Abstract
Innate memory-like CD8 thymocytes develop and acquire effector function during maturation in the absence of encounter with antigens. Here, we demonstrate that enhanced function of transcription factors T Cell Factor (TCF)-1 and β-catenin regulate the frequency of promyelocytic leukemia zinc finger (PLZF) expressing IL-4 producing thymocytes that promote the generation of Eomesodermin-expressing memory-like CD8 thymocytes in trans. By contrast, TCF1-deficient mice do not have PLZF-expressing thymocytes and Eomesodermin-expressing memory-like CD8 thymocytes. Generation of TCF1 and β-catenin-dependent memory-like CD8 thymocytes is non-cell intrinsic and requires the expression of IL-4 and IL-4 receptor. CD8 memory-like thymocytes migrate to the peripheral lymphoid organs and the memory-like CD8 T cells rapidly produce IFNγ. Thus, TCF1 and β-catenin regulate the generation of PLZF-expressing thymocytes and thereby facilitate the generation of memory-like CD8 T cells in the thymus.
Keywords: β-catenin, TCF1, IL4, IL4Rα, memory-like thymocytes
Introduction
In addition to conventional αβ T cells, recent studies have demonstrated the presence of subsets of thymocytes that acquire effector functions in the thymus as a result of the maturation process. A subset of CD8 single positive (SP) mature thymocytes, referred to as “memory-like” or “innate-like” has been described in mice with mutations in Krüppel-like factor 2 (KLF2), inducible T cell kinase (Itk), CREB binding protein (CBP), inhibitor of DNA binding 3 (Id3) and Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP76:Y145F) genes (1–9). Non-mutant Balb/c mice also show an increase in the frequency of CD8 memory-like cells (1, 8, 10). Memory-like CD8 thymocytes share characteristics with conventional memory CD8 T cells, including higher expression of surface markers CD44 and CD122, intracellular Eomesodermin (Eomes) and rapid production of cytokines after T cell receptor activation (5). In addition to a memory-like phenotype, memory-like CD8 thymocytes respond to TCR signals by rapidly producing interferon-γ (IFN-γ) and therefore appear competent to provide immune protection (8, 11). Generation of memory-like CD8 thymocytes is dependent on thymocytes that express transcription factor promyelocytic leukemia zinc finger (PLZF) (1). PLZF expressing thymocytes are expanded in the thymuses of KLF2, Itk and Id3 gene-deficient mice and produce interleukin 4 (IL-4), which is required for the generation of memory-like CD8 thymocytes (1, 8, 9). However, the mechanisms that regulate the increase in the accumulation of PLZF-expressing and IL-4 producing thymocytes and consequent increase in memory-like CD8 thymocytes remain to be fully understood.
TCF1 critically regulate T cell development at multiple developmental stages (12, 13). Despite this we, and others, have demonstrated that normal naïve T cells develop in TCF1-deficient mice and migrate to populate the spleen and the lymph nodes (13–15). Early events in TCR-activated TCF1-deficient T cells are also comparable to control T cells (16, 17). TCF1-deficient CD4 T cells fail to make IL-4 due to lack of GATA-3 expression and produce enhanced IFN-γ and IL-17 because TCF1 directly negatively regulates IFN-γ and IL-17 genes (16, 17). TCF1 also plays a definitive role in the generation and maintenance of conventional antigen driven CD8 memory T cells (18–22). Interestingly, TCF1 is highly and constitutively expressed in thymocytes whereas expression of β-catenin is intrathymically regulated in a developmentally sensitive manner suggesting that signal-dependent expression of β-catenin might significantly regulate thymocyte development (15, 23, 24). We have previously shown that enforced expression of β-catenin from the Lck-proximal promoter results in an increase in memory-like CD8SP thymocytes (25). However, transgenic expression of β-catenin from the CD4 promoter failed to induce the generation of memory-like CD8SP thymocytes (26). The reason for this difference in phenotype of the two mouse strains that expressed ectopic β-catenin in thymocytes might be explained based on two hypotheses. First, the level of transgene expression might be different. Second, the stage of thymocyte development at which, expression of transgenic β-catenin is initiated plays an important role in regulating the generation of memory-like CD8SP thymocytes. These studies outline the scope of the role played by TCF1 and co-factors β- and γ-catenin in T cell function during an immune response.
In this report we provide a mechanism by which TCF1 and β-catenin dependent gene expression regulates the generation of memory-like mature CD8 thymocytes. Enforced expression of β-catenin, from the LCK-promoter, in TCF1-sufficient mice (β-CAT-Tg), increases the generation of CD4+αβ+ thymocytes that express PLZF and produce IL-4. Enhanced IL-4 production by PLZF expressing thymocytes induces the generation of Eomes expressing memory-like CD8 thymocytes. By contrast, memory-like CD8 thymocytes are absent in TCF1-deficient mice, which shows that TCF1-dependent transcription is required. Deletion of IL-4 or IL-4R expression abrogates memory-like CD8 thymocytes, demonstrating that IL-4R-dependent signals regulate their generation in trans. Finally, we show that the thymic CD8 memory-like cells migrate to the periphery and rapidly produce interferon (IFN)-γ ex-vivo.
Materials and Methods
Animals
β-CAT-Tg mice, expressing β-catenin in thymocytes and T cells from the proximal Lck-promoter, have been described previously (25). TCF1-deficient mice (12) were provided by H. Clevers (Hubrect Institute, Utrecht). IL-4-deficient mice were obtained from The Jackson Laboratory and were bred with β-CAT-Tg mice to generate IL-4KO-β-CAT-Tg mice. IL-4Rα-deficient mice (27) were provided by Z. Zhu (The Johns Hopkins Asthma and Allergy Center, MD) and were bred with β-CAT-Tg mice to generate IL-4RαKO-β-CAT-Tg mice. RAG2-deficient mice were purchased from Jackson Laboratory. All mice were on C57BL/6 genetic background. Age-matched littermate controls or C57BL/6 mice were used in all experiments. All mice were bred and maintained in animal facility at the NIA according to NIH regulations and were in compliance with the guidelines of NIA animal resources facility, which operates under the regulatory requirements of the U.S. Department of Agriculture and Association for Assessment and Accreditation of Laboratory Animal Care.
