The changes in mineral metabolism in chronic kidney disease (CKD) are well established as being major contributors to the morbidity and mortality of CKD patients, both early and established. The known factors involved were considered to be phosphate (P), calcium, parathyroid hormone (PTH), vitamin D and metabolic acidosis. A number of epidemiological studies have shown that high P associates with mortality; but more recently, the field has been invigorated by a new player, namely fibroblast growth factor 23 (FGF23). We shall review the exciting developments in research on FGF23 and its interplay with other factors that are known to be altered in CKD, particularly P and calcium, PTH and vitamin D. In particular, we will consider new findings on the effect of FGF23 on the heart.
Phosphorus metabolism in CKD
One of us (E.S.) showed in 1966 that there is a control system that governs P excretion in uremia. This system appears to be designed to preserve normal P concentrations in the plasma as long as possible, and it expresses itself by a progressive increase in P excretion per nephron as the number of nephrons diminishes. The efferent limb of this control system was PTH. In experiments in dogs with experimental uremia and regulated phosphorus intake when glomerular filtration rate (GFR) diminished due to nephron destruction, P excretion decreases transiently and transient hyperphosphatemia ensues [1, 2]. The latter would result in reciprocal hypocalcemia. They hypothesized that the decrease in ionized calcium rather than the elevation of P per se stimulates the parathyroid glands to increase the rate of hormone secretion. This hypothesis remains the state of the art and has been enhanced by studies showing an independent effect of phosphate on PTH gene expression and secretion independent of changes in ionized calcium and serum calcitriol [3–5]. The effect of changes in phosphate on parathyroid gland function demands an intact parathyroid gland architecture in that in vitro they were present only in intact glands or tissue slices but not in isolated cells. Changes in dietary phosphorus also regulated parathyroid cell proliferation [6, 7]. The cellular mechanisms involved in these responses are still not defined. Prokaryotes such as bacteria and unicellular eukaryotes such as yeast also respond by changing the expression of a large number of genes in response to growth in media with different phosphorus concentrations and their sensing mechanisms have yet to be defined [8].
At the level of the kidney, the response of the renal P transporters, NaPi2a and c, to changes in serum P is well defined. In response to a low P, there is a rapid transfer of the NaPi transporters to the brush border membrane, followed by increased NaPi messenger RNA (mRNA) levels and NaPi protein synthesis [9–12]. The Na+-Pi cotransporter PiT-2 (SLC20A2) is also expressed in the apical membrane of rat renal proximal tubules and regulated by dietary phosphorus [13]. All of these result in the increased uptake of filtered P. These effects occur both in vitro and in vivo. Therefore, renal P conservation is regulated directly by the kidney as well as by secondary changes in serum PTH.
PTH acts at the level of the proximal renal tubule via the PTHR1 expressed at the basolateral and apical membrane to activate Gsα/PKA and Gq/PLC/PKC, respectively [14]. Activation of both signaling pathways leads to internalization of the Na+-Pi cotransporters NaPi2a and c, resulting in renal P wasting and hypophosphatemia [9].
In addition, there is compelling evidence for changes in oral phosphorus being recognized by a small intestine factor which then acts directly on the kidney to increase urinary P excretion [15]. Identification of the intestinal phosphaturic factor remains elusive. There is another factor involved in renal P and vitamin D homeostasis, namely FGF23.
FGF23 and klotho
The recent discovery that osteocytes and osteoblasts are the principal source for FGF23 identifies the bone not only as the major reservoir for calcium and phosphorus but as an endocrine organ that communicates with other organs involved in mineral homeostasis [16, 17]. FGF23 acts on its receptor complex, klotho-FGFR1, in the kidney to increase P excretion and to decrease calcitriol synthesis [14, 18–20]. This action of FGF23 allows bone to respond to P mobilization and changes in bone turnover by secreting FGF23 and thereby removing the excess of extracellular P as well as protecting the organism from vitamin D effects to increase the intestinal absorption of calcium and phosphorus.
