Abstract
Purpose.
Stimulation to the cornea via noxious chemical and mechanical means evokes tearing, blinking, and pain. In contrast, mild cooling of the ocular surface has been reported to increase lacrimation via activation of corneal cool primary afferent neurons. The purpose of our study was to determine whether menthol induces corneal cool cell activity and lacrimation via the transient receptor potential melastatin-8 (TRPM8) channel without evoking nociceptive responses.
Methods.
Tear measurements were made using a cotton thread in TRPM8 wild type and knockout mice after application of menthol (0.05–50 mM) to the cornea. In additional studies, nocifensive responses (eye swiping and lid closure) were quantified following cornea menthol application. Trigeminal ganglion electrophysiologic single unit recordings were performed in rats to determine the effect of low and high concentrations of menthol on corneal cool cells.
Results.
At low concentrations, menthol increased tear production in TRPM8 wild type and heterozygous animals, but had no effect in TRPM8 knockout mice, while nocifensive responses remained unaffected. At the highest concentration, menthol (50 mM) increased tearing and nocifensive responses in TRPM8 wild type and knockout animals. A low concentration of menthol (0.1 mM) increased cool cell activity, yet a high concentration of menthol (50 mM) had no effect.
Conclusions.
These studies indicated that low concentrations of menthol can increase lacrimation via TRPM8 channels without evoking nocifensive behaviors. At high concentrations, menthol can induce lacrimation and nocifensive behaviors in a TRPM8 independent mechanism. The increase in lacrimation is likely due to an increase in cool cell activity.
This study examined the effect of low and high concentrations of menthol on tearing, nocifensive behaviors, and on the activity of trigeminal ganglion corneal cool cells. Concentration-dependent effects of menthol were found, with a high concentration producing effects that were not TRPM8-mediated.
Introduction
Dry eye syndrome (DES) is an ocular disorder characterized by the inability to produce a tear film of sufficient quantity and quality to allow for adequate lubrication and protection of the corneal surface.1,2 The pathophysiologic basis of DES typically involves either inadequate or altered tear film due to a dysfunction of the lacrimal and/or meibomian glands, whereby they are unable to produce an adequate tear film, or a dysfunction in the neural regulation of tear production.3–6 While the mechanisms of glandular tear production and release have been examined in some detail, relatively less is known regarding the sensory neuronal regulation of these glands.
Reflex tear secretion increases in response to a variety of stimulants applied to the ocular surface. Most of these stimuli are subjectively noxious or irritating; mechanical stimulation, low pH, capsaicin, and other chemicals cause copious tearing.6,7 The purpose of such noxious stimulation-evoked tear production is to rid the cornea, conjunctiva, and eyelids of substances that likely will cause injury to the eye. During normal waking periods, however, the cornea usually is exposed to air and not to irritating substances. A cornea exposed to ambient air will dry (evaporation is the main factor in tear film thinning and break-up8) and, therefore, basal tear production is required to maintain a tear film with optimal osmolarity and temperature.3
The cornea is innervated by three classes of physiologically characterized primary afferent neurons that terminate as free nerve endings.9,10 Mechanoreceptive neurons respond exclusively to mechanical stimulation of the cornea surface; polymodal neurons respond to mechanical, thermal, and noxious chemical stimulation (including low pH); and cool cells respond to innocuous cooling.11 Activation of mechanoreceptive and polymodal neurons evokes irritation and pain as well as reflex tearing.7,12,13 In contrast, activation of cool neurons may induce tearing without producing irritation or pain.14
In addition to cool temperatures, cool cell activity is increased by the application of menthol, the transient receptor potential melastatin-8 (TRPM8) agonist, and by the application of hypertonic artificial tears to the cornea.10,12,15,16 The TRPM8 channel is a thermally gated, nonselective cation channel that is activated by moderate cold (15–30°C) and by compounds, such as menthol.17 TRPM8 channels are expressed in trigeminal ganglion neurons, and found on unmyelinated and lightly myelinated A-delta and C primary afferent neurons, including corneal afferent fibers.18–20
Previously, our laboratory reported that cool cells are activated when the ocular surface fluid status is altered by reducing the layer of artificial tears covering cornea, leading to the hypothesis that these neurons contribute to basal tearing.15 More recently, cooling of the ocular surface in humans and mice has been shown to increase tearing.14 Furthermore, it has been reported that TRPM8 knockout mice have a lower level of basal tear flow.14 In our study, we investigated the role of TRPM8 and corneal cool cells on the regulation of menthol-induced tearing and nocifensive ocular reflexes.
Materials and Methods
Animals
Adult (>6 weeks) wild-type (TRPM8+/+), TRPM8−/−, and TRPM8+/− mice were used to measure tear production and ocular nocifensive behaviors. Breeding pairs of TRPM8 knockout mice kindly were provided by David Julius (University of California San Francisco, San Francisco, CA). Animals were genotyped by PCR screening using tail DNA and the experimenter was blind to the animals' genotypes. Corneal responsive neurons were recorded from the trigeminal nucleus in male Sprague-Dawley rats (330–400 g; Charles River Laboratories International, Inc., Wilmington, MA). Animals were housed in an environment with a controlled 12-hour light/dark cycle, and were allowed free access to food and water. Temperature and humidity in the experimental room were 22 ± 1°C and 40–50%, respectively. All procedures were approved by the Committee on Animal Research at the University of New England, and animals were treated according to the policies and recommendations of the National Institutes of Health guidelines for the handling and use of laboratory animals, and in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Tear Measurements
Tear measurements were made using the cotton thread test in unanesthetized mice.21 Using fine forceps, cotton phenol red threads (Zone-Quick; Odyssey Medical, Memphis, TN) were placed in the lateral canthus of the eye for 30 seconds. After removal, the length of color change on the phenol red threads was measured under a microscope to the nearest 0.1 mm. To take the tear measurements, animals were placed in a decapicone (MDC-200; Braintree Scientific, Braintree, MA) restraint, modified so that the head of the mouse was isolated outside of the cone. The mouse was allowed to acclimate for five minutes before a series of three baseline measurements were taken, with five minutes between each measurement. Following baseline measurements, solutions (10 μL) were applied directly to the surface of the eye with a micropipette. Treatments consisted of menthol (0.05, 0.1, 50 mM), 5% mannitol, 50 mM cyclohexanol, and vehicle controls. After two minutes, the fluid was wicked away with a Kimwipe (Fisher Scientific, Pittsburgh, PA) by lightly touching the tear meniscus at the lateral canthus. Immediately afterwards, a cotton phenol thread was placed in the lateral canthus of the eye for 30 seconds to remove any remaining excess fluid. Tear production then was measured at 5, 10, and 15 minutes after removal of the fluid.