Antibodies and flow cytometry
Cells were harvested, stained and analyzed on a FACS Calibur (Becton Dickinson). Dead cells were excluded by forward light scatter or forward light scatter plus propidium iodide. All the data were acquired and are presented on log scale. The following antibodies conjugated to FITC, PE, peridinin chlorophyll protein-cyanine 5.5, or allophycocyanin (all from BD Biosciences) were used for staining: anti-CD4 (GK1.5), anti-CD8α (53-6.7), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-CD122 (TM-β1), anti-IL-4Rα (mIL4R-M1), anti-TCRβ (H57-597), anti-TCRγδ (GL3), anti-STAT6 (pY641) (J71-773.58.11), anti-integrin β7 chain (M293) and anti-CD103 (M290), Vβ 2 TCR (B20.6), Vβ 3 TCR (KJ25), Vβ 4 TCR (KT4), Vβ 5.1, 5.2 TCR (MR9-4), Vβ 6 TCR (RR4-7), Vβ 8.1, 8.2 TCR (MR5-2), Vβ 9 TCR (MR10-2), and Vβ 14 TCR (14-2). FITC- or PE- conjugated anti-IL-7Rα (A7R34), anti-CXCR3 (CXCR3-173), anti-IFN-γ (XMG1.2), anti-IL-4 (11B11), anti-Eomes (Dan11mag) and anti-T-bet (eBio4B10) antibodies were purchased from eBioscience. PE-conjugated mouse CD1d tetramers loaded with glycolipid PBS-57 were obtained from the tetramer facility of the US National Institutes of Health. Purified anti-CD3 (145-2C11) and anti-CD28 (37.51) were from BD Pharmingen. PLZF staining was done as described (28). In brief, cell surfaces were stained with antibodies, and then cells were fixed with the Foxp3 Staining Buffer set (eBioscience). Permeable cells were incubated with antibody to PLZF (D-9; Santa Cruz) followed by anti–mouse immunoglobulin G1 (A85-1; BD Biosciences) alone or with anti-IL-4 (BVD4-1D11) where appropriate. For Eomes staining and T-bet staining, cells were stained for 1 h with anti-Eomes (Dan11mag) and anti-T-bet (eBio4B10) with the Foxp3 Staining Buffer set (eBioscience).
In vitro cytokine production
1 × 106 cells per ml in were were incubated for 5 h in RPMI medium plus 10% (vol/vol) FCS with or without PMA (50 ng/ml) and ionomycin (1.5 μM; Sigma-Aldrich). GolgiStop (BD Pharmingen) was added for the final 3 h. Cells were then harvested and stained for intracellular cytokines with BD Cytofix/Cytoperm kit.
Cell sorting and Quantitative (real-time) RT-PCR
Cell suspensions of total thymocytes were surface-stained with anti-CD4 and anti-CD8 followed by electronic cell sorting on a Dako-Cytomation Moflo. Total mRNA was reversed transcribed using poly (dT) and Superscript III reverse transcriptase (Invitrogen). SYBR green quantitative real-time RT-PCR was performed, using PCR Master Mix (Applied Biosystems), for 40 cycles with annealing and extension at 60 °C. Primer sequences are provided upon request. The expression of target gene was determined relative to GAPDH and calculated as 2−(CtTarget gene − CtGAPDH).
Mixed bone marrow (BM) chimera
BM was isolated from femurs and tibias of β-CAT-Tg and IL-4RαKO-β-CAT-Tg mice. T cells were depleted and β-CAT-Tg BM cells were mixed with IL-4RαKO-β-CAT-Tg BM cells at 1:1 ratio and injected intravenously into sub-lethally irradiated Rag2-deficient mice. Chimeric mice were sacrificed and analyzed 16–18 weeks after transplant.
Retroviral infection
CD8+ T cells were purified using mouse CD8+ T cell isolation kit (Miltenyi). Purified CD8+ T cells were activated by plate-bound anti-CD3 and CD28 antibodies for 2 days, and infected with murine stem cell virus (MSCV)-based retroviruses that express human CD8 as a marker (MSCV-Vector) or coexpress stabilized mouse β-catenin (MSCV-β-CAT). huCD8+ populations were electronically sorted and analysed for mRNA.
Stimulation of CD8 T cells with cytokines
Electronically sorted CD122-CD44lo naïve CD8 T cells from C57BL/6 mice were treated with 10ng/ml of IL-2, 50 ng/ml of IL-4, 5 ng/ml of IL-7, 100 ng/ml of IL-15 or the combination of cytokines as indicated for 20 h. For in-vitro CD8 T cell differentiation lymph node cells were stimulated with 1 μg/ml of soluble anti-CD3 for 3 d and then rested in cultures that contained 5 ng/ml of IL-7 and 10 ng/ml of IL-4 for another 6 d. CD8 T cells were purified by positive selection using biotin-anti-CD8 and anti-biotin-magnetic beads (Miltenyi). All cytokines were purchased from R&D Systems.
Preparation of small intestine intraepithelial lymphocytes (IEL)
IEL from small intestines of control and β-CAT-Tg mice were prepared according to a modification of a previously published procedure (29). Briefly, small intestine was removed and carefully cleaned from its mesentery. Peyer’s patches were removed and fecal contents flushed out from small intestine. The intestinal tissue was cut longitudinally and then into 1 cm pieces and shaken three times at 200 rpm for 20 mins at 37°C in RPMI 1640 media with 5 mM EDTA and 0.145 mg/ml DTT. The cells in suspension were collected and centrifuged in a discontinuous 40%/70% Percoll (GE Healthcare BioSciences AB, Uppsala, Sweden) gradient at 1600 rpm for 20 min. Cells from the 40%/70% interface were collected, washed, and re-suspended in complete RPMI media supplemented with 10% FBS.
Statistics
Statistical significance was determined by the Student’s t-test.
