Abstract
Purpose.
Extraocular muscle afferent signals contribute to oculomotor control and visual localization. Prompted by the close links between the oculomotor and attention systems, it was investigated whether these proprioceptive signals also modulated the allocation of attention in space.
Methods.
A suction sclera contact lens was used to impose an eye rotation on the nonviewing, dominant eye. With their viewing, nondominant eye, participants (n = 4) fixated centrally and detected targets presented at 5° in the left or right visual hemifield. The position of the viewing eye was monitored throughout the experiment. As a control, visual localization was tested using finger pointing without visual feedback of the hand, whereas the nonviewing eye remained deviated.
Results.
The sustained passive rotation of the occluded, dominant eye, while the other eye maintained central fixation, resulted in a lateralized change in the detectability of visual targets. In all participants, the advantage in speed and accuracy for detecting right versus left hemifield targets that occurred during a sustained rightward eye rotation of the dominant eye was reduced or reversed by a leftward eye rotation. The control experiment confirmed that the eye deviation procedure caused pointing errors consistent with an approximately 2° shift in perceived eye position, in the direction of rotation of the nonviewing eye.
Conclusions.
With the caveat of the small number of participants, these results suggest that extraocular muscle afferent signals modulate the deployment of attention in visual space.
Using a sclera lens we induced a sustained rotation of the nonviewing, dominant eye, stimulating the extraocular muscle proprioceptors. Although participants viewed a display with the nondominant eye, this procedure improved visual detection in the hemifield located in the direction of this rotation.
Introduction
Humans can sense the direction of the passive rotation of their eyes in darkness1 and interfering with the signal from the extraocular muscles (EOMs) causes errors in visual localization.2–6 In adults, EOM afferent signals contribute to locating retinal objects in relation to the body and to the long-term maintenance of ocular alignment (for reviews, see Steinbach,7 Gauthier et al.,8 Weir,9 and Donaldson10), whereas during development, they support the emergence of orientation selective columns and binocular stereopsis.11
It is well established that planned eye movements influence the allocation of attention in space. For instance, visual detectability increases at the location toward which a saccade is planned12–14 or, conversely, a lack of ability to move the eyes in one direction is accompanied by a failure to improve visual perception in that direction after a predictive cue.15–17 Less is known about whether EOM afferent signals can affect visual detectability. Observations in the auditory domain suggest that eye position modulates the allocation of attention, given that sounds presented in the direction of gaze have a perceptual advantage.18,19 Theoretically, EOM afferent signals could contribute to such an eye position effect. More specifically, in the visual domain it has been found that interfering with the cortical eye proprioceptive signal causes not only a change in perceived eye position, but also a change in visual sensitivity.6,20 After decreasing the excitability of the eye proprioceptive area with a 1-Hz repetitive transcranial stimulation (rTMS), a target presented at approximately 3° to the left is perceived to be straight in front of the nose, corresponding to a shift in perceived eye position rightward.6 The same manipulation causes an increase of detectability of the targets in the right visual field and a decrease of detectability in the left visual field when participants fixate straight ahead.20 Because the shift in visual sensitivity cooccurred and was spatially congruent with the shift in the perceived direction of gaze, it was suggested that eye proprioception and visuospatial attention may be functionally linked.20
If this is the case, then an alteration of the proprioceptive signal in the periphery would be expected to have the same effect on visual detection as that observed after rTMS of the eye proprioceptive area. The aim of the present study was to investigate this prediction. To this end, participants performed in monocular vision the same detection task as in the previous rTMS study,20 while their nonviewing eye was passively rotated. This sustained rotation was achieved by using an opaque contact lens attached to the sclera by light suction.2,21 During fixation, this manipulation changes the perceived position in the other, viewing, eye, causing errors in visual localization in the direction of rotation.2 In this study the participants' dominant eye was rotated. This choice was motivated by previous research showing stronger effects on visual localization after perturbing eye proprioception in the dominant compared with the nondominant eye.22 Participants viewing with the nondominant eye were asked to detect briefly flashed targets in the left and right visual hemifields. Based on the previous observations,20 we predicted that a visual target will be better detected if presented in the direction of the shift in eye position than opposite to it.