The classical endocrine action of FGF23 is dependent upon its binding and activation of the klotho-FGFR1 complex. Klotho exists as a transmembrane form as well as a secreted form either due to alternative RNA splicing or the product of the α-secretase, sheddases, ADAM (a disintegrin and metalloprotease) 10 and 17 and β-secretase, β-APP (amyloid precursor protein) cleaving enzyme 1 [21]. Klotho transcript is highest in the kidney and parathyroid gland, but it is also expressed in the brain, heart, pancreatic islet β-cell and placenta with lesser abundance.
The presence of both FGF23 and PTH are necessary for normal P homeostasis
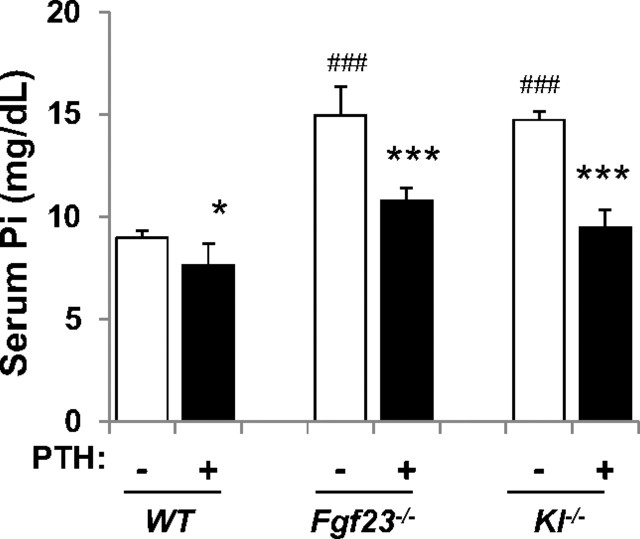
FGF23 and PTH both act on the kidney independently to inhibit the activity of the Na-Pi cotransporters 2a and 2c [14]. Excess of either humoral factor leads to an enhanced phosphaturia with increased calcium reabsorption [22], but only PTH leads to an increase in serum calcium. This is because the PTH receptor, PTH1R, is expressed in bone and kidney unlike klotho that is only expressed in the kidney. Furthermore, PTH stimulates calcitriol synthesis that further increases serum calcium and FGF23 has the opposed effect on vitamin D and calcium. It is of interest why the excess of PTH is not compensated for by a decrease in FGF23 and in turn, why an increase in FGF23 is not compensated for by a decrease in PTH. This is also intriguing in the opposite situation, where both hypoparathyroidism and tumoral calcinosis are characterized by hyperphosphatemia. In hypoparathyroidism, there is a modest increase in serum FGF23, which correlates with the hyperphosphatemia but these patients also receive calcitriol that would itself increase FGF23 [23, 24]. Why is there no profound increase in serum FGF23 to maintain phosphorus homeostasis? Similarly, in tumoral calcinosis where FGF23 levels are undetectable, why is there no dramatic increase in serum PTH to correct the serum P [25]? The answers are not clear; particularly, when one considers that there is a well-defined PTH-FGF23 feedback loop, which is described below. Contributing factors may be changes in concentrations of serum calcitriol and of course any small change in serum Ca2+ to which the parathyroid is exquisitely sensitive. FGF23 decreases and PTH increases serum calcitriol. FGF23 decreases serum PTH in the physiological state and PTH increases serum FGF23. Lanske's laboratory showed that mice with deletion of the genes for either FGF23 or klotho still respond to administered PTH with a significant phosphaturia (Figure 1) [26]. So, PTH can regulate serum P without FGF23 but the responses in disease states are often not as clear-cut as one would expect. Bone remodeling is particularly important in regulating FGF23 secretion and this topic is discussed below [27]. What is clear is that FGF23 and PTH are both necessary for normal P homeostasis and PTH remains the prime factor for calcium homeostasis.
Fig. 1.
FGF23/klotho signaling is not essential for the phosphaturic action of PTH. Injection of PTH decreases serum P levels in wild type (WT) mice and mice with genetic deletion of FGF23 (Fgf23−/−) and klotho (Kl−/−) indicating that the phosphaturic action of PTH in mice is independent of FGF23/klotho [26]. Reproduced with permission of the Journal of Bone and Mineral Research.