Results
TCF1 and β-catenin regulate memory-like CD8 T-cell generation in the thymus
We have noted that enforced expression of stabilized form of β-catenin from the proximal Lck-promoter in β-CAT-Tg mice results in an increase of TCRβhi CD8SP thymocytes that exhibit a memory phenotype (25) (Fig. 1A). By contrast, we find (Fig. 1A) as had been reported before (12) that TCF1-deficient CD8SP thymocytes contain a high representation of immature single positive (ISP) cells and a lower proportion of TCRβhi mature CD8 thymocytes compared to control. Further analysis of the TCRβhi CD8SP population revealed that majority of β-CAT-Tg CD8SP thymocytes expressed high levels of Eomes (Fig. 1B). By contrast, majority of CD8SP TCF1-deficient thymocytes showed very low Eomes expression (with a minor population showing intermediate expression) (Fig. 1B). The absolute numbers of these Eomes-expressing TCRβhi mature CD8 thymocytes were significantly (p=0.004) lower in TCF1-deficient mice (0.087±0.016×106) compared to control mice (0.15±0.012×106). Eomes expression was regulated at the mRNA level as the relative abundance was greatly increased in β-CAT-Tg CD8 memory-like cells compared to control CD8SP thymocytes (data not shown). These data are congruent with impaired TCF1-dependent regulation of Eomes expression in conventional antigen-driven CD8 memory cells (21). β-CAT-Tg CD8SP thymocytes also expressed higher levels of IL-4Rα chain compared to control or TCF1-deficient CD8SP thymocytes (Fig. 1B). We examined expression of other surface memory markers and found that β-CAT-Tg CD8SP thymocytes express high levels of memory markers including CD44, CD122 and CXCR3 (Fig. 1C). Finally, ex-vivo β-CAT-Tg CD8 memory-like cells produced higher levels of IFN-γ compared to control CD8SP thymocytes (Fig. 1D). Together these data demonstrate that TCF1 and β-CAT-Tg regulate the generation of Eomes-expressing CD8SP thymocytes that acquire memory like phenotype and function during their development in the thymus.
Figure 1.
Memory-like CD8 T cell development in the thymus is regulated by β-catenin and TCF1.
A) Flow-cytometric analysis of surface CD4, CD8 expression on total thymocytes and TCR-β expression on the gated CD8SP population from control, β-CAT-Tg and TCF1-deficient mice. Numbers show the percentage of cells. Data are representative of eight independent analyses. The graph on upper right panel shows total thymic cellularity and the graph on lower right panel shows absolute numbers of TCRβhi CD8SP thymocytes from control, β-CAT-Tg and TCF1-deficient mice.
B) Flow-cytometric analysis of Eomes and IL-4Rα expression on the gated TCRβhi CD8SP thymocytes from control, β-CAT-Tg and TCF1-deficient mice. Data are representative of six independent analyses.
C) Flow-cytometric analysis of cell surface memory markers expression on the gated CD8SP thymocytes from control and β-CAT-Tg mice. Data are representative of eight independent analyses.
D) Flow-cytometric analysis of intracellular IFN-γ expression on gated CD8SP thymocytes after 5 h PMA plus ionomycin stimulation of total thymocytes from control and β-CAT-Tg mice is shown. Data are representative of three independent analyses with total five to six mice in each group.
TCF1 and β-catenin regulate the generation of PLZF-expressing thymocytes
Several recent studies have indicated that generation of memory-like CD8 thymocytes is dependent on the presence of PLZF expressing thymocytes (1, 8, 9). To determine if generation of TCF1 and β-catenin-dependent memory-like CD8SP thymocytes was regulated by PLZF expressing thymocytes, we assayed for PLZF-expressing thymocytes in β-CAT-Tg and TCF1-deficient mice. Co-staining with PLZF and TCRβ chain showed that β-CAT-Tg thymocytes had a significantly (p=5.5×10−6) higher percentage and number of TCRβ+ cells that also expressed PLZF compared to control thymocytes (Fig. 2A). By contrast, the frequency and number of TCRβ+ thymocytes expressing PLZF was significantly decreased (p=1.8×10−14) in TCF1-deficient mice (close to none) compared to control mice (Fig. 2A). These data show that TCF1 and β-catenin regulate the generation of PLZF-expressing TCRβ+ thymocytes. Since major PLZF-expressing thymocytes are known to be invariant natural killer (iNKT) cells, defined by their binding to CD1d tetramers loaded with the glycolipids, we stained with CD1d tetramers loaded with glycolipid α-galactosylceramide analogue PBS-57 and PLZF. We found that β-CAT-Tg thymocytes had a significantly (p=7.9×10−5) higher percentage and number of PLZF-expressing CD1dPBS-57-tetramer binding NKT cells compared to control thymocytes (Fig. 2B). The data suggest that increased PLZF expression in β-CAT-Tg mice is related to increased induction of NKT cells. Further characterization of PLZF-expressing thymocytes in β-CAT-Tg mice showed that they express CD4 but not CD8 (Fig. 2C). Finally, the relative abundance of PLZF mRNA was found to be higher in CD4SP but not CD8SP thymocytes showing that CD4SP thymocytes contained PLZF-expressing cells (Fig. 2D). Together these data show that TCF1 and β-catenin promote the generation of PLZF-expressing thymocytes and support the hypothesis that the increase in memory-like CD8 thymocytes was along the principles outlined in other mutant mice that show an increase in this population.
Figure 2.
TCRβ+ and CD1dPBS-57+ PLZF-expressing thymic population is increased in β-CAT-Tg mice.
A) Flow-cytometric analysis of expression of PLZF and TCRβ on total thymocytes from control, β-CAT-Tg and TCF1-deficient mice. Numbers adjacent to outlined areas (upper panels) and graphs (lower panels) show percentage and absolute numbers of PLZF+TCRβ+ cells as indicated. Data are representative of five independent analyses with total eight to ten mice per group.
B) Flow-cytometric analysis of expression of PLZF and CD1dPBS-57 on total thymocytes from control and β-CAT-Tg mice. Numbers adjacent to outlined areas (left panels) and graphs (right panels) show percentage and absolute numbers of PLZF+CD1dPBS-57+ cells as indicated. Data are representative of five independent analyses with total eight to ten mice per group.
C) Flow-cytometric analysis of expression of PLZF on gated CD4 and CD8SP populations of total thymocytes from control and β-CAT-Tg mice. Numbers adjacent to outlined areas show percent of PLZF+ CD4SP and PLZF+ CD8SP thymocytes as indicated. Data are representative of five independent analyses.
