Abstract
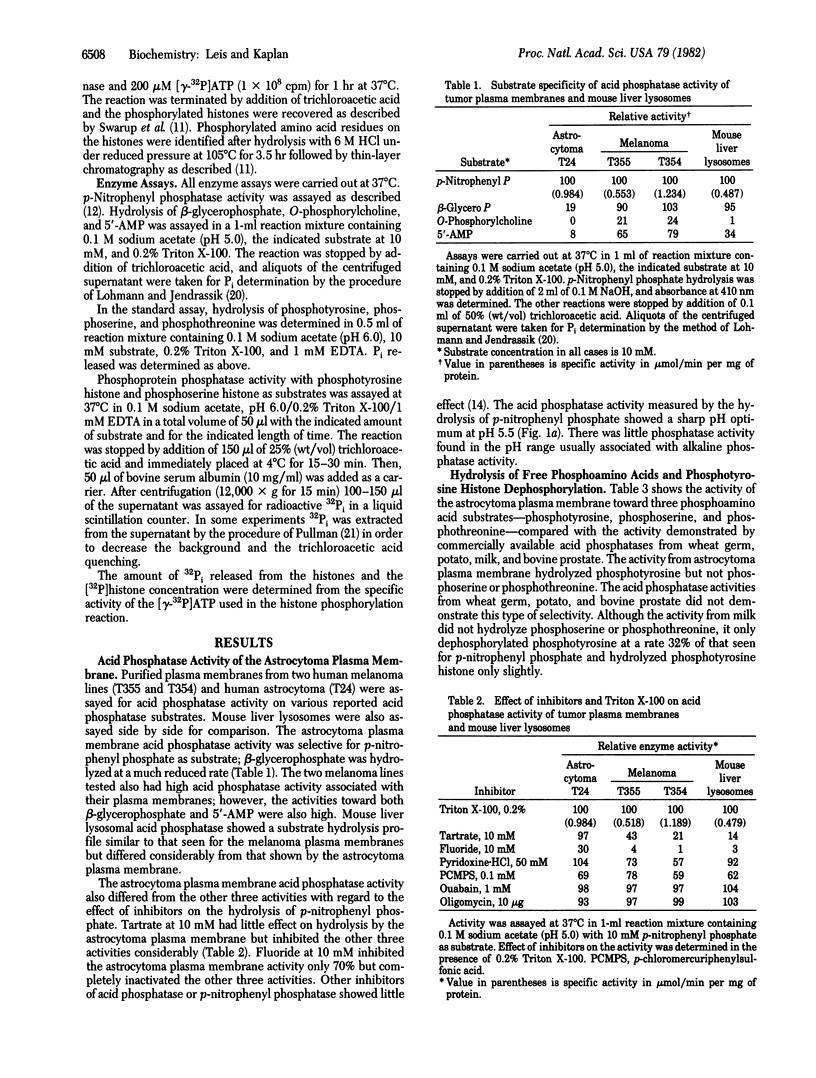
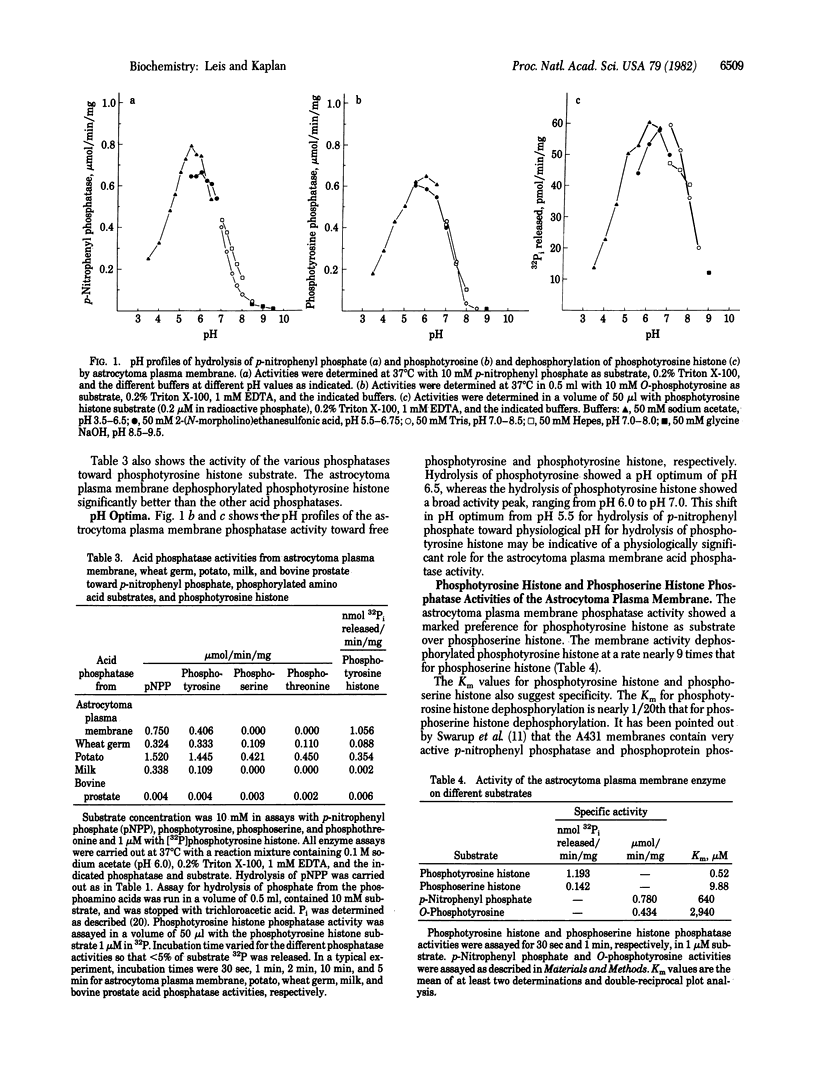
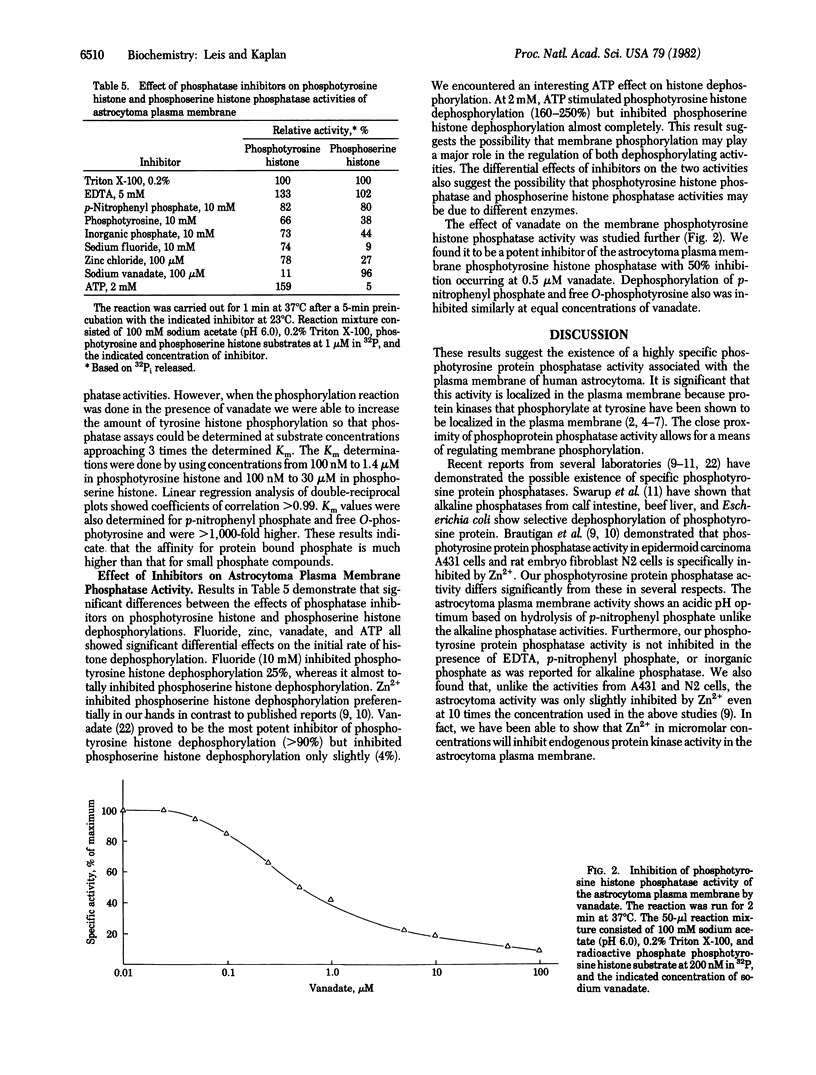
The plasma membrane from the human tumor astrocytoma contains an active acid phosphatase activity based on hydrolysis of p-nitrophenyl phosphate. Other acid phosphatase substrates--beta-glycerophosphate, O-phosphorylcholine, and 5'-AMP--are not hydrolyzed significantly. The phosphatase activity is tartrate insensitive and is stimulated by Triton X-100 and EDTA. Of the three known phosphoamino acids, only free O-phosphotyrosine is hydrolyzed by the membrane phosphatase activity. Other acid phosphatases tested from potato, wheat germ, milk, and bovine prostate did not show this degree of specificity. The plasma membrane activity also dephosphorylated phosphotyrosine histone at a much greater rate than did the other acid phosphatases. pH profiles for free O-phosphotyrosine and phosphotyrosine histone showed a shift toward physiological pH, indicating possible physiological significance. Phosphotyrosine histone dephosphorylation activity was nearly 10 times greater than that seen for phosphoserine histone dephosphorylation, and Km values were much lower for phosphotyrosine histone dephosphorylation (0.5 microM vs. 10 microM). Fluoride and zinc significantly inhibited phosphoserine histone dephosphorylation. Vanadate, on the other hand, was a potent inhibitor of phosphotyrosine histone dephosphorylation (50% inhibition at 0.5 microM) but not of phosphoserine histone. ATP stimulated phosphotyrosine histone dephosphorylation (160-250%) but inhibited phosphoserine histone dephosphorylation (95%). These results suggest the existence of a highly specific phosphotyrosine protein phosphatase activity associated with the plasma membrane of human astrocytoma.