Abstract
Printed research consent forms serve to legally document what has been disclosed, but are usually suboptimal as a means of actually communicating that information to potential participants. We conducted a preliminary study of web-based multimedia consent. Participants included 19 patients with schizophrenia and 16 normal comparison (NC) subjects randomly assigned to a routine or web-media consent. Although comprehension among NCs was excellent regardless of consent condition, the web-based consent was associated with better comprehension and satisfaction among patients with schizophrenia. Findings suggest web-aided multimedia consent is feasible and potentially more effective than printed consent forms in schizophrenia research.
Keywords: Decisional capacity, web-aided consent, schizophrenia, multimedia consent
1. INTRODUCTION
Schizophrenia is often associated with cognitive deficits that may hinder the informed consent for research in some contexts (Appelbaum, 2006). Cognitive deficits are the strongest correlates of impaired decisional capacity (Palmer & Savla, 2007), but the quality of the consent process may also affect participant comprehension. Thus, there has been growing interest in the potential of multimedia tools in the consent process (Ryan et al., 2008). We previously demonstrated improved understanding of disclosed information through a DVD (Jeste et al., 2009). Although DVD confers advantages of multimedia learning, it is difficult to adjust the pace and sequence of information to the needs of individual participants. The automatic flow of information may engender a relatively passive interaction between participant and researcher during the consent disclosure. These limitations may be avoided by compiling the information into conceptual units that can be individually accessed via webpage hyperlinks, thereby enabling a more flexible presentation and alternation between interpersonal dialogue, printed and bullet point text, figures/graphics and video.
The present study was conducted as a preliminary evaluation of the feasibility and potential effectiveness of a web-media approach to consent, i.e., to determine whether development of such web-media based tools warrants further pursuit. Our hypothesis was that web-media aided consent would result in better comprehension of disclosed information relative to routine consent. Assuming better understanding confers a more satisfying experience, we also hypothesized that the web-media approach would be associated with more satisfaction with the consent procedures.
2. METHODS
2.1 Participants
19 outpatients with schizophrenia and 16 normal comparison subjects (NCs) were recruited through a registry at the University of California San Diego's (UCSD) Advanced Center for Innovation in Services and Interventions Research. Inclusion criteria included DSM-IV-TR diagnosis of schizophrenia (established through review of research records) or (for NCs) a lifetime absence of psychiatric illness (established with the Mini International Neuropsychiatric Interview (Sheehan, Lecrubier, & Sheehan, 1998)). Exclusion criteria included diagnosis of dementia or other neurological/medical conditions known to affect cognition. The study was approved by the UCSD Human Research Protections Program; all participants provided written informed consent to participate.
2.2 Simulated consent procedures
We investigated participants’ comprehension of consent information in reference to a hypothetical clinical trial of an experimental cognition enhancing medication that was developed for our earlier study of DVD-aided consent (Jeste, et al., 2009). Participants were randomly assigned to receive a routine or web-media enhanced consent simulation for the clinical trial. For routine consent, a research associate (RA) met with each participant and reviewed the printed consent form. Participants were encouraged to stop the RA with questions, and the RA also stopped after major conceptual units (e.g. “purpose of the study”) and checked if the participant had questions.
Participants randomized to web-media consent also met with an RA, but their review of the study information was conducted with a web-media prototype. This involved a computer-aided presentation intermixed with video clips, static images/graphics, and bullet pointed text to explain the main points from the consent form. Content from the printed consent form was presented on the screen in organized sections covering: a study introduction, a time-line showing when visits would take place, a description of procedures during each visit, risks/discomforts, possible benefits, compensation, who to contact if injured, and voluntary participation information. Throughout these sections, links and graphics were provided on important points. Participants were told to stop the RA with questions, and that video segments could be paused or replayed at any time. The web-media tool included questions with corrective feedback after each key thematic section to ensure recently disclosed information was understood.