D) Real-time PCR analysis for Zbtb16 mRNA in purified CD4SP and CD8SP thymocytes, presented relative to Hprt. Data are from four independent samples.
PLZF-expressing β-CAT-Tg thymocytes produce IL-4
PLZF-expressing thymocytes have been shown to produce IL-4 (1, 8, 9). To determine if PLZF-expressing β-CAT-Tg thymocytes produce IL-4, we stimulated total thymocytes with PMA and ionomycin and measured IL-4 production by intra-cellular staining followed by flow cytometric analysis. We found that β-CAT-Tg thymocytes contained 5 to 10-fold higher percentage of PLZF+ IL-4 producing cells after a 5 h in vitro stimulation compared to control thymocytes (Fig. 3A). Double staining with CD4 and intracellular IL-4 antibodies confirmed that β-CAT-Tg CD4SP thymocytes contained higher percentage of IL-4 producing cells as compared to their control counterparts (Fig. 3B). Thus, a subset of TCRβ+ CD4+ thymocytes express PLZF and produce IL4 in β-CAT-Tg mice. By contrast, β-CAT-Tg CD8SP thymocytes did not show enhanced IL-4 production compared to control thymocytes (Fig. 3B). Accordingly, Il4 transcripts were increased in β-CAT-Tg CD4SP thymocytes compared to control CD4SP thymocytes showing regulation at the transcription level (Fig. 3C). Thus, TCRβ+PLZF+ subset of CD4SP thymocytes is the major source of IL-4. Parenthetically, KLF2 expression in β-CAT-Tg CD4 or CD8SP thymocytes was comparable to control thymocytes (data not shown), suggesting that the effect of TCF1 and β-catenin-dependent regulation may not go through KLF2. Together these data suggest that the memory-like or effector phenotype of β-CAT-Tg CD8 thymocytes is not cell intrinsic but a direct consequence of increased IL-4 production by PLZF-expressing CD4SP thymocytes. We propose that β-catenin expression in developing thymocytes enhances the generation of PLZF-expressing thymocytes that over-produce IL-4 and promote the generation of memory-like CD8 thymocytes.
Figure 3.
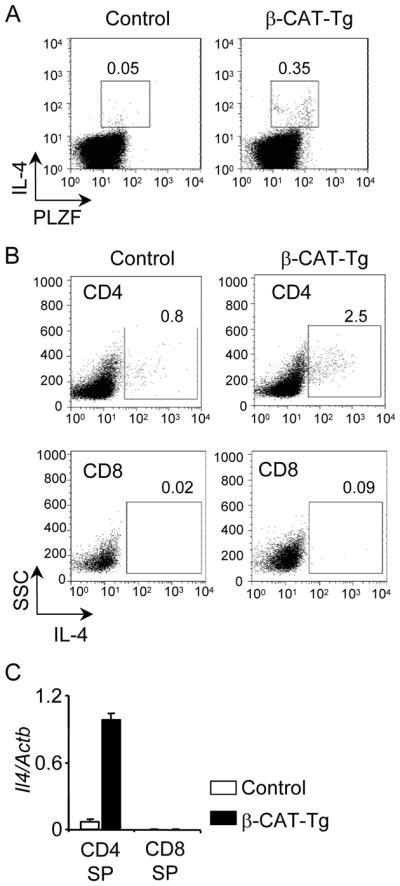
β-CAT-Tg PLZF+ CD4SP thymocytes over-produce IL-4.
A) Flow-cytometric analysis of intracellular expression of IL-4 and PLZF in control and β-CAT-Tg thymocytes stimulated for 5 h with PMA and ionomycin. Numbers adjacent to outlined areas show percent IL-4+PLZF+ cells. Data are representative of four independent analyses.
B) Flow-cytometric analysis of intracellular IL-4 expression on gated CD4 and CD8SP thymocytes after 5h PMA plus ionomycin stimulation of total thymocytes from control and β-CAT-Tg mice. Numbers adjacent to outlined areas show percent of IL-4+ CD4SP and IL-4+ CD8SP thymocytes as indicated. Data are representative of five independent analyses.
C) Real-time PCR analysis for Il4 mRNA in purified CD4SP and CD8SP thymocytes, presented relative to Actb. Data are from four independent samples.
