Abstract
Background.
Patients with acute kidney injury (AKI) requiring initiation of renal replacement therapy (RRT) have poor short- and long-term outcomes, including the development of dialysis dependence. Currently, little is known about what factors may predict renal recovery in this population.
Methods.
We conducted a single-center, retrospective analysis of 170 hospitalized adult patients with AKI attributed to acute tubular necrosis who required inpatient initiation of RRT. Data collection included patient characteristics, laboratory data, details of hospital course and degree of fluid overload at RRT initiation. The primary outcome was recovery of renal function to dialysis independence.
Results.
Within 1 year of RRT initiation, 35.9% (61/170) of patients reached the primary end point of renal recovery. The median (interquartile range) duration of RRT was 11 (3–33) days and 83.6% (51/61) recovered prior to hospital discharge. Recovering patients had significantly less fluid overload at the time of RRT initiation compared to non-recovering patients (3.5 versus 9.3%, P = 0.004). In multivariate Cox proportional hazard regression analysis, a rise in percent fluid overload at dialysis initiation remained a significant negative predictor of renal recovery (hazard ratio 0.97, 95% confidence interval 0.95–1.00, P = 0.024).
Conclusions.
In patients with AKI, a higher degree of fluid overload at RRT initiation predicts worse renal recovery at 1 year. Clinical trials are needed to determine whether interventions targeting fluid overload may improve patient and renal outcomes.
Keywords: acute kidney injury, dialysis, fluid overload, renal recovery
Introduction
Severe acute kidney injury (AKI) requiring initiation of renal replacement therapy (RRT) occurs in ∼1% of hospitalized patients and up to 5% of intensive care unit (ICU) patients [1–4]. Such patients have a hospital mortality rate of 45–70% [1–5], and patients surviving to hospital discharge continue to carry a high risk for long-term morbidity and mortality [6, 7]. The development of chronic kidney disease, including end-stage renal disease (ESRD), is likely to contribute to the poor long-term outcomes [8, 9]. Several studies have shown that between 10 and 30% of hospitalized patients with AKI who required RRT initially will remain dialysis dependent at discharge [5–12]. However, there remains a paucity of data on the long-term renal outcomes of these patients, and the factors that predict recovery of renal function (alive without need for RRT) have not been well characterized.
Fluid overload is a potentially modifiable factor contributing to increased mortality among patients with AKI. Studies in the pediatric population demonstrated that greater degrees of fluid overload at RRT initiation were associated with higher mortality [13–15], and recent studies in adult cohorts also support the association between fluid overload and increased mortality in hospitalized AKI patients [16–18]. In many instances, early intervention may reduce the severity of fluid overload in the setting of AKI and thus fluid overload may be an important marker for timing of RRT initiation. Apart from its impact on outcomes, fluid overload complicates the medical management of AKI. For example, greater degrees of fluid overload may require higher fluid removal rates during RRT, the latter being associated with systemic hypotension and potential exacerbation of ischemic injury to the kidneys [19, 20]. To date, few studies have examined renal recovery in relation to fluid overload and the results have been inconsistent [17, 21].
The aim of this study was to characterize survival and renal outcomes of hospitalized patients with AKI requiring RRT, both during hospitalization and up to 1 year following RRT initiation. The primary outcome of interest was the recovery of renal function to the point of no longer necessitating maintenance dialysis in patients who initially required RRT due to AKI during the index hospitalization. We analyzed clinical variables associated with risk of dialysis dependency following AKI. We hypothesized that an increasing degree of fluid overload at RRT initiation would be associated with a decreased likelihood of renal recovery.
Materials and methods
Subjects
This retrospective study was conducted in patients admitted to a tertiary medical center between November 2007 and October 2008. Patients were identified by review of the inpatient dialysis log. Inclusion criteria were (i) age ≥18 years, (ii) a diagnosis of AKI secondary to acute tubular necrosis (ATN) and (iii) receipt of at least one inpatient RRT treatment. The diagnosis of ATN was established by the consulting nephrologist; at our institution, this process typically includes microscopic examination of the urinary sediment and analysis of urinary indices (such as fractional excretion of sodium), in addition to correlation with the clinical presentation. Exclusion criteria were (i) diagnosis of ESRD, (ii) diagnosis other than ATN as primary cause of AKI, (iii) patients hospitalized for >1 month prior to RRT initiation, (iv) patients transferred from an outside institution after RRT initiation and (v) prior history of AKI requiring RRT. This study was approved by the institutional review board and the need for informed consent was waived.
