Abstract
BACKGROUND
Damage control surgery is a staged approach to the trauma patient in extremis that improves survival, but leads to open abdominal wounds that are difficult to manage. We evaluated whether directed peritoneal resuscitation (DPR) when used as a resuscitation strategy in severely injured trauma patients with hemorrhagic shock requiring damage control surgery would affect the amount of and timing of resuscitation and/or show benefits in time to abdominal closure and reduction of intra-abdominal complications.
STUDY DESIGN
A retrospective case-matched study of patients undergoing damage control surgery for hemorrhagic shock secondary to trauma between January 2005 and December 2008 was performed. Twenty patients undergoing standardized wound closure and adjunctive DPR were identified and matched to 40 controls by Injury Severity Score, age, gender, and mechanism of injury. A single early death was excluded because of inability to control ongoing hemorrhage.
RESULTS
There were no differences in age, gender, or mechanism of injury between the groups. Injury Severity Score (35.07 ± 17.1 versus DPR 34.95 ± 16.95; p = 0.82) and packed red blood cell administration in 24 hours (23.8 ± 14.35 U versus DPR 26.9 ± 14.1 U; p = 0.43) were similar between the groups. Presenting pH was similar between the study group and the DPR group (7.24 ± 0.13 d versus DPR 7.26 ± 0.11; p = 0.8). Time to definitive abdominal closure was significantly less in the DPR group compared with controls (DPR: 4.35 ± 1.6 d versus 7.05 ± 3.31; p = 0.003). DPR also allowed for a higher rate of primary fascial closure, lower intra-abdominal complication rate, and lower rate of ventral hernia formation at 6 months. Adjunctive DPR afforded a definitive wound closure advantage compared with Wittmann patch closure techniques (DPR 4.35 ± 1.6 versus Wittmann patch 6.375 ± 1.3; p = 0.004).
CONCLUSIONS
The addition of adjunctive DPR to the damage control strategy shortens the interval to definitive fascial closure without affecting overall resuscitation volumes. As a result, this mitigates intra-abdominal complications associated with open abdomen and damage control surgery and affords better patient outcomes.
The advent of damage control surgery (DCS) has led to a staged approach to the patient in extremis with intra-abdominal hemorrhage and shock that has undoubtedly saved lives. However, massive resuscitation associated with severe hemorrhagic shock involves fluid administration in volumes far in excess of estimated blood loss because of the shift of fluid from the intravascular to the extravascular space. This massive volume load usually results in substantial tissue edema, which can delay abdominal closure.1–3 Acute tissue edema with swelling of the interstitial space secondary to resuscitation is a dominant factor in the inability to close many DCS patients.4 As the experience with DCS has evolved, the major long-term problems of this approach often involve complications of the abdominal wall. Ventral hernias, fistulas, and difficult to reconstruct abdominal wall defects are often the sequelae of the inability to achieve primary fascial closure.
Our group has extensive experience studying the physiologic effects of direct peritoneal resuscitation (DPR), which consists of suffusing the peritoneal cavity with a hypertonic glucose-based peritoneal dialysis solution. In these experimental studies using a variety of animal models, we have demonstrated that the suffusion of a 2.5% glucose-based peritoneal dialysis solution concurrent with intravenous resuscitation from hemorrhagic shock causes microvascular vasodilation and increases visceral and hepatic blood flow5; reverses endothelial cell dysfunction6; improves survival and downregulates the inflammatory response7; reverses established microvascular constriction8; normalizes capillary perfusion density9; and normalizes systemic water compartments.10 In addition to these observed effects on microcirculation, we have noted a marked ability to decrease visceral edema and normalize body water ratios. We have undertaken this case-control study to determine if DPR could ameliorate the deleterious effects of massive fluid resuscitation and visceral edema, alter the volume of resuscitation required for correction of shock, and facilitate early primary fascial closure.
METHODS
The study was conducted at the University of Louisville Hospital, a 414-bed tertiary care facility. The hospital has an American College of Surgeons Level I trauma center designation and is the major adult trauma referral center for metropolitan Louisville, Western and Central Kentucky, and Southern Indiana. The study period for cases extended from January 1, 2004 to June 30, 2008, and encompassed all patients admitted in hemorrhagic shock requiring DCS for management of their injuries by the University of Louisville Hospital Trauma Service. All patients were cared for by the trauma service, consisting primarily of 5 core trauma/critical care faculty at the University of Louisville Hospital.