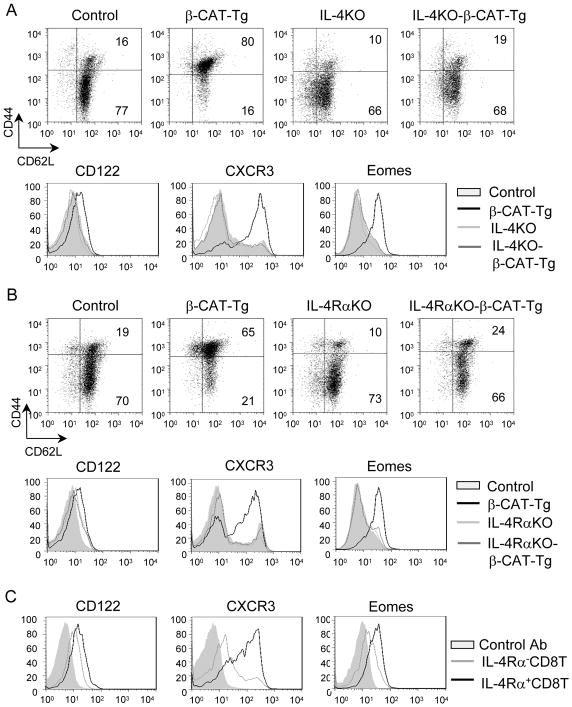
IL-4/IL-4Rα expression is required for generation of β-CAT-Tg memory-like CD8SP thymocytes
To demonstrate that IL-4 production by PLZF+ cells in β-CAT-Tg mice was required for the generation of memory-like CD8 thymocytes, we generated IL-4KO-β-CAT-Tg and IL-4Rα-KO-βCAT-Tg mice by breeding β-CAT-Tg mice with IL-4KO and IL-4Rα-KO mice respectively. Analysis of thymocytes from these mice showed that in contrast to β-CAT-Tg CD8SP thymocytes, which were CD44hiCD62Lhi, IL-4KO-β-CAT-Tg (Fig. 4A) and IL-4Rα-KO-β-CAT-Tg (Fig. 4B) CD8SP thymocytes were CD44loCD62Lhi, similar to control, IL-4KO and IL-4Rα-KO CD8SP thymocytes. In addition, cell surface expression of memory-phenotype markers CD122 and CXCR3 also showed that IL-4KO-β-CAT-Tg and IL-4Rα-KO-β-CAT-Tg CD8SP thymocytes failed to acquire the memory-like phenotype noted in β-CAT-Tg CD8SP thymocytes (Fig. 4C). Increased expression of Eomes noted in β-CAT-Tg CD8SP thymocytes was also abolished in IL-4KO-β-CAT-Tg and IL-4Rα-KO-β-CAT-Tg CD8SP thymocytes (Fig. 4C). Thus, expression of IL-4 and IL-4Rα is required to promote generation of CD8SP thymocytes with a memory-like phenotype in β-CAT-Tg mice. We confirmed that transgenic β-catenin protein was expressed at the same level in β-CAT-Tg, IL4-KO-β-CAT-Tg and IL-4Rα-KO-β-CAT-Tg thymocytes (data not shown). Finally, in contrast to memory-like β-CAT-Tg CD8SP thymocytes, which produced IFN-γ ex-vivo, IL-4-deficient β-CAT-Tg CD8SP thymocytes failed to produce IFN-γ (Fig. 4D). These data demonstrate that IL4 is also required for the memory-like function of β-CAT-Tg CD8SP thymocytes. We conclude that the generation of memory-like CD8SP phenotype in β-CAT-Tg thymus was dependent on IL-4 produced by PLZF+ T cells in a cell-extrinsic manner.
Figure 4.
The memory-like CD8 phenotype in β-CAT-Tg mice is dependent on IL-4 and IL-4Rα.
A) Flow-cytometric analysis of the cell surface memory markers CD44 and CD62L on the gated CD8SP population of total thymocytes from control, β-CAT-Tg, IL-4KO and IL-4KO-β-CAT-Tg mice. Numbers show the percentage of cells. Data are representative of four independent analyses.
B) Flow-cytometric analysis of the cell surface memory markers CD44 and CD62L on the gated CD8SP population of total thymocytes from control, β-CAT-Tg, IL-4RαKO and IL-4RαKO-β-CAT-Tg mice. Numbers show the percentage of cells. Data are representative of four independent analyses.
C) Flow-cytometric analysis of the surface CD122, CXCR expression and intracellular Eomes expression in the gated CD8SP thymocytes from control, β-CAT-Tg, IL-4KO, IL-4RαKO, IL-4KO-β-CAT-Tg and IL-4RαKO-β-CAT-Tg mice. Data are representative of four independent analyses.
D) Flow cytometric analysis of intracellular IFN-γ expression on gated CD8SP thymocytes after 5 h PMA plus ionomycin stimulation of total thymocytes from control, β-CAT-Tg, and IL-4KO-β-CAT-Tg mice is shown. Data are representative of four independent analyses.
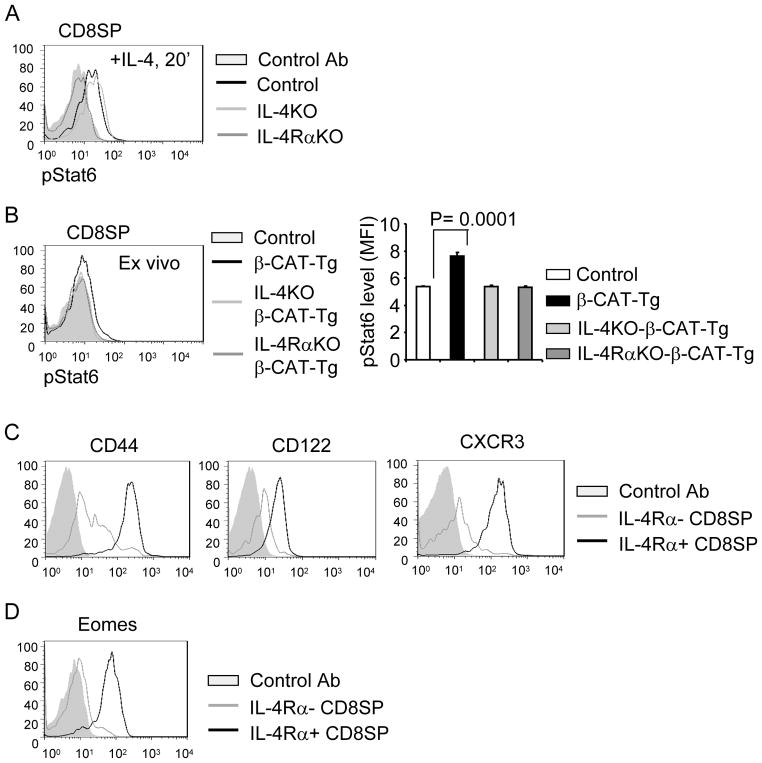
β-CAT-Tg CD8SP thymocytes experience higher IL-4/IL-4Rα signals
To determine if β-CAT-Tg memory-like CD8SP thymocytes, that express higher levels of IL-4Rα (Fig. 1B), receive higher IL-4/IL-4Rα signaling we examined STAT6 phosphorylation levels. Transient stimulation with IL-4 resulted in a measurable increase in pSTAT6 levels in control and IL-4KO CD8SP thymocytes but not in IL-4Rα-deficient CD8SP thymocytes (Fig. 5A). Next, we assayed pSTAT6 levels in freshly isolated β-CAT-Tg CD8SP thymocytes. We found that ex-vivo β-CAT-Tg CD8SP thymocytes showed significantly increased levels of pSTAT6 compared to control CD8SP thymocytes without stimulation with IL-4 in-vitro (Fig. 5B). Indeed, the increase in pSTAT6 levels in β-CAT-Tg CD8SP thymocytes was abolished in thymocytes from IL-4-deficient or IL-4Rα-deficient β-CAT-Tg mice (Fig. 5B). These data show that β-CAT-Tg CD8SP thymocytes receive higher IL-4/IL4Rα signaling in-vivo.
