Abstract
Background and Aims
IL-is important in gastric damage, mucosal repair and gastric cancer progression. We analysed IL-11 expression in H.pylori infected mouse stomach, the site of gastric IL-11 expression in mice and humans, and the effect of exogenous IL-11 on gastric mucosal homeostasis.
Methods
IL-11 protein was localised in mouse and human stomach. The impact of chronic, exogenous IL-11 on normal mouse stomach was examined Histologically and transcriptionally by microarray, confirmed by mRNA and protein analysis. Functional impact of IL-11 on gastric acid secretion was determined.
Results
In mice infected with H.pylori, IL-11 was increased in fundic mucosa with temporal expression similar to IL-1b. IL-11 protein was localised predominantly to parietal cells in mouse and human stomach. Application of exogenous IL-11 to resulted in fundic parietal and chief cell loss, hyperplasia, mucous cell metaplasia and inflammation. Coincident with cellular changes were an increased gastric pH, altered parietal cell ultrastructure and altered gene expression, particularly genes involved in immune response and ion transport which could result in compromised acid secretion. We confirmed that a single dose of IL-11 effectively ablated the gastric response to histamine.
Conclusions
IL-11 is a parietal cell cytokine that blocks gastric acid secretion, likely via reducing expression of parietal cell ion transport genes, CCKb and histamine H2 receptors. IL-11 expression is increased in H. pylori infected mouse stomach and treatment of wild type mice with IL-11 induced changes in the gastric fundic mucosa reminiscent of chronic atrophic gastritis, a precursor to gastric cancer.
Recent evidence suggests that the cytokine IL-11 may play a pivotal role in gastric cancer development. Gastric cancer has a very high mortality rate, largely due to diagnosis post-metastasis,1,2 and so it is crucially important to define precancerous characteristics and identify transitional markers to allow for screening of at-risk individuals. Gastric cancer occurs as a result of chronic Helicobacter pylori infection.3 Most infections are asymptomatic, but susceptible individuals develop progressive gastric pathology including atrophic gastritis, metaplasia, dysplasia, carcinoma in situ and metastatic carcinoma.4 Host genetic factors,5–10 environmental triggers and dietary factors11,12 contribute to an individual’s susceptibility, on the background of chronic inflammation.
IL-11 is a multifunctional cytokine regulating haematopoiesis, 13 bone function and cytoprotective abilities in the gut.14–21 It belongs to the IL-6 cytokine family and initiates signal transduction by binding to the IL-11 receptor alpha (IL-11Rα) thereby recruiting the signal transducing receptor gp130.14,22,23 IL-6 and IL-11 are prevalent in the stomach where they modulate the inflammatory response, angiogenesis, proliferation and programmed cell death in the context of neoplastic progression. 15,16 Although IL-11 induction is not associated with early H pylori inflammation, chronic bacterial infection and the attendant atrophic gastritis and intestinal metaplasia are accompanied by increased IL-11, particularly in the fundic mucosa.17,18 Atrophic gastritis and intestinal metaplasia are precancerous lesions, requisites in intestinal-type adenocarcinoma, the most common gastric cancer in humans.4 Elevated IL-11 expression is also associated with tumour grade and invasion.19–21
Elevated IL-11 expression occurs in most murine models of gastric pathology,17,24 and unlike IL-625 is indispensable for tumour development in the gp130757FF mouse.17,26 This mouse has a single base pair substitution at position 757 of gp130, which simultaneously blocks downstream ERK/MAPK signalling, while STAT1/3 is constitutively activated, resulting in antral stomach tumour development with complete penetrance. 27,28 IL-11 is relevant in other models of gastric tumorigenesis and damage including gastrin-driven fundic hypertrophy29 and ulceration;24 however, how its temporal expression relates to H pylori infection is unclear. Gastric mucosal structure and function are uncompromised in the absence of IL-11Rα,13 so while IL-11 is implicated in gastric damage, it is not absolutely required for normal stomach function.
Atrophic gastritis is marked by altered gastric differentiation programmes, such that parietal and chief cells in particular are lost and partly replaced in a reduced glandular structure by a diffuse mucous metaplasia.30–33 The mechanisms of induction of atrophy have not been defined, but their delineation might provide early therapeutic targets to prevent irreversible tumorigenesis. Here we demonstrate that IL-11 is expressed at high levels specifically in the parietal cells of the fundic mucosa, and that chronically elevated IL-11 in normal mice causes significant fundic damage that closely models human chronic atrophic gastritis including increased proliferation, loss of parietal and chief cells, mucous metaplasia and inflammation. Furthermore, we demonstrate that IL-11 can block gastric acid secretion via gastric IL-1β and key ion transport genes. We have discovered that IL-33, important in regulating mucosal T-helper (Th) type 1/2 immune response, is a novel IL-11 target. These data support the view that IL-11 is a key regulator of gastric damage acting to initiate chronic atrophic gastritis.
MATERIALS AND METHODS
Mice
Wild-type (WT) mice were 129X1(Sv-J)/C57BL/6 background, 10–12 weeks old. HKβ−/− mice,34 10–12 weeks old and on either a BALB/cCrSlc or C57BL/6 background, respectively. Mice were genotyped by multiplex PCR, free of H pylori. Approval was obtained from Murdoch Children’s Research Institute (A583) and Bio21 Institute (0809107).
Human gastric biopsies
Selection and processing of gastric biopsies from disease-free individuals was undertaken as previously described.18,35 Written informed consent was obtained and studies were approved by Melbourne Health (#2004.176).
Helicobacter infection of mice
WT (C57BL6) mice were infected with H pylori Sydney strain 1 (SS1) as described.11
Cytokine treatment
WT mice (n≥5) were injected intraperitoneally with 5 µg recombinant human IL-11 (des-Pro hIL-11, 19.05 kDa, from Dr Lorraine Robb, Walter and Eliza Hall Institute (WEHI), Australia) or saline every 6 h and killed 3 h post-injection at 3, 6 and 24 h or 5 and 7 days. A recovery group was treated for 7 days and rested for 4 weeks. The saline-dosed controls were included in all subsequent analysis to determine any changes that occurred as a result of IL-11 administration.
Tissue preparation
Mouse stomachs were prepared and analysed as previously described.17 Briefly, stomachs were excised and cut along the lesser curvature, pinned out and bisected from forestomach to duodenum. Antrum and fundus from one half was dissected and snap frozen in liquid nitrogen for protein and RNA extraction. For histological examination, bisected tissue was fixed in 4% paraformaldehyde in phosphate-buffered saline for a minimum of 16 h at 4°C. Stomachs were cut into approximately 4 mm wide strips (two or more per mouse), processed to paraffin wax and embedded.
Immunohistochemistry
Paraffin sections (4 µm) on 3-aminopropyltriethoxysilane slides were subject to immunohistochemistry according to supplementary table 1 (available online only). Antigen retrieval was in 10 mM citric acid at 100°C for 30 min, followed by 30 min cooling. Staining was completed with biotinylated secondary antibodies, avidin and biotinylated horseradish peroxidase complex (Vector Laboratories, CA, USA), 3,3′-diaminobenzidine and haematoxylin counterstained, or Alexa-fluor 488/594 conjugated secondary antibodies. Ki-67 immunohistochemistry was counterstained with periodic acid Schiff reagent (PAS). For all staining reactions a control was performed with secondary antibody alone. For all immunofluorsecence, images were captured from all groups with the same microscope setting to allow for direct comparison between images. Representative images from each treatment group are shown.
IL-11 antibody adsorption
IL-11 antibody (2.5 µg per slide) was adsorbed overnight at 4°C with 0, 1, 2 or 5 µg of rhIL-11 in a final volume of 5 µl. Staining was completed as above with the addition of a 5 µg rhIL-11-only control.
Quantitative morphometry
All quantitative morphometry was performed by a blinded observer. At least six representative photographs per animal (n≥5) of histochemically or immunohistochemically stained sections were captured using a Coolpix 4500 digital camera (Nikon Instruments, Melville, New York, USA) attached to a light microscope. Lengths or relevant cells were manually traced on these images using ImageJ software for Windows v1.38 (http://rsb.info.nih.gov/ij/index.html) to generate measurements. Measurements were converted to millimeters after comparison with a calibrated graticule.
Electron microscopy
For electron microscopy, 1 mm cubes of fundic tissue (two or more per mouse) were fixed overnight at 4°C in 4% paraformaldehyde, 4% sucrose and 2% gluteraldehyde in 0.1 M phosphate buffer, pH 7.4, then processed for electron microscopy.
Immunoblotting
Proteins (n≥5 animals/group) were prepared with TRIzol (Life Technologies, NY, USA) and 20 µg of extract subjected to sodium dodecylsulphate polyacrylamide gel electrophoresis. Membranes were incubated with antibodies specific for; IL-11 (WEHI Antibody facility), STAT3, phosphorylatedTyr757-STAT3 (pSTAT3), ERK1/2, p-ERK1/2, AKT, p-AKT (Cell Signalling, MA, USA), IL-33, Tenascin-C or GAPDH (Abcam, Cambridge, UK), peroxide-conjugated secondary antibody and visualised by enhanced chemiluminescence (Amersham, NJ, USA). Quantification was using Quantity 1 software (Bio-Rad Laboratories, NSW, Australia) and phosphorylated:total protein ratios determined from duplicate membranes.