We performed a retrospective case-control study with 2:1 matching. Twenty patients in refractory hemorrhagic shock with substantial tissue edema requiring DCS were selected for this trial. These 20 patients undergoing standardized wound closure and adjunctive DPR were matched to 40 controls by Injury Severity Score (ISS), age, gender, mechanism of injury, presenting systolic blood pressure, and presenting pH. Lack of random patient selection in the trial group was the primary reason to select a larger control section. Additionally, head injuries with an Abbreviated Injury Score >3 were excluded because no patients in the experimental group had a traumatic brain injury this severe. A single early death was excluded from the adjunctive DPR group because of the inability to obtain surgical control of ongoing low pelvic hemorrhage and total time for DPR of <2 hours. Therefore, 19 patients were available for analysis.
Abdominal closure technique was standardized in the DPR group to the following: a 19F silicone elastomer round Blake drain (Ethicon) was placed in the left upper lateral quadrant and directed around the root of the mesentery along the left pericolic gutter and down into the pelvis. A sterile x-ray cassette cover was placed over the abdominal contents but under the fascia. A sterile operating room towel was placed over the plastic cover and another drain was placed within the towel. The entirety of the abdomen was covered with an Ioban (3M) occlusive dressing. The towel drain was placed to low-pressure suction and the DPR solution was instilled using the left upper quadrant drain, causing a continuous lavage within the abdomen until suctioned out the top of the wound through the towel drain. DPR was initiated using commercially available 2.5% glucose-based peritoneal dialysis solution (Delflex; Fresenius USA) (25 g/L D-glucose, 0.567 g/L sodium chloride, 0.392 g/L sodium lactate, 0.0257 g/L calcium chloride, 0.0152 g/L magnesium chloride at a pH of 6, osmolality of 486 mOsm/L). The 500 mL Delflex fluid was instilled initially and at a rate of 1.5 mL/kg/h thereafter until definitive abdominal closure. Intravenous blood and crystalloid resuscitation was conducted at the discretion of the treating physicians, with an aim toward restoring hemodynamic stability in both the study and control patient groups. There was no standardized abdominal wound closure method in the control group.
The following variables were collected: age, gender, presenting heart rate, presenting systolic blood pressure, ISS, presenting arterial pH, pH 24 hours postadmission, presenting base deficit, presenting international normalized ratio and at 24 hours after admission, presenting and 24-hour liver transaminases, serum BUN and creatinine at presentation and 24 hours after admission, total IV fluid administration in the first 24 hours of admission, and total blood products administered in the first 24 hours after admission. Additionally, dates of admission and discharge from the ICU; dates of admission and of discharge from the hospital; number and type of complications, both intra-abdominal and extra-abdominal; and total number of ventilator days were recorded. The number and timing of abdominal operations, time to definitive abdominal closure, and type of temporary and definitive abdominal closure were identified. Finally, outcome data on discharge status, disposition, and long-term complications identified at 6-month follow-up appointments were collected.
Variables were expressed as mean ± SD. A post hoc p value ≤0.05 in a 2-tailed test was considered to indicate statistical significance. Levene’s test for equality of variance was used to determine homogeneity of group data. Percentages were compared with use of the chi-square test and means with t-test. Odds ratios with 95% confidence intervals were determined where appropriate with significance evaluated with Fisher’s exact test. The Institutional Review Board of the University of Louisville and the Human Subjects Protection Committee at University of Louisville Hospital approved the study.
RESULTS
Comparison of DPR group with controls is noted in Table 1. As shown, there were no appreciable differences between the 2 groups, with the exception of presenting international normalized ratio, which was higher in the DPR group. These groups were compared in univariate analysis using post hoc p value significant at 0.05. Management of the open abdomen in these control patients was determined by the treating surgeon with ~83% (33 of 40 patients) managed using a similar homemade vacuum dressing as described for the DPR patients. The remaining 17% were managed with a variety of techniques, including Bogota bag (3 of 40 patients), absorbable mesh (1 of 40), and permanent mesh (2 of 40). Total operative time and initial injuries were similar, with major hepatic injuries quite common in both groups suffering blunt trauma (~54%) and great vessel or pelvic vessel injury common in those patients suffering penetrating trauma. Overall, patients were severely injured with an ISS >32 and required >20 U blood products for resuscitation within the first 24 hours. The amount of resuscitative fluid and blood required to correct the physiologic variables identified were no different between the groups. Also, variables dictating the end points of resuscitation were also not substantially different between the groups.