Figure 5.
Enhanced IL-4 signaling and IL-4Rα expression regulates β-CAT-Tg CD8 memory-like phenotype.
A) Flow-cytometric analysis of intracellular pSTAT6 in gated CD8SP thymocytes from ex vivo control, IL-4KO and IL-4RαKO total thymocytes transiently stimulated with IL-4 for 20 min. Data are representative of two independent analyses.
B) Flow-cytometric analysis of intracellular pSTAT6 on gated CD8SP thymocytes from ex vivo total thymocytes from control, β-CAT-Tg, IL-4KO-β-CAT-Tg and IL-4RαKO-β-CAT-Tg mice. Data are representative of two independent analyses. Graph on right side represents pSTAT6 levels as mean fluorescence intensity (MFI).
C) Flow-cytometric analysis of the cell surface memory markers on the gated IL-4Rα− and IL-4Rα+ CD8SP population of total thymocytes from β-CAT-Tg and IL-4RαKO-β-CAT-Tg 1:1 bone marrow chimera. Data are representative of two independent analyses with total 5 chimera mice.
D) Flow-cytometric analysis of Eomes expression on the gated IL-4Rα− and IL-4Rα+ CD8SP thymocytes from β-CAT-Tg and IL-4RαKO-β-CAT-Tg 1:1 bone marrow chimera. Data are representative of two independent analyses with total 5 chimera mice.
TCF1 and β-Catenin-dependent CD8 memory-like phenotype requires IL-4Rα expression
To further substantiate that IL-4Rα expression on CD8 thymocytes was required for the generation of memory-like CD8SP thymocytes, we generated mixed BM chimeras in RAG2-deficient mice using a mix of equal numbers of BM cells from IL-4Rα-sufficient β-CAT-Tg and IL-4Rα-deficient β-CAT-Tg mice. After sixteen weeks we assayed for the memory-phenotype of CD8SP thymocytes. We found that only IL-4Rα expressing, but not IL-4Rα-deficient β-CAT-Tg, CD8SP thymocytes showed up-regulation of surface memory markers CD44, CD122, CXCR3 (Fig. 5C) and intracellular Eomes (Fig. 5D). We conclude that memory-like-CD8 T cell generation in β-CAT-Tg mice is dependent on IL-4Rα expression on the CD8SP thymocytes. These data further substantiate the notion that development of memory-like CD8SP thymocytes in β-CAT-Tg mice is not cell autonomous and requires signals from the IL-4Rα in addition to enhanced β-catenin expression in a TCF1-sufficient background.
CD8 memory-like thymocytes migrate to the periphery and are functional
The phenotypic description of β-CAT-Tg CD8 T cells in the periphery showed the same memory-like phenotype as the CD8SP thymocytes with high levels of memory marker CD44 and CD62L (Fig. 6A). CD62L and β7 integrin regulate the entry of mature T cells into peripheral lymphoid tissues (30, 31). Memory-like CD8 T cells in the LN express high levels of other surface memory markers and CD62L (L-selectin) and lower levels CD103 and β7 integrin (Fig. 6B). β-Cat-Tg CD4 and CD8 T cells were found to represent a TCR repertoire that was comparable to control mice (data not shown). These studies rule out expansion of a sub-population in β-CAT-Tg mice. Intracellular staining showed that β-CAT-Tg memory-like CD8 T cells expressed high levels of Eomes compared to control CD8 T cells while T-bet protein levels are comparable to control (Fig. 6C). Abundance of Eomes and T-bet mRNA expression using real-time RT-PCR showed that Eomes mRNA expression was markedly higher, while T-bet mRNA level was moderately lower, in peripheral β-CAT-Tg CD8 T cells than control CD8 T cells (Fig. 6D). Additionally, β-CAT-Tg CD8 T cells express higher levels of mRNA for Prf1 (Perforin) and Prdm1 (Blimp-1) (Fig. 6D), which is consistent with the fact that Eomes is a critical regulator of perforin expression in CD8 T cells (32). To determine if memory-like CD8 T cells were functional, we stimulated freshly isolated ex-vivo CD8 T cells with PMA and ionomycin to assess IFN-γ production. We found a greater percentage of β-CAT-Tg CD8 T cells produced IFN-γ as compared to control CD8 T cells (Fig. 6E). These data suggest that β-CAT-Tg memory-like CD8 T cells rapidly produce IFN-γ upon stimulation. We also studied the phenotype of CD8 T cells in the small intestine of β-CAT-Tg mice and found that the β-CAT-Tg IEL CD8 T cells showed increased CD44 (but not CD62L) expression compared to control suggesting an increase in memory phenotype (Fig. 6F). These data show that the memory-like CD8 thymocytes generated in the thymus in the absence of antigenic stimulation migrate to the peripheral lymphoid organs and are functional.
Figure 6.
Peripheral CD8 T cells in β-CAT-Tg also have memory-like features and function.
A) Flow-cytometric analysis of the cell surface memory markers CD44 and CD62L on the gated CD8 T population of lymph node cells from control and β-CAT-Tg mice; Numbers show the percentage of cells. Data are representative of ten independent analyses.
B) Flow-cytometric analysis of the cell surface memory markers CD122, IL-7Rα, IL-4Rα, CXCR3, CD103 and β7 expression on the gated CD8 T population of lymph node cells from control and β-CAT-Tg mice. Data are representative of ten independent analyses.
C) Flow-cytometric analysis of Eomes and T-bet expression on the gated CD8 T population of lymph node cells from control and β-CAT-Tg mice. Data are representative of six independent analyses.
D) Real-time PCR analysis for mRNA abundance of Eomes, Tbx21, Perforin and Prdm-1 in purified ex vivo CD8 T cells from lymph nodes of control and β-CAT-Tg mice, presented relative to Actb. Data are from four independent samples.
E) Flow cytometric analysis of intracellular IFN-γ expression on gated CD8 T cells after 5 h PMA plus ionomycin stimulation of lymph node cells from control and β-CAT-Tg mice is shown. Data are representative of four independent analyses.