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Brautigan D. L., Bornstein P., Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J Biol Chem. 1981 Jul 10;256(13):6519–6522. [PubMed] [Google Scholar]
- Brockes J. P., Lemke G. E., Balzer D. R., Jr Purification and preliminary characterization of a glial growth factor from the bovine pituitary. J Biol Chem. 1980 Sep 25;255(18):8374–8377. [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Rapid enhancement of protein phosphorylation in A-431 cell membrane preparations by epidermal growth factor. J Biol Chem. 1979 Jun 10;254(11):4884–4891. [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Four different classes of retroviruses induce phosphorylation of tyrosines present in similar cellular proteins. Mol Cell Biol. 1981 May;1(5):394–407. doi: 10.1128/mcb.1.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrota M., Hinton R. H., El-Aaser A. A., Fitzsimons T. R., Reid E. Membrane association may limit the use of acid phosphatase as a lysosomal marker [proceedings]. Biochem Soc Trans. 1978;6(1):291–293. doi: 10.1042/bst0060291. [DOI] [PubMed] [Google Scholar]
- Gallis B., Bornstein P., Brautigan D. L. Tyrosylprotein kinase and phosphatase activities in membrane vesicles from normal and Rous sarcoma virus-transformed rat cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6689–6693. doi: 10.1073/pnas.78.11.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson L., Cantley L. C., Jr Isolation of a potent (Na-K)ATPase inhibitor from striated muscle. Biochemistry. 1977 Oct 18;16(21):4572–4578. doi: 10.1021/bi00640a006. [DOI] [PubMed] [Google Scholar]
- Knowles A. F., Leis J. F., Kaplan N. O. Isolation and characterization of plasma membranes from transplantable human astrocytoma, oat cell carcinoma, and melanomas. Cancer Res. 1981 Oct;41(10):4031–4038. [PubMed] [Google Scholar]
- Li H. C., Hsiao K. J., Sampathkumar S. Characterization of a novel alkaline phosphatase activity which co-purifies with a phosphorylase (phosphoprotein) phosphatase of Mr = 35,000 cardiac muscle. J Biol Chem. 1979 May 10;254(9):3368–3374. [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Selective dephosphorylation of proteins containing phosphotyrosine by alkaline phosphatases. J Biol Chem. 1981 Aug 10;256(15):8197–8201. [PubMed] [Google Scholar]
- Swarup G., Speeg K. V., Jr, Cohen S., Garbers D. L. Phosphotyrosyl-protein phosphatase of TCRC-2 cells. J Biol Chem. 1982 Jul 10;257(13):7298–7301. [PubMed] [Google Scholar]
- Thom D., Powell A. J., Lloyd C. W., Rees D. A. Rapid isolation of plasma membranes in high yield from cultured fibroblasts. Biochem J. 1977 Nov 15;168(2):187–194. doi: 10.1042/bj1680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouet A. Isolation of modified liver lysosomes. Methods Enzymol. 1974;31:323–329. doi: 10.1016/0076-6879(74)31034-8. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]



