Abstract
Corticotropin-releasing hormone (CRH) is secreted under stress and regulates the hypothalamic-pituitary-adrenal (HPA) axis. However, CRH is also secreted outside the brain where it exerts pro-inflammatory effects through activation of mast cells, which are increasingly implicated in immunity and inflammation. Substance P (SP) is also involved in inflammatory diseases. Human LAD2 leukemic mast cells express only CRHR-1 mRNA weakly. Treatment of LAD2 cells with SP (0.5–2 µM) for 6 hr significantly increases CRHR-1 mRNA and protein expression. Addition of CRH (1 µM) to LAD2 cells “primed” with SP for 48 hr and then washed, induces synthesis and release of IL-8, tumor necrosis factor (TNF) and vascular endothelial growth factor (VEGF) 24 hr later. These effects are blocked by pretreatment with an NK-1 receptor antagonist. Treatment of LAD2 cells with CRH (1 µM) for 6 hr induces gene expression of NK-1 as compared to controls. However, repeated stimulation of mast cells with CRH (1 µM) leads to downregulation of CRHR-1 and upregulation in NK-1 gene expression. These results indicate that SP can stimulate mast cells and also increase expression of functional CRHR-1, while CRH induces NK-1 gene expression. These results may explain CRHR-1 and NK-1 expression in lesional skin of psoriatic patients.
Introduction
Mast cells are ubiquitous in the human body and are critical for allergic reactions (Theoharides and Kalogeromitros, 2006), but are also involved in innate (Abraham and St John, 2010) and acquired immunity (Galli et al., 2005) during which they secrete numerous vasoactive molecules, cytokines and proteases. Increasing evidence implicates mast