Methods
Participants
Four right-handed, healthy adults (age range: 28–47 years; median: 35 years; 2 female; 3 right eye dominant), who had normal vision, participated in this study after giving written informed consent. Eye dominance was established using the “hole in the card” test.23 This test identifies the eye preference during sighting tasks24 and has previously been used to investigate how eye dominance influences the effect of eye proprioception on visual localization.22 Two of the participants were naïve to the purpose of the study; the others were authors (DB and PCK). The study adhered to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee at the University of Liverpool (RETH000171).
Eye Rotation
To interfere with EOM afferent signals, one eye was passively deviated using a method described by Gauthier and colleagues2 as well as by ourselves.21 The procedure was performed by a Consultant Ophthalmologist (author WN) who also, at the end of each session, examined the participants' eye on a slit lamp. After instillation of a few drops of local anesthetic (Proxymetacaine 0.5%), a scleral lens was applied to the dominant eye, which was the right eye in three participants and the left eye in one. The lenses were custom-made from fenestrated haptic lenses (Innovative Sclerals, Hertford, UK) by attaching a stalk to the center of the lens and a suction tube to the fenestration. Light suction (0.2 bar) applied through the tube using a 20-mL plastic syringe ensured that the lens was firmly attached to the sclera while standing clear of the cornea. The lens and its attachments prevented vision in the dominant eye, so all tasks were performed monocularly, using the nondominant eye. To position the dominant eye, the participant fixated a visual target requiring 10° of rotation. The stalk of the lens was then fixed in a static holder. The participant then fixated centrally, causing the viewing eye to rotate back to the primary position, whereas the nonviewing eye remained deviated. This deviation was confirmed by visual inspection. The lens was removed after a maximum of 5 minutes. Each participant performed the experiment twice. Two lateral directions of rotation, left or right, were tested on different days. Our study did not include a control condition for the effect of the anesthetic alone, which theoretically could have altered the EOM signal. However, the anesthetic was applied topically, to the cornea, so the risk of penetration within the orbit to the eye muscles was low. Furthermore, even if the anesthetic reached the eye muscles, its effect would not explain the observed lateralized effects on visual detection.
Tasks
Visual Detection.
Spatial attention can be defined as the selection of a location for preferential processing.25 To investigate the role of eye deviation in spatial attention, participants were asked to detect targets presented to the left or right of fixation at equal retinal eccentricity. In addition, to test whether eye deviation affected the ability to shift attention, the task was designed with a cue that preceded the target. The cue could appear at the location of the target (50% valid trials) or at the contralateral location (50% invalid trials). Spatially uninformative cues test stimulus-driven, exogenous orienting (as opposed to endogenous, voluntary orienting),26 the component of the spatial attention system that has been suggested to be most tightly linked to eye movements.27 The task used here was similar to that used in the rTMS study20 (Fig. 1). Participants were seated 57 cm from a 36 × 29 cm cathode ray tube screen with their head stabilized using a chin rest and cheek pads. The midsagittal plane through the viewing eye was aligned to the central fixation square (black solid, 0.2° × 0.2°). Each trial started with presentation of a fixation square for a random period of between 20 and 500 ms. A spatially nonpredictive cue (red square frame, 0.4° × 0.4°) then appeared for 40 ms at one of two locations centered either 5° to the left or right of fixation. After a 100-ms delay, this was followed by a barely visible target (a gray solid square, 0.1° × 0.1°) presented for 80 ms. The target could appear either at the cued location, 5° from fixation (valid condition, 50% of the trials), or at the uncued location (invalid condition, 50% of the trials). A mask (red square frame, 0.4° × 0.4°) was then presented bilaterally at both possible target positions for 100 ms. The mask reduces the processing of the target,28 increasing the difficulty of visual detection. The mask was presented bilaterally, and was therefore uninformative as to the location of the target. The participants had 1000 ms to respond before the start of a new trial. They held a small response box in their hands underneath the table, corresponding to their body midline and responded by pressing a button on the left of the box with their left thumb for targets on the left, and the right button with their right thumb for targets on the right. We instructed the participants to try to be as accurate as possible and to refrain from pressing any key if they were unsure. We calculated mean reaction time across correct responses. The hit rate was calculated as the percentage of correct responses relative to the total number of trials of that type, regardless of the participant's response (e.g., number of trials when the participant responded “left” divided by the total number of trials when the target was presented to the left side). The false positive rate was the percentage of responses indicating left when the target was presented to the right relative to the total number of trials where a target was presented to the right. Because the task was performed during sustained eye rotation using a suction scleral lens, we limited task duration to 4 minutes. Within this interval each participant completed 136 trials, 34 trials for each of the four conditions: valid cue, left target; invalid cue, left target; valid cue, right target; invalid cue, right target. Before the experiment, the participants practiced the task in monocular vision, with their dominant eye patched.