Data collection
The health system electronic medical record was reviewed to collect demographics, comorbidity information and reason for hospitalization. Additional variables included laboratory data both at admission and at the time of RRT initiation, dialysis treatment characteristics and details of hospital course (e.g. use of vasopressor agents, need for mechanical ventilation). The cause of renal failure and indication for RRT initiation was obtained by review of renal consultation notes. Time between renal consultation and RRT initiation was recorded in days, with a value of 0 assigned if RRT initiation occurred on the same day as the initial consultation.
Percent fluid overload was calculated based on the following formula: [(Weight at dialysis initiation – Baseline weight)/Baseline weight] × 100%. Recognizing that weight changes (gain or loss) may begin to occur pre-hospitalization, we defined baseline weight as the average outpatient weight recorded within 3 months preceding hospitalization. If this was not available, then the patient’s self-reported usual weight was used. If this was not recorded, then hospital admission weight was used as the baseline weight.
The primary outcome of renal recovery was defined as recovery of adequate renal function to discontinue dialysis for at least 2 weeks within 1 year of dialysis initiation. Patients withdrawn from dialysis for comfort care reasons were counted as non-recovery. Patients were assessed from RRT initiation to the first of renal recovery, death or 1-year post-initiation. Time to recovery was defined as the interval in days between first and last hemodialysis session or day on continuous renal replacement therapy (CRRT).
Statistical analysis
Demographic, admission and initiation characteristics were compared using χ2 and Mann–Whitney tests as appropriate. Non-parametric tests were chosen due to non-normality in several continuous variables. Percent weight change was assessed separately as both a continuous and categorical covariate. For the categorical analysis, categories of <10 and ≥10% were chosen based on prior literature suggesting the clinical significance of this cut-off [17, 18, 22]. Kaplan–Meier curves were used to estimate time to renal recovery in the entire cohort and by categories of percent weight change. Patients were censored at whichever event came first: death, discharge from hospital to hospice/comfort care or 1-year follow-up. Multivariate Cox proportional hazards models assessed the impact of percent weight change on time to renal recovery after adjusting for patient characteristics chosen using a best subset selection approach. The leaps and bound algorithm [23] was used to consider different combinations of predictors and to find the models of various sizes with the highest global chi-square statistics. The final model was then selected from these candidates based on Akaike’s Information Criterion. The proportionality assumption was checked using interaction terms with the log of time. An additional model predictive of mortality was developed using a similar approach; because of the lack of reliable data on dates of death, a multiple logistic regression approach was used to assess association of risk factors with the likelihood of survival. Analyses were conducted in SAS 9.2, with statistical significance set at a two-sided α ≤ 0.05.
Results
Patient characteristics
A total of 208 patients were identified as undergoing inpatient RRT initiation for a diagnosis of AKI during the study period. Among these, 38 patients met exclusion criteria resulting in a study cohort of 170 patients that were included in the final analysis. The most common reason for exclusion was a primary renal diagnosis other than ATN (hepatorenal syndrome in eight patients, acute obstructive nephropathy in six patients, glomerulonephritis in three patients, multiple myeloma in three patients, ESRD in three patients). Additional patient exclusions were: dialysis for non-AKI indications (six patients), patients receiving RRT prior to transfer from an outside hospital (five patients) and patients in hospital for >1 month prior to RRT initiation (four patients).
Patient characteristics overall and stratified by recovery status are presented in Table 1. In the total cohort, median (interquartile range) age was 60 (50, 71) years, 56.5% were males and 72.4% were Caucasian. The majority of patients had at least one major comorbidity and 27.7% had diabetes mellitus. Median baseline serum creatinine was 1.0 (0.8, 1.5) mg/dL, while the median creatinine at RRT initiation was 3.9 (2.9, 5.0) mg/dL. Most patients (138/170, 81.2%) underwent care in an ICU for at least part of their hospitalization, and 60.6% of patients underwent CRRT as the initial dialysis modality.
Table 1.