Ocular Nocifensive Responses
The eye wipe test was used in the presence of increasing concentrations of menthol applied to the eye in mice.22–24 Each animal was placed in a clear Plexiglas testing chamber (24 × 45 × 20 cm) and allowed to acclimate for 15 minutes. Menthol (0.05, 0.1, 50 mM) or vehicle solutions (10 μL) were pipetted directly into one eye. The time each mouse spent either wiping at the ocular region with either hind- or forepaws, or with complete lid closure was recorded for a period of 15 minutes following drug application.
Spontaneous Blink Measurements
Blinks were monitored in TRPM8+/+ and TRPM8−/− mice. Mice were placed in a 24 × 45 × 20 cm chamber and acclimated for 15 minutes, and spontaneous blinks in the left eye were counted over a 10-minute period.
Fluorescein Staining
Corneal fluorescein staining was performed to assess the cornea in TRPM8+/+ and TRPM8−/− mice. A 10 μL drop of 1% fluorescein solution was applied to the cornea in isoflurane anesthetized animals. After 3 minutes, the eye was flushed with artificial tears to remove excess fluorescein and examined using cobalt blue light from a slit-lamp (PocketScope; Welch Allyn, Scaneateles Falls, NY).
Electrophysiologic Recordings
Rats initially were anesthetized with isoflurane (2%–3%) and prepared for electrophysiologic recordings in the trigeminal ganglion.15 Briefly, the femoral artery and vein were catheterized to monitor blood pressure and deliver drugs, and a tracheotomy was performed for ventilation. End-tidal CO2 was monitored continuously and maintained between 3.5 and 4.5%. Following surgery, urethane/chloralose anesthetics (500 mg/kg urethane and 50 mg/kg chloralose, intravenously) were delivered to replace the isoflurane. After placing rats in a stereotaxic apparatus, a partial craniotomy was performed to allow for electrode penetration of the trigeminal ganglion (approximately 0.8–1.2 mm posterior and 0.6 mm medial to Bregma). Extracellular single unit recordings were carried out using tungsten electrodes (5 MΩ) or platinum-coated tungsten microelectrodes (500 KΩ; FHC, Inc, Bowdoinham, ME).
Corneal cool cells were identified by responses evoked by the placement of a cold metal probe (tip diameter ∼1 mm) placed near the receptive field. Controlled thermal stimulation with a 5 mm2 contact thermode was used to examine responses to cooling stimuli (TSAII; Medoc Ltd., Ramat Yishai, Israel). As reported previously, neurons responded to innocuous cooling, starting from a holding temperature of 35°C and ramping down to 15–31°C. In addition, these neurons were sensitive to changes in the ocular surface fluid status.15 To examine responses to changes in ocular fluid status, responses were recorded following removal of the artificial tears from the bath. While sometimes referred to as a “dry condition,” neuronal discharge under this condition likely represents activity evoked by evaporative cooling and/or increased osmolarity.15,25,26
Neuronal responses to menthol and vehicle (mineral oil) were examined by replacing artificial tears with 20 μL of the test solutions. In most experiments, vehicle and drugs were tested on the same neuron. In these cases, vehicle was applied first, followed by several rinses with artificial tears, followed by low dose menthol (0.1 mM), and lastly, after several more rinses with artificial tears, high dose menthol (50 mM). A minimum interval of 30 minutes was allowed between test solution applications. All data were acquired by CED Micro 1401 and isolated neurons were analyzed with Spike2 (Cambridge Electronic Design, Cambridge, England).
Drugs
Tear measurements, ocular nocifensive behaviors, and neuronal discharge were determined following application of menthol (W266523; Sigma-Aldrich, St. Louis, MO) dissolved in mineral oil vehicle. In addition, the effect of 0.1 mM menthol in a vehicle of 0.4% ethanol in artificial tears was determined to be consistent with previous electrophysiologic studies.15 A solution of 5% mannitol in artificial tears was used to determine the effect of hyperosmolar conditions on tearing. Osmolarity of solutions was measured using an Osmomat 030 (Gonotec, Berlin, Germany). Cyclohexanol (105899; Sigma-Aldrich) was dissolved in mineral oil. Artificial tears were composed of (in mM): NaCl 106.5, NaHCO3 26.1, KCl 18.7, MgCl2 1.0, NaH2PO4 0.5, CaCl2 1.1, HEPES 10; pH 7.45.
Data Analysis
Data were represented as the treatment group mean ± SEM and for analysis comparing treatment groups, P < 0.05 was considered to be statistically significant. Group sample sizes were determined by power analyses based on anticipated effect sizes and variability of the various types of data as determined by pilot experiments and prior work. ANOVA tests were performed after testing the data set for normality and equal variances (sigma Stat 3.5; SYSTAT, Chicago, IL). To analyze the time-course in evoked tearing, data were analyzed using a two-way ANOVA with repeated measures followed by Tukey's multiple comparison post-hoc test. In additional analysis, the change in tearing was calculated by subtracting the final baseline tear measurement from the measurement taken at 10 minutes post-treatment (which represented the peak time point in evoked tearing). Comparisons between treatment groups were made using a two-way ANOVA followed by Tukey's multiple comparison post-hoc test. For nocifensive behaviors, the total times recorded over the 15-minute test period were compared using a two-way ANOVA followed by Tukey's multiple comparison post-hoc test. Spontaneous blinks over a 10-minute period in TRPM8+/+ and TRPM8−/− mice were compared using an unpaired Student t-test. To determine the effect of menthol on the activity of corneal units, the average ongoing baseline activity recorded for 30 seconds before drug application was subtracted from the average number of spikes recorded over the first 10 seconds following drug application and from 10 to 300 seconds following drug application. Treatment groups were compared using the Kruskal-Wallis test. This nonparametric alternative to the one-way ANOVA was chosen because of a significant difference in the variances and the large differences in sample sizes. When overall significance was reached, post-hoc comparisons were performed using the Mann-Whitney U test.
