Hierarchical functional modularity in the resting-state human brain. Hum Brain Mapp. 2009;30(7):2220–31. doi: 10.1002/hbm.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Distler C, et al. Cortical connections of inferior temporal area TEO in macaque monkeys. J Comp Neurol. 1993;334:125–150. doi: 10.1002/cne.903340111. [DOI] [PubMed] [Google Scholar]
- 36.Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3(12):2563–86. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4(2):157–65. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 38.Mesulam M. Representation, inference, and transcendent encoding in neurocognitive networks of the human brain. Ann Neurol. 2008;64(4):367–78. doi: 10.1002/ana.21534. [DOI] [PubMed] [Google Scholar]
- 39.Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6Pt 6):1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 40.Pandya DN, Kuypers HG. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13(1):13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- 41.Achard S, et al. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26(1):63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salvador R, et al. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- 43.Eguíluz VM, et al. Scale-free brain functional networks. Phys Rev Lett. 2005;14:018102. doi: 10.1103/PhysRevLett.94.018102. [DOI] [PubMed] [Google Scholar]
- 44.Bassett DS, et al. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28(37):9239–48. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meunier D, et al. Hierarchical modularity in human brain functional networks. Front Neuroinformatics. 2009;3:37. doi: 10.3389/neuro.11.037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Mars RB, et al. Connectivity-Based Subdivisions of the Human Right “Temporoparietal Junction Area”: Evidence for Different Areas Participating in Different Cortical Networks. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr268. This paper describes the network pivotal role of the temporoparietal junction area of the human brain. [DOI] [PubMed] [Google Scholar]
- 47*.Leech R, et al. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31(9):3217–24. doi: 10.1523/JNEUROSCI.5626-10.2011. This paper describes the network pivotal role of the PCC area of the human brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Doucet G, et al. Brain activity at rest: a multiscale hierarchical functional organization. J Neurophysiol. 2011;105(6):2753–63. doi: 10.1152/jn.00895.2010. This paper provides recent findings about the hierarchical structure of the human brain. [DOI] [PubMed] [Google Scholar]
- 49*.Li R, et al. Large-scale directional connections among multi resting-state neural networks in human brain: a functional MRI and Bayesian network modeling study. Neuroimage. 2011;56(3):1035–42. doi: 10.1016/j.neuroimage.2011.03.010. This paper provides recent findings about the hierarchical structure of the human brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–78. doi: 10.1016/j.neuron.2011.09.006. This paper provides recent findings about the hierarchical structure of the human brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uddin LQ, et al. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31(50):18578–89. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spreng RN, et al. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–17. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao W, et al. The Synchronization within and Interaction between the Default and Dorsal Attention Networks in Early Infancy. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitzbichler MG, et al. Broadband criticality of human brain network synchronization. PLoS Comput Biol. 2009;5(3):e1000314. doi: 10.1371/journal.pcbi.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao W, et al. Evaluating the effective connectivity of resting state networks using conditional Granger causality. Biol Cybern. 2010;102(1):57–69. doi: 10.1007/s00422-009-0350-5. [DOI] [PubMed] [Google Scholar]
- 58.Feldt S, Bonifazi P, Cossart R. Dissecting functional connectivity of neuronal microcircuits: experimental and theoretical insights. Trends Neurosci. 2011;34(5):225–36. doi: 10.1016/j.tins.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 59**.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. This is an excellent review paper that summarizes network methods for characterizing aberrant brain networks in several diseases and provides novel interpretations about the dysfunctional brain. [DOI] [PubMed] [Google Scholar]
- 60**.Seeley WW, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. This outstanding paper demonstrates that neurodegenerative disorders such as dementias target large-scale functional and structural networks in the human brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61**.Mesulam M. The evolving landscape of human cortical connectivity: Facts and inferences. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.12.033. This is an excellent opinion paper that highlight the risk of generating a soft and “parallel” network science” if structural and anatomical validation is not conducted by functional neuroimaging researchers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Downar J, et al. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3(3):277–83. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- 63.De Nooy W, Mrvar A, Batageli V. Exploratory Network Analysis with Pajek. Cambridge University Press; 2005. [Google Scholar]
- 64.Kamada T, Kawai S. An Algorithm for Drawing General Undirected Graphs. Information Processing Letters. 1989;31:7–15. [Google Scholar]