Table 1.
Comparison of Study Groups
| Variables | Control group (n = 40), mean ± SD | DPR group (n = 19), mean ± SD | p Value | W |
|---|---|---|---|---|
| Age, y | 30.7 ± 12.8 | 30.9 ± 12.5 | 0.96 | 0.89 |
| HR (bpm), presenting | 107 ± 36 | 109 ± 35 | 0.91 | 0.86 |
| SBP (mmHg), presenting | 90 ± 28 | 88 ± 28 | 0.72 | 0.51 |
| Injury Severity Score | 34 ± 16 | 36 ± 17 | 0.63 | 0.93 |
| pH, presenting | 7.26 ± 0.14 | 7.25 ± 0.12 | 0.74 | .095 |
| pH, 24 h | 7.36 ± 0.06 | 7.38 ± 0.04 | 0.83 | 0.22 |
| Base deficit, presenting | 7.8 ± 4 | 8.0 ± 2.6 | 0.89 | 0.21 |
| INR, presenting | 1.4 ± 0.5 | 1.7 ± 0.5 | 0.026 | 0.88 |
| INR, 24 h | 1.2 ± 0.4 | 1.1 ± 0.4 | 0.27 | 0.07 |
| ALT (IU), presenting | 508 ± 943 | 742 ± 1,296 | 0.43 | 0.24 |
| ALT (IU), 24 h | 762 ± 1,329 | 717 ± 934 | 0.89 | 0.55 |
| AST (IU), presenting | 757 ± 1,250 | 1,200 ± 1,900 | 0.28 | 0.09 |
| AST (IU), 24 h | 1025 ± 98 | 984 ± 1,172 | 0.92 | 0.49 |
| BUN, presenting | 12 ± 4 | 13 ± 5 | 0.49 | 0.75 |
| BUN, 24 h | 15 ± 7 | 16 ± 6 | 0.82 | 0.36 |
| Creatinine, presenting | 1.01 ± 0.40 | 1.11 ± 0.35 | 0.30 | 0.65 |
| Creatinine, 24 h | 1.23 ± 0.58 | 1.32 ± 0.51 | 0.56 | 0.31 |
| IV fluid (L), first 24 h (L) | 23 ± 7 | 25 ± 11 | 0.51 | 0.15 |
| Blood products, U, first 24 h | 22 ± 12 | 27 ± 14 | 0.24 | 0.49 |
ALT, alanine transaminase; AST, aspartate transaminase; bpm, beats per minute; DPR, directed peritoneal resuscitation; HR, heart rate; INR, international normalized ratio; IU, international units; SBP, systolic blood pressure.
Mortality, hospital length of stay, ICU length of stay, and ventilator days were similar between the groups. The odds ratio for death for patients who underwent DPR as an adjunct to shock resuscitation was 0.82; however, this did not achieve statistical significance with the power of this study (p = 0.97). All deaths in both groups occurred before definitive closure (<7 days into hospital course), with the exception of a single patient in the control group. That patient did not get definitive abdominal closure after the family elected for palliative care secondary to substantial cerebrovascular accident following blunt carotid injury. That patient died on hospital day 10. These patients were excluded from analysis for evaluation of definitive closure because no attempt was made to definitively address their abdominal wounds (Table 2).
Table 2.
Group Outcomes Data
| Variable | Control (n = 40) | DPR (n = 19) | p Value | W |
|---|---|---|---|---|
| Hospital LOS, d, mean ± SD | 25 ± 15 | 24 ± 16 | 0.79 | 0.75 |
| ICU LOS, d, mean ± SD | 16 ± 12 | 16 ± 11 | 0.98 | 0.80 |
| Ventilator, d, mean ± SD | 10 ± 7 | 12 ± 9 | 0.063 | 0.28 |
| Mortality, n (%) | 5 (12.5) | 2 (10.5) | NA | NA |
DPR, directed peritoneal resuscitation; LOS, length of stay.