F) Flow-cytometric analysis of the expression of cell surface memory markers CD44 and CD62L on the gated CD8 T population of IEL from small intestines of control and β-CAT-Tg mice. Numbers show the percentage of cells.
TCF1 and β-Catenin-dependent CD8 memory-like T cells require IL-4 and IL-4Rα expression
To determine if memory-like CD8 T cells found in β-CAT-Tg mice were present in the peripheral compartment of mutant mice we compared the CD8 T cells in β-CAT-Tg mice with CD8 T cells from IL-4-KO-β-CAT-Tg and IL-4Rα-KO-β-CAT-Tg mice. We found that memory-like CD8 T cells were absent from β-catenin expressing mice that lacked IL-4 or IL-4R (Fig. 7A and B). To confirm the requirement for the expression of IL-4R on the memory-like CD8 T cells we analyzed peripheral CD8 T cells from mixed BM chimeric mice described above. We found that when CD8 T cells lacked IL-4R expression memory-like phenotype was absent (Fig. 7C). Together these data show that TCF1 and β-catenin dependent IL-4 regulates the generation and peripheral migration of memory-like CD8 T cells.
Figure 7.
IL-4 or IL-4Rα chain deficiency results in loss of memory-like phenotype of β-CAT-Tg CD8 T cells.
A) Flow-cytometric analysis of the cell surface memory markers and Eomes expression by the gated CD8T cells from lymph nodes from control, β-CAT-Tg, IL-4KO and IL-4KO-β-CAT-Tg mice; CD44 and CD62L expression (upper panel) and CD122, CXCR3 and Eomes expression (lower panel). Numbers show the percentage of cells. Data are representative of four independent experiments.
B) Flow-cytometric analysis of the cell surface memory markers and Eomes expression by the gated CD8T cells from lymph nodes from control, β-CAT-Tg, IL-4Rα-KO and IL-4RαKO-β-CAT-Tg mice; CD44 and CD62L expression (upper panel) and CD122, CXCR3 and Eomes expression (lower panel). Numbers show the percentage of cells. Data are representative of four independent experiments.
C) Flow-cytometric analysis of the cell surface memory markers on and Eomes expression in the gated IL-4Rα- and IL-4Rα+ CD8 T population of spleenocytes from β-CAT-Tg and IL-4RαKO-β-CAT-Tg 1:1 bone marrow chimera. Data are representative of two independent analyses with total 5 chimera mice.
Finally, we wanted to determine if over-expression of β-catenin or treatment with cytokines promoted memory-like features. We expressed β-catenin using retroviral expression system in control naïve CD8 T cells. We found that memory-like features were not induced upon expression of β-catenin in naïve CD8 T cells from control mice (Fig. 8A). Instead, we found that treatment of naïve CD8 T cells with IL-4, but not other cytokines, promoted memory-like features (Fig. 8B). Thus IL-4R-dependent signaling regulated the generation of memory-like CD8 T cells in β-CAT-Tg mice. Interestingly, when activated TCF1-deficient CD8T cells are treated with IL-4 expression of Eomes, Perforin, IL-4R and CD122 is not induced whereas expression of T-bet remains unchanged (Fig. 8C). These data support the notion that there may be a cell-intrinsic aspect to the development of CD8 memory-like features.
Figure 8.
IL-4 signals induce memory-like gene expression in naïve CD8 T cells.
A) CD8 T cells were infected with control MSCV-human CD8 vector or MSCV-β-CAT-human-CD8 construct. Human CD8+ infected CD8 T cells were sorted and the relative abundance of mRNA for transcription factors and effector molecules was analyzed by real time RT-PCR. Data are presented relative to Actb and are from six independent samples.
B) CD122−CD44lo naïve CD8 T cells that were treated with various cytokines and the relative abundance of mRNA for transcription factors and effector molecules was analyzed by real time RT-PCR. Data are presented relative to Actb and are from four to six independent samples.
C) Lymph node cells from control and TCF1-deficient mice were stimulated with anti-CD3 for 3 d and then rested in culture that contained IL-7 and IL-4 for 6 d. After this in vitro stimulation CD8 T cells were purified and transcription factors and effector molecules analyzed by real-time RT-PCR. Data are presented relative to Actb and are from three independent samples.
Discussion
In this report we demonstrate that enforced expression of β-catenin in a TCF1-sufficient background facilitates the generation of PLZF expressing CD4+ TCRαβ+ T cells and TCRγδ+ thymocytes, which in turn induce the generation of memory-like CD8SP thymocytes. We show that expression of both IL-4 and IL-4Rα is required for the generation of memory-like CD8SP thymocytes. Finally, we demonstrate that CD8 memory-like thymocytes migrate to the periphery and rapidly produce high levels of IFN-γ ex-vivo suggesting they are functional.
We have previously reported that transgenic expression of β-catenin from the proximal Lck promoter in a TCF1-sufficient background results in increased number of CD8SP thymocytes that acquire a memory-like phenotype in the thymus (25). However, when β-catenin was over-expressed using the CD4-promoter a similar phenotype was not noted (26). Data shown in this paper provide an explanation for this discrepancy. One major difference between expression from the proximal Lck-promoter and the CD4-promoter is the timing of gene-expression during thymocyte development. Proximal Lck-promoter initiates gene expression as early as the DN2 stage whereas CD4-promoter dependent expression is first noted in post-β-selection DN4/pre-DP thymocytes. We propose that timing of β-catenin stabilization during T cell development is essential to facilitate the development of PLZF expressing thymocytes and thereby to promote the generation of memory-like CD8 thymocytes. Signals that stabilize β-catenin at these developmental stages remain to be defined. However, two possible candidates that regulate β-catenin expression might be considered. First, in light of previous reports from our laboratory that TCR (33) and pre-TCR (34) signals stabilize β-catenin, one might consider that the strength of lineage decision signals transmitted through the TCR might stabilize β-catenin to different extent allowing for PLZF expression in a subset of thymocytes. The second possibility is the involvement of Wnt-dependent signals for β-catenin stabilization in a developmentally sensitive manner. Further research will be useful in resolving these issues.