The FGF and FGFR family
The FGF family is one of the largest growth factor families, consisting of 23 members sharing 13–71% sequence similarity in mammals [28]. FGFs possess a large range of activities in embryonic development and physiological functions in the adult. In the embryo, FGFs often signal across mesenchymal–epithelial boundaries, where they regulate organogenesis and pattern formation. In the adult, FGFs play important roles in regulating homeostasis, wound healing and tissue repair. Unregulated expression of FGFs can cause cancer. FGFs bind and activate alternatively spliced forms of four tyrosine kinase FGF receptors (FGFRs 1–4). The spatial and temporal expression patterns of FGFs and FGFRs and the ability of specific ligand–receptor pairs to actively signal are important factors regulating FGF activity in a variety of biological processes [29]. FGF signaling activity is regulated by the binding specificity of ligands and receptors and is modulated by extrinsic cofactors such as heparan sulfate proteoglycans [28, 30]. Each FGFR contains an extracellular ligand-binding domain, a single transmembrane domain and an intracellular tyrosine kinase domain. The extracellular ligand-binding domain of the FGFR contains two or three Ig-like domains. Alternative RNA splicing that utilizes one of two unique exons results in two different versions of Ig-like domain III (referred to as domains IIIb and IIIc) in FGFRs 1–3. This alternative splicing is an essential determinant of ligand-binding specificity. The IIIb (‘b’) and IIIc (‘c’) splice forms are regulated in a tissue-specific manner, such that the b isoform is restricted to epithelial lineages and the c isoform is preferentially expressed in mesenchymal lineages [28].
Regulation of FGF23 synthesis and secretion
Healthy individuals injected with PTH (1-34) develop hypophosphatemia and elevated calcitriol levels, along with an increase of serum FGF23 [31], suggesting that calcitriol or PTH are important regulators of FGF23 synthesis independent of the serum P concentration. However, increased circulating FGF23 levels were likewise reported in some patients with hypoparathyroidism [23], suggesting that the hyperphosphatemia observed under these conditions can modulate FGF23 despite low or normal calcitriol levels [32]. P-independent regulation of FGF23 by calcitriol may also explain why FGF23 is unable to compensate in patients with hyper- or hypoparathyroidism, i.e. conditions in which calcitriol is elevated to inappropriately stimulate or decreased to inappropriately reduce FGF23 levels, respectively [32, 33]. Overexpression of high molecular weight FGF2 increases FGF23/FGFR/KLOTHO signaling to downregulate NPT2a, causing P wasting, osteomalacia and decreased bone mineral density [34]. Microarray gene expression analysis showed that canonical activation of FGFR pathways by addition of recombinant FGF1 and FGF2 to osteoblast cultures stimulated FGF23 promoter activity [35] and selective inhibition of FGFR blocks FGF23 transcription in bone suggesting that FGFR signaling is essential for FGF23 expression in bone [36, 37]. Moreover, PHEX and DMP1 control a common pathway regulating bone mineralization and FGF23 production, the latter involving activation of the FGFR signaling in osteocytes [37].