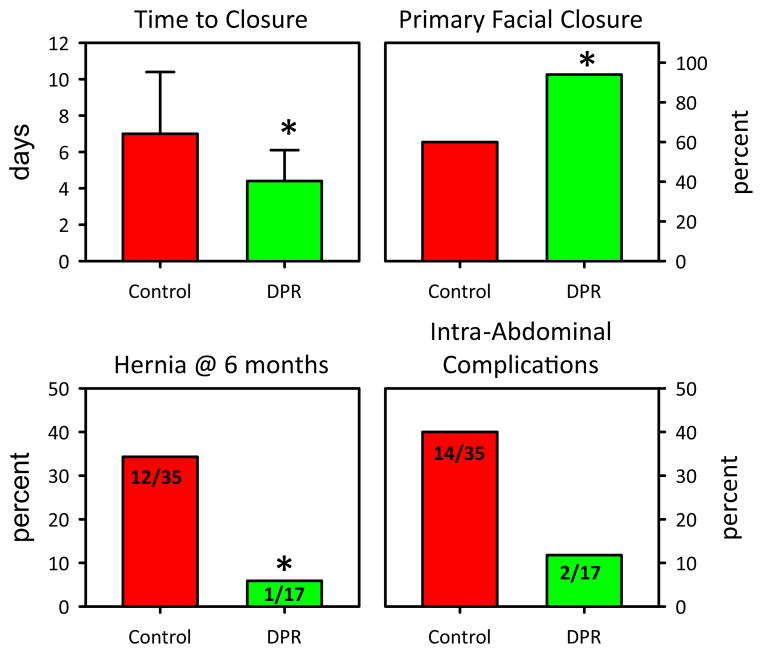
A substantial decrease in the time to closure was identified in patients receiving adjunctive DPR compared with conventional resuscitation alone, as shown in Figure 1. Patients receiving adjunctive DPR were closed in 4.4 ± 1.7 days compared with 7.0 ± 3.4 days in the control patients. The percent of patients undergoing primary fascial closure was also considerably increased in the group of patients receiving adjunctive peritoneal resuscitation. The odds ratio for primary fascial closure was 10.7:1 for those undergoing DPR (p = 0.01), as opposed to traditional management. Method of definitive abdominal closure of the control group varied. The majority of these patients were definitively closed primarily, as shown in Table 3. Absorbable mesh was used in 11 of 35 (31%) and 4 of 35 (11%) were closed definitively with biologic mesh.
Figure 1.
Demonstrating the significant differences between the control group and the directed peritoneal resuscitation (DPR) group. *indicates statistical significance.
Table 3.
Comparison of DPR with Wittman Patch Technique
| Variable | Wittman patch (n = 8) | DPR (n = 19) | p Value |
|---|---|---|---|
| Age, y, mean ± SD | 30.4 ± 11.8 | 30.9 ± 12.5 | 0.92 |
| HR (bpm), presenting, mean ± SD | 121 ± 17 | 109 ± 35 | 0.34 |
| SBP (mmHg), presenting, mean ± SD | 95 ± 11 | 88 ± 28 | 0.46 |
| ISS, mean ± SD | 34 ± 12 | 36 ± 17 | 0.45 |
| pH, presenting, mean ± SD | 7.30 ± 0.02 | 7.25 ± 0.12 | 0.19 |
| Time to closure, d, mean ± SD | 6.4 ± 1.3 | 4.4 ± 1.7 | 0.003* |
| Primary fascial closure, % (n) | 87.5 (7/8) | 94.1 (16/17) | — |
| Intra-abdominal complications, % (n) | 37.5 (3/8) | 11.7 (2/17) | — |
Statistically significant.
bpm, beats per minute; DPR, directed peritoneal resuscitation; HR, hazard ratio; ISS, Injury Severity Score; SBP, systolic blood pressure.
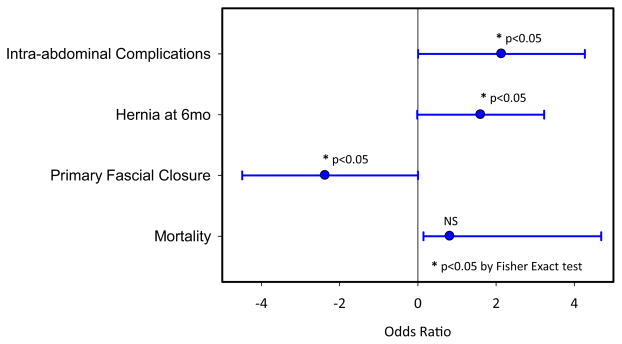
The number of abdominal complications was considerably less in the DPR group as compared with the control group. Abdominal complications identified included wound infection, intra-abdominal abscess/infection, enterocutaneous fistula, biloma, and dehiscence or evisceration. The odds ratio for intra-abdominal complications after DCS was 5:1 in favor of those patients receiving DPR as compared with controls (p = 0.05). Overall complication rate, however, was unchanged between groups, with 34 of 40 (85%) patients suffering complications (death included in the complication list) in the control group and 15 of 19 (79%) suffering a complication in the DPR group. There were 6 enterocutaneous fistulae reported in the control group as opposed to 0 in the DPR group. Follow-up on the patients was noted out to 6 months after discharge and only a single patient in the DPR group developed a ventral hernia. This was the patient who was not closed primarily at the time of definitive abdominal closure. This is significantly different than the control group (p = 0.034), in which ventral hernia developed at 6 months in 34% of control patients managed with traditional means. Odds ratio for development of a ventral hernia after open abdomen was 8.5:1 in favor of DPR (p = 0.04). This is shown in Figure 2.