TCF1 and β-catenin have been shown to be required for the generation of long-lived antigen-driven CD8 memory T cells (18–22). In the absence of TCF1 antigen-driven memory CD8 T cells expressed lower Eomes and failed to provide adequate long-term memory whereas double transgenic mice over-expressing TCF1 and β-catenin show enhanced generation of memory CD8 T cells. One difference between antigen driven CD8 memory cells and CD8 memory-like cells generated in the thymus is that the former are clonal whereas the latter are polyclonal. However, observations studies with TCF1 and β-catenin mutant mice suggest that the requirement of Eomes expression is a common feature. Future studies will be required to determine the extent of functional overlap between these cell populations.
The memory features in bystander CD8SP thymocytes in various gene knockout models have been shown to be dependent on PLZF+ thymocyte population (1, 8–10, 35). PLZF expressing thymocytes were shown to produce IL-4, which was essential for the development of the memory-like CD8SP thymocytes in the absence of encounter with antigen (1, 8, 9). Studies presented in this paper, using mice that have enforced expression of β-catenin in IL-4 sufficient and deficient background, show that IL-4 is required for the generation of TCF1 and β-catenin induced memory-like CD8SP. Experiments with mixed bone marrow chimeras using a mix of equal numbers of bone marrow cells from IL-4Rα-sufficient-β-CAT-Tg and IL-4Rα-deficient-β-CAT-Tg mice in RAG2-deficient mice showed that in the same background only IL-4Rα chain expressing β-CAT-Tg CD8 thymocytes acquire the memory-like phenotype. Thus IL-4 signals are required for the generation of memory-like CD8SP thymocytes generated when expression of β-catenin is enforced at the appropriate developmental stage.
Thus data provided in this paper show that β-catenin induction is limiting for the generation of PLZF-expressing thymocytes, which in turn promote the generation of CD8 memory-like thymocytes that migrate to the periphery and rapidly produce IFN-γ.
Acknowledgments
We thank NIA animal facility for maintaining animals, S. Luo and his team for genotyping, tetramer facility of the US National Institutes of Health for providing PE-conjugated mouse CD1d tetramers loaded with glycolipid PBS-57, H. Clevers (Hubrecht Institute, Utrecht) for providing TCF1-deficient mice and Z. Zhu (The Johns Hopkins Asthma and Allergy Center, MD) for providing IL-4Rα-deficient mice. We are grateful to Drs. Alycia Williams and Mark Soloski for help with isolation of IELs.
Footnotes
This research was supported by the Intramural Research Program of the National Institute on Aging at the NIH.
References
- 1.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011;32:50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, Aschenbrenner AC, Koretzky GA. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28:359–369. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prince AL, Yin CC, Enos ME, Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate conventional versus innate T-cell development. Immunol Rev. 2009;228:115–131. doi: 10.1111/j.1600-065X.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuyama T, Kasper LH, Boussouar F, Jeevan T, van Deursen J, Brindle PK. Histone acetyltransferase CBP is vital to demarcate conventional and innate CD8+ T-cell development. Mol Cell Biol. 2009;29:3894–3904. doi: 10.1128/MCB.01598-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics. Immunity. 2010;33:203–215. doi: 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai D, Zhu J, Wang T, Hu-Li J, Terabe M, Berzofsky JA, Clayberger C, Krensky AM. KLF13 sustains thymic memory-like CD8(+) T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. J Exp Med. 2011;208:1093–1103. doi: 10.1084/jem.20101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 13.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- 14.Staal FJ, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur J Immunol. 2008;38:1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Q, Sharma A, Sen JM. TCF1 and beta-catenin regulate T cell development and function. Immunol Res. 2010;47:45–55. doi: 10.1007/s12026-009-8137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, Zhu Z, Sen JM. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat Immunol. 2009;10:992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Q, Sharma A, Ghosh A, Sen JM. T cell factor-1 negatively regulates expression of IL-17 family of cytokines and protects mice from experimental autoimmune encephalomyelitis. J Immunol. 2011;186:3946–3952. doi: 10.4049/jimmunol.1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao DM, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue HH. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paley MA, Wherry EJ. TCF-1 flips the switch on Eomes. Immunity. 2010;33:145–147. doi: 10.1016/j.immuni.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Yu Q, Sen JM. Beta-catenin regulates positive selection of thymocytes but not lineage commitment. J Immunol. 2007;178:5028–5034. doi: 10.4049/jimmunol.178.8.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu M, Sharma A, Hossain MZ, Wiest DL, Sen JM. Sustained expression of pre-TCR induced beta-catenin in post-beta-selection thymocytes blocks T cell development. J Immunol. 2009;182:759–765. doi: 10.4049/jimmunol.182.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulroy T, Xu Y, Sen JM. beta-Catenin expression enhances generation of mature thymocytes. Int Immunol. 2003;15:1485–1494. doi: 10.1093/intimm/dxg146. [DOI] [PubMed] [Google Scholar]
- 26.Xie H, Huang Z, Sadim MS, Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. J Immunol. 2005;175:7981–7988. doi: 10.4049/jimmunol.175.12.7981. [DOI] [PubMed] [Google Scholar]
- 27.Noben-Trauth N, Shultz LD, Brombacher F, Urban JF, Jr, Gu H, Paul WE. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci U S A. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camerini V, Panwala C, Kronenberg M. Regional specialization of the mucosal immune system. Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J Immunol. 1993;151:1765–1776. [PubMed] [Google Scholar]
- 30.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 31.Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G, Rajewsky K, Muller W. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 32.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 34.Xu M, Sharma A, Wiest DL, Sen JM. Pre-TCR-induced beta-catenin facilitates traversal through beta-selection. J Immunol. 2009;182:751–758. doi: 10.4049/jimmunol.182.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon SM, Carty SA, Kim JS, Zou T, Smith-Garvin J, Alonzo ES, Haimm E, Sant’Angelo DB, Koretzky GA, Reiner SL, Jordan MS. Requirements for eomesodermin and promyelocytic leukemia zinc finger in the development of innate-like CD8+ T cells. J Immunol. 2011;186:4573–4578. doi: 10.4049/jimmunol.1100037. [DOI] [PMC free article] [PubMed] [Google Scholar]