cells in inflammatory diseases, especially those exacerbated by stress (Theoharides and Cochrane, 2004). Corticotropin-releasing hormone (CRH) is secreted under stress and activates the hypothalamic-pituitary-adrenal (HPA) axis (Chrousos, 1995). CRH, CRH receptors (CRHR), other neuropeptides such as substance P (SP), mast cells and other cells communicate as part of a local network mimicking the HPA axis in the skin (Slominski and Wortsman, 2000).
SP exerts its effects by binding to two specific G protein-coupled cell surface receptors, CRHR-1 and CRHR-2 (Chen et al., 1993;Liaw et al., 1996). Human mast cells express CRHR-1, stimulation of which by CRH leads to selective release of VEGF without degranulation from human mast cells (Cao et al., 2005).
SP was originally isolated and characterized from the brain (Carraway and Leeman, 1973), but is now known to be widely distributed in many tissues, and is induced in many inflammatory processes (O'Connor et al., 2004). SP was known to be a potent mast cell trigger of rodent mast cells (Fewtrell et al., 1982), but less was known of its effect on human mast cells. We have shown that SP induces release of vascular endothelial growth factor (VEGF) from human mast cells, an action augmented by IL-33 (Theoharides et al., 2010b).
Here we report that SP induces expression of functional CRHR-1, while CRH induces NK-1 gene expression on LAD2 human leukemic mast cells. Repeated stimulation of mast cells with CRH leads to downregulation of CRHR-1 and upregulation of NK-1 genes.
Results
Effect of SP on CRHR-1 expression on LAD2 cells
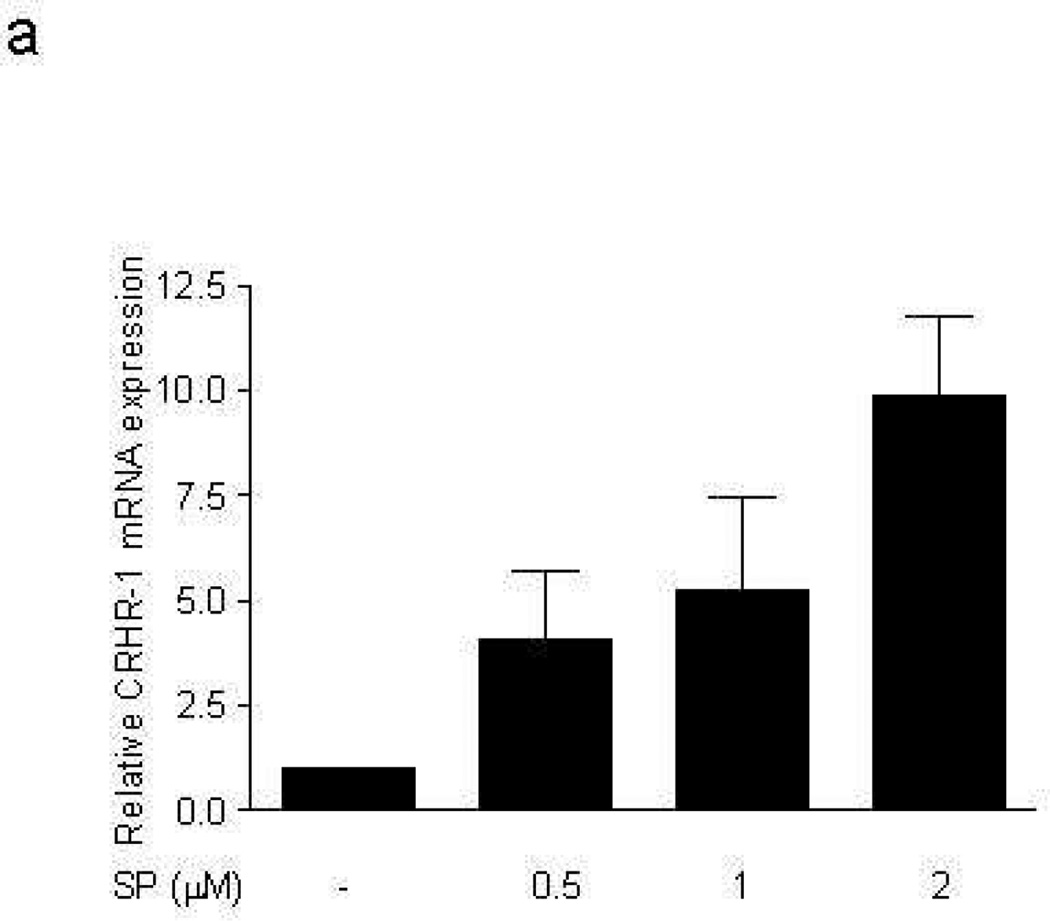
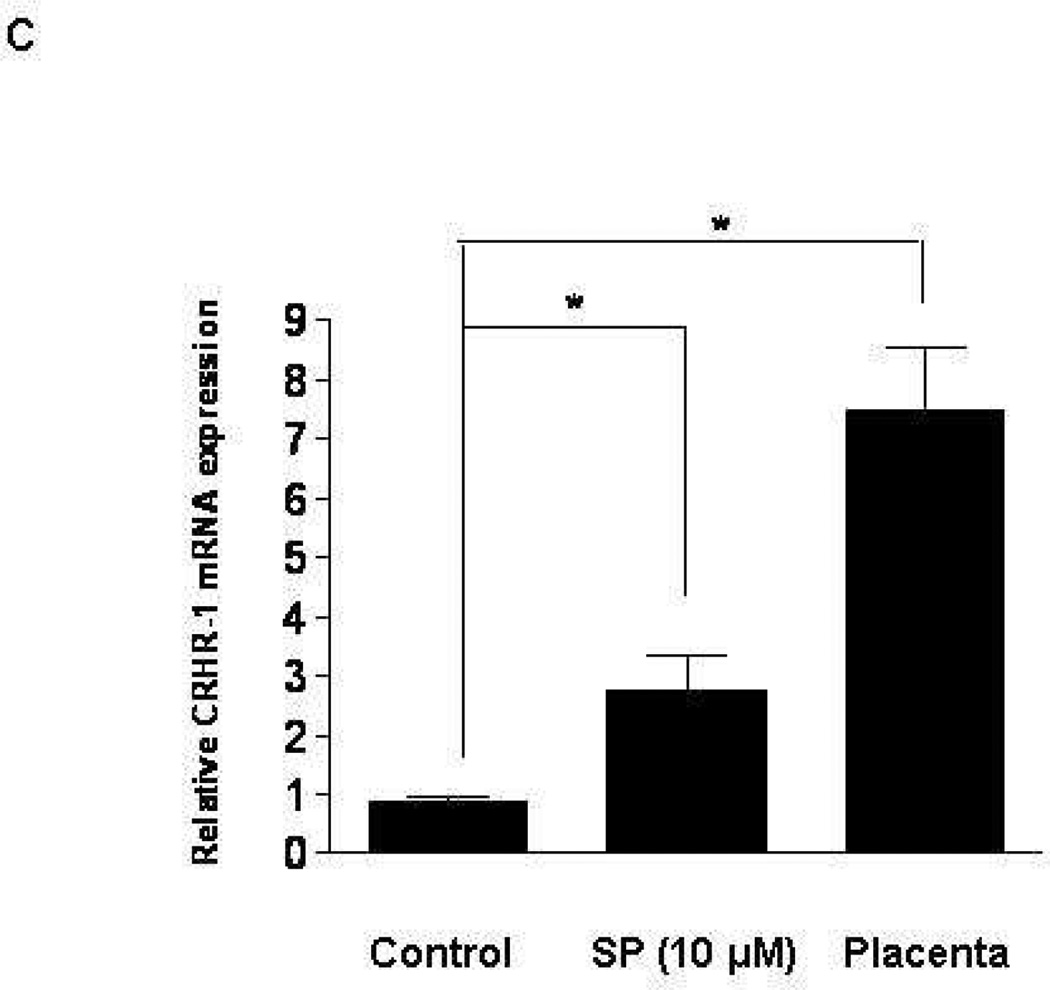