Quantitiative RT–PCR
Total RNA was harvested using TRIzol reagent (Life Technologies). RNA (3 µg) (n≥5 animals/group) was reverse transcribed into complementary DNA using Moloney murine leukaemia virus reverse transcriptase (Promega) primed with oligo(dT). Quantitative reverse transcription (RT)–PCR primers were designed using PRIMER EXPRESS (Applied Biosystems) (see supplementary table 2, available online only). SYBR green chemistry was used with rL32 as the internal reference gene. quantitative RT–PCR conditions were 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 15 s (Applied Biosystems AB7500, VIC, Australia). Results were analysed using sequence detector software, relative fold differences were determined using the ΔΔCt method.
Microarray
Illumina MouseWG-6 V2 arrays were used to hybridise the 12 messenger RNA samples. Three biological replicates of fundic gastric mucosa from saline and IL-11 treatments were performed at 24-h and 7-day time points (n=3 animals/group). Data were analysed in R using Bioconductor packages Lumi36 (for VST, quantile normalisation and quality control) and Limma37 (for differential expression analysis, multiple testing correction by the Benjamini and Hochberg method). GOstat38 was utilised to identify significant gene ontologies.
In-vivo acid secretion analysis
Analysis was performed on mice 8–12 weeks of age as previously described39 (details in supplementary methods, available online only) (n=5 animals/group). Once basal acid secretion was established mice were given intraperitoneal treatments including histamine (10 mg/kg) and IL-11 (5 µg).
Statistical analysis
All data were expressed as mean±SEM and statistical analysis was performed using one-way analysis of variance and the appropriate parametric or nonparametric statistical test using Sigmastat (Jandel Scientific, San Rafael, California, USA). p Values of 0.05 or less were considered statistically significant.
RESULTS
IL-11 is increased in H pylori infection and expressed by parietal cells in normal fundic mucosa
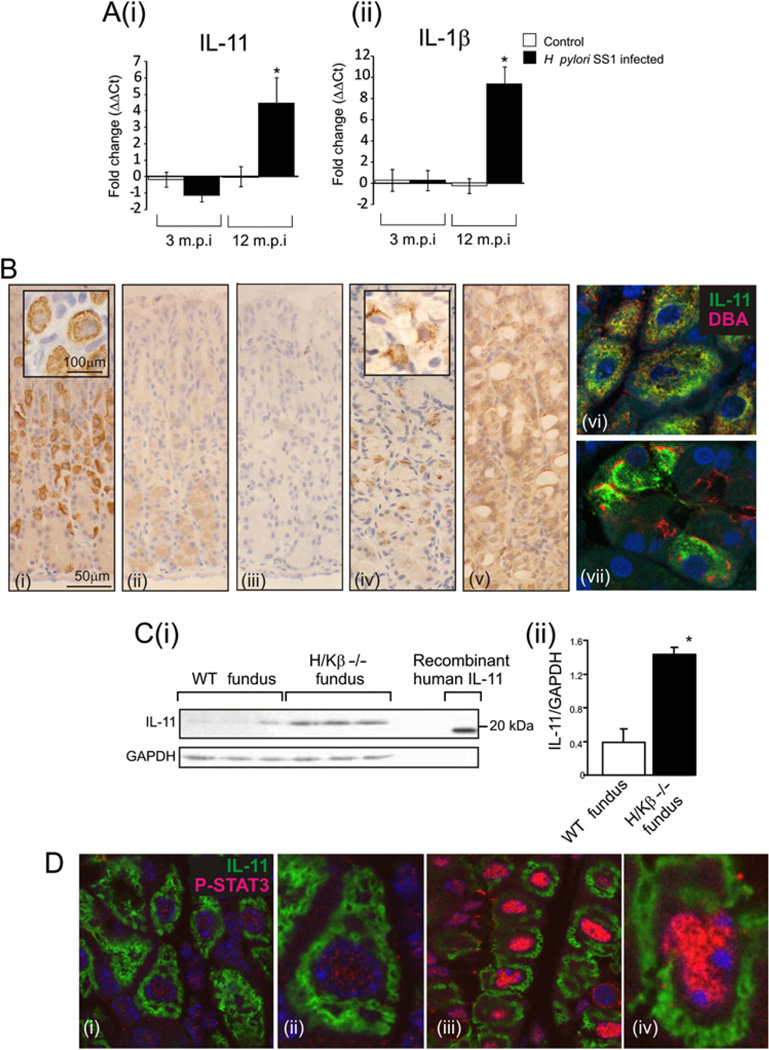
Recent studies have indicated an important role for IL-11 and IL-1β in gastric damage, including a potential role in tumorigenesis. To investigate this we analysed the expression of IL-1β and IL-11 in the fundic mucosa of mice infected with mouse-adapted H pylori SS1 strain. At 3 months post-infection we saw no change in the expression of either IL-11 or IL-1β (figure 1A); however, at 12 months post-infection, when IL-1β expression was increased, IL-11 was also increased 4.5-fold (figure 1A). The overlapping temporal expression of IL-11 and IL-1β suggests that IL-11 might also have a role to play in gastric atrophy.
Figure 1.
mRNA for IL-11 (Ai) and IL-1β (Aii) in stomachs of H pylori SS1-infected or control mice, killed at 3 or 12 months post-infection, standardized against rL32 and expressed as fold change compared to controls (ΔΔCt method). Immunolocalisation of IL-11 in fundic mucosa from wild-type (WT) mice (Bi-iii), human (iv) and H/K-ATPase β subunit deficient (H/Kβ−/−) mice (v). To confirm antibody specificity, antibody was adsorbed with rhIL-11 (Bii) or peptide was substituted (Biii). Immunolocalisation of IL-11 (green) and the parietal cell lectin, DBA (red) in WT mouse (Bvi) and human (Bvii) tissue. Immunoblotting for IL-11 and GAPDH was performed on gastric fundic tissue from WT and H/Kβ−/− mice and compared with rhIL-11 (19.05 kDa) (C), quantitative densitometry was performed of IL-11/GAPDH. Immunolocalisation of IL-11 (green) and pSTAT3 (red) (phosphorylated STAT3) in the fundus of WT (Di and ii) and IL-11 treated mice (Diii and iv). Bars refer to mean±SEM; *p≤0.05.
A major outstanding issue with regard to IL-11 is its role in normal gastric mucosa. To this end we examined IL-11 peptide expression in the normal mouse and human stomach. IL-11 peptide strongly and specifically localised to parietal cells in the mouse fundic mucosa (figure 1Bi). Staining specificity was demonstrated by adsorption of the antibody with peptide (figure 1Bii) or staining with peptide alone (figure 1Biii). All staining was performed with secondary antibody alone to confirm the specificity of staining (data not shown). Parietal cell-specific staining was also present in human gastric biopsies (figure 1Biv). Staining for IL-11 was also performed on fundus from H/Kβ−/− mice. These mice lack the H/K-ATPase β subunit expressed in parietal cells and develop gastric atrophy with near complete loss of parietal and zymogenic cells and mucous cell hyperplasia. 34 H/Kβ−/− mice have increased expression of IL-11 mRNA in the fundus.29 In H/Kβ−/− mice, the intensity of IL-11 staining in the few remaining but abnormal parietal cells was reduced and there was increased staining in other epithelial cells (figure 1Bv). Staining mouse and human tissues with an anti-body for IL-11 and DBA, the lectin specific for parietal cells, confirmed the parietal cell localisation of IL-11 (figure 1Bvi and vii). These data demonstrate the specificity of IL-11 parietal cell staining and, importantly, that other epithelial cells have the capacity to produce IL-11 during damage.
Immunoblotting confirmed the IL-11 staining. A 23 kDa band that co-migrates with rhIL11 was apparent in mouse fundic mucosa (figure 1C). In the fundus of H/Kβ−/− mice there was more IL-11 than in WT mice (figure 1Ci and ii).
To determine if fundic IL-11 was acting in an autocrine and/or paracrine manner we treated mice with rhIL-11 then stained stomach sections for IL-11 and pSTAT3. STAT3 signalling is one pathway activated by IL-11 signalling through the IL-11Rα/gp130 complex. In saline-treated mice there was intense IL-11 staining but only limited nuclear staining for pSTAT3 (figure 1Di and ii). In the presence of exogenous IL-11, nuclear pSTAT3 was present at markedly greater levels both in the cells that expressed IL-11 and in those that did not (figure 1Diii and iv), demonstrating that IL-11 can act in both an autocrine and paracrine manner.