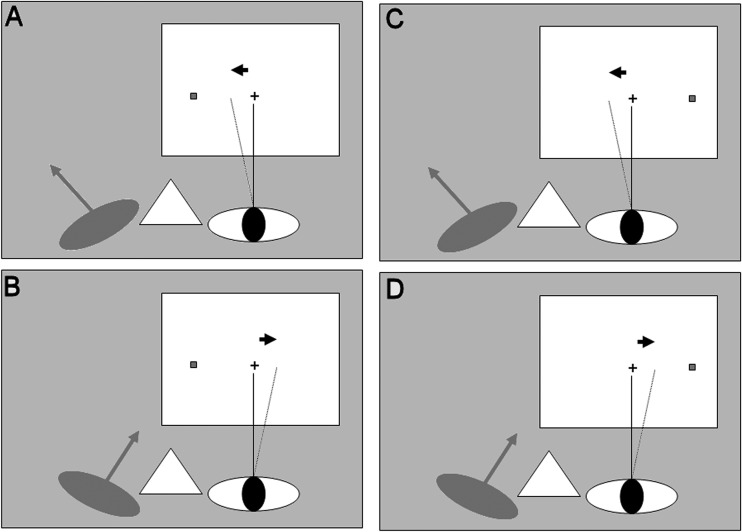
Figure 1. .
The experiment design. The design was 2 × 2 factorial with factors: (1) The side of rotation of the nonviewing eye, left (A, C) or right (B, D); (2) The visual hemifield of target presentation, left (A, B) or right (C, D). The dashed line indicates the perceived direction of gaze in the viewing eye, shifted approximately 2° in the direction of rotation of the nonviewing eye (see Results section). The hypothesis was that visual sensitivity will increase in the same direction (arrow).
Lateral asymmetry in visual detection was measured using a laterality index. A hit rate index was calculated as (HR − HL)/(HR + HL), where HR and HL were hit rates for right and left targets, respectively, calculated as an average across the valid and invalid trials. A similar laterality index was calculated for the false positive rate (FPR − FPL)/(FPR + FPL). These laterality indices have values between +1 and −1. The more positive the value, the larger the rightward bias and the more negative, the larger the leftward bias.
We computed similar laterality indices in the hit rates for the benefit added by the cue: [(HRV − HRI) − (HLV − HLI)]/[(HRV − HRI) + (HLV − HLI)], where the V and I subscripts denote the validly and invalidly cued conditions, respectively. The more positive the value of this laterality index, the larger the benefit of the cue for a right hemifield target compared with a left hemifield target.
We compared the laterality indices for the hit rate and false positive rate across the two directions of eye rotation, leftward and rightward. To avoid the assumption of normality, nonparametric, Wilcoxon signed-rank tests were used. One-tailed tests investigated the a priori hypothesis that the laterality index was larger after a rightward versus leftward eye rotation. In addition, the same test was used to compare the benefit in hit rate due to the cueing across the two directions of eye rotation.
Open-Loop Pointing (Control Task).
To verify that the eye deviation changed the proprioceptive signal used for visual localization, immediately after the visual detection task participants pointed to a visual target without visual feedback.2 A transparent acetate sheet was overlaid on the monitor screen, its center marked and aligned with a target presented at the center of the screen. With the lens still in place, participants viewed this target, then shut the viewing eye and pointed to the target using a marker pen, making a mark on the sheet. They kept their eye closed, while a new acetate sheet was placed for the next trial. They pointed with the hand contralateral to the deviated eye, which was the left hand in three participants and the right hand in the fourth. Each participant completed five trials. Pointing error was taken to be the horizontal distance between the position of the central target and the participants' mark.
Eye Tracking.