Results
Tear Measurements
A total of three baseline tear measurements was performed before application of drugs to the eye. Analysis of the three baseline measurements revealed no differences between wild-type, TRPM8 heterozygous, and TRPM8 knockout mice (F[2, 43] = 1.51, P > 0.05). However, a significant effect of time was found, with tear measurements increasing over the three trials (F[2,86] = 7.42, P = 0.001, see Table).
Table. .
Baseline Tear Measurements Taken Every 5 Minutes before Application of Drugs to the Cornea
|
|
Baseline 1 |
Baseline 2 |
Baseline 3 |
| TRPM8+/+ | 2.8 ± 0.4 | 3.6 ± 0.2 | 4.5 ± 0.4* |
| TRPM8+/− | 3.7 ± 0.5 | 3.6 ± 0.4 | 4.7 ± 0.5† |
| TRPM8−/− | 4.2 ± 0.7 | 4.0 ± 0.3 | 4.7 ± 0.5 |
Phenol red cotton threads were placed in the posterior canthus of the eye for 30 seconds before measuring the length (mm) of color change (see Methods for details). N = 16, 14, and 17 mice for TRPM8+/+, TRPM8+/−, and TRPM8−/− groups, respectively.
P < 0.05 versus baseline 1.
P < 0.05 versus baseline 2.
To determine the contribution of TRPM8 channels to the secretion of tears, the TRPM8 agonist menthol was applied to the eye in TRPM8+/+, TRPM8+/−, and TRPM8−/− mice. After application of 0.1 mM menthol, a significant increase in lacrimation was recorded in TRPM8+/+ mice, whereas the same concentration of menthol did not affect tear secretions in TRPM8−/− mice (Fig. 1A). A 2-way ANOVA revealed a significant difference for time (F[3,59] = 9.33, P < 0.001) and animal genotype (F[2, 21] = 8.68, P < 0.001). Post-hoc analysis determined that TRPM8+/+ mice showed increased tear secretions at 5 and 10 minutes after menthol solution application compared to baseline controls (P < 0.05) and TRPM8−/− mice at similar post-drug time points (P < 0.05). Data shown are for menthol dissolved in a mineral oil vehicle; however, similar results were observed when 0.1 mM menthol was dissolved in artificial tears with 0.4% EtOH (data not shown).
Figure 1. .
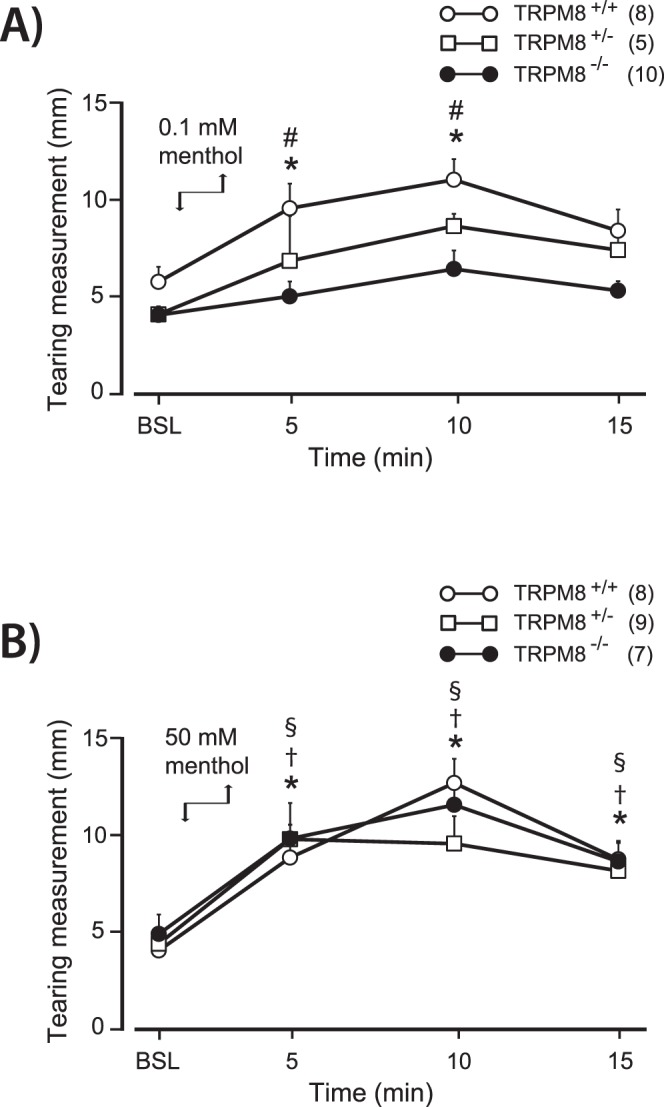
Tearing evoked by menthol applied to the cornea in TRPM8+/+, TRPM8+/−, and TRPM8−/− mice. Evoked tears were measured over a 15-minute period after drug removal. Menthol was applied directly to the eye (downward arrow) and removed with a Kimwipe after 2 minutes (upward arrow). Measurements were taken using cotton phenol red threads. (A) 0.1 mM menthol produced an increase in tearing in TRPM8+/+ mice with peak tearing occurring at 10 minutes after menthol application. The same concentration of menthol did not change tearing in TRPM8−/− mice. (B) 50 mM menthol produced an increase in tearing in TRPM8+/+, TRPM8+/−, and TRPM8−/− mice. At this concentration, no significant difference was observed between genotypes at any time point. BSL, values from the third and final baseline tear measurement before application of menthol to the eye. *P < 0.05 for the TRPM8+/+ group compared to baseline values. #P < 0.05 for the TRPM8+/+ group compared to TRPM8−/− mice at the same time point. †P < 0.05 for the TRPM8+/− group compared to baseline values. §P < 0.05 for the TRPM8−/− group compared to baseline values. Number of animals in each group is indicated in parentheses.