Chronic IL-11 treatment results in continuous STAT3 activation and numerous changes in the transcriptome
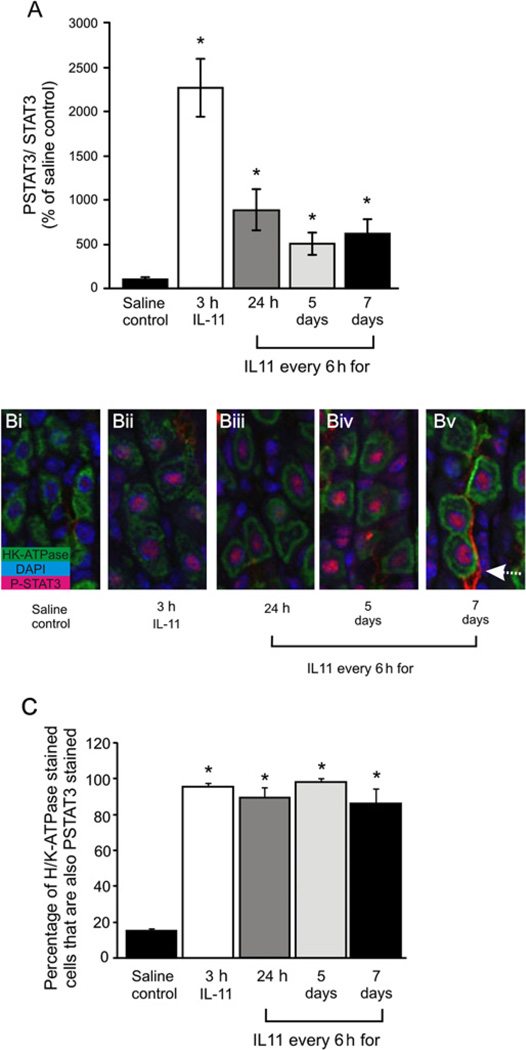
To determine the effect of IL-11 on the normal mouse fundus, mice were injected intraperitoneally with either rhIL-11 or saline every 6 h. The 6-hourly interval was chosen as this was the maximal time following IL-11 administration that STAT3 activation was sustained (data not shown). Fundic mucosa from treated mice at all time points examined had significantly greater pSTAT3 than saline controls (figure 2A), an observation reiterated by immunohistochemical staining (figure 2B). Three hours following a single IL-11 dose most of the parietal cells (stained for H/K-ATPase), showed strong nuclear pSTAT3 staining (figure 2Bii), while other cell types were unstained, demonstrating that the dominant mechanism of acute IL-11 action is on parietal cells. After 24 h of IL-11 treatment, staining for pSTAT3 was still present in all parietal cells but was less intense (figure 2Biii). The parietal cell staining pattern after 5 days (figure 2Biv) or 7 days (figure 2Bv) was very similar to 24 h, but increased in other epithelial cells, particularly after 7 days (figure 2Biv and v). At this time, pSTAT3 was also associated with blood vessels (figure 2Bv). At all time points almost all parietal cells were immunoreactive for pSTAT3 (figure 2C).
Figure 2.
Wild-type (WT) mice treated with IL-11 or saline. pSTAT3 and STAT3 in fundus were measured by immunoblotting on duplicate blots. Quantitative densitometry was performed of pSTAT3/STAT3 (A). pSTAT3 (red) and H/K-ATPase (green) were immunolocalised in fundus from WT mice (B) treated with saline (i), IL-11 for 3 h (ii) or IL-11 every 6 h for 24 h (iii), 5 days (iv) or 7 days (v). The proportion of H/K-ATPase and pSTAT3-positive parietal cells was quantified (C). Bars refer to mean±SEM; *p≤0.05.
The IL-11 regulated transcriptome was assessed by microarray analysis of RNA isolated from the fundic stomach of mice treated for 24 h or 7 days. Altered expression in a subset of genes was observed 24 h following IL-11 treatment, with the majority of genes increased in expression compared with saline controls. These changes were maintained after 7 days with varied intensity, but an additional set of genes was also altered. An arbitrary gene list was constructed of transcripts with significantly altered expression and a known or inferred role in gastric biology. These were clustered into six functional groups: immune response, signal transduction, proliferation/apoptosis/differentiation, protein degradation and endocrine (table 1).
Table 1.
Expression microarray analysis of the effects of IL-11 on the mouse fundic mucosa
| Symbol | 24 h IL-11 vs control | 7 day IL-11 vs control |
|---|---|---|
| Immune response | ||
| Serpina3n | 10.9 | 16.7 |
| Hp | * | 12.9 |
| Dmbt1 | 6.4 | 8.1† |
| Lcn2 | * | 7.1 |
| Ly6d | * | 5.8 |
| Serpina3g | 3.1 | 6.6 |
| Spp1 | 4.3 | 5.3 |
| C4a | * | 5.0 |
| Ifitm1 | 2.9 | 5.0 |
| Pigr | 2.5 | 4.3 |
| Serpina3m | * | 3.7 |
| Cxcl17 | * | 3.4 |
| Ifitm3 | 2.6 | 3.4 |
| Il33 | 2.4† | 3.4† |
| Cxcl13 | * | 3.2 |
| Osmr | 1.8 | 2.8 |
| Ly6a | * | 2.4 |
| Cf1 | * | 2.3 |
| Cxcl14 | * | 2.2 |
| Serpinb1a | 2.2 | 2.2 |
| Serpinf2 | * | 2.1 |
| Irf1 | 2.1 | * |
| Icam1 | * | 1.8 |
| Tnfrsf12a | * | 1.7 |
| Myd88 | 1.9 | 1.7 |
| Proliferation/apoptosis/differentiation | ||
| Reg3b | 64.3 | 146.7 |
| Reg1 | 24.7 | 63.2 |
| Reg3g | 13.4 | 39.9 |
| Wfdc2 | * | 6.0† |
| Reg3a | 1.5 | 5.5 |
| Igfbp4 | 2.1 | 3.7† |
| Bmp1 | * | 3.3 |
| Gadd45g | 2.6 | 2.5 |
| Gsdmc1 | 1.9 | 2.1 |
| Grem1 | 2.0 | 2.0 |
| Bcl3 | 1.6 | 1.9 |
| Muc1 | 1.9 | * |
| Sfrp1 | * | 1.8 |
| Reg3d | * | 1.8 |
| Klf5 | * | 1.7 |
| Mki67 | * | 1.6 |
| Shh | * | 1.5 |
| Gdf9 | * | −6.1 |
| Blm | −4.0 | −5.6 |
| Mist1 | * | −2.3 |
| Bmpr1b | * | −2.1 |
| Tob1 | * | −2.0 |
| Egf | * | −2.0 |
| Transport | ||
| Aqp5 | 2.1 | 5.2† |
| Kcnk1 | * † | 1.7† |
| Kcng4 | 1.7 | * |
| Slc38a5 | * | −3.9 |
| Slc26a9 | * † | −2.7† |
| Kcnj16 | * † | −2.6† |
| Kcnk5 | * | −2.5 |
| Kcnf1 | * † | −2.3† |
| Kcnj15 | * † | −2.0† |
| Aqp4 | −2.0 | * |
| Atp2c1 | * | −1.7 |
| Slc5a8 | * † | −1.7† |
| Slc16a5 | * | −1.6 |
| Slc25a12 | * | −1.6 |
| Slc26a6 | * † | −1.5† |
| Slc24a3 | 1.5† | * † |
| Protein degradation | ||
| Timp1 | 5.4† | 6.9† |
| Mmp3 | 1.8 | 2.1† |
| Adam28 | 1.9 | 2.0 |
| Adamdec1 | * | 1.8 |
| Efemp2 | 1.6 | 1.7 |
| Mmp23 | * | 1.7 |
| Mmp13 | * | 1.6 |
| Try10 | * | −33.1 |
| Try4 | * | −21.1 |
| Amy2 | * | −22.3 |
| Cpm | −1.9 | −2.3 |
| Endocrine | ||
| Chgb | * | 1.9 |
| Car11 | * | 1.5 |
| Gper | −2.8 | −5.2 |
| Sstr2 | * | −2.2 |
| Cckbr | −1.8† | −2.0† |
| Ddc | −1.6 | −1.8 |
| Signal transduction | ||
| Socs3 | 5.2 | 6.6 |
| Stat3 | * | 2.0 |
| Socs2 | * | 1.8 |
| Jak3 | 1.8 | 1.7 |
| Junb | 1.7 | 1.6 |
| Il-11ra1 | * | −1.6 |
Listed are transcripts of interest that had a fold changed 1.5 or greater and an adjusted p value of 0.05 or less for either 24 h or 7 days treatment with IL-11. Transcripts are clustered into six distinct functional groups; immune response, signal transduction, proliferation/apoptosis/differentiation, transport, protein degradation and endocrine.
Indicates expression at this time point not significantly altered by IL-11 expression.
Indicates transcriptional regulation confirmed by quantitative PCR analysis.