Figure 2.
Odds ratio of significant variable between control and directed peritoneal resuscitation (DPR) group showing a decrease rate of hernia formation, increased primary fascial closure rate, and lower number of intra-abdominal complications.
Overall range of complications was broad, with a considerable majority associated with extra-abdominal injuries. We did find that patients undergoing DPR had a slightly lower incidence of pulmonary complications, particularly ventilator-associated pneumonia diagnosed by bronchial alveolar lavage. This did not reach significance and did not affect either ventilator days or ICU length of stay as shown in Table 2. Length of stay, both ICU and hospital, and ventilator days were consistent across both groups and related more to type and severity of illness than method of resuscitation.
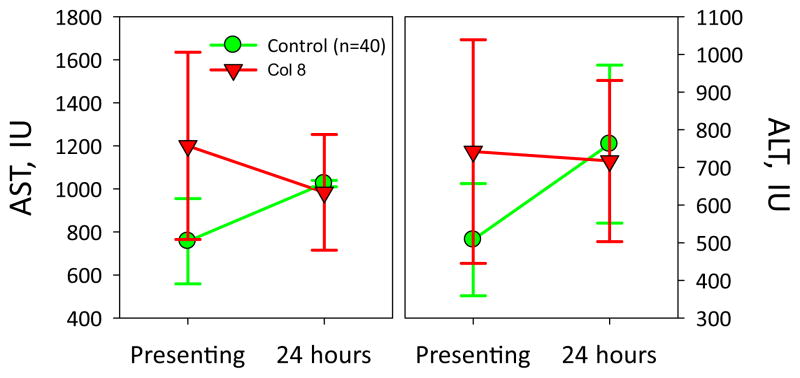
We have also shown a substantial increase in visceral blood flow after instituting DPR in rodents.11 In assessing the impact of DPR in our rodent model, we have seen a substantial increase in the hepatic blood flow after initiation of DPR in rodents being resuscitated from hemorrhagic shock. We could not assess liver blood flow in this retrospective study, however, we were able to evaluate liver function using measurements of hepatocellular enzymes. The aspartate transaminase and alanine transaminase levels at presentation and 24 hours after injury were similar between the 2 groups. However, we found a substantial difference in the rate of improvement in these values 24 hours postresuscitation, as shown in Figure 3. The change in aspartate transaminase and alanine transaminase in 24 hours points toward a normalization of splanchnic perfusion in the DPR group, despite undergoing the same volume of resuscitation of both IV fluid and blood products.
Figure 3.
Liver enzymes levels in the control versus directed peritoneal resuscitation (DPR) groups showing a trend toward improvement in the DPR group compared with worsened aspartate transaminase (AST)/alanine transaminase (ALT) levels at 24 hours in the control group. IU, international units.
In an effort to standardize the closure technique used in the control group to be similar to the technique used in the DPR group, we initially excluded a small group of patients that were managed using a Wittman patch. However, a small secondary group analysis was also performed on 8 patients undergoing closure with a Wittman patch that met our initial inclusion criteria. The findings of this comparison are noted in Table 3. The groups were similar, however, again a significant difference was noted in the DPR group with regard to time to closure. The study populations for this comparison are very small and not powered to identify differences (Table 3).