In contrast to the results obtained with 0.1 mM menthol, a higher concentration of menthol, 50 mM, increased lacrimation irrespective of the mouse genotype (Fig. 1B). While there was statistical significance for time (F[3, 63] = 37.07, P < 0.001), there was no difference based on mouse genotype (F[2, 21] = 0.14, P > 0.05). Compared to baseline controls, all groups of animals demonstrated increased tearing at the 5- and 10-minute time-points and remained elevated at 15 minutes post-menthol (P < 0.05). Additionally, as a control for the 50 mM menthol, we applied 50 mM cyclohexanol to the ocular surface in TRPM8+/+ mice. The baseline tear measurement before application of cyclohexanol did not differ from the peak tears measured at 10 minutes post-drug application (6.2 ± 1.0 vs. 7.9 ± 1.7 mm, n = 8, P > 0.05, paired t-test).
Previous studies have shown that corneal cool cells are activated by hyperosmotic solutions. We examined whether TRPM8 channels were necessary for the sensitivity of corneal afferents hyperosmotic conditions. Application of 5% mannitol in artificial tears (a 495 mOsm solution) induced lacrimation in TRPM8+/+ and TRPM8−/− animals compared to groups that received the artificial tear vehicle control (a 295 mOsm solution). While a significant difference was found between treatment groups (F[1,25] = 16.32, P < 0.001), there was no difference between the mouse genotypes (F[1,25] = 0.015, P = 0.9). Ten minutes after application of hyperosmotic artificial tears, tear secretion measurements increased 6.4 ± 1.4 mm above baseline in TRPM8+/+ mice (n = 8), which was significantly greater than tears measured after application of artificial tears (1.3 ± 1.3 mm, n = 8, P < 0.05). TRPM8−/− mice responded similarly to hyperosmotic conditions, with increased tear production measured at 7.2 ± 1.5 mm (n = 8), compared to −0.7 ± 1.6 mm after application of artificial tears (n = 5, P < 0.05).
Nocifensive Responses
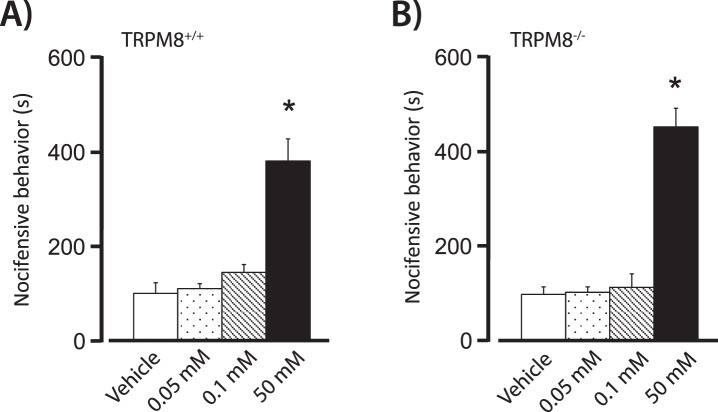
After determining that 0.1 mM menthol can induce lacrimation in TRPM8+/+ but not TRPM8−/− animals, we examined whether similar concentrations of menthol evoked nocifensive responses. The eye-wipe assay was used to assess the behavioral effect of menthol application to the cornea.22 Nocifensive responses consisted of either swiping the eye or full closure of the eyelids, and were recorded for a total of 15 minutes after drug application. A two-way ANOVA revealed a significant treatment effect (F[3,51] = 39.03, P < 0.001). Post-hoc analysis indicated that in TRPM8+/+ and TRPM8−/− mice, only the highest concentration of menthol (50 mM) produced an immediate nocifensive response that was significantly greater than vehicle controls (Figs. 2A, 2B). Notably, the reaction times did not differ between the wild-type and TRPM8 knockout mice for any of the concentrations of menthol (F[1, 51] = 0.103, P > 0.05).
Figure 2. .
Ocular nocifensive behaviors quantified after application of menthol to the eye in (A) TRPM8+/+ mice and (B) TRPM8−/− mice. Increased nocifensive behaviors were evoked by 50 mM menthol in TRPM8+/+ and TRPM8−/− mice. Eye-wiping or eye closure behaviors were assessed for 15 minutes after application of mineral oil vehicle, or 0.05 mM, 0.1 mM, and 50 mM menthol. Nocifensive behaviors are reported as the total amount of time spent executing the behaviors. In TRPM8+/+ animals, n = 10/group and in TRPM8−/− animals, n = 6, 4, 5, 4 for mineral oil vehicle, 0.05 mM, 0.1 mM, and 50 mM menthol, respectively. *P < 0.05 versus all other treatment groups.
Spontaneous Blink Measurements
No difference was found in the number of spontaneous blinks in TRPM8+/+ and TRPM8−/− mice over a 10-minute observation period (3.0 ± 0.5 vs. 3.2 ± 0.4 blinks, respectively, n = 5 animals/group).
Fluorescein Staining
The TRPM8−/− mice (n = 6) did not show any signs of corneal abrasions upon examination after fluorescein staining.