The position of the viewing, nondominant eye, was monitored using a high-speed infrared eye tracker (Skalar IRIS; Skalar Medical BV Cambridge Research Systems, Cambridge, UK), the output of which was digitized at 1 kHz. A five-point calibration routine was performed immediately after the removal of the lens at the end of each open-loop pointing task. Eye position data were analyzed offline. Trials in which a saccade (velocity > 20°/s) or any other eye movement with an amplitude > 2° occurred within 200 ms of target presentation, or trials with a blink at the moment of target presentation, were excluded from data analysis. To investigate any directional change in eye movements in response to the changes in the proprioceptive input from the nonviewing eye we compared saccade frequency and amplitude across the two directions of eye rotation. Saccades were recorded throughout the fixation periods of the visual detection task. The laterality index for frequency was calculated as (FR − FL)/(FR + FL) and for saccade amplitude as (SAR − SAL)/(SAR + SAL), then the indices were compared across conditions. Finally, to check whether the fixation position of the viewing eye was affected by the direction of rotation of the nonviewing eye, we compared the mean eye position between leftward and rightward rotation conditions. Horizontal eye position was calibrated then averaged over the 100-ms interval immediately before target appearance during the visual detection task. We compared the laterality indices for amplitude and frequency, as well as the eye position at fixation across the two rotation conditions, using the Wilcoxon signed-rank test.
Results
Eye Manipulation
Slit-lamp examination at the end of each session, in which we deviated one eye, showed no pathologic findings and none of the participants reported any pain during or after the procedure.
Visual Detection Task
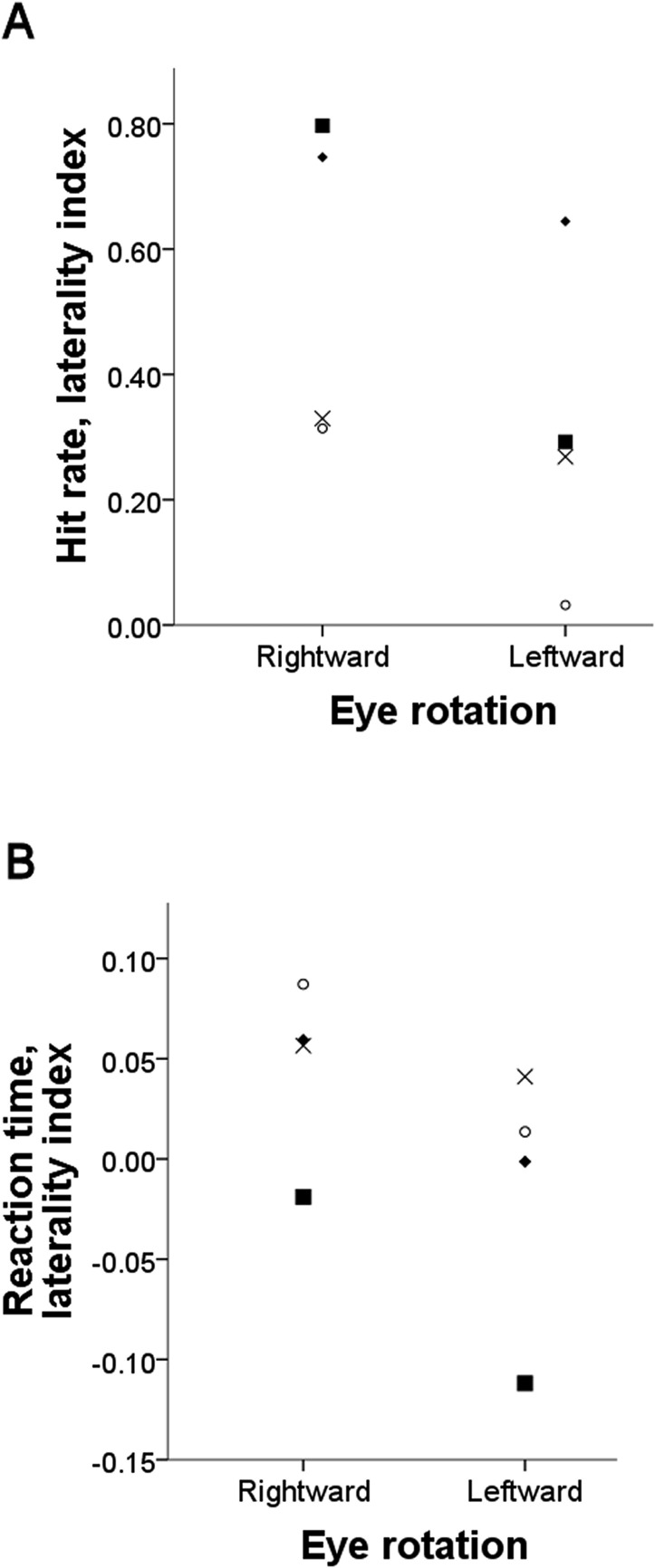
Participants showed a larger right versus left visual hemifield advantage in hit rate after rightward eye rotation (median difference right minus left: 31.47%; range: 27.17–39.29%) compared with left eye rotation (median difference right minus left: 18.03%; range: 3.05–21.73%) (Table 1). Thus for hit rate, the laterality index was significantly larger after a rightward than that after a leftward eye rotation (one-tailed Wilcoxon signed-rank test, P = 0.034; Fig. 2A). No similar advantage was found for false positives (Table 2, rightward eye rotation median: 11.37%; range: 1.98–22.01% and leftward eye rotation median: 6.24%; range: −0.42 to 33.33%; no statistically significant difference between laterality indices, one-tailed Wilcoxon signed-rank test, P = 0.5), so a difference in the tendency to respond “right” versus “left” across conditions cannot explain the difference in hit rate. As expected, in all participants the visual targets were detected more accurately if preceded by a spatially valid versus invalid cue (one-tailed Wilcoxon signed-rank test, P = 0.034, Table 1). There was no difference, however, in the benefit for visual detection added by the cue across the two eye rotation conditions (one-tailed Wilcoxon signed-rank test, P = 0.23).
Table 1. .
Hit Rate and Mean Reaction Time in the Visual Detection Task for Each Individual Participant and Condition
|
Participant |
Direction of Eye Rotation |
|||||||
|
Rightward |
Leftward |
|||||||
|
Target Hemifield |
Target Hemifield |
|||||||
|
RV |
RI |
LV |
LI |
RV |
RI |
LV |
LI |
|
| Hit rate, % | ||||||||
| 1 | 44.83 | 20.69 | 7.41 | 0.00 | 35.71 | 37.04 | 32.14 | 7.69 |
| 2 | 55.56 | 36.36 | 13.33 | 0.00 | 41.67 | 13.79 | 12.00 | 0.00 |
| 3 | 86.67 | 50.00 | 29.63 | 39.29 | 44.44 | 48.15 | 25.81 | 27.59 |
| 4 | 71.88 | 41.94 | 43.33 | 16.13 | 62.96 | 35.71 | 46.43 | 46.15 |
| Reaction time, s | ||||||||
| 1 | 0.600 | 0.681 | 0.616 | 0.640 | 0.714 | 0.594 | 0.488 | |
| 2 | 0.555 | 0.592 | 0.645 | 0.592 | 0.614 | 0.602 | ||
| 3 | 0.565 | 0.572 | 0.661 | 0.612 | 0.609 | 0.652 | 0.691 | 0.678 |
| 4 | 0.425 | 0.543 | 0.512 | 0.642 | 0.491 | 0.523 | 0.469 | 0.573 |
Note: Participants 3 and 4 were naïve to the purpose of the experiment. L, left; R, right; V, validly cued; I, invalidly cued. Participant 4 was left eye dominant; all the other participants were right eye dominant.
Figure 2. .
Reduced or reversed right minus left gradient in visual detection when the nonviewing eye is rotated to the left compared with the right. The laterality index was calculated as (HR − HL)/(HR + HL), where HR and HL were hit rates for right and left targets, respectively, and for reaction times (RTL − RTR)/(RTR + RTL). The index has values between +1 and −1; the more positive the value, the larger the rightward bias and the more negative, the larger the leftward bias. The symbols correspond to each participant 1, █, 2, ⧫, 3, ×, 4, ○. Participants 3 and 4 were naïve to the purpose of the experiment.
Table 2. .
Rate of a False Left or Right Response for the Visual Detection Task for Each Individual Participant
|
Participant |
Direction of Eye Rotation |
|||
|
Rightward |
Leftward |
|||
|
Target Hemifield |
Target Hemifield |
|||
|
R |
L |
R |
L |
|
| False positive response, % | ||||
| 1 | 3.70 | 1.72 | 6.25 | 6.67 |
| 2 | 9.38 | 0.00 | 5.00 | 0.00 |
| 3 | 25.45 | 3.45 | 33.33 | 0.00 |
| 4 | 21.31 | 7.94 | 11.11 | 3.64 |
Note: For a false positive response, L denotes a response “left” when the target was presented to the right (and vice versa for R). Conventions are like those in Table 1.