Patient characteristics presented overall and by renal recovery statusa
| Overall (N = 170) | Non-recovered (N = 109) | Recovered (N = 61) | P-valueb | |
| Age (years) | 60.0 (50.0, 71.0) | 60.0 (50.0, 71.0) | 59.0 (50.0, 67.0) | 0.492 |
| Gender: male (%) | 96 (56.5) | 63 (57.8) | 33 (54.1) | 0.641 |
| Race | 0.105 | |||
| Caucasian (%) | 123 (72.4) | 82 (75.2) | 41 (67.2) | |
| African-American (%) | 31 (18.2) | 15 (13.8) | 16 (26.2) | |
| Other (%) | 16 (9.4) | 12 (11.0) | 4 (6.6) | |
| Primary reason for hospitalization | ||||
| Renal (%) | 16 (9.4) | 10 (9.2) | 6 (9.8) | 0.887 |
| Sepsis (%) | 10 (5.9) | 6 (5.5) | 4 (6.6) | 0.747 |
| Cardiac (%) | 64 (37.7) | 39 (35.8) | 25 (41.0) | 0.502 |
| Pulmonary (%) | 8 (4.7) | 3 (2.8) | 5 (8.2) | 0.108 |
| Liver (%) | 29 (17.1) | 25 (22.9) | 4 (6.6) | 0.007 |
| Oncologic (%) | 13 (7.7) | 9 (8.3) | 4 (6.6) | 0.689 |
| Other (%) | 34 (20.0) | 21 (19.3) | 13 (21.3) | 0.749 |
| Comorbidities | ||||
| ≥1 comorbidity (%) | 134 (78.8) | 93 (85.3) | 41 (67.2) | 0.006 |
| Diabetes mellitus (%) | 47 (27.7) | 28 (25.7) | 19 (31.2) | 0.445 |
| Vascular disease (%) | 26 (15.3) | 16 (14.7) | 10 (16.4) | 0.766 |
| Congestive heart failure (%) | 71 (41.8) | 47 (43.1) | 24 (39.3) | 0.632 |
| Liver cirrhosis (%) | 27 (15.9) | 24 (22.0) | 3 (4.9) | 0.004 |
| Cancer (%) | 31 (18.2) | 21 (19.3) | 10 (16.4) | 0.642 |
| RIFLE category | 0.465 | |||
| R (%) | 18 (10.6) | 11 (10.1) | 7 (11.5) | |
| I (%) | 21 (12.4) | 16 (14.7) | 5 (8.2) | |
| F (%) | 131 (77.1) | 82 (75.2) | 49 (80) | |
| Serum creatinine (mg/dL) | ||||
| Baseline | 1.0 (0.8, 1.5) | 1.1 (0.8, 1.5) | 1.0 (0.8, 1.3) | 0.410 |
| Dialysis initiation | 3.9 (2.9, 5.0) | 3.7 (2.7, 4.8) | 4.2 (2.7, 4.8) | 0.073 |
| BUN at initiation (mg/dL) | 74.5 (51.0, 94.0) | 77 (53, 94) | 71 (45, 91) | 0.276 |
| Admission hemoglobin (mg/dL) | 10.9 (9.4, 12.8) | 10.7 (9.4, 12.9) | 11.1 (9.5, 12.8) | 0.650 |
| Admission albumin (g/dL) | 3.3 (2.8, 3.8) | 3.2 (2.8, 3.5) | 3.5 (3.0, 3.8) | 0.022 |
| Consult time (days) | 0.0 (0.0, 2.0) | 1.0 (0.0, 3.0) | 0.0 (0.0, 1.0) | 0.066 |
| Length of ICU stay (days) | 8.0 (2.0, 22.0) | 8.0 (2.0, 22.0) | 9.0 (3.0, 23.0) | 0.869 |
| Modality: CRRT (%) | 103 (60.6) | 72 (66.1) | 31 (50.8) | 0.051 |
| Use of vasopressors (%) | 119 (70.0) | 84 (77.1) | 35 (57.4) | 0.007 |
| Use of mechanical ventilation (%) | 115 (67.7) | 79 (72.5) | 36 (59.0) | 0.072 |
| % Fluid overload at dialysis initiation | 7.5 (0.7, 14.8) | 9.3 (2.8, 17.9) | 3.5 (0.0, 11.1) | 0.004 |
| ≥10% overload (%) | 72 (42.4) | 53 (48.6) | 19 (31.1) | 0.027 |
Values are presented as median (interquartile range).