Effect of Menthol on Cool Cell Activity
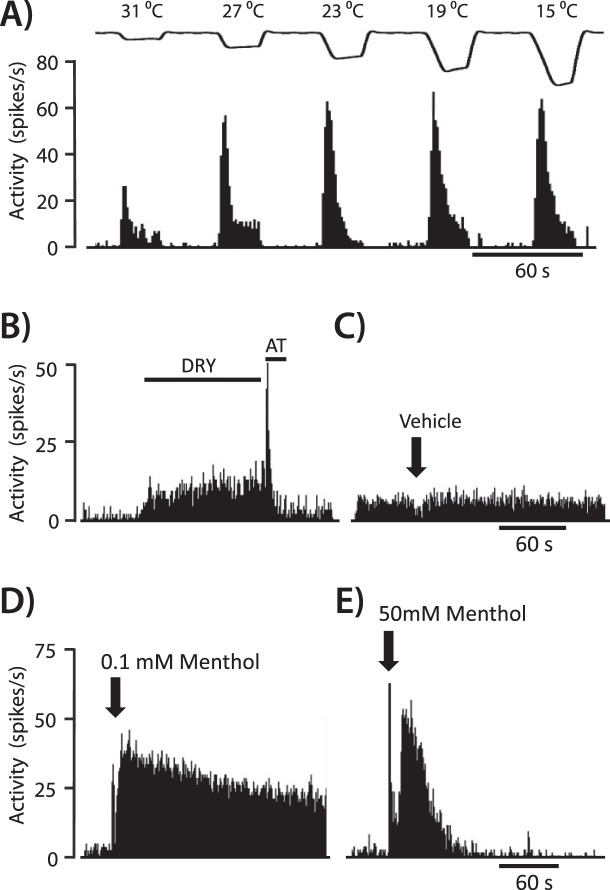
We sought to determine whether menthol evoked lacrimation and nocifensive responses correlated with its effect on corneal cool cells. While menthol at low concentrations has been demonstrated to increase the activity of corneal cool cells, the effect of high concentrations of menthol has not been examined. Corneal cool cell responses to vehicle (n = 17), 0.1 mM menthol (n = 14), and 50 mM menthol (n = 5) were examined while recording in the rat trigeminal ganglion. As expected, corneal cool cells increased their activity in response to successive cooling ramps from a holding temperature of 35°C to successively colder temperatures (for example unit, see Fig. 3A). In addition, cool cells responded to changes in the ocular fluid status (Fig. 3B), confirming our previous findings.15 Application of the mineral oil vehicle did not affect ongoing activity of these neurons, while the response to menthol was concentration-dependent (Figs. 3C–3E).
Figure 3. .
An example of a corneal cool cell, demonstrating increased neuronal discharge evoked by (A) cooling and (B) drying of the ocular surface. The “DRY” condition was created by removal of excess artificial tears (AT) with a Kimwipe. (C) Activity was not affected after application of the menthol vehicle solution (mineral oil). (D) Application of 0.1 mM menthol caused an immediate and sustained increase in activity. (E) In contrast, 50 mM menthol evoked an initial increase in activity that quickly returned to baseline values.
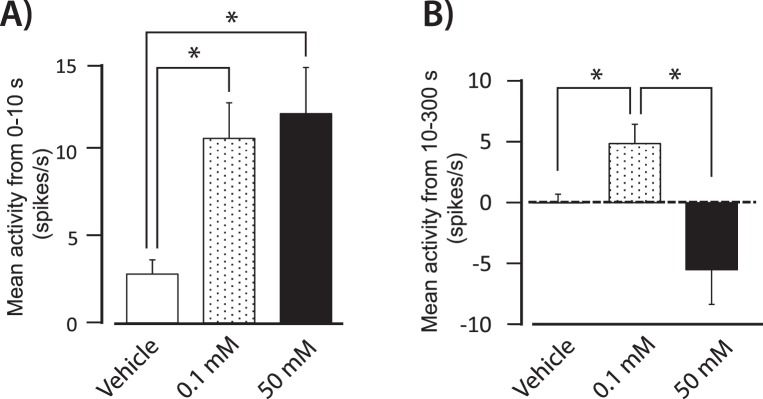
A low concentration of menthol, 0.1 mM, produced an immediate increase in activity that lasted for several minutes (Fig. 3D). In contrast, 50 mM menthol evoked an initial burst of relatively short duration that often was followed by partial or complete inactivation of the neuron (Fig. 3E). To illustrate these concentration-dependent effects, unit activity over the first 10 seconds after drug application and activity from 10 to 300 seconds after drug application were analyzed (Fig. 4). Over the first 10 seconds, 0.1 mM and 50 mM menthol evoked similar increases in neuronal activity compared to the vehicle control group (Fig. 4A, P < 0.05, Kruskal-Wallis test). In contrast, mean neuronal activity from 10 to 300 seconds was significantly greater after 0.1 mM menthol compared to the vehicle control and 50 mM menthol (Fig. 4B, P < 0.05, Kruskal-Wallis test). While the decrease in activity observed after 50 mM menthol during this time period was not significantly lower than the vehicle control group, activity after 50 mM menthol application was significantly lower compared to their baseline level of activity (10.4 ± 1.8 spikes/s vs. 4.2 ± 1.0 spikes/s, P < 0.05, paired t-test).
Figure 4. .
(A) Average neuronal activity during the first 10 seconds after application of mineral oil (n = 17), 0.1 mM menthol (n = 14), and 50 mM menthol (n = 5) to the eye. The 0.1 and 50 mM menthol evoked greater activity compared to the vehicle control. (B) Average activity from 10 to 300 seconds after vehicle, and 0.1 and 50 mM menthol. Actvity after 0.1 mM menthol was greater compared to vehicle and 50 mM menthol. The change in activity was determined by subtracting the average baseline activity for 30 seconds before drug application from the average activity following drug application. *P < 0.05.
Discussion
In these studies, a low concentration of menthol applied to the eye induced TRPM8-dependent tear secretions, as evidenced by the absence of menthol-evoked tears in TRPM8 knockout mice. At a high concentration, however, menthol produced tear secretions even in mice lacking TRPM8 channels. This higher concentration of menthol also evoked ocular nocifensive behaviors in the eye wipe test irrespective of the mouse genotype, while low concentrations of menthol had no effect on nocifensive behaviors. In parallel experiments, a low concentration of menthol increased the activity of corneal cool cells, whereas a high concentration caused these neurons to inactivate after a brief period of activity. Taken together, these results indicate that menthol activation of cool cells via TRPM8 channels can evoke tearing without eliciting nociceptive responses.