A FGF23-PTH endocrine loop
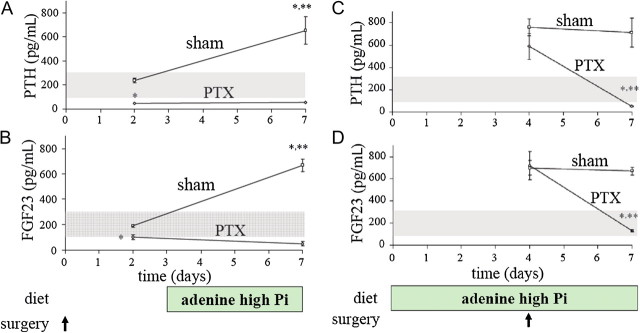
FGF23 acts on its receptor klotho-FGFR1 in the parathyroid as part of a bone-parathyroid axis whereby FGF23 decreases PTH gene expression, secretion and parathyroid cell proliferation [38, 39]. In turn, PTH acts on bone cells both in vivo and in vitro to increase FGF23 expression [40, 41]. Furthermore, Lavi-Moshayoff et al. [40] showed that PTH is necessary for the high FGF23 levels of early kidney failure due to an adenine high-phosphorus diet. Parathyroidectomy before the diet totally prevented the 5-fold increase in FGF23 levels in kidney failure rats (Figure 2). Moreover, parathyroidectomy of early kidney failure rats corrected their high FGF23 levels (Figure 2) [40]. Therefore, in early kidney failure, the high FGF23 levels are dependent on the high PTH levels. PTH infusion for 3 days to mice with normal renal function increased serum FGF23 and calvaria FGF23 mRNA levels. To demonstrate a direct effect of PTH on FGF23, they added PTH to rat osteoblast-like UMR106 cells [40]. PTH increased FGF23 mRNA levels (4-fold) and this effect was mimicked by a PKA activator, forskolin. They showed that the PTH increase in FGF23 expression involves the PKA and Wnt pathways. Importantly, hemodialysis patients treated with the calcimimetic cinacalcet had a pronounced decrease not only in serum PTH, calcium and phosphorus but also serum FGF23 [42] as did total parathyroidectomy [43]. The effect of PTH on FGF23 completes a bone-parathyroid endocrine feedback loop. Importantly, secondary hyperparathyroidism is essential for the high FGF23 levels in early CKD.
Fig. 2.
Parathyroidectomy (PTX) prevents and corrects the increased FGF23 levels of adenine high Pi-induced early kidney failure [40]. PTX before the adenine high-Pi diet (A–B). Rats underwent PTX or sham operation. At Day 3, they were fed the adenine diet for four additional days as indicated below the graphs. Blood was sampled at Days 2 and 7 and results are shown for PTH (above) and FGF23 (below). Adenine diet before PTX (C–D). Rats were fed an adenine high-Pi diet for seven days and at Day 4, underwent PTX or sham operation as indicated below the graphs. Reproduced with permission of the American Journal of Physiology.
In CKD, serum levels of both FGF23 and PTH are increased implying resistance of the parathyroid to FGF23, which we and others have shown is due to downregulation of klotho-FGFR1 in the parathyroid [44–46]. There is a similar downregulation of klotho in the kidneys of rats with experimental CKD [47] and interestingly, the administration of klotho has been shown to improve renal function by binding to TGFβ in the kidney [48]. The increase in FGF23 in early CKD may be responsible, at least in part, for the early decrease in calcitriol levels in CKD patients.
Lopez et al. [41] showed that renal klotho protein expression was decreased in parathyroidectomized rats and the administration of calcitriol led to an increase not only in serum calcium but also normalized the klotho expression with no change in serum P. These results suggest that calcitriol or calcium regulate renal klotho and emphasize the tight interactions among the minerals, their hormones and receptors.
FGF23, klotho and the cardiovascular system
The FGF23 renal coreceptor klotho has been shown to be an early biomarker for CKD in man and mouse, and klotho deficiency contributes to the soft tissue calcification in CKD [49]. Soluble klotho ameliorates vascular calcification by enhancing phosphaturia, preserving glomerular filtration and directly inhibiting P uptake by vascular smooth muscle [49]. FGF23 has now been shown to be not only associated with an increased mortality in patients with CKD but also to directly cause left ventricular hypertrophy (LVH) [50].