For comparisons between non-recovered and recovered groups.
Patient outcomes
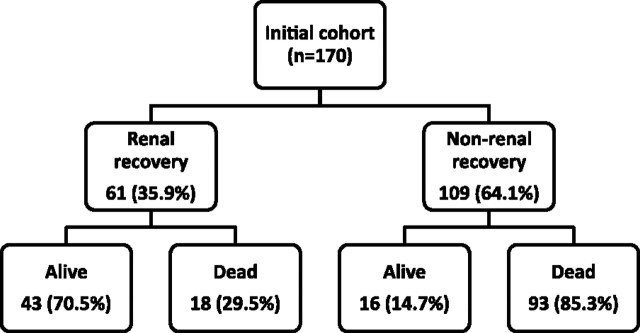
Within 1 year of RRT initiation, 35.9% (61/170) of patients reached the primary end point of renal recovery no longer requiring maintenance dialysis (Figure 1). The median time between RRT initiation and discontinuation was 11 (3, 33) days, and no patient restarted dialysis after renal recovery during the study period. The majority (51/61, 83.6%) of cases of renal recovery occurred prior to hospital discharge.
Fig. 1.
One year outcomes among patients with AKI requiring RRT.
The overall 1-year mortality was 65.3% (111/170). The 1-year mortality of patients experiencing recovery of renal function was 29.5% (18/61) compared to 85.3% (93/109) in patients that never recovered renal function (P < 0.001).
Predictors of renal recovery
In the univariate analyses (Table 1), there were no differences in age, gender or race by renal recovery status. Compared to recovering patients, more patients in the non-recovery group had underlying cirrhosis (22.0 versus 4.9%, P = 0.004) or were hospitalized for liver-related diseases (22.9 versus 6.6%, P = 0.007). More patients in the non-recovery group received vasopressor agents during hospitalization than in the recovery group (77.1 versus 57.4%, P = 0.007). Baseline creatinine was similar between the recovery and non-recovery groups [1.0 (0.8, 1.3) versus 1.1 (0.8, 1.5), P = 0.410]. Serum creatinine and blood urea nitrogen (BUN) at the time of RRT initiation did not significantly differ between the recovery and the non-recovery groups.
Fluid overload at RRT initiation was significantly greater among patients who did not recover renal function compared to the recovery patients [9.3% (2.8, 17.9) versus 3.5% (0, 11.1), P = 0.004]. When considered as a categorical variable, more patients in the non-recovery group had fluid overload ≥10% than in the recovery group (48.6 versus 31.2%, P = 0.027) (Table 1).
In multivariate Cox proportional hazard regression analysis, five variables were included in the final model (Table 2). A rise in percent fluid overload at RRT initiation remained a significant negative predictor of risk for renal recovery [hazard ratio (HR) for recovery 0.97, 95% confidence interval (CI) 0.95–1.00, P = 0.024]. This relationship persisted after adjusting for source of baseline weight in the same model. Higher baseline creatinine, one or more major comorbidities and use of vasopressors were each associated with a decreased relative risk for renal recovery. Increasing time between renal consultation and RRT initiation was also associated with a decreased relative risk of renal recovery.
Table 2.
Cox regression model of risk for renal recovery within 1 year of dialysis initiation (n = 170)a
| Predictor | Hazard ratio | 95% CI | P-value |
| % FO at initiation (per 1%) | 0.97 | (0.95–1.00) | 0.024 |
| ≥1 comorbidity | 0.51 | (0.30–0.89) | 0.018 |
| Baseline serum creatinine (per 1 mg/dL) | 0.56 | (0.37–0.87) | 0.009 |
| Use of vasopressors | 0.49 | (0.28–0.85) | 0.011 |
| Time between consult and initiation (per day) | 0.84 | (0.72–0.98) | 0.025 |
FO, Fluid overload.