The TRPM8 channel is a nonselective cation channel found on lightly myelinated and unmyelinated A-delta and C primary afferent neurons.18–20 Although results are not completely consistent between studies, they are expressed in trigeminal and dorsal root ganglion (DRG) neurons, including corneal afferents,18 and the majority do not appear to express markers typical of the two main classes of unmyelinated nociceptive neurons; TRPM8 expressing neurons do not appear to include IB-4 positive neurons, and the majority of them are not peptidergic (do not express the peptides substance P or calcitonin gene-related peptide).27 TRPM8 channels are activated by moderate cold (15–30°C) and by cooling compounds, such as menthol and icilin.28–30 (reviewed by McCoy et al.17 and Nilius and Voets31). Menthol and icilin appear to modulate TRPM8 through different mechanisms that may have unique downstream consequences,32–34 and various TRPM8 independent effects have been reported for menthol.35–37 One study in particular found evidence for activation of nociceptive neurons following hind paw injection of 40 mM menthol in TRMP8 knockout mice.37 This result is consistent with our finding that 50 mM menthol induced tearing and eye-wipe behaviors in TRPM8 knockout animals. The mechanism of action for the effect of high concentrations of menthol remain unknown, it appears unlikely to involve TRPA1, the receptor for wasabi and other related noxious chemicals.37 However, other studies have shown that menthol can activate TRPA1 receptors.38
Over the course of the three baseline tear measurements taken before adding solutions to the eye a small, yet significant, increase in tearing was noted, which may have been caused by mild irritation produced by the cotton thread used for measuring tears. In contrast to an earlier report on tearing in TRPM8−/− mice,14 we did not find a decrease in baseline tearing in these animals. In addition, we did not find evidence of corneal abrasions after fluorescein was applied to the ocular surface, or differences in the rate of spontaneous blinks. A simple methodologic difference may explain the discrepancy between the results found in basal tear levels. While our tear measurements were performed in awake animals, the previous study measured tears under ketamine/xylazine anesthesia. Measuring tears in awake animals may affect tearing by increasing stress, and it is well established that general anesthesia reduces tearing.39,40 The absence of any difference in baseline tearing between TRPM8+/+ and TRPM8−/− mice was not entirely surprising, since cold-evoked activity likely involves additional cold-sensitive channels.41 Corneal cool cells also are sensitive to hyperosmotic stimuli,10,15 which may initiate tear secretions upon evaporation of the tear film even in the absence of a cooling response. Our data indicated that TRPM8 knockout mice retain the ability to respond to hyperosmotic tears.
The increase in tear secretions after the low concentration of menthol application likely is due to the activation of corneal cool cells. In this and in previous studies, menthol at relatively low concentrations (≤0.2 mM) has been demonstrated to increase the ongoing activity of cool cells innervating the cornea and depolarize cool sensitive corneal afferent nerve terminals.10,14,15 While different species of animals were used in the recording and behavioral experiments, previous studies have found similar electrophysiologic properties in multiple species of animals, and consistent correlations between the activity of these neurons and sensory function in humans.11 The results of our study are consistent with earlier findings that demonstrated increased tear production by cooling the cornea in TRPM8+/+, but not TRPM8−/− mice.14 In addition, decreased corneal temperatures in human subjects led to an increase in tearing in human subjects.14 However, it should be noted that an additional study found that non-noxious cooling of the cornea did not alter tearing, perhaps because of the relatively brief nature (3 seconds) of the stimulus.7
Several additional lines of evidence indicate that corneal cool cell activity is involved in regulating tear secretions. Cool cells are the only known corneal primary afferent neuron with spontaneous activity at room temperature10,12,42–44 and topical anesthesia of the ocular surface decreases tear secretions,45 indicating that this spontaneous activity is responsible for basal tearing. In addition, diabetes and LASIK surgery, conditions that result in damage to corneal afferents, can cause dry eye conditions.46–55 Furthermore, dry eye prevalence increases with age, which is correlated with a loss of corneal sensation.56,57
The utility of targeting cool cells as a means to increasing tear production depends on the selectivity of the response to cool cell activation. In addition to regulating tear secretions, corneal afferents can elicit nocifensive responses, such as blinking, irritation, and pain. This constellation of responses is initiated by the activation of polymodal and mechanoreceptive neurons to protect the eye from potentially damaging stimuli. In contrast, mild cooling of the ocular surface in humans (<2.0°C decrease), which selectively activates cool cells, does not appear to elicit irritation or pain.12,13
While increasing cool cell activity may increase tearing without producing irritation or pain, the effectiveness of TRMP8 agonists as potential therapies would depend on its ability to activate cool cells selectively. Subtypes of corneal polymodal neurons, some of which respond to cold stimuli, have been reported. In one study of corneal afferents in cats, 6/26 polymodal neurons responded to cold stimuli with a low frequency of activity evoked only at temperatures lower than that needed to evoke activity in cool cells.12 These corneal temperatures also produced irritation of pain in human subjects.12 This cold-evoked activity in polymodal neurons would be consistent with the finding in cultured DRG neurons of cells sensitive to menthol and capsaicin.58,59 Those neurons responsive to menthol and capsaicin were less responsive to menthol than those that were sensitive only to menthol.58,59 These neurons may represent polymodal nociceptive neurons. Alternatively, it has been suggested that TRPV1 and TRPM8 co-expression in DRG neurons may explain the “paradoxical” heat responses in cool cells.60
The effect of menthol in corneal polymodal nociceptive neurons remains to be described in detail. However, one study reported no changes in thermal or chemical responsiveness after application of menthol to seven polymodal corneal units.12 We used the eye wipe test to gain insight into potential nociceptive effects of menthol. This test has been used previously to examine nociceptive responses produced by application of capsaicin and hyperosmotic solutions to the ocular surface.22–24,61 The amount of time spent wiping at the eye correlates with the concentration of these irritants and is reduced by analgesic drugs. The absence of eye wipe behaviors after low concentrations of menthol suggests that TRPM8 activation may increase tearing selectively without producing irritation or pain. However, it still is possible that the behavioral measure was not sensitive enough to detect the possible activation of a small subpopulation of menthol-sensitive corneal nociceptors. In a previous report, a similar concentration of menthol (0.2 mM) produced a feeling of cooling and in some cases slight discomfort in human volunteers.12
Unlike the absence of nociceptive responses in response to low concentrations of menthol, the highest concentration evoked an increase in eye wipe behaviors in TRPM8+/+ and TRPM8−/− mice. Similarly, the same concentration of menthol induced tearing that was unaffected in the TRPM8−/− mice. These responses are unlikely to be the result of cool cell activity. While low and high concentrations of menthol increased cool cell activity over the first 10 seconds, only the low concentration of menthol produced a prolonged increase in cool cell activity. The high concentration of menthol tended to inactivate cool cells after a brief period of activity. These results indicated that this higher concentration of menthol activates nociceptive neurons in a TRPM8-independent fashion. A previous study also has demonstrated that a relatively high concentrations of menthol (6.4–64 mM) enhanced cold-evoked activity in trigeminal subnucleus caudalis nociceptive neurons with intraoral receptive fields.62 Similarly, intraoral menthol (19 mM) enhanced lingual cold pain in human subjects,63 and even higher concentrations of menthol have been used to model cold pain in humans.64–66 The results of studies using these high concentrations of menthol should be interpreted with caution, since such high concentrations produce effects that are not mediated by TRPM8 channels.