An unexpected observation was the higher hit rate for targets appearing in the right visual hemifield in all participants and conditions (Table 1).
Task instructions emphasized accuracy rather than speed of the response. However, we also analyzed reaction time data. Following the same trend as that in the hit rates, participants responded faster to right versus left hemifield targets after a rightward eye rotation (median difference in reaction time left minus right: 70 ms; range: −20 to 90 ms) compared with leftward eye rotation (median difference in reaction time left minus right: 10 ms; range: −140 to 50 ms). Thus, again, the laterality index for mean reaction time (RTL − RTR)/(RTR + RTL) showed larger values in the condition where the eye was rotated rightward versus leftward (one-tailed Wilcoxon signed-rank test, P = 0.034; Fig. 2B and Table 1), indicating a larger bias in visual sensitivity toward the right visual hemifield.
The benefit of the cue for reaction time could not be computed in two participants because of the lack of data in the invalid condition (Table 1). In the other two participants where this index could be calculated, it varied in opposite directions across the two conditions (increasing from left to right rotation in one participant and decreasing in the other).
Open-Loop Pointing Task
Open-loop pointing to a visual landmark confirmed that rotating the dominant eye changed the apparent position of a central visual target by approximately 2° in the direction of rotation of the nonviewing eye. For rightward rotation the mean pointing error was 2.18° (range from −0.4 to 2.96°), whereas for leftward rotation it was −2.15° (range from −1.28 to −3.87°), where a negative value denotes an error to the left of the center, and a positive value an error toward the right. The difference in pointing error across conditions was statistically significant (one-tailed Wilcoxon signed-rank test, P = 0.034).
Eye Tracking
Breaks of fixation occurred in 12.84 ± 4.83% (intersubject mean ± SD) of trials. These trials were excluded from the analysis. The direction of deviation of the dominant eye did not affect the frequency or amplitude of saccades/microsaccades directed to the left versus right visual hemifield. The comparison of the laterality indices across the two eye rotation conditions was not statistically significant for either frequency (one-tailed Wilcoxon signed-rank test, P = 0.23) or amplitude (one-tailed Wilcoxon signed-rank test, P = 0.5) of these eye movements. Finally, there was no statistically significant difference between the left and right eye deviation with respect to the mean position of the viewing eye during fixation (one-tailed Wilcoxon signed-rank test, P > 0.2).
Discussion
This study has found that altering EOM afferent signals in the periphery not only induced an approximately 2° error in visual localization, but also altered the accuracy and speed of visual detection. A sustained passive rotation of one, occluded, eye, whereas the other, viewing eye, maintained central fixation, resulted in a change in the detectability of visual targets presented in the left or right hemifield. We found that the advantage in speed and accuracy for detecting right versus left hemifield targets with the nondominant eye that occurred during a sustained rightward rotation of the dominant eye was reduced or reversed by a leftward rotation. This variation in visual detection after the manipulation of the extraocular muscles argues for a functional coupling between eye proprioception and visuospatial attention.
There are two major limitations of the current study dictated by safety concerns. First, only a small number of participants (n = 4) were included, and of them only two were naïve. In this small sample, however, the nonparametric tests showed a statistically significant effect in hit rate and reaction time, which are indicators of visual detection, but not in the false positive rate, which measures the bias in response. Second, each participant completed only a small number of sessions (two) with the scleral lens, where the eye was deviated in opposite directions, leftward or rightward. So the experiment lacked a baseline condition where the lens was applied but no eye deviation was imposed. Therefore, these results cannot provide an absolute measure of the effect caused by each of the two conditions separately. Nevertheless, the relative difference in performance between conditions in the hypothesized direction argues for an effect of the eye deviation procedure on visual detection.
One could object that the changes in visual detection after EOM manipulation in the current study reflect an alteration in the ability to shift attention to a location in response to a predictive cue, which occurs in conditions when the eyes cannot move to that location.15–17,29 However, this interpretation is not consistent with the null result when the benefit in hit rate added by a valid cue was compared across the two eye rotation conditions. It is also not consistent with the lack of evidence for a lateral bias in the frequency or amplitude of saccades. These null results mirror those found after rTMS in the eye proprioceptive area in the somatosensory cortex.20 Based on these negative results we suggest that the effect of deviating the nonviewing eye on visual detection is unlikely to reflect an impairment in moving the viewing eye or in shifting attention toward one hemifield.