Klotho expression and action on blood vessels
Klotho is not only an essential component of the FGF23 receptor complex but also has functions of its own. These are now starting to be unraveled. In human and experimental CKD, there is less klotho expression in the kidney and the parathyroid as well as lower levels in the serum and urine [49]. Correction of tissue klotho levels in mice with experimental CKD, either by administration of klotho protein or the generation of transgenic mice expressing the klotho gene, improves not only renal function but also the vascular calcification evident in the CKD mice. The CKD mice had a 10-fold increase in serum PTH, which was decreased to control levels in the transgenic klotho CKD mice [49]. This last result emphasizes the importance of klotho in the parathyroid in mediating a decrease in serum PTH after the ligand FGF23 and now alone. Klotho is a phosphaturic substance in its own right independent of FGF23 [51]. The beneficial effect of klotho on vascular calcification was a result of more than its effect on renal function and phosphatemia, suggesting a direct effect of klotho on the vasculature. In vitro, klotho suppressed sodium-dependent uptake of P and mineralization induced by high P and preserved differentiation in vascular smooth muscle cells (VSMC) [49]. Klotho inhibits Na-coupled P cotransport when directly added to cultured proximal tubule-like cells and in cell-free brush border membrane vesicles (BBMV) without FGF23 [49]. Therefore, klotho opposes soft tissue calcification in CKD. Klotho ameliorates vascular calcification by enhancing phosphaturia, preserving glomerular filtration and directly inhibiting P uptake by VSMC [48]. In addition, klotho proteins interact with the VEGFR-2/TRPC-1 complex in the surface of endothelial cells to maintain endothelial integrity(c) [52]. So these studies suggest that klotho exerts a beneficial effect in other cells of the vascular wall besides VSMC.
Serum FGF23 levels are associated with increased mortality in dialysis patients
Serum FGF23 levels increase markedly as GFR decreases reaching >10 000-fold increases in dialysis patients. Levels of FGF23 directly correlate with mortality in these patients [53–55]. The order of magnitude of the increase in mortality risk in association with high serum FGF23 levels is by a factor of 5–6, whereas the increase in mortality risk in association with high serum phosphorus is only by a factor of 1.3–2. Moreover, the increased mortality risk with high FGF23 levels was essentially independent of increased phosphorus levels. The high levels of FGF23 are related to hyperphosphatemia, vitamin D therapy and secondary hyperparathyroidism. How FGF23 causes or is related to the increased mortality has been enigmatic but new work sheds light on the subject.
FGF23 directly induces LVH [50]
Patients with CKD have a remarkably high prevalence of LVH. There are a number of factors that contribute to the LVH and recently it has been shown by a combination of epidemiological, in vitro and in vivo, studies that FGF23 directly induces LVH [50]. FGF23 levels were measured in baseline plasma samples from 3000 individuals who underwent echocardiography 1 year later as part of the prospective Chronic Renal Insufficiency Cohort (CRIC) Study. Elevated FGF23 levels were independently associated with LVH in patients with varying degrees of CKD. Moreover, elevated FGF23 levels at baseline were associated with increased future risk of new-onset LVH. They then compared the response of isolated neonatal rat ventricular cardiomyocytes (NRVMs) to 48 h of treatment with FGF23 versus FGF2, the prototypical FGF. FGF23-induced hypertrophy of isolated NRVMs via FGFR activation but through a klotho-independent pathway. They then showed that the PLCγ-calcineurin-NFAT axis is the dominant signaling pathway of FGF23-mediated hypertrophy of NRVMs and not the MAPK pathway, which is activated by FGF23 activation of klotho-FGFR1. In vivo, FGF23 directly injected into the myocardium or given intravenously led to LVH in rodents and the administration of an FGF-receptor blocker to rats with 5/6 nephrectomy-induced kidney failure attenuated LVH. Therefore, these studies have provided solid evidence for an effect of FGF23 to directly induce LVH (Figure 3).
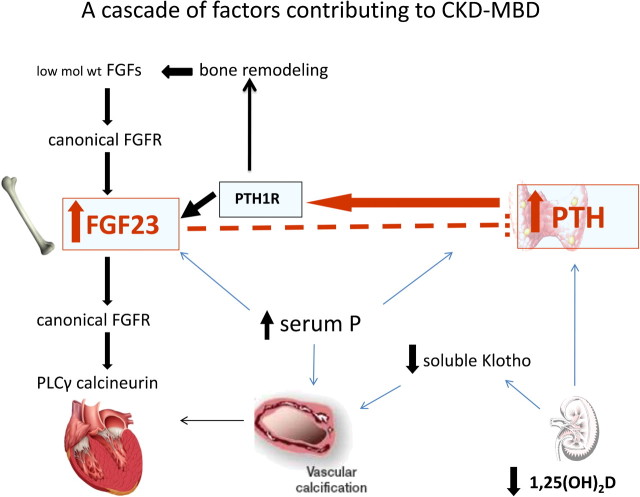
Fig. 3.