In summary, these results indicated that TRPM8 and potentially other channels expressed on corneal cool cells represent potential targets for increasing tearing without eliciting nociceptive responses. In this way, new therapies for treating DES could include TRPM8-mediated activation of corneal cold cells to drive the basal tearing reflex loop. Furthermore, our study raised an additional consideration regarding a common current therapy for DES: not only is repeated application of artificial tears often inadequate, it also may be detrimental to the overall disease progression. Repeated application of artificial tears would reduce the afferent drive for endogenous tear production.3 This decrease in afferent drive would reduce the release of trophic factors from the parasympathetic neurons that innervate the lacrimal gland, which may result in atrophy of the gland. Such a consequence would be consistent with previous studies that have reported atrophy of the salivary glands following denervation.67–69 Thus, increasing the activity of neurons involved in basal tearing would be beneficial to the overall health of the tear-producing glands.
Footnotes
Supported by National Eye Institute Grant R01EY021230 (IDM).
Disclosure: A. Robbins, None; M. Kurose, None; B.J. Winterson, None; I.D. Meng, None
References
- 1.Barabino S, Dana MR. Dry eye syndromes. Chem Immunol Allergy. 2007;92:176–184 [DOI] [PubMed] [Google Scholar]
- 2.Abelson MB, Ousler GW III, Maffei C. Dry eye in 2008. Curr Opin Ophthalmol. 2009;20:282–286 [DOI] [PubMed] [Google Scholar]
- 3.Mathers WD. Why the eye becomes dry: a cornea and lacrimal gland feedback model. CLAO J. 2000;26:159–165 [PubMed] [Google Scholar]
- 4.van Bijsterveld OP, Kruize AA, Bleys RL. Central nervous system mechanisms in Sjögren's syndrome. Br J Ophthalmol. 2003;87:128–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dartt DA. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. Ocul Surf. 2004;2:76–91 [DOI] [PubMed] [Google Scholar]
- 6.Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acosta MC, Peral A, Luna C, Pintor J, Belmonte C, Gallar J. Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers. Invest Ophthalmol Vis Sci. 2004;45:2333–2336 [DOI] [PubMed] [Google Scholar]
- 8.King-Smith PE, Nichols JJ, Nichols KK, Fink BA, Braun RJ. Contributions of evaporation and other mechanisms to tear film thinning and break-up. Optom Vis Sci. 2008;85:623–630 [DOI] [PubMed] [Google Scholar]
- 9.Zander E, Weddell G. Observations on the innervation of the cornea. J Anat. 1951;85:68–99 [PMC free article] [PubMed] [Google Scholar]
- 10.Gallar J, Pozo MA, Tuckett RP, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat's cornea. J Physiol. 1993;468:609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–525 [DOI] [PubMed] [Google Scholar]
- 12.Acosta MC, Belmonte C, Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol. 2001;534:511–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001;42:2063–2067 [PubMed] [Google Scholar]
- 14.Parra A, Madrid R, Echevarria D, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16:1396–1399 [DOI] [PubMed] [Google Scholar]
- 15.Hirata H, Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci. 2010;51:3969–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madrid R, Donovan-Rodriguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci. 2006;26:12512–12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy DD, Knowlton WM, McKemy DD. Scraping through the ice: uncovering the role of TRPM8 in cold transduction. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1278–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bautista DM, Siemens J, Glazer JM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208 [DOI] [PubMed] [Google Scholar]
- 19.Abe J, Hosokawa H, Okazawa M, et al. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res. 2005;136:91–98 [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi K, Fukuoka T, Obata K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606 [DOI] [PubMed] [Google Scholar]
- 21.Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638 [PubMed] [Google Scholar]
- 22.Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol. 2004;141:1118–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farazifard R, Safarpour F, Sheibani V, Javan M. Eye-wiping test: a sensitive animal model for acute trigeminal pain studies. Brain Res Brain Res Protoc. 2005;16:44–49 [DOI] [PubMed] [Google Scholar]
- 24.Urban L, Campbell EA, Panesar M, et al. In vivo pharmacology of SDZ 249-665, a novel, non-pungent capsaicin analogue. Pain. 2000;89:65–74 [DOI] [PubMed] [Google Scholar]
- 25.Hirata H, Okamoto K, Tashiro A, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci. 2004;24:4224–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirata H, Oshinsky ML. Ocular dryness excites two classes of corneal afferent neurons implicated in basal tearing in rats: involvement of transient receptor potential channels. J Neurophysiol. 2012;107:1199–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4, 5-bisphosphate. J Biol Chem. 2009;284:1570–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715 [DOI] [PubMed] [Google Scholar]
- 30.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58 [DOI] [PubMed] [Google Scholar]
- 31.Nilius B, Voets T. Neurophysiology: channelling cold reception. Nature. 2007;448:147–148 [DOI] [PubMed] [Google Scholar]
- 32.Kuhn FJ, Kuhn C, Luckhoff A. Inhibition of TRPM8 by icilin distinct from desensitization induced by menthol and menthol derivatives. J Biol Chem. 2009;284:4102–4111 [DOI] [PubMed] [Google Scholar]
- 33.Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron. 2004;43:859–869 [DOI] [PubMed] [Google Scholar]