2.3 Measures
Comprehension/decisional capacity and satisfaction
Following the simulated consent procedure, the RA administered the UCSD Brief Assessment of Capacity to Consent (UBACC) (Jeste et al., 2007), a 10-item scale with each item scored from 0 (inadequate) to 2 points (adequate). Next, participants met with a second RA (blind to consent condition) who administered the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR) (Appelbaum & Grisso, 2001) which provides a more detailed assessment of Understanding (scores range from 0-26), Appreciation (0-6), Reasoning (0-8), and Expression of a choice (0-2). Participants subsequently rated the quality of consent procedure (better, worse, or equivalent relative to past research experiences) as part of a brief self-administered questionnaire.
2.4 Statistical analyses
Analyses focused on the effects of consent condition within each diagnostic group. Due to skewed distributions among the UBACC and MacCAT-CR scores, Mann- Whitney U tests were used to examine effects of consent condition. The small sample size was dictated by the intent of the present study, i.e. exploring whether the web-media approach warrants further development. We also calculated effect sizes (d) (Cohen, 1988) to describe the magnitude of differences between the two consent methods.
3. RESULTS
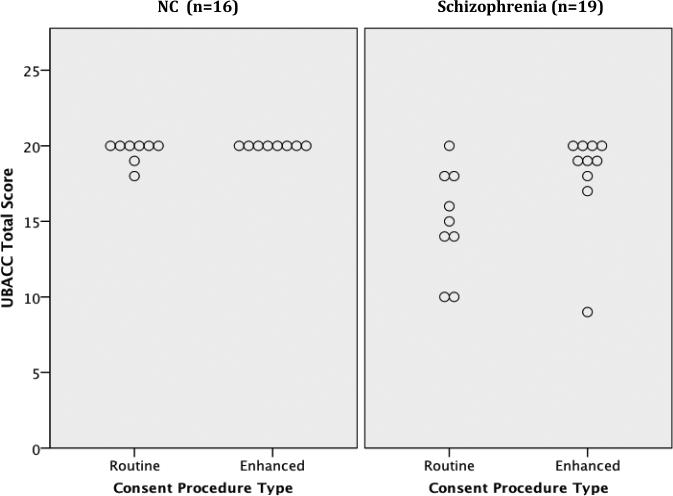
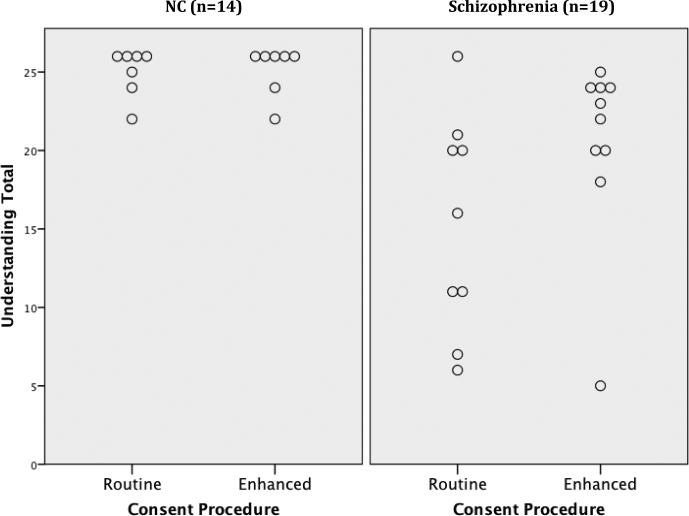
NCs reported higher levels of education than patients, mean (SD) = 15.4(1.8) versus 12.1(1.5), t(33) = 5.96, p<.001. However, there were no other significant differences in age, gender, or ethnicity. NCs had good comprehension regardless of consent condition, but there were no significant effects of consent condition (Table 1). Among patients, however, relative to those receiving the routine consent procedure, those receiving the web-media consent evidenced better UBACC scores, U = 19, z =-2.15, p = 0.03 (d= 0.94; “large” effect size). In a prior study we found a good balance of sensitivity and specificity with a UBACC cut-score of 14.5 (scores ≤ 14 defining the incapable range) (Jeste, et al., 2007). All but one patient (90%) in the web-media condition, but only five (56%) patients in the routine consent condition, earned a UBACC score above 14 (Figure 1a). None of the differences on the MacCAT-CR subscales reached statistical significance, but the overall pattern on Understanding, Appreciation, and Reasoning subscales was consistently toward better performance in the patients receiving web-media consent. These differences were in the large effect size range for the Understanding subscale (d = 0.81), and small to medium effect size range for Appreciation and Reasoning (ds =0.33 and 0.45, respectively). A good balance of sensitivity and specificity was reported in the NIMH CATIE-study using a cut-score of 16 on the MacCAT-CR Understanding subscale (scores ≤ 15 defining the incapable range) (Kim et al., 2007). All but one patient (90%) in the web-media consent condition, but only 5 of those in the routine consent condition, earned a MacCAT-CR Understanding score above 15 (Figure 1b).