DISCUSSION
The technique of wound packing for hemorrhage control has been used throughout the history of surgery, however, complications arising from recurrent bleeding at pack removal and late infections led to the eventual abandonment of this technique in the late 1940s and early 1950s.12 Drs Lucas and Ledgerwood, in a prospective trial from 1969 to 1973 at Wayne State University in Detroit, began to reintroduce this technique.13 Subsequent series by various surgeons demonstrated the use of packing and proved superior to historical controls.14–16 Stone and colleagues17 reported their experience with 31 patients noted to have a coagulopathy with onset during an operation. In the first 14 patients, the procedure continued with hematologic replacement and completion of all facets of the operation. Only 1 patient survived, yielding a mortality rate of 93%. In the subsequent 17 patients, the operation was aborted once a coagulopathy was noted and abdominal tamponade was achieved with an average of 9 laparotomy pads. Reoperation was performed an average of 27 hours later after the correction of the coagulopathy. Eleven patients survived, reducing the mortality rate from 93% to 35%. Dr Rotondo and colleagues demonstrated the use of this technique in their landmark 1993 article in the Journal of Trauma and coined the term damage control surgery.18 Our article demonstrates a novel technique in management of the open abdomen that leads to more rapid primary closure with better long-term abdominal wall outcomes.
In this retrospective clinical experience, we have demonstrated statistically significant decreased time to definitive abdominal closure in patients receiving adjunctive DPR despite having no substantial difference in the resuscitative volume required. We speculate this difference in time to closure is a result of the reduction in visceral tissue edema so often noted after conventional crystalloid resuscitation from hemorrhagic shock. Conventional resuscitation from hemorrhagic shock that targets restoration and maintenance of central hemodynamics leads to tissue fluid sequestration and edema formation causing compromise of tissue perfusion.19 Hemorrhagic shock and cellular hypoxia alter the ability of cell membranes to regulate the interchange of ions between the cell and its immediate microenvironment. Our previous studies indicated that endothelial cell swelling, resulting from the hemorrhage-stimulated Na+/H+ exchanger, injures the endothelial cell and results in end-organ tissue hypoperfusion. Cells, in an effort to mitigate the growing intracellular acidosis from anaerobic metabolism, force H+ out and draw Na+ into the cell. Water subsequently follows the ions causing cellular swelling and endothelial dysfunction. We have demonstrated and quantified the reduction in visceral edema in both the intracellular space and extracellular space after adjunctive DPR in our rodent model of hemorrhagic shock. We postulate that the reduction of endothelial cell swelling and dysfunction modulates the inflammatory response in the rodent model. However, this study is not designed to assess this question. Regardless of the mechanism, any measure to reduce ischemia-reperfusion injury and the subsequent inflammatory response can reduce bowel edema and could be the explanation for earlier primary fascial closure.
The reduction in the time to definitive closure cannot be underestimated in this patient population. Miller and colleagues20 has shown that definitive abdominal closure in <8 days is associated with fewer overall complications and better outcomes. Six of the 17 patients in the Miller study who developed complications in the late-closure group died, with 5 of the 6 deaths directly related to intra-abdominal complications.20 In this study, the patients who were closed after 8 days had a considerably higher complication rate. We demonstrated a similar finding with a decreased rate of intra-abdominal complications in patients undergoing adjunctive DPR. This cannot be directly related to time to closure, however. Miller and colleagues additionally noted that patients unable to undergo a primary fascial closure had a considerable increase in the infectious complication rate (52 of 96; 54%). The patients undergoing DPR in our study had a substantial decrease in the intra-abdominal complication rate and a higher rate of primary fascial closure compared with control subjects.
One of the more dreaded complications of DCS and the open abdomen is an enterocutaneous fistula. Miller and colleagues20 reported a fistula rate of 12% and Mayberry and colleagues21 reported a rate of 7.1%. Fischer and colleagues22 found an 8% fistula rate in their extensive experience and a spontaneous closure rate of 37%, which is higher than other reports of 25%. Regardless, a substantial proportion of patients in whom this complication will develop require complex operative management. Earlier studies have linked late abdominal closure and closure with prosthetic mesh to increased fistula formation.23 There were no fistulas in the DPR group and 4 enterocutaneous fistulas in the control group. All enterocutaneous fistulas were in patients who did not undergo primary fascial closure as a definitive closure procedure. Early primary closure of the patient’s native tissue afforded by DPR should allow for an overall reduction in this morbid complication.
Another late complication requiring considerable resources and time to treat is abdominal hernia formation after definitive closure. Primary fascial closure, as long as it is not under tension, has repeatedly been shown to afford the patient the best possible abdominal wall repair.24 Many other series have documented a fascial closure rate of only 50% to 70% in DCS.25–27 Adjunctive DPR was associated with a high rate of primary fascial closure (94%). We noted a considerable difference in hernia formation at 6 months between the control group and the patients undergoing DPR. The reason for this is unclear but, as noted, we speculate this is related to the higher primary fascial closure rate in DPR group.