A cascade of factors contributing to CKD-MBD. In advanced CKD dietary, the phosphorus load is tropic to both PTH and FGF23 and FGF23 itself acts to decrease serum calcitriol that is a further stimulus to PTH secretion. FGF23 normally acts to decrease PTH but not in CKD where its receptor is downregulated in both the parathyroid and kidneys. PTH acts directly on bone cells to increase FGF23 expression and secretion and is essential for the high FGF23 levels of CKD. The high PTH also leads to enhanced bone remodeling thereby releasing low-molecular weight FGFs that act on canonical FGFRs to increase FGF23. The high FGF23 levels act on FGFRs in the heart activating PLCγ calcineurin contributing to the LVH in these patients. The high serum P and the low levels of soluble klotho are factors involved in the vascular calcification of these patients.
We should not forget that FGF23 is by no means the only factor that is altered in CKD where there are direct effects on the myocardium. For instance, PTH directly affects rat myocardial cells, causes early death of cells by increasing calcium entry into heart cells [56] and has long been considered a uremic toxin [57]. There is renewed interest in the contribution of vitamin D deficiency to heart disease and the effects of hyperphosphatemia, hypertension, volume overload and changes in lipoproteins are among a myriad of other real or potential uremic toxins which are well studied and beyond the scope of this review. But there is now substantial epidemiological and laboratory data that FGF23 is of prime importance as a cause of myocardial damage.
Unanswered questions
There are still many more questions to be answered. For instance, the proof provided that klotho is not expressed in the myocardium was only shown for klotho mRNA. It needs to be shown that the protein is also not present due to translation from an alternatively transcribed form of klotho. The in vitro studies are convincing but klotho is expressed in blood vessels and it is possible that the in vivo results reflect an effect of FGF23 on klotho in blood vessels, which then affects the myocardium. For that matter, klotho is strongly expressed in the sinoatrial node of the heart [58]. Was that relevant here? Which FGFR binds FGF23 in the myocardium and what cofactors such as heparin compounds are needed for binding?
Whatever it is, Faul et al. have opened up an exciting new avenue of research in the field of CKD-MBD and for that we are grateful. Targeting FGF23 by decreasing the serum PTH in patients with secondary hyperparathyroidism [42] as well as maintaining a serum P in target range and a more judicious use of vitamin D analogues seem prudent advice for our patients at this stage of the game. FGF23 antibodies have been developed and they may one day become tools in the management of CKD patients.
Acknowledgments
Work in the authors' laboratories is supported in part by the Harold and Ethel Pupkewitz Renal Research Fund (J.S.), Amgen (J.S. and M.R.); Spanish Government PI 070315, 080127,1102055, Regional Government PO-0127/2008, CTS5205, CVI7925, SAS111221 (M.R.) and Fresenius (M.R.); Washington University Research in Renal Diseases Grant 31030 and WUCKDR O'Brian Center Grant P30DK079333 (E.S.).
Conflict of interest statement. None of the authors have any share holdings in companies with research or products related to this manuscript. J.S. has received grants from Amgen, consultant fees from Teva and honoraria and travel support from Amgen and Fresenius; M.R. has received lectures fees from Abbot, Amgen, Fresenius and Shire and serves on the advisory boards for Vifor, Abbott, Shire and Amgen and has received grants from Amgen, Abbott and Fresenius; E.S. has received honoraria and grants from Abbott and Genzyme.