The 244 differentially expressed probes in the day 7 IL-11 treatment group were also analysed for gene ontologies using GOstat.38 IL-11 treatment induced statistically significant changes in the acute inflammatory response, protease inhibitor, wounding and extracellular space pathways. From this analysis, it is clear that IL-11, either directly or indirectly, alters the transcriptional activity of genes mainly involved in the immune response, ion transport and differentiation that can also impact damage.
Chronic treatment of WT mice with IL-11 induces gastric fundic atrophy
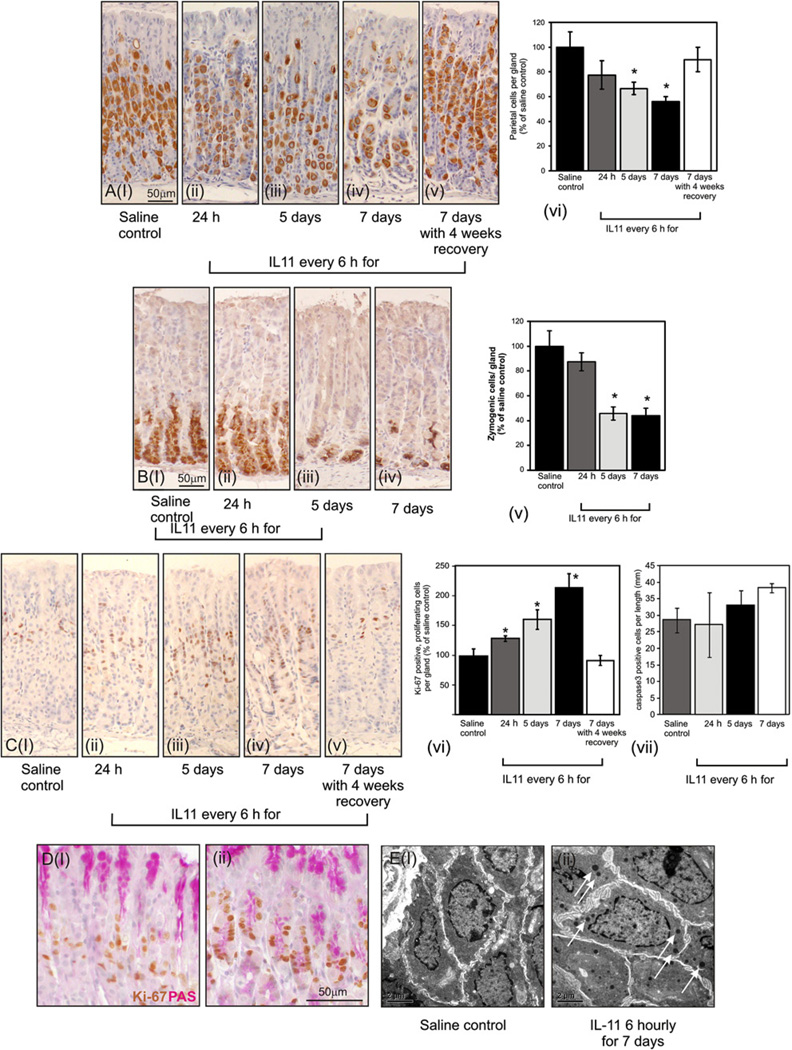
Exogenous IL-11 caused severe fundic atrophy, with a progressive reduction in parietal cell numbers to a maximum of 40% by 7 days, as quantified by H/K-ATPase immunostaining (figure 3A). Parietal cell atrophy was fully reversible 4 weeks after IL-11 cessation (figure 3Av, vi). Likewise, using an intrinsic factor as a marker, IL-11 administration caused a 60% reduction in chief cell numbers by 5 days and 7 days (figure 3Bi–v). These data suggest that IL-11 may suppress both parietal and chief cell differentiation to promote fundic atrophy.
Figure 3.
Parietal (A), zymogenic (B), proliferating (Ci-vi) and apoptotic (Cvii) cells were immunolocalised in saline control mice (i) and each of the IL-11 treatment groups (ii–v) using antibodies for H/KATPase, intrinsic factor, Ki-67 and activated caspase 3, respectively. Stained cells were expressed relative to control. Saline (Di) or IL-11-treated (Dii) sections were stained with Ki-67 and periodic acid Schiff reagent. Proliferating zone cells from fundus of saline (Ei) or IL-11-treated (Eii) mice were examined by electron microscopy. Arrows in 3Eii indicate the presence of electron dense granules. Bars refer to mean±SEM; *p≤0.05.
Chronic IL-11 treatment causes increased cell proliferation and induces immature mucous cell metaplasia
IL-11 treatment of WT mice resulted in a progressive increase in fundic cell proliferation, with cells staining for the cell division-associated antigen Ki-67 in gland isthmi increased by twofold after 7 days (figure 3Ci–vi). Cessation of IL-11 treatment followed by 4 weeks of recovery normalised proliferation to WT levels (figure 3vi). In contrast, treatment with IL-11 did not affect the number of apoptotic cells in the fundic mucosa as assessed by staining for activated caspase 3 (figure 3Cvii; all comparisons with saline were not significant p>0.1). In saline-treated mice Ki-67 and mucous (PAS-stained) cells were distinct (figure 3Di), demonstrating that only the most immature of cells, which do not express mucous, proliferate. In IL-11-treated mice, a significant proportion of Ki-67-stained cells also express PAS (figure 3Dii) suggesting that proliferation of pre-pit cells is increased. This was confirmed by electron microscopy showing a paucity of immature gastric epithelial cells (figure 3Ei) and numerous cells with a large nuclear:cytoplasmic ratio, reminiscent of immature gastric epithelial cells but with scattered electron-dense mucous granules (figure 3Eii see arrows). These cells most likely correspond to the PAS/Ki-67-stained proliferating pre-pit cells.
Mucous metaplasia were further analysed histochemically. Alcianophilia indicative of SPEM metaplasia was absent after IL-11 treatment, but glandular PAS-staining cells were progressively increased over 7 days (see supplementary figure 1A,B, available online only). Four weeks after the cessation of IL-11 treatment PAS-stained cells had returned to normal (see supplementary figure 1v, available online only). PAS-expressing cells were further characterised using MUC5AC or GSII. Both markers were strongly reduced after IL-11 treatment (see supplementary figure 1Ci–ii, Di–ii, available online only), as were mRNA for pit and mucous neck cells peptides, TFF1 and TFF2, respectively (see supplementary figure 1E, F, available online only).
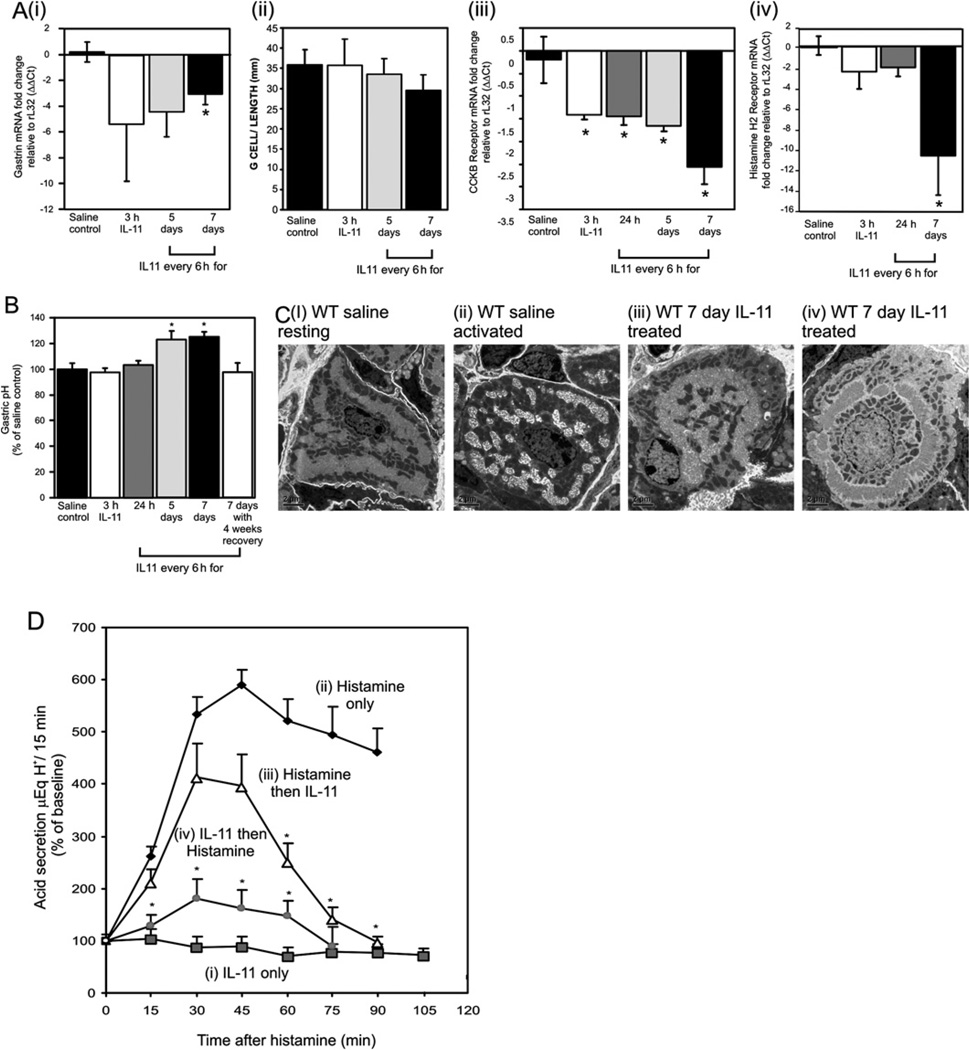
IL-11 inhibits endocrine regulators of parietal cell activation
As gastrin production is implicated in gastric damage, particularly involving parietal cell atrophy, gastrin mRNA was quantified after IL-11 treatment. Gastrin mRNA was reduced 3 h after IL-11 exposure and this effect was sustained at 7 days (figure 4Ai). Despite this decrease, the number of gastrin-expressing G cells was unchanged (figure 4Aii). IL-11 also caused an immediate and sustained reduction in the gastrin receptor CCKBR (figure 4Aiii) and the histamine H2 receptor (figure 4Aiv). Reduction of gastrin and CCKB receptor mRNA levels preceded any changes in gastric pH and fundic atrophy, suggesting that while IL-11 may be responsible for changes in gastrin and CCKB receptor, epithelial proliferation resulting from IL-11 treatment is gastrin independent.