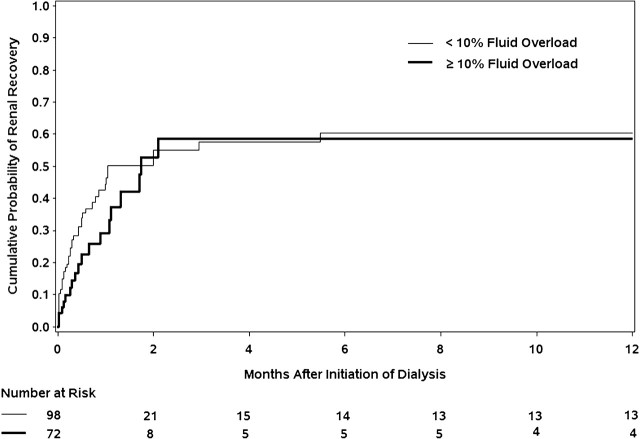
In a separate multivariate model assessing fluid overload as a categorical variable, ≥10% fluid overload was associated with a lower relative risk of renal recovery although this failed to reach significance (HR 0.66, 95% CI 0.37–1.15, P = 0.143). Kaplan–Meier curves of time to renal recovery by fluid overload status (≥10 or <10%) are presented in Figure 2. In the first 2 months following dialysis initiation, there appeared to be a trend towards lower renal recovery in the >10% fluid overload group, but this effect was no longer observed through the remainder of the follow-up. Notably, there was loss of power at later follow-up time points due to fewer patients remaining at risk.
Fig. 2.
Kaplan–Meier curves of time to renal recovery, stratified by % fluid overload.
Predictors of survival
In the multivariate analysis for mortality, five variables were included in the final model (Table 3). A higher percent of fluid overload was predictive of lower likelihood of 1-year survival (OR 0.96, 95% CI 0.92–0.99, P = 0.010). Older age, one or more major comorbid conditions, lower admission albumin and use of vasopressors were each associated with lower survival.
Table 3.
Multiple logistic regression model for 1-year survival (n = 170)a
| Predictor | Odds ratio | 95% CI | P |
| % FO at initiation (per 1%) | 0.96 | (0.92–0.99) | 0.010 |
| Age (per year) | 0.98 | (0.95–1.00) | 0.036 |
| ≥1 comorbidity | 0.36 | (0.15–0.86) | 0.022 |
| Use of vasopressors | 0.16 | (0.07–0.37) | < 0.001 |
| Admission albumin (per 1 g/dL) | 1.98 | (1.05–3.72) | 0.035 |
FO, Fluid overload.
Discussion
Fluid overload is recognized as an important prognostic factor in patients with AKI. Our results are consistent with prior literature demonstrating a relationship between fluid overload and survival, and we have further contributed by showing that this association endured up to 1 year following an episode of AKI. By including a general hospitalized population, our results also demonstrate that the clinical significance of fluid overload extends to non-ICU level patients. A novel finding of this study is that a greater degree of fluid overload predicts a lower likelihood of renal recovery to dialysis independence.
The ability to predict renal recovery in AKI is an important clinical tool as failure to recover renal function is associated with a grave prognosis and significant morbidity. Previous studies have identified several non-modifiable predictive factors such as baseline renal function [10, 24], comorbidity [10] and age [25]. Consideration of these factors may be useful when counseling patients on prognosis and treatment options but are not subject to intervention. Unfortunately, studies have not been consistent in identifying factors amenable to intervention. Our study is among the first to demonstrate an association between fluid overload and long-term risk of dialysis dependence. In a pediatric study, Hayes et al. found that fluid overload >20% was associated with a prolonged time to recovery; notably, all patients in that study eventually recovered renal function [21]. In an analysis of the PICARD database, Bouchard et al. [17] failed to find an association between fluid overload and renal recovery. However, fluid status was only assessed for 3 days prior to renal consultation, which likely underestimated the degree of fluid overload, and renal recovery was only assessed at the time-point of hospital discharge, which therefore excluded any post-discharge renal recovery events. In our study, >15% of patients recovering renal function did so after hospital discharge. By following patients for renal recovery up to 1 year after RRT initiation, we were able to capture the vast majority of renal recovery episodes that were likely to occur and to establish the relationship with fluid overload.