References
- 1.Slatolposky E, Gradowsky L, Kashemsa C, et al. Control of phosphate excretion in uremia. J Clin Invest. 1966;45:672–677. doi: 10.1172/JCI105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slatopolsky E, Caglar S, Pennell JP, et al. On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J Clin Invest. 1971;50:492–499. doi: 10.1172/JCI106517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Hilker S, Dusso AS, Rapp NS, et al. Phosphorus restriction reverses hyperparathyroidism in uremia independent of changes in calcium and calcitriol. Am J Physiol. 1990;259:F432–F437. doi: 10.1152/ajprenal.1990.259.3.F432. [DOI] [PubMed] [Google Scholar]
- 4.Kilav R, Silver J, Naveh-Many T. Parathyroid hormone gene expression in hypophosphatemic rats. J Clin Invest. 1995;96:327–333. doi: 10.1172/JCI118038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almaden Y, Canalejo A, Hernandez A, et al. Direct effect of phosphorus on parathyroid hormone secretion from whole rat parathyroid glands in vitro. J Bone Miner Res. 1996;11:970–976. doi: 10.1002/jbmr.5650110714. [DOI] [PubMed] [Google Scholar]
- 6.Naveh-Many T, Rahamimov R, Livni N, et al. Parathyroid cell proliferation in normal and chronic renal failure rats: the effects of calcium, phosphate and vitamin D. J Clin Invest. 1995;96:1786–1793. doi: 10.1172/JCI118224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denda M, Finch J, Slatopolsky E. Phosphorus accelerates the development of parathyroid hyperplasia and secondary hyperparathyroidism in rats with renal failure. Am J Kidney Dis. 1996;28:596–602. doi: 10.1016/s0272-6386(96)90473-4. [DOI] [PubMed] [Google Scholar]
- 8.Silver J, Dranitzki-Elhalel M. Sensing phosphate across the kingdoms. Curr Opin Nephrol Hypertens. 2003;12:357–361. doi: 10.1097/00041552-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Murer H, Hernando N, Forster I, et al. Regulation of Na/Pi transporter in the proximal tubule. Annu Rev Physiol. 2003;65:531–542. doi: 10.1146/annurev.physiol.65.042902.092424. [DOI] [PubMed] [Google Scholar]
- 10.Levi M, Arar M, Kaissling B, et al. Role of microtubules in the rapid regulation of renal phosphate transport in response to acute alterations in dietary phosphate content. J Clin Invest. 1997;99:1302–1312. doi: 10.1172/JCI119289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moz Y, Levi R, Lavi-Moshayof V, et al. Calcineurin A beta is central to the expression of the renal type II Na/Pi-cotransporter gene and to the regulation of renal phosphate transport. J Am Soc Nephrol. 2004;15:2972–2980. doi: 10.1097/01.ASN.0000144207.44469.BE. [DOI] [PubMed] [Google Scholar]
- 12.Moz Y, Silver J, Naveh-Many T. Characterization of cis-acting element in renal NaPi-2 cotransporter mRNA that determines mRNA stability. Am J Physiol Renal Physiol. 2003;284:F663–F670. doi: 10.1152/ajprenal.00332.2002. [DOI] [PubMed] [Google Scholar]
- 13.Villa-Bellosta R, Ravera S, Sorribas V, et al. The Na+-Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi. Am J Physiol Renal Physiol. 2009;296:F691–F699. doi: 10.1152/ajprenal.90623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prie D, Urena TP, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009;75:882–889. doi: 10.1038/ki.2008.643. [DOI] [PubMed] [Google Scholar]
- 15.Berndt T, Thomas LF, Craig TA, et al. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci U S A. 2007;104:11085–11090. doi: 10.1073/pnas.0704446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White KE, Carn G, Lorenz-Depiereux B, et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 17.Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 19.Sitara D, Razzaque MS, St Arnaud R, et al. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol. 2006;169:2161–2170. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razzaque MS, Sitara D, Taguchi T, et al. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurosu H, Kuro-o M. The Klotho gene family and the endocrine fibroblast growth factors. Curr Opin Nephrol Hypertens. 2008;17:368–372. doi: 10.1097/MNH.0b013e3282ffd994. [DOI] [PubMed] [Google Scholar]