Figure 4.
mRNA levels of antral gastrin (Ai) and fundic cholecystokinin B (CCKB) receptor (Aiii) and histamine H2 receptor (Aiv) were measured following IL-11 or saline treatment by quantitative PCR, standardised against rL32 and expressed as fold change compared with controls (ΔΔCt method). G cells (Aii) were localised in control mice and treatment groups. Stained cells/mm were quantified. Gastric content pH was measured from wild-type (WT) mice treated with IL-11 or saline (B). Electron microscopic analysis of parietal cells from saline (Ci–ii) or IL-11-treated mice (Ciii–iv). Gastric acid secretion (D) was measured at 15 min intervals in mice treated with (i) IL-11, (ii) histamine, (ii) histamine plus IL-11, 15 min later (iii) IL-11 plus histamine 15 min later. Acid secretion was expressed as percentage change from baseline. Bars refer to mean±SEM; *p≤0.05.
IL-11 induces morphological changes to parietal cells and blocks acid secretion
A consequence of IL-11-induced parietal cell atrophy was a reversible and time-dependent decrease of 20% in basal acid secretion in vivo comparedwith controls (figure 4B). IL-11-induced morphological changes to parietal cells were further analysed in vitro by acute IL-11 treatment and electron microscopy. The membranes of parietal cells have two morphological conformations depending on whether they are secreting acid. In the resting state the membranes resemble tubulovesicles and in the activated state they resemble an open canalicular structure. Saline-treated mice had parietal cells with membranes in both conformations (figure 4Ci and ii). In contrast, parietal cells after IL-11 treatment were less numerous with atypical morphology. The membranes either resembled resting parietal cells but with circumferential tubulovesicle-type membrane structures around the nucleus (figure 4Ciii), or parallel tubules forming a very defined nuclear ring structure (figure 4Civ). The lack of electron density of this cell population suggested early senescence (figure 4Civ).
To confirm that IL-11 was able to influence acid secretion and that the increased pH of the IL-11-treated stomach did not result from parietal cell atrophy, we measured gastric acid output directly in WT mice after a single dose of IL-11 (figure 4D). The acid secretagogue histamine induced acid secretion that was maximal at 45 min and IL-11 given alone did not alter basal acid secretion. Strikingly, IL-11 administration 15 min after histamine impaired the response by approximately 30%, while IL-11 given 15 min before histamine reduced acid secretion by more than 70% demonstrating that IL-11 can directly inhibit histamine-induced acid secretion.
IL-11 alters expression of fundic ion transporters with potential implications for acid secretion
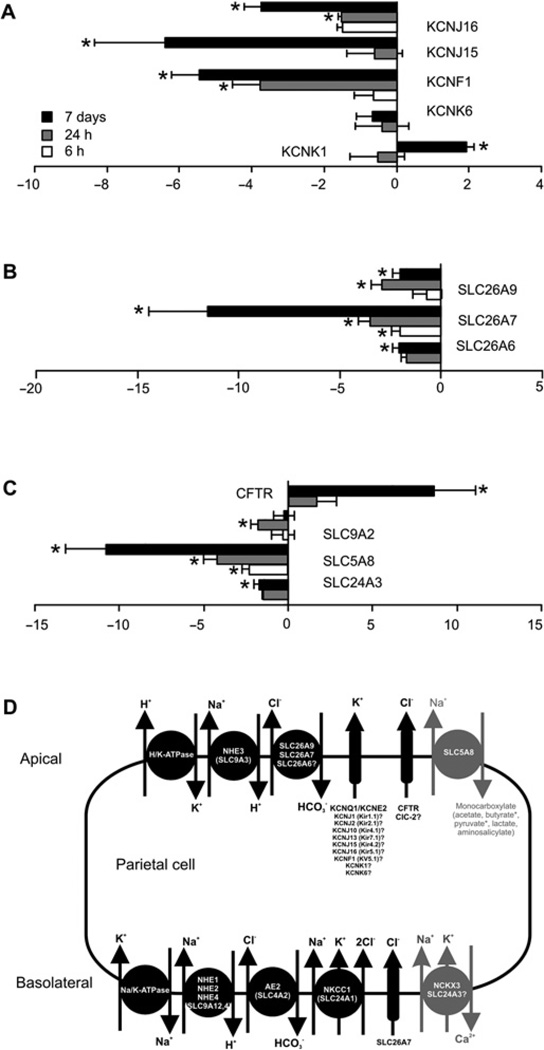
Microarray analysis of IL-11-treated stomach showed altered expression of numerous genes involved in ion transport (table 1). After IL-11 administration a subset of mRNA corresponding to genes involved in potassium (figure 5A), sodium/bicarbonate (figure 5B) or other ion transport (figure 5C) were significantly decreased. The exceptions were the potassium channel subunit, KCNK1, and the chloride channel, CFTR, both of which increased in expression (figure 5A,C). Many of these ion transporters are required for parietal cell-mediated acid secretion; SLC26A7, SLC26A9 and SLC9A2 or have activities that implicate roles in this process; SLC26A7, SLC24A3, CFTR, KCNJ16, KCNJ15, KCNF1, KCNK6 and KCNK1 (figure 5D) suggesting a mechanism by which IL-11 can block acid secretion.
Figure 5.
mRNA expression of potassium channels (KCN) (A), sodium-bicarbonate transporters (SLC26) (B) and miscellaneous transporters (C) were measured in the fundus of mice treated with IL-11 by quantitative PCR, standardised against rL32 and expressed as fold change compared with control mice (ΔΔCt method). Model of ion transport in the parietal cell (D). Bars refer to mean±SEM; *p≤0.05.