- 34.Rawls SM, Gomez T, Ding Z, Raffa RB. Differential behavioral effect of the TRPM8/TRPA1 channel agonist icilin (AG-3-5). Eur J Pharmacol. 2007;575:103–104 [DOI] [PubMed] [Google Scholar]
- 35.Mahieu F, Owsianik G, Verbert L, et al. TRPM8-independent menthol-induced Ca2+ release from endoplasmic reticulum and Golgi. J Biol Chem. 2007;282:3325–3336 [DOI] [PubMed] [Google Scholar]
- 36.Kim SH, Nam JH, Park EJ, et al. Menthol regulates TRPM8-independent processes in PC-3 prostate cancer cells. Biochim Biophys Acta. 2009;1792:33–38 [DOI] [PubMed] [Google Scholar]
- 37.Braz JM, Basbaum AI. Differential ATF3 expression in dorsal root ganglion neurons reveals the profile of primary afferents engaged by diverse noxious chemical stimuli. Pain. 2010;150:290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28:9640–9651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cross DA, Krupin T. Implications of the effects of general anesthesia on basal tear production. Anesth Analg. 1977;56:35–37 [DOI] [PubMed] [Google Scholar]
- 40.Krupin T, Cross DA, Becker B. Decreased basal tear production associated with general anesthesia. Arch Ophthalmol. 1977;95:107–108 [DOI] [PubMed] [Google Scholar]
- 41.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf. 2004;2:248–253 [DOI] [PubMed] [Google Scholar]
- 43.Carr RW, Pianova S, Fernandez J, Fallon JB, Belmonte C, Brock JA. Effects of heating and cooling on nerve terminal impulses recorded from cold-sensitive receptors in the guinea-pig cornea. J Gen Physiol. 2003;121:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brock JA, Pianova S, Belmonte C. Differences between nerve terminal impulses of polymodal nociceptors and cold sensory receptors of the guinea-pig cornea. J Physiol. 2001;533:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herreras JM, Perez S, Perez H, Calonge M, Pastor JC. Influence of topical anesthesia on tests diagnostic of blepharitis-associated dry eye syndrome. Ocul Immunol Inflamm. 1997;5:33–41 [DOI] [PubMed] [Google Scholar]
- 46.Konomi K, Chen LL, Tarko RS, et al. Preoperative characteristics and a potential mechanism of chronic dry eye after LASIK. Invest Ophthalmol Vis Sci. 2008;49:168–174 [DOI] [PubMed] [Google Scholar]
- 47.Ambrosio R Jr, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24:396–407 [DOI] [PubMed] [Google Scholar]
- 48.Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol. 2000;84:19–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grus FH, Sabuncuo P, Dick HB, Augustin AJ, Pfeiffer N. Changes in the tear proteins of diabetic patients. BMC Ophthalmol. 2002;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain S. Dry eyes in diabetes. Diabetes Care. 1998;21:1375–1376 [DOI] [PubMed] [Google Scholar]
- 51.Kaiserman I, Kaiserman N, Nakar S, Vinker S. Dry eye in diabetic patients. Am J Ophthalmol. 2005;139:498–503 [DOI] [PubMed] [Google Scholar]
- 52.Liang L, Zhang M, Zou W, Liu Z. Aggravated dry eye after laser in situ keratomileusis in patients with Sjögren syndrome. Cornea. 2008;27:120–123 [DOI] [PubMed] [Google Scholar]
- 53.Liu C, Hille K, Tan D, Hicks C, Herold J. Keratoprosthesis surgery. Dev Ophthalmol. 2008;41:171–186 [DOI] [PubMed] [Google Scholar]
- 54.Liu X, Gu YS, Xu YS. Changes of tear film and tear secretion after phacoemulsification in diabetic patients. J Zhejiang Univ Sci B. 2008;9:324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:179–193 [DOI] [PubMed] [Google Scholar]
- 57.Rocha EM, Alves M, Rios JD, Dartt DA. The aging lacrimal gland: changes in structure and function. Ocul Surf. 2008;6:162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarria I, Gu J. Menthol response and adaptation in nociceptive-like and nonnociceptive-like neurons: role of protein kinases. Mol Pain. 2010;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xing H, Ling J, Chen M, Gu JG. Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J Neurophysiol. 2006;95:1221–1230 [DOI] [PubMed] [Google Scholar]
- 60.Okazawa M, Inoue W, Hori A, Hosokawa H, Matsumura K, Kobayashi S. Noxious heat receptors present in cold-sensory cells in rats. Neurosci Lett. 2004;359:33–36 [DOI] [PubMed] [Google Scholar]
- 61.Tamaddonfard E, Khalilzadeh E, Hamzeh-Gooshchi N, Seiednejhad-Yamchi S. Central effect of histamine in a rat model of acute trigeminal pain. Pharmacol Rep. 2008;60:219–224 [PubMed] [Google Scholar]
- 62.Zanotto KL, Merrill AW, Carstens MI, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. J Neurophysiol. 2007;97:966–978 [DOI] [PubMed] [Google Scholar]
- 63.Albin KC, Carstens MI, Carstens E. Modulation of oral heat and cold pain by irritant chemicals. Chem Senses. 2008;33:3–15 [DOI] [PubMed] [Google Scholar]
- 64.Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol--a human model for cold pain by activation and sensitization of C nociceptors. Brain. 2004;127:1159–1171 [DOI] [PubMed] [Google Scholar]
- 65.Hatem S, Attal N, Willer JC, Bouhassira D. Psychophysical study of the effects of topical application of menthol in healthy volunteers. Pain. 2006;122:190–196 [DOI] [PubMed] [Google Scholar]
- 66.Binder A, Stengel M, Klebe O, Wasner G, Baron R. Topical high-concentration (40%) menthol-somatosensory profile of a human surrogate pain model. J Pain. 2011;12:764–773 [DOI] [PubMed] [Google Scholar]
- 67.Wells H, Peronace AA. Functional hypertrophy and atrophy of the salivary glands of rats. Am J Physiol. 1967;212:247–251 [DOI] [PubMed] [Google Scholar]
- 68.Mansson B, Nilsson BO, Ekstrom J. Effects of repeated infusions of substance P and vasoactive intestinal peptide on the weights of salivary glands subjected to atrophying influences in rats. Br J Pharmacol. 1990;101:853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall HD, Schneyer CA. Role of autonomic pathways in disuse atrophy of rat parotid. Proc Soc Exp Biol Med. 1973;143:19–23 [DOI] [PubMed] [Google Scholar]