There are several potential explanations for the association between progressive fluid overload and lack of renal recovery. Firstly, the fluid overload may contribute directly to renal injury. Potential effects include direct renal interstitial edema causing organ dysfunction as well as the indirect effects of intra-abdominal hypertension causing vascular congestion and impaired organ perfusion, especially in encapsulated organs such as the kidneys [26, 27]. In addition, fluid overload can contribute to injury in other organs, which may indirectly result in renal injury through reciprocal organ ‘cross-talk’ [26]. Secondly, progressive fluid overload may promote a more aggressive approach to fluid removal during dialysis, which may in turn exacerbate renal ischemia. Thirdly, degree of fluid overload may be a clinically important marker for timing of RRT initiation. Previous studies have suggested that earlier initiation of RRT may be associated with improved outcomes of survival and renal recovery [28]. However, these studies have been inconsistent in the definition of timing, which has included BUN or creatinine at RRT initiation, urine output changes and timing relative to ICU admission [28–30]. In this study, neither BUN nor creatinine at RRT initiation were significant predictors of either survival or renal recovery. Future studies examining timing of RRT should consider inclusion of fluid overload status.
A fourth explanation for our findings is that the degree of fluid overload may simply be a marker of disease severity, as more critically ill patients are likely to receive greater degrees of fluid resuscitation. Observational studies cannot rule out this possibility, and clinical trials are needed to determine if interventions targeting fluid overload will prove beneficial. Few prospective clinical trials have attempted to address this question, and none have included patients with renal failure (acute or chronic). Most studies have focused on peri-operative fluid management, with one study suggesting benefit of a restrictive fluid approach [31], while others suggest either no benefit [32] or even potential harm [33, 34]. To our knowledge, only one prospective randomized controlled trial has focused on fluid management strategies in non-surgical patients. The Fluid and Catheter Treatment Trial [35] randomized patients with acute respiratory distress syndrome to a conservative versus liberal fluid strategy for the first 7 days of ICU stay. This study found improved lung function and shorter ICU stay in patients with the conservative fluid strategy, although overall mortality was the same. Importantly, this strategy was not associated with any greater degree of renal failure. While our study results suggest that a conservative fluid approach may also provide benefit in AKI patients, prospective clinical trials involving this population are needed to confirm this finding before any management recommendations can be made.
The optimal definition of fluid overload remains uncertain, and several definitions have been reported in the literature [13, 16–18]. We chose a weight-based definition due to lack of reliable intake–output data, particularly in non-ICU patients. Summing the intake–output data is highly sensitive to any missing data, which will result in cumulative errors, whereas weight determination eliminates reliance on previous measurements other than baseline weight. Furthermore, fluid balance calculations do not typically account for insensible losses, which can be quite significant in critically ill patients, and therefore, such calculations will tend to progressively overestimate fluid overload over time. Limitations of a weight-based method are also important to note. Weights may be difficult to accurately obtain in immobilized critically ill patients. In addition, weight changes may reflect body composition changes other than fluid administration. However, this is unlikely to occur acutely on a day-to-day basis, and we excluded patients hospitalized for an extended period prior to RRT initiation in whom baseline weight may be unreliable as a surrogate for dry weight. On the whole, we believe that weight-based determination of fluid overload is both more practical and potentially more accurate than measuring intake–output balance, and the results of this study demonstrate that weight-based assessment of fluid overload can provide important prognostic information.
Some limitations of this study should be noted. As a single-center retrospective study, the results may be difficult to generalize and further studies are needed to confirm our findings. The definition of renal recovery as dialysis independence is practical but may be subject to clinical variation. Unfortunately, we were not able to obtain data on residual renal function at the time of recovery, which may provide a more robust outcome measure.