IL-11 induces an inflammatory response in the fundic mucosa
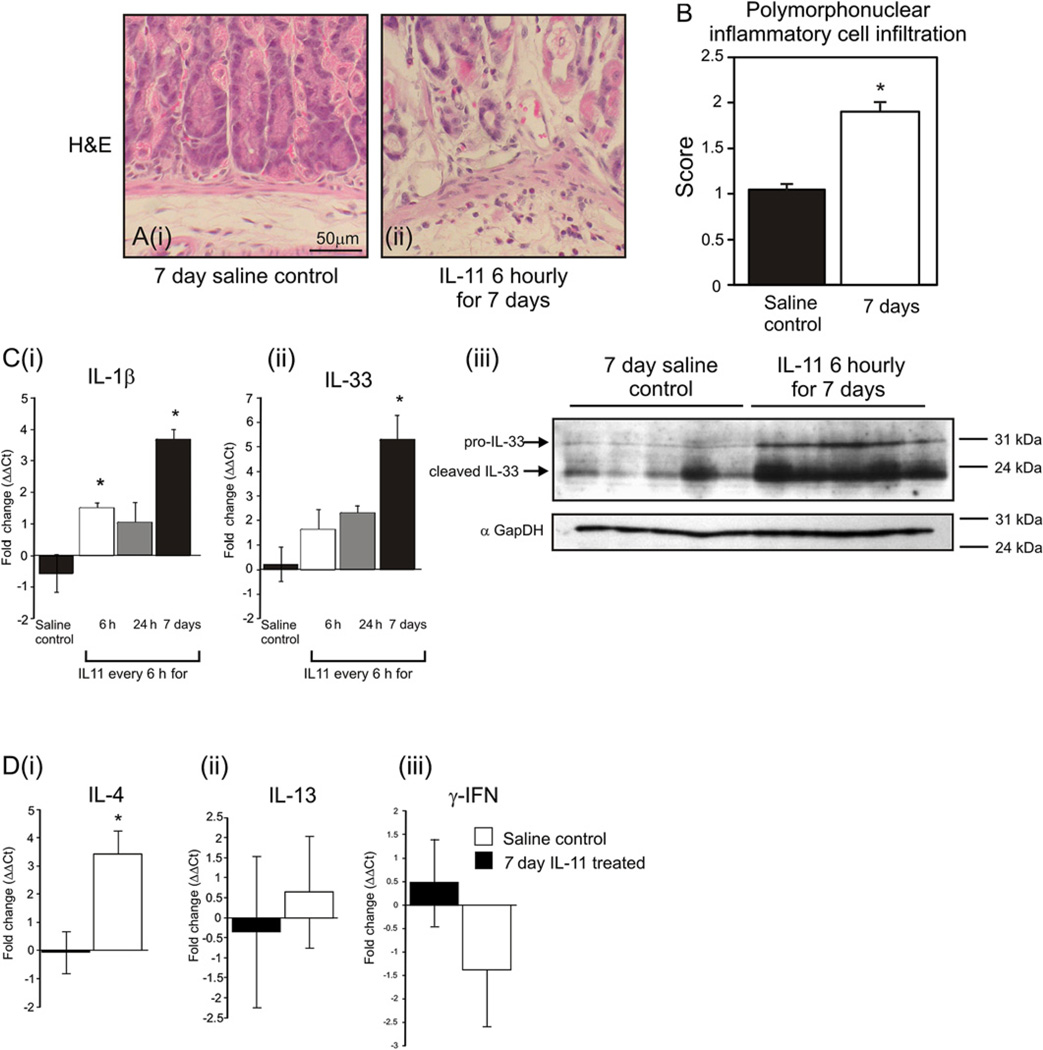
IL-11 treatment elicited a twofold increase in poly-morphonuclear cell infiltrate (figure 6A,B), but did not alter lymphoplasmocytic infiltrate. Coincident increases were observed in the expression of the pro-inflammatory cytokines IL-1β mRNA and IL-33 mRNA and protein (figure 6C). IL-33 can regulate Th1/Th2 cytokine balance in epithelia,40 and coincident with IL-11-induced IL-33 expression, IL-4 mRNA, but not IL-13 or γ-IFN was increased (figure 6Di–iii).
Figure 6.
Polymorphonuclear cells were quantitated on stomach sections of mice treated with saline (Ai) or IL-11 (Aii) (B). IL-1β (Ci) and IL-33 (Cii) mRNA from gastric fundus of mice treated with IL-11 by quantitative PCR, standardised against the rL32 and expressed as fold change compared with controls (ΔΔCt method). IL-33 (Ciii) protein measured by immunoblotting and compared with GAPDH. mRNA levels of IL-4 (Di), IL-13 (Dii) and IFNγ (Diii) were measured as above. Bars refer to mean±SEM; *p≤0.05.
DISCUSSION
Chronic atrophic gastritis induced by H pylori infection is a prerequisite for the development of gastric cancer.3,4,30,33,41–44 We demonstrated that chronic exposure of mice to exogenous IL-11 over 7 days, such that activated gastric STAT3 remains continuously elevated, in the absence of H pylori or any other mitigating factors, caused pathological changes that accurately recapitulate human chronic atrophic gastritis, with progressive parietal and chief cell loss, focal mucosal inflammation, increased proliferation, achlorhydria and the development of immature mucous cell metaplasia.
A striking feature of the IL-11-induced atrophy is the reduction in parietal cell numbers as well as their abnormal phenotype and associated achlorhydria. Parietal cells are responsible for acidification of the gastric lumen via H/K-ATPase, localised to a complex intracellular membrane network.45,46 These membrane structures were profoundly perturbed following IL-11 treatment, such that tubulovesicular structures were reduced, and ordered canalicular structures predominated. These changes are reminiscent of Hip1r−/−,47 SLC26A7−/− 48 and SLC26A9−/− 49 mice in which acid secretion is strongly suppressed due to genetic ablation of key ion transporters or structural proteins. IL-11 can alter the membrane potential difference of the colon and small intestine,50 and the expression of SLC26A6, 7, 9, KCNF1, KCNJ15, 16, SLC5A8, SLC24A3 and SLC9A2 was decreased following IL-11 treatment. SLC9A2,51 SLC26A748 and SLC26A949 are absolutely required for acid secretion while the other ion transporters are implicated. Therefore, we suggest that IL-11 directly regulates the transcription of ion transport genes, which leads to disordered parietal cell intracellular membrane structures and their reduced capacity to acidify the gastric lumen. Interestingly, despite parietal cell loss, IL-11 does not regulate HK-ATPase expression, demonstrating both its transcriptional specificity and that observed gene changes are independent of parietal cell loss.
Gastric acid secretion is regulated physiologically by hormonal (gastrin),45,52 local53 regulatory (histamine)45 and neuronal (acetylcholine) feedback circuits.45 Gastrin regulates acid secretion both directly via the gastrin (CCKB) receptor, and indirectly by the release of histamine.52 We demonstrate for the first time that a single dose of IL-11 can inhibit gastric acid secretion. Moreover, IL-11 treatment immediately decreased gastrin, CCKB and histamine H2 receptor mRNA in a time-dependent fashion, suggesting that IL-11 inhibits acid secretion both by ion channel, and gastrin and H2 receptor inhibition.
The IL-11-mediated changes in gene expression are not due to acid feedback inhibition because luminal pH was unchanged for 24 h and the mRNA changes were observed after 3 h. These data suggest that IL-11 regulation of acid secretion and cell proliferation are mediated through the inhibition of gastrin-dependent and independent pathways.
Another striking feature of the IL-11-treated fundus was the loss of chief cells; this was accompanied by reduced expression of active protease products of chief cells54,55; trypsin 4/10, amylase 2, pancreatic lipase-related protein 1 and furin, whereas pepsinogen C expression and localisation of the intrinsic factor were unchanged. It is unclear from the present study whether chief cell loss was secondary to a reduction in parietal cells, or whether IL-11 acts directly on the chief cell. However, carbonic anhydrase IX-deficient mice, with altered gastric pH and marked chief cell loss,56,57 have reduced expression of digestive enzymes as well as Bhlhb8, a paralogue of Mist 1,58 and we have established that the transcription factor Mist1, which promotes chief cell differentiation,59 was negatively regulated by IL-11, suggesting a direct role.
IL-11-induced atrophy also resulted in increased proliferation of gastric epithelial cells after only 24 h of treatment. IL-11 has cytoprotective activity in the colon53,60 and mitogenic activity during gastric mucosal repair.24 Given that expression of the established gastric mitogen, gastrin, was reduced following IL-11 treatment, and proliferation was induced before parietal or chief cell depletion was evident, these data implicate IL-11 directly in inducing gastric cell proliferation. The majority of proliferative cells in IL-11-treated fundus contained mucous granules and there was an accumulation of undifferentiated cells with mucous type granules evident by electron microscopy. This suggests that cell differentiation was impaired, and newly dividing progenitor cells were accumulating in an undifferentiated state in the presence of high IL-11. This was confirmed by the reduced expression of mature mucous cell markers GSII, Muc5AC and TFF2. Significantly, treatment of mice with IL-11 followed by a 4-week recovery period allowed the complete reconstitution of the gastric cell population, and baseline levels of proliferation were restored. This is consistent with other models of acute gastric parietal cell atrophy,61 and demonstrates that while high levels of IL-11 can alter proliferation and differentiation programmes, stem cells are not lost following treatment.
In spite of the clear atrophy, especially loss of parietal and zymogenic cells, which occurs following IL-11 treatment, we did not observe intestinalisation of the gastric mucosa, a hallmark of human atrophic gastritis. However, it is worth noting that only under very exceptional circumstances do mice develop true intestinalisation including goblet cell development, the best described being mice with ectopic expression of Cdx2.62 Clearly, the factors required to cause intestinalisation in humans do not occur in murine models and this model of atrophic gastritis is no exception.
Coincident with IL-11-induced gastric atrophy was a modest influx of polymorphonuclear cells, accompanied by elevated expression of IL-1β and IL-33. IL-33, a member of the IL-1 family, can drive epithelial Th2 responses that maintain the balance between host immune homeostasis and pathogen defence.63 IL-1β regulates expression of the HK-ATPaseα subunit64 and inhibits gastrin-dependent acid secretion.65 IL-1β polymorphisms alter gastro-oesophageal reflux disease susceptibility, 9,66 and gastric-specific transgenic IL-1β overexpression induces spontaneous gastric inflammation, atrophy and cancer.67,68 Moreover, we show that both IL-11 and IL-1β are temporally induced by H pylori SS1 in mice, coincident with the previously reported induction of chronic inflammation and atrophy, but before significant dysplasia and carcinoma.69 Here we show that in the gastric mucosa the expression of IL-33 and IL-1 β is increased with IL-11 treatment, suggesting that pathological outcomes arising from chronic H pylori infection may be due to both direct IL-11 action and indirect action via IL-1β induction.