We acknowledge the weaknesses inherent within this study. This was a retrospective study and patients were selected for DPR in a manner that was not randomized or controlled. Therefore, we cannot exclude the possibility of bias in patients selected to receive DPR. We used case-control methodology to try and make our groups as comparable as possible and several variables, such as amount of resuscitative volume, ISS, mortality, and presenting base deficit were identical between groups. Because selection was not randomized, our groups might not be identical and a randomized controlled trial would be necessary. It is worth noting that our rate of primary fascial closure in the conventionally treated group is similar to that published by others.28–30 The high rate of primary fascial closure and the accelerated time course to primary fascial closure in the DPR group is noteworthy and suggests that this technique has promise for the multiply injured patient undergoing DCS.
In conclusion, we present the first reported use of adjunctive DPR in DCS patients. With the addition of adjunctive DPR to our standard treatment regimen, we were able to achieve a rapid, long-lasting primary fascial closure in these patients and often a considerable reduction in the intra-abdominal complication rate.
Abbreviations and Acronyms
- DCS
damage control surgery
- DPR
direct peritoneal resuscitation
- ISS
Injury Severity Score
Footnotes
Disclosure Information: Nothing to disclose.
Presented at Southern Surgical Association 121st Annual Meeting, Hot Springs, VA, December 2009.
Author Contributions
Study conception and design: Smith, Garrison, Matheson, Richardson
Acquisition of data: Smith, Franklin
Analysis and interpretation of data: Smith, Garrison, Matheson, Harbrecht
Drafting of manuscript: Smith, Franklin
Critical revision: Smith, Garrison, Harbrecht, Matheson, Richardson
References
- 1.Shires GT. Shock and metabolism. Surg Gynecol Obstet. 1967;124:284–287. [PubMed] [Google Scholar]
- 2.Brand ED, Shuh Tk, Avery MC. Reversal of postoligemic shock in the cat by hypervenobaric massive fluid therapy. Am J Physiol. 1966;211:1232–1240. doi: 10.1152/ajplegacy.1966.211.5.1232. [DOI] [PubMed] [Google Scholar]
- 3.Wolfman EF, Neill SA, Heaps DK. Donor blood and isotonic salt solution. Effect on survival after hemorrhagic shock and operation. Arch Surg. 1963;86:869–873. doi: 10.1001/archsurg.1963.01310110179023. [DOI] [PubMed] [Google Scholar]
- 4.Foy H. Reinforced silicone elastomer sheeting, an improved method of temporary abdominal closure in damage control laparotomy. Am J Surg. 2003;185:498–501. doi: 10.1016/s0002-9610(03)00059-x. [DOI] [PubMed] [Google Scholar]
- 5.Hurt RT, Zakaria ER, Matheson PJ, et al. Hemorrhage-induced hepatic injury and hypoperfusion can be prevented by direct peritoneal resuscitation. J Gastrointest Surg. 2009;13:587–594. doi: 10.1007/s11605-008-0796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zakaria ER, Li N, Garrison RN. Mechanisms of direct peritoneal resuscitation-mediated splanchnic hyperperfusion following hemorrhagic shock. Shock. 2007;27:436–442. doi: 10.1097/01.shk.0000245017.86117.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrison RN, Conn AA, Harris PD, Zakaria ER. Direct peritoneal resuscitation as adjunct to conventional resuscitation from hemorrhagic shock: a better outcome. Surgery. 2004;136:900–908. doi: 10.1016/j.surg.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Zakaria ER, Garrison RN, Kawabe T, Harris PD. Direct peritoneal resuscitation from hemorrhagic shock: effect of time delay in therapy initiation. J Trauma. 2005;58:499–506. doi: 10.1097/01.TA.0000152892.24841.54. discussion 506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakaria ER, Li N, Matheson PJ, Garrison RN. Cellular edema regulates tissue capillary perfusion after hemorrhage resuscitation. Surgery. 2007;142:487–496. doi: 10.1016/j.surg.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zakaria ER, Matheson PJ, Flessner MF, Garrison RN. Hemorrhagic shock and resuscitation-mediated tissue water distribution is normalized by adjunctive peritoneal resuscitation. J Am Coll Surg. 2008;206:970–983. doi: 10.1016/j.jamcollsurg.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svoboda JA, Peter ET, Dang CV, et al. Severe liver trauma in the face of coagulopathy: a case for temporary packing and early re-exploration. Am J Surg. 1982;144:717–721. doi: 10.1016/0002-9610(82)90557-8. [DOI] [PubMed] [Google Scholar]