Table 1.
Decisional capacity scores and quality of consent ratings by diagnosis and consent condition
| NC Group (n=16) | Schizophrenia Group (n=19) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Routine (n=8) | Enhanced (n=8) | Test Statistic | p-value | Routine (n=9) | Enhanced (n=10) | Test Statistic | p-value |
| Age in years, mean (SD) | 52.5 (12.1) | 48.6 (15.9) | t14=0 .55 | 0.59 | 57.0 (10.0) | 57.9 (8.9) | t17= -0.12 | 0.90 |
| Education in years, mean (SD) | 14.9 (1.6) | 16.0 (1.9) | t14=-1.29 | 0.22 | 12.4(0.9) | 11.8 (1.9) | t17= 0.92 | 0.37 |
| Gender, % women | 50.0 | 62.5 | X2= 0.25 | 0.61 | 11.0 | 40.0 | X2= 2.04 | 0.15 |
| Ethnic minorities (%) | 25.0 | 25.0 | X2= 1.33 | 0.51 | 22.2 | 30.0 | X2= 0.95 | 0.81 |
| UBACC total (potential range 0-20) | 19.6 (0.7) | 20.0 (0.0) | U = 24.00 | 0.14 | 15.0 (3.5) | 18.2 (3.3) | U = 19 | 0.03 |
| MacCAT- CR | ||||||||
| Understanding (potential range 0-26) | 25.0 (1.5) | 25.1 (1.6) | U = 22.00 | 0.71 | 15.3 (6.9) | 20.5 (5.9) | U = 25 | 0.10 |
| Appreciation (potential range 0-6) | 6.0 (0.0) | 5.7 (0.6) | U = 21.00 | 0.31 | 4.8 (1.0) | 5.2 (1.4) | U = 32.5 | 0.25 |
| Reasoning (potential range 0-8) | 7.4 (1.0) | 7.6 (1.5) | t12 = -0.34 | 0.74 | 5.6 (2.0) | 6.3 (0.9) | t17= -1.05 | 0.31 |
| Expression of a choice (potential range 0-2) | 2.0 (0.0) | 2.0 (0.0) | U = 24.50 | 1.00 | 2.0 (0.0) | 1.9 (0.3) | U = 40.5 | 0.34 |
| Quality of consent relative to past experience | ||||||||
| Better | 17% | 60% | N/A | N/A | 25% | 44% | N/A | N/A |
Note. UBACC= UCSD Brief Assessment of Capacity to Consent; MacCAT-CR= MacArthur Competence Assessment Tool for Clinical Research
Figure 1a.
UCSD Brief Assessment of Capacity to Consent (UBACC) total score for participants by diagnostic status and consent condition
Figure 1b.
MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR) Understanding scores for participants by diagnostic status and consent condition
When participants were queried about satisfaction with the consent process compared to prior consent experiences, many participants rated the quality of the enhanced consent procedure as “better” and no participant reported their current experience as worse (Table 1).
4. DISCUSSION
The web-media consent procedure was well received by the participants despite some of its drawbacks including its increased length of administration and the fact that computer-based approaches may be intimidating to people with limited computer experience. Although NCs had adequate comprehension regardless of consent condition, the present results suggest that for patients with schizophrenia, web-media consent approaches may be more efficacious in promoting comprehension of consent relevant information. Prior studies of video aided research consent have shown mixed results (reviewed in Ryan et al. 2008), but the present study illustrates the feasibility of incorporating audio-visual materials on a computer/web platform, thereby enabling a more interactive and flexible presentation than is typical with presentation on a videotape/DVD. Such presentation may enable researchers to capitalize on the benefits of audiovisual learning, while circumventing the limitations of video/DVD presentation.