In general, IL-11 is considered to have anti-inflammatory actions, for which it has been considered as a potential therapeutic agent in a number of immune disorders.60,70–77 Here, in the fundic stomach we have shown that IL-11 is pro-inflammatory and in the antral mucosa IL-11 is required to initiate inflammatory tumorigenesis.17 Our data argue that a link between IL-11, IL-33 and IL-1β is crucial in mediating the gastric mucosal response to H pylori infection, perhaps by skewing the mucosal immunity response towards a Th2 bias, which would be less effective at clearing infection. We hypothesise that the stomach as the primary line of innate defence to ingested pathogens utilises IL-11 as part of its defence mechanism.
We have demonstrated that IL-11 is a parietal cell cytokine that acts in an autocrine manner to regulate acid secretion, and as such can influence gastric epithelial cell homeostasis. We have further demonstrated that IL-11 is a key cytokine mediating epithelial cell proliferation and inflammatory responses in the gastric fundic mucosa. Increased exposure to IL-11 both increases the luminal pH and promotes a Th2-biased immune response. Novel therapies that specifically block the IL-11 response may have utility in mucosal clearance of H pylori by facilitating a Th1 response and in preventing the development of atrophic gastritis.
Supplementary Material
Significance of this study.
What is already known about this subject?
-
►
IL-11 is a pleiotrophic cytokine with known anti-inflammatory actions.
-
►
Expression of IL-11 mRNA and protein is increased in human gastric precancer and cancer, and in mouse models of gastric damage including atrophic gastritis and cancer.
-
►
IL-11 is protective in mouse models of experimental colitis.
What are the new findings?
-
►
IL-11 is upregulated in chronic murine H pylori infection with temporal expression similar to IL-1β.
-
►
IL-11 is expressed by the acid-secreting parietal cells of the normal human and mouse stomach.
-
►
Acute administration of IL-11 causes activation of the downstream transcription factor STAT3 in parietal cells, demonstrating that these cells are most responsive to IL-11.
-
►
IL-11 causes inhibition of histamine stimulated acid secretion through reduced expression of associated ion transporters.
-
►
Chronic administration of IL-11 results in reversible atrophic gastritis characterised by a loss of parietal and chief cells, mucous cell metaplasia, epithelial cell proliferation and Th2 skewed inflammatory response.
How might it impact on clinical practice in the foreseeable future?
-
►
Here we demonstrate that IL-11 can cause atrophic gastritis, as such it could be used as a diagnostic marker in humans to indicate progression to H pylori-induced intestinal meta-plasia, an early marker of progression to cancer.
-
►
Understanding the signalling pathways down-stream of IL-11 and the mechanisms of action could lead to more targeted therapies to halt the progression from atrophic gastritis to gastric cancer.
Acknowledgments
Funding This study was supported by the Victorian Government’s Operational Infrastructure Support Program and NH&MRC Australia #APP1008776.
Footnotes
Additional supplementary materials are published online only. To view these files please visit the journal online (http://gut.bmj.com/content/early/recent).
Competing interests None.
Contributors MH, TRM, ASG and LMJ conceived and designed the experiments; MH, JNB, HVC, NN and LMJ performed the experiments; MH, KMB and LMJ analysed the data; IRvD, JGF and ED contributed reagents/materials; MH, LMJ and ASG wrote the paper.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Shibuya K, Mathers CD, Boschi-Pinto C, et al. Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer. 2002;2:37. doi: 10.1186/1471-2407-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verdecchia A, Mariotto A, Gatta G, et al. Comparison of stomach cancer incidence and survival in four continents. Eur J Cancer. 2003;39:1603–1609. doi: 10.1016/s0959-8049(03)00360-5. [DOI] [PubMed] [Google Scholar]
- 3.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Correa P. The biological model of gastric carcinogenesis. IARC Sci Publ. 2004;157:301–310. [PubMed] [Google Scholar]
- 5.Kim TY, Lee HJ, Hwang KS, et al. Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Lab Invest. 2004;84:479–484. doi: 10.1038/labinvest.3700060. [DOI] [PubMed] [Google Scholar]
- 6.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 7.Gatti LL, Burbano RR, Zambaldi-Tunes M, et al. Interleukin-6 polymorphisms, Helicobacter pylori infection in adult Brazilian patients with chronic gastritis and gastric adenocarcinoma. Arch Med Res. 2007;38:551–555. doi: 10.1016/j.arcmed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Hwang IR, Hsu PI, Peterson LE, et al. Interleukin-6 genetic polymorphisms are not related to Helicobacter pylori-associated gastroduodenal diseases. Helicobacter. 2003;8:142–148. doi: 10.1046/j.1523-5378.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 9.Hwang IR, Kodama T, Kikuchi S, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793–1803. doi: 10.1053/gast.2002.37043. [DOI] [PubMed] [Google Scholar]
- 10.Kamangar F, Abnet CC, Hutchinson AA, et al. Polymorphisms in inflammation-related genes and risk of gastric cancer (Finland) Cancer Causes Control. 2006;17:117–125. doi: 10.1007/s10552-005-0439-7. [DOI] [PubMed] [Google Scholar]
- 11.Fox JG, Dangler CA, Taylor NS, et al. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59:4823–4828. [PubMed] [Google Scholar]
- 12.Nozaki K, Shimizu N, Inada K, et al. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res. 2002;93:1083–1089. doi: 10.1111/j.1349-7006.2002.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nandurkar HH, Robb L, Tarlinton D, et al. Adult mice with targeted mutation of the interleukin-11 receptor (IL11Ra) display normal hematopoiesis. Blood. 1997;90:2148–2159. [PubMed] [Google Scholar]
- 14.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo K, Oka M, Murase K, et al. Expression of interleukin 6 and its receptor in human gastric and colorectal cancers. J Int Med Res. 2003;31:69–75. doi: 10.1177/147323000303100202. [DOI] [PubMed] [Google Scholar]
- 16.Howlett M, Menheniott TR, Judd LM, et al. Cytokine signalling via gp130 in gastric cancer. Biochim Biophys Acta. 2009;1793:1623–1633. doi: 10.1016/j.bbamcr.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Howlett M, Giraud AS, Lescesen H, et al. The interleukin-6 family cytokine interleukin-11 regulates homeostatic epithelial cell turnover and promotes gastric tumor development. Gastroenterology. 2009;136:967–977. doi: 10.1053/j.gastro.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Jackson CB, Judd LM, Menheniott TR, et al. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J Pathol. 2007;213:140–151. doi: 10.1002/path.2218. [DOI] [PubMed] [Google Scholar]
- 19.Ellmark P, Ingvarsson J, Carlsson A, et al. Identification of protein expression signatures associated with Helicobacter pylori infection and gastric adenocarcinoma using recombinant antibody microarrays. Mol Cell Proteomics. 2006;5:1638–1646. doi: 10.1074/mcp.M600170-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama T, Yoshizaki A, Izumida S, et al. Expression of interleukin-11 (IL-11) and IL-11 receptor alpha in human gastric carcinoma and IL-11 upregulates the invasive activity of human gastric carcinoma cells. Int J Oncol. 2007;30:825–833. [PubMed] [Google Scholar]
- 21.Yakata Y, Nakayama T, Yoshizaki A, et al. Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis. Int J Oncol. 2007;30:437–442. [PubMed] [Google Scholar]
- 22.Bravo J, Heath JK. Receptor recognition by gp130 cytokines. EMBO J. 2000;19:2399–2411. doi: 10.1093/emboj/19.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 2004;20:23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Judd LM, Ulaganathan M, Howlett M, et al. Cytokine signalling by gp130 regulates gastric mucosal healing after ulceration and, indirectly, antral tumour progression. J Pathol. 2009;217:552–562. doi: 10.1002/path.2479. [DOI] [PubMed] [Google Scholar]
- 25.Howlett M, Judd LM, Jenkins B, et al. Differential regulation of gastric tumor growth by cytokines that signal exclusively through the coreceptor gp130. Gastroenterology. 2005;129:1005–1018. doi: 10.1053/j.gastro.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 26.Ernst M, Najdovska M, Grail D, et al. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–1738. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst M, Inglese M, Waring P, et al. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194:189–203. doi: 10.1084/jem.194.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Judd LM, Alderman BM, Howlett M, et al. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology. 2004;126:196–207. doi: 10.1053/j.gastro.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 29.Franic TV, van Driel IR, Gleeson PA, et al. Reciprocal changes in trefoil 1 and 2 expression in stomachs of mice with gastric unit hypertrophy and inflammation. J Pathol. 2005;207:43–52. doi: 10.1002/path.1811. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson NK, Karkkainen PA, Farkkila MA, et al. Prevalence and distribution of gastric intestinal metaplasia and its subtypes. Dig Liver Dis. 2008;40:355–360. doi: 10.1016/j.dld.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Lu B, Chen MT, Fan YH, et al. Effects of Helicobacter pylori eradication on atrophic gastritis and intestinal metaplasia: a 3-year follow-up study. World J Gastroenterol. 2005;11:6518–6520. doi: 10.3748/wjg.v11.i41.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh JD, Kling-Backhed H, Giannakis M, et al. Interactions between gastric epithelial stem cells and Helicobacter pylori in the setting of chronic atrophic gastritis. Curr Opin Microbiol. 2006;9:21–27. doi: 10.1016/j.mib.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Sepulveda AR, Patil M. Practical approach to the pathologic diagnosis of gastritis. Arch Pathol Lab Med. 2008;132:1586–1593. doi: 10.5858/2008-132-1586-PATTPD. [DOI] [PubMed] [Google Scholar]