We have shown that in patients with AKI, the degree of fluid overload at RRT initiation predicts both the mortality and the likelihood of renal recovery to dialysis independence up to 1 year later. By demonstrating this relationship using a weight-based determination of fluid overload status, we offer a more pragmatic approach to defining this variable. Future clinical trials are needed to determine whether interventions targeting fluid restriction or fluid removal may impact prognosis in patients with AKI.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Hoste EAJ, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med. 2008;36(Suppl):S146–S151. doi: 10.1097/CCM.0b013e318168c590. [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 4.Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Andrikos E, Tseke P, Balafa O, et al. Epidemiology of acute renal failure in ICUs: a multi-center prospective study. Blood Purif. 2009;28:239–244. doi: 10.1159/000231986. [DOI] [PubMed] [Google Scholar]
- 6.Coca S. Long-term outcomes of acute kidney injury. Curr Opin Nephrol Hypertens. 2010;19:266–272. doi: 10.1097/MNH.0b013e3283375538. [DOI] [PubMed] [Google Scholar]
- 7.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg R, Dennen P. Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis. 2008;15:297–307. doi: 10.1053/j.ackd.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 10.Bagshaw SM, Mortis G, Godinez-Luna T, et al. Renal recovery after severe acute renal failure. Int J Artif Organs. 2006;29:1023–1030. doi: 10.1177/039139880602901102. [DOI] [PubMed] [Google Scholar]
- 11.Uchino S, Bellomo R, Kellum JA, et al. Patient and kidney survival by dialysis modality in critically ill patients with acute kidney injury. Int J Artif Organs. 2007;30:281–292. doi: 10.1177/039139880703000402. [DOI] [PubMed] [Google Scholar]
- 12.Van Berendoncks AM, Elseviers MM, Lins RL. Outcome of acute kidney injury with different treatment options: long-term follow-up. Clin J Am Soc Nephrol. 2010;5:1755–1762. doi: 10.2215/CJN.00770110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein SL, Currier H, Graf JM, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107:1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie RS, Seidel K, Symons JM. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol. 2004;19:1394–1399. doi: 10.1007/s00467-004-1655-1. [DOI] [PubMed] [Google Scholar]
- 15.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 16.Payen D, de Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 18.Fulop T, Pathak MB, Schmidt DW, et al. Volume-related weight gain and subsequent mortality in acute renal failure patients treated with continuous renal replacement therapy. ASAIO J. 2010;56:333–337. doi: 10.1097/MAT.0b013e3181de35e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzen D, Rupprecht C, Hauri D, et al. Predicting outcomes in critically ill patients with acute kidney injury undergoing intermittent hemodialysis—a retrospective cohort analysis. Int J Artif Organs. 2010;33:15–21. [PubMed] [Google Scholar]
- 20.Zsom L, Zsom M, Fulop T, et al. Treatment time, chronic inflammation, and hemodynamic stability: the overlooked parameters in hemodialysis quantification. Semin Dial. 2008;21:395–400. doi: 10.1111/j.1525-139X.2008.00488.x. [DOI] [PubMed] [Google Scholar]
- 21.Hayes LW, Oster RA, Tofil NM, et al. Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care. 2009;24:394–400. doi: 10.1016/j.jcrc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furnival GM, Wilson RW. Regression by leaps and bounds. Technometrics. 1974;16:499–511. [Google Scholar]
- 24.Hsu CY, Chertow GM, McCulloch CE, et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt R, Coca S, Kanbay M, et al. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008;52:262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Prowle JR, Bellomo R. Fluid administration and the kidney. Curr Opin Crit Care. 2010;16:332–336. doi: 10.1097/MCC.0b013e32833be90b. [DOI] [PubMed] [Google Scholar]
- 27.Yerram P, Karuparthi PR, Misra M. Fluid overload and acute kidney injury. Hemodial Int. 2010;14:348–354. doi: 10.1111/j.1542-4758.2010.00498.x. [DOI] [PubMed] [Google Scholar]
- 28.Palevsky PM. Indications and timing of renal replacement therapy in acute kidney injury. Crit Care Med. 2008;36(Suppl):S224–S228. doi: 10.1097/CCM.0b013e318168e3fb. [DOI] [PubMed] [Google Scholar]
- 29.Liu KD, Himmelfarb J, Paganini E, et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1:915–919. doi: 10.2215/CJN.01430406. [DOI] [PubMed] [Google Scholar]
- 30.Bagshaw SM, Uchino S, Bellomo R, et al. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care. 2009;24:129–140. doi: 10.1016/j.jcrc.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacKay G, Fearon K, McConnachie A, et al. Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. Br J Surg. 2006;93:1469–1474. doi: 10.1002/bjs.5593. [DOI] [PubMed] [Google Scholar]
- 33.Vermeulen H, Hofland J, Legemate DA, et al. Intravenous fluid restriction after major abdominal surgery: a randomized blinded clinical trial. Trials. 2009;10:50. doi: 10.1186/1745-6215-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wenkui Y, Ning L, Jianfeng G, et al. Restricted peri-operative fluid administration adjusted by serum lactate level improved outcome after major elective surgery for gastrointestinal malignancy. Surgery. 2010;147:542–552. doi: 10.1016/j.surg.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 35.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2563–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]