Our ability to draw definitive conclusions from the present data is limited by the small sample size. This sample size reflected the intended goal as a preliminary evaluation of the feasibility and potential effectiveness of this web-media approach. Yet even in the context of the small sample, the difference for patients receiving the web-media aided consent procedure versus routine consent was statistically significantly for the UBACC, and although not statistically significant, in the large effect size range for the MacCAT-CR Understanding subscale. Thus, these results suggest utility in further development and validation of such web-media based consent tools.
Another potential limitation is that use of hypothetical rather than actual clinical trial consent might affect ecological validity. However, the described procedures, safety measures, and risk/benefits in the hypothetical protocol were adapted from an actual clinical trial of the effects of a cholinomimetic agent on cognitive deficits in schizophrenia. Moreover, use of a hypothetical protocol enabled us greater control over the specific content permitting appropriate tailoring of the consent to include procedures/risks common in current schizophrenia research, and thereby potentially enhancing generalizability relative to that achieved with any one particular actual protocol.
In short, this study introduced a viable and easily integrated web-media design that shows promise in producing greater comprehension of consent information relative to routine consent procedures, particularly in patients with schizophrenia, and circumvents some of the limitations inherent in videotape /DVD-based consent. Further research is warranted to develop and validate web-media tools that can provide consumer knowledge and be easily disseminated to neuropsychiatric and medical research populations.
Acknowledgement
We would like to thank Faraz Yaghouti for programming the web-based consent tool and Nha Nguyen for preparing the video materials.
Role of funding source
This work was supported, in part, by NIH grants T32MH019934, 5P30MH066248, MH64722, and the Stein Institute for Research on Aging. The NIMH and Stein Institute for Research on Aging had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Alexandrea Harmell conducted some of the literature searches and review, conducted data analyses, interpreted the findings, and prepared the first draft of the manuscript for publication.
Dr. Palmer was involved in designing the study, assisted in data analyses, as well as assisted in the data interpretation and manuscript revisions
Dr. Jeste was involved in designing the study, assisted in data analyses, as well as assisted in the data interpretation and manuscript revisions
Conflict of Interest
All of the authors declare that they have no conflicts of interest.
REFERENCES
- Appelbaum PS. Decisional capacity of patients with schizophrenia to consent to research: taking stock. Schizophr Bull. 2006;32(1):22–25. doi: 10.1093/schbul/sbi063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum PS, Grisso T. MacCAT-CR: MacArthur Competence Assessment Tool for Clinical Research. Professional Resource Press; Sarasota, FL: 2001. [Google Scholar]
- Brink S. A web approach to well-informed consent. Good Clinical Practice Journal. 2006;13(11):25–28. [Google Scholar]
- Getz KA. Informed consent process: A survey of subjects assesses strengths and weeknesses. Applied Clinical Trials. 2002:30–36. [Google Scholar]
- Jeste DV, Palmer BW, Appelbaum PS, Golshan S, Glorioso D, Dunn LB, et al. A new brief instrument for assessing decisional capacity for clinical research. Archives of General Psychiatry. 2007;64:966–974. doi: 10.1001/archpsyc.64.8.966. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Palmer BW, Golshan S, Eyler LT, Dunn LB, Meeks T, et al. Multimedia consent for research in people with schizophrenia and normal subjects: A randomized controlled trial. Schizophr Bull. 2009;35(4):719–729. doi: 10.1093/schbul/sbm148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Appelbaum PS, Swan J, Stroup TS, McEvoy JP, Goff DC, et al. Determining when impairment constitutes incapacity for informed consent in schizophrenia research. Br J Psychiatry. 2007;191:38–43. doi: 10.1192/bjp.bp.106.033324. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Savla GN. The association of specific neuropsychological deficits with capacity to consent to research or treatment. Journal of the International Neuropsychological Society. 2007;13:1047–1059. doi: 10.1017/S1355617707071299. [DOI] [PubMed] [Google Scholar]
- Ryan RE, Prictor MJ, McLaughlin KJ, Hill SJ. Audio-visual presentation of information for informed consent for participation in clinical trials. Cochrane Database of Systematic Reviews. 2008;(1) doi: 10.1002/14651858.CD003717.pub2. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]