- 34.Scarff KL, Judd LM, Toh BH, et al. Gastric H(+), K(+)-adenosine triphosphatase beta subunit is required for normal function, development, and membrane structure of mouse parietal cells. Gastroenterology. 1999;117:605–618. doi: 10.1016/s0016-5085(99)70453-1. [DOI] [PubMed] [Google Scholar]
- 35.Giraud AS, Jackson C, Menheniott TR, et al. Differentiation of the Gastric Mucosa IV. Role of trefoil peptides and IL-6 cytokine family signaling in gastric homeostasis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1–G5. doi: 10.1152/ajpgi.00382.2006. [DOI] [PubMed] [Google Scholar]
- 36.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 37.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article 3. [DOI] [PubMed] [Google Scholar]
- 38.Beissbarth T, Speed TP. GOstat: find statistically overrepresented Gene Ontologies within a group of genes. Bioinformatics. 2004;20:1464–1465. doi: 10.1093/bioinformatics/bth088. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen NV, Gleeson PA, Courtois-Coutry N, et al. Gastric parietal cell acid secretion in mice can be regulated independently of H/K ATPase endocytosis. Gastroenterology. 2004;127:145–154. doi: 10.1053/j.gastro.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Pastorelli L, Garg RR, Hoang SB, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A. 2010;107:8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crowe SE. Helicobacter infection, chronic inflammation, and the development of malignancy. Curr Opin Gastroenterol. 2005;21:32–38. [PubMed] [Google Scholar]
- 42.Holmes K, Egan B, Swan N, et al. Genetic mechanisms and aberrant gene expression during the development of gastric intestinal metaplasia and adenocarcinoma. Curr Genomics. 2007;8:379–397. doi: 10.2174/138920207783406460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machado JC, Figueiredo C, Canedo P, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364–371. doi: 10.1016/s0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- 44.Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–248. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 45.Schubert ML. Gastric exocrine and endocrine secretion. Curr Opin Gastroenterol. 2009;25:529–536. doi: 10.1097/MOG.0b013e328331b62a. [DOI] [PubMed] [Google Scholar]
- 46.Miller ML, Judd LM, Van Driel IR, et al. The unique ultrastructure of secretory membranes in gastric parietal cells depends upon the presence of H+, K+ -ATPase. Cell Tissue Res. 2002;309:369–380. doi: 10.1007/s00441-002-0606-z. [DOI] [PubMed] [Google Scholar]
- 47.Jain RN, Al-Menhali AA, Keeley TM, et al. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J Clin Invest. 2008;118:2459–2470. doi: 10.1172/JCI33569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Song P, Nakamura S, et al. Deletion of the chloride transporter slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion. J Biol Chem. 2009;284:29470–29479. doi: 10.1074/jbc.M109.044396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J, Song P, Miller ML, et al. Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach. Proc Natl Acad Sci U S A. 2008;105:17955–17960. doi: 10.1073/pnas.0800616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greenwood-Van Meerveld B, Tyler K, Keith JC., Jr Recombinant human interleukin-11 modulates ion transport and mucosal inflammation in the small intestine and colon. Lab Invest. 2000;80:1269–1280. doi: 10.1038/labinvest.3780135. [DOI] [PubMed] [Google Scholar]
- 51.Schultheis PJ, Clarke LL, Meneton P, et al. Targeted disruption of the murine Na +/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest. 1998;101:1243–1253. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dockray GJ, Varro A, Dimaline R, et al. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 53.Du X, Liu Q, Yang Z, et al. Protective effects of interleukin-11 in a murine model of ischemic bowel necrosis. Am J Physiol. 1997;272:G545–G552. doi: 10.1152/ajpgi.1997.272.3.G545. [DOI] [PubMed] [Google Scholar]
- 54.Davis BP, Hammer RE, Messing A, et al. Selective expression of trypsin fusion genes in acinar cells of the pancreas and stomach of transgenic mice. J Biol Chem. 1992;267:26070–26077. [PubMed] [Google Scholar]
- 55.Fujisawa T, Kamimura H, Hosaka M, et al. Functional localization of proprotein-convertase furin and its substrate TGFbeta in EGF receptor-expressing gastric chief cells. Growth Factors. 2004;22:51–59. doi: 10.1080/0897719042000201659. [DOI] [PubMed] [Google Scholar]
- 56.Gut MO, Parkkila S, Vernerova Z, et al. Gastric hyperplasia in mice with targeted disruption of the carbonic anhydrase gene Car9. Gastroenterology. 2002;123:1889–1903. doi: 10.1053/gast.2002.37052. [DOI] [PubMed] [Google Scholar]
- 57.Leppilampi M, Karttunen TJ, Kivela J, et al. Gastric pit cell hyperplasia and glandular atrophy in carbonic anhydrase IX knockout mice: studies on two strains C57/BL6 and BALB/C. Transgenic Res. 2005;14:655–663. doi: 10.1007/s11248-005-7215-z. [DOI] [PubMed] [Google Scholar]
- 58.Kallio H, Hilvo M, Rodriguez A, et al. Global transcriptional response to carbonic anhydrase IX deficiency in the mouse stomach. BMC Genomics. 2010;11:397. doi: 10.1186/1471-2164-11-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramsey VG, Doherty JM, Chen CC, et al. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 60.Orazi A, Du X, Yang Z, et al. Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab Invest. 1996;75:33–42. [PubMed] [Google Scholar]
- 61.Goldenring JR, Ray GS, Coffey RJ, et al. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 62.Silberg DG, Sullivan J, Kang E, et al. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 63.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2- associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Saha A, Hammond CE, Gooz M, et al. The role of Sp1 in IL-1beta and H. pylori-mediated regulation of H, K-ATPase gene transcription. Am J Physiol Gastrointest Liver Physiol. 2008;295:G977–G986. doi: 10.1152/ajpgi.90338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallace JL, Cucala M, Mugridge K, et al. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol. 1991;261:G559–G564. doi: 10.1152/ajpgi.1991.261.4.G559. [DOI] [PubMed] [Google Scholar]
- 66.Chourasia D, Achyut BR, Tripathi S, et al. Genotypic and functional roles of IL-1B and IL-1RN on the risk of gastroesophageal reflux disease: the presence of IL-1B-511*T/IL-1RN*1 (T1) haplotype may protect against the disease. Am J Gastroenterol. 2009;104:2704–2713. doi: 10.1038/ajg.2009.382. [DOI] [PubMed] [Google Scholar]
- 67.Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waghray M, Zavros Y, Saqui-Salces M, et al. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology. 2010;138:562–572. 572.e1–572.e2. doi: 10.1053/j.gastro.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fox JG, Wang TC, Rogers AB, et al. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 70.Heinrich PC, Behrmann I, Muller-Newen G, et al. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hermann JA, Hall MA, Maini RN, et al. Important immunoregulatory role of interleukin-11 in the inflammatory process in rheumatoid arthritis. Arthritis Rheum. 1998;41:1388–1397. doi: 10.1002/1529-0131(199808)41:8<1388::AID-ART7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 72.Keith JC, Jr, Albert L, Sonis ST, et al. IL-11, a pleiotropic cytokine: exciting new effects of IL-11 on gastrointestinal mucosal biology. Stem Cells. 1994;(12 Suppl 1):79–89. discussion 89–90. [PubMed] [Google Scholar]
- 73.Lgssiar A, Hassan M, Schott-Ohly P, et al. Interleukin-11 inhibits NF-kappaB and AP-1 activation in islets and prevents diabetes induced with streptozotocin in mice. Exp Biol Med (Maywood) 2004;229:425–436. doi: 10.1177/153537020422900511. [DOI] [PubMed] [Google Scholar]
- 74.Nicoletti F, Zaccone P, Conget I, et al. Early prophylaxis with recombinant human interleukin-11 prevents spontaneous diabetes in NOD mice. Diabetes. 1999;48:2333–2339. doi: 10.2337/diabetes.48.12.2333. [DOI] [PubMed] [Google Scholar]
- 75.Opal SM, Keith JC, Jr, Jhung J, et al. Orally administered recombinant human interleukin-11 is protective in experimental neutropenic sepsis. J Infect Dis. 2003;187:70–76. doi: 10.1086/345864. [DOI] [PubMed] [Google Scholar]
- 76.Ropeleski MJ, Tang J, Walsh-Reitz MM, et al. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology. 2003;124:1358–1368. doi: 10.1016/s0016-5085(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 77.Trepicchio WL, Bozza M, Pedneault G, et al. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996;157:3627–3634. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.