- 22.Chang Q, Hoefs S, van der Kemp AW, et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A, Winer K, Econs MJ, et al. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89:4489–4492. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 25.Araya K, Fukumoto S, Backenroth R, et al. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:5523–5527. doi: 10.1210/jc.2005-0301. [DOI] [PubMed] [Google Scholar]
- 26.Yuan Q, Sato T, Densmore M, et al. FGF-23/Klotho signaling is not essential for the phosphaturic and anabolic functions of PTH. J Bone Min Res. 2011;26:2026–2035. doi: 10.1002/jbmr.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Ibrahimi OA, Olsen SK, et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149:121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goetz R, Beenken A, Ibrahimi OA, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnett-Bowie SA, Henao MP, Dere ME, et al. Effects of hPTH(1-34) infusion on circulating serum phosphate, 1,25-Dihydroxyvitamin D, and FGF23 levels in healthy men. J Bone Miner Res. 2009;24:1681–1685. doi: 10.1359/JBMR.090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishida Y, Taketani Y, Yamanaka-Okumura H, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 34.Xiao LP, Naganawa T, Lorenzo J, et al. Nuclear isoforms of fibroblast growth factor 2 are novel inducers of hypophosphatemia via modulation of FGF23 and KLOTHO. J Biol Chem. 2010;285:2834–2846. doi: 10.1074/jbc.M109.030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu SG, Tang W, Fang JW, et al. Novel regulators of Fgf23 expression and mineralization in hyp bone. Mol Endocrinol. 2009;23:1505–1518. doi: 10.1210/me.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wohrle S, Bonny O, Beluch N, et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J Bone Min Res. 2011;26:2486–2497. doi: 10.1002/jbmr.478. [DOI] [PubMed] [Google Scholar]
- 37.Martin A, Liu S, David V, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25:2551–2562. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krajisnik T, Bjorklund P, Marsell R, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 40.Lavi-Moshayoff V, Wasserman G, Meir T, et al. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 41.Lopez I, Rodriguez-Ortiz ME, Almaden Y, et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011;80:475–482. doi: 10.1038/ki.2011.107. [DOI] [PubMed] [Google Scholar]
- 42.Koizumi M, Komaba H, Nakanishi S, et al. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27:784–790. doi: 10.1093/ndt/gfr384. [DOI] [PubMed] [Google Scholar]
- 43.Sato T, Tominaga Y, Ueki T, et al. Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis. 2004;44:481–487. [PubMed] [Google Scholar]
- 44.Galitzer H, Ben Dov IZ, Silver J, et al. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–218. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 45.Komaba H, Goto S, Fujii H, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232–238. doi: 10.1038/ki.2009.414. [DOI] [PubMed] [Google Scholar]
- 46.Canalejo R, Canalejo A, Martinez-Moreno JM, et al. FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol. 2010;21:1125–1135. doi: 10.1681/ASN.2009040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu MC, Shi MJ, Zhang JN, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang CL, Moe OW. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch. 2011;462:185–193. doi: 10.1007/s00424-011-0950-5. [DOI] [PubMed] [Google Scholar]
- 49.Hu MC, Shi MJ, Zhang JN, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu MC, Shi MJ, Zhang JN, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kusaba T, Okigaki M, Matui A, et al. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci U S A. 2010;107:19308–19313. doi: 10.1073/pnas.1008544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jean G, Terrat JC, Vanel T, et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–2796. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 54.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isakova T, Xie HL, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bogin E, Massry SG, Harary I. Effect of parathyroid-hormone on rat-heart cells. J Clin Invest. 1981;67:1215–1227. doi: 10.1172/JCI110137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Massry SG. Parathyroid hormone: a uremic toxin. Adv Exp Med Biol. 1987;223:1–17. doi: 10.1007/978-1-4684-5445-1_1. [DOI] [PubMed] [Google Scholar]
- 58.Takeshita K, Fujimori T, Kurotaki Y, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]