An unexpected finding was the larger hit rate for targets that appeared in the right versus left hemifield regardless of the direction of eye rotation. One possible reason for this baseline bias could be that the eye deviation method applied an unpleasant somatic stimulus to the dominant eye, which in three of the four participants was the right eye, located nearer to the right relative to the left hemifield target. All participants reported a mild sensation of foreign object in the eye during the procedure. Perhaps this somatic stimulus was sufficiently salient to bias perception toward its location. This interpretation would be in line with previous observations that spatially uninformative, but unpleasant, somatic stimuli facilitate visual detection in their vicinity.30
In common with the study by Gauthier and colleagues2 we found an error in open-loop pointing of approximately 2°, even though the procedure deviated the eye by 10°. One trivial explanation for this difference may be slippage in the mechanical grip between the lens and the eye. More likely is that a large alteration in proprioceptive input results in only a relatively modest change in visual localization relative to the body because the estimate of eye position relies not only on eye proprioception, but also, and possibly more heavily, on the efference copy of the motor command31,32 and visual signals.33 Indeed the gain of the proprioceptive signal has been estimated at 0.25.3
In conclusion, the current results suggest that eye proprioception modulates the prioritization of visual space for perception. This adds to the previous evidence that body posture can shape the allocation of attention in space34 and that eyes and attention are tightly coupled.27,35 Furthermore, it suggests that EOM afferent signals, in addition to their role in visual localization2–6 and oculomotor control,21 may also play a role in the deployment of visual attention. This conclusion should be regarded as preliminary because it is based on a small number of participants and experimental conditions.
Footnotes
Supported by a Marie Curie Intra-European Fellowship within the 7th European Community Framework Programme, the Danish Medical Research Councils Grant 09-072209, and a Training Visit under Value in People Awards Scheme from Wellcome Trust (DB).
Disclosure: D. Balslev, None; W. Newman, None; P.C. Knox, None
References
- 1.Skavenski A. Inflow as a source of extraretinal eye position information. Vision Res. 1972;12:221–229 [DOI] [PubMed] [Google Scholar]
- 2.Gauthier GM, Nommay D, Vercher JL. The role of ocular muscle proprioception in visual localization of targets. Science. 1990;249:58–61 [DOI] [PubMed] [Google Scholar]
- 3.Bridgeman B, Stark L. Ocular proprioception and efference copy in registering visual direction. Vision Res. 1991;31:1903–1913 [DOI] [PubMed] [Google Scholar]
- 4.Allin F, Velay JL, Bouquerel A. Shift in saccadic direction induced in humans by proprioceptive manipulation: a comparison between memory-guided and visually guided saccades. Exp Brain Res. 1996;110:473–481 [DOI] [PubMed] [Google Scholar]
- 5.Lennerstrand G, Tian S, Han Y. Effects of eye muscle proprioceptive activation on eye position in normal and exotropic subjects. Graefes Arch Clin Exp Ophthalmol. 1997;235:63–69 [DOI] [PubMed] [Google Scholar]
- 6.Balslev D, Miall RC. Eye position representation in human anterior parietal cortex. J Neurosci. 2008;28:8968–8972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinbach MJ. Inflow as a long-term calibrator of eye position in humans. Acta Psychol. 1986;63:297–306 [DOI] [PubMed] [Google Scholar]
- 8.Gauthier GM, Vercher JL, Blouin J. Egocentric visual target position and velocity coding: role of eye muscle proprioception. Ann Biomed Eng. 1995;23:423–435 [DOI] [PubMed] [Google Scholar]
- 9.Weir CR. Proprioception in extraocular muscles. J Neuroophthalmol. 2006;26:123–127 [DOI] [PubMed] [Google Scholar]