- 12.Walt AJ. The surgical management of hepatic trauma and its complications. Ann R Coll Surg Engl. 1969;45:319–339. [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas CE, Ledgerwood AM. Prospective evaluation of hemostatic techniques for liver injuries. J Trauma. 1976;16:442–451. doi: 10.1097/00005373-197606000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Calne RY, McMaster P, Pentlow BD. The treatment of major liver trauma by primary packing with transfer of the patient for definitive treatment. Br J Surg. 1979;66:338–339. doi: 10.1002/bjs.1800660512. [DOI] [PubMed] [Google Scholar]
- 15.Feliciano DV, Mattox KL, Jordan GL., Jr Intra-abdominal packing for control of hepatic hemorrhage: a reappraisal. J Trauma. 1981;21:285–290. doi: 10.1097/00005373-198104000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Cue JI, Cryer HG, Miller FB, et al. Packing and planned reexploration for hepatic and retroperitoneal hemorrhage: critical refinements of a useful technique. J Trauma. 1990;30:1007–1013. doi: 10.1097/00005373-199008000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Stone HH, Strom PR, Mullins RJ. Management of coagulopathy with onset during laparotomy. Ann Surg. 1983;197:532–535. doi: 10.1097/00000658-198305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotondo MF, Schwab CW, McGonigal MD, et al. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375–383. [PubMed] [Google Scholar]
- 19.Balogh Z, McKinley BA, Holcomb JB, et al. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848–859. doi: 10.1097/01.TA.0000070166.29649.F3. [DOI] [PubMed] [Google Scholar]
- 20.Miller RS, Morris JA, Jr, Diaz JJ, Jr, et al. Complications after 344 damage-control open celiotomies. J Trauma. 2005;59:1365–1374. doi: 10.1097/01.ta.0000196004.49422.af. [DOI] [PubMed] [Google Scholar]
- 21.Mayberry JC, Burgess EA, Goldman RK, et al. Enterocutaneous fistula and ventral hernia after absorbable mesh prosthesis closure for trauma: the plain truth. J Trauma. 2004;57:157–163. doi: 10.1097/01.ta.0000102411.69521.80. [DOI] [PubMed] [Google Scholar]
- 22.Fischer PE, Fabian TC, Magnotti LJ, et al. A ten-year review of enterocutaneous fistulas after laparotomy for trauma. J Trauma. 2009;67:924–928. doi: 10.1097/TA.0b013e3181ad5463. [DOI] [PubMed] [Google Scholar]
- 23.Vargo D, Richardson JD. Management of the open abdomen: from initial operation to definitive closure. Am Surg. 2009;75(Suppl):S1–S22. [PubMed] [Google Scholar]
- 24.Cheatam ML, Safcsak K. Longterm impact of abdominal decompression: a prospective comparative analysis. J Am Coll Surg. 2008;207:573–579. doi: 10.1016/j.jamcollsurg.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Barker DE, Kaufman HJ, Smith LA, et al. Vacuum pack technique of temporary abdominal closure: a seven year experience with 112 patients. J Trauma. 2000;48:201–207. doi: 10.1097/00005373-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Smith PC, Tweddell JS, Bessey PQ. Alternative approaches to abdominal wound closure in severely injured patients with massive visceral edema. J Trauma. 1992;32:16–20. doi: 10.1097/00005373-199201000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Fabian TC, Croce MA, Pritchard FE, et al. Planned ventral hernia: staged management for acute abdominal wall defects. Ann Surg. 1994;219:643–653. doi: 10.1097/00000658-199406000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bee TK, Croce MA, Magnotti LJ, et al. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J Trauma. 2008;65:337–344. doi: 10.1097/TA.0b013e31817fa451. [DOI] [PubMed] [Google Scholar]
- 29.Barker DE, Green JM, Maxwell RA, et al. Experience with vacuum-pack temporary abdominal wound closure in 258 trauma and general and vascular surgical patients. J Am Coll Surg. 2007;204:784–793. doi: 10.1016/j.jamcollsurg.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 30.Miller PR, Meredith JW, Johnson JC, Chang MC. Prospective evaluation of vacuum-assisted fascial closure after open abdomen: planned ventral hernia rate is substantially reduced. Ann Surg. 2004;239:608–616. doi: 10.1097/01.sla.0000124291.09032.bf. [DOI] [PMC free article] [PubMed] [Google Scholar]