- 10.Donaldson IML. The functions of the proprioceptors of the eye muscles. Philos Trans R Soc Lond B Biol Sci. 2000;355:1685–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buisseret P. Influence of extraocular muscle proprioception on vision. Physiol Rev. 1995;75:323–338 [DOI] [PubMed] [Google Scholar]
- 12.Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837 [DOI] [PubMed] [Google Scholar]
- 13.Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916 [DOI] [PubMed] [Google Scholar]
- 14.Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57:787–795 [DOI] [PubMed] [Google Scholar]
- 15.Craighero L, Nascimben M, Fadiga L. Eye position affects orienting of visuospatial attention. Curr Biol. 2004;14:331–333 [DOI] [PubMed] [Google Scholar]
- 16.Smith DT, Rorden C, Jackson SR. Exogenous orienting of attention depends upon the ability to execute eye movements. Curr Biol. 2004;14:792–795 [DOI] [PubMed] [Google Scholar]
- 17.Gabay S, Henik A, Gradstein L. Ocular motor ability and covert attention in patients with Duane Retraction Syndrome. Neuropsychologia. 2010;48:3102–3109 [DOI] [PubMed] [Google Scholar]
- 18.Morais J, Cary L, Vanhaelen H, Bertelson P. Postural determinants of frontal-position advantage in listening to speech. Perception. 1980;27:141–148 [DOI] [PubMed] [Google Scholar]
- 19.Pavani F, Driver J. Gaze direction modulates auditory spatial deficits in stroke patients with neglect. Cortex. 2005;41:181–188 [DOI] [PubMed] [Google Scholar]
- 20.Balslev D, Gowen E, Miall RC. Decreased visual attention further from the perceived direction of gaze for equidistant retinal targets. J Cogn Neurosci. 2011;23:661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knox PC, Weir CR, Murphy PJ. Modification of visually guided saccades by a nonvisual afferent feedback signal. Invest Ophthalmol Vis Sci. 2000;41:2561–2565 [PubMed] [Google Scholar]
- 22.Velay JL, Roll R, Lennerstrand G, Roll JP. Eye proprioception and visual localization in humans: influence of ocular dominance and visual context. Vision Res. 1994;34:2169–2176 [DOI] [PubMed] [Google Scholar]
- 23.Crider B. A battery of tests for the dominant eye. J Gen Psychol. 1944;31:179–190 [Google Scholar]
- 24.Mapp AP, Ono H, Barbeito R. What does the dominant eye dominate? A brief and somewhat contentious review. Percept Psychophys. 2003;65:310–317 [DOI] [PubMed] [Google Scholar]
- 25.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennett S, Spence C, Driver J. Visuo-tactile links in covert exogenous spatial attention remap across changes in unseen hand posture. Percept. Psychophys. 2002;64:1083–1094 [DOI] [PubMed] [Google Scholar]
- 27.Smith DT, Schenk T. The premotor theory of attention: time to move on? Neuropsychologia. 2012;50:1104–1114 [DOI] [PubMed] [Google Scholar]
- 28.Enns JT, Lollo VD. What's new in visual masking? Trends Cogn Sci. 2000;4:345–352 [DOI] [PubMed] [Google Scholar]
- 29.Craighero L, Carta A, Fadiga L. Peripheral oculomotor palsy affects orienting of visuospatial attention. Neuroreport. 2001;12:3283–3286 [DOI] [PubMed] [Google Scholar]
- 30.Van Damme S, Crombez G, Lorenz J. Pain draws visual attention to its location: experimental evidence for a threat-related bias. J Pain. 2007;8:976–982 [DOI] [PubMed] [Google Scholar]
- 31.Lewis RF, Gaymard BM, Tamargo RJ. Efference copy provides the eye position information required for visually guided reaching. J Neurophysiol. 1998;80:1605–1608 [DOI] [PubMed] [Google Scholar]
- 32.Guthrie BL, Porter JD, Sparks DL. Corollary discharge provides accurate eye position information to the oculomotor system. Science. 1983;221:1193–1195 [DOI] [PubMed] [Google Scholar]
- 33.Blouin J, Amade N, Vercher JL, Teasdale N, Gauthier GM. Visual signals contribute to the coding of gaze direction. Exp Brain Res. 2002;144:281–292 [DOI] [PubMed] [Google Scholar]
- 34.Reed CL, Grubb JD, Steele C. Hands up: attentional priorization of space near the hand. J Exp Psychol Hum Percept Perform. 2006;32:166–177 [DOI] [PubMed] [Google Scholar]
- 35.Rizzolatti G, Riggio L, Dascola I, Umiltà C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40 [DOI] [PubMed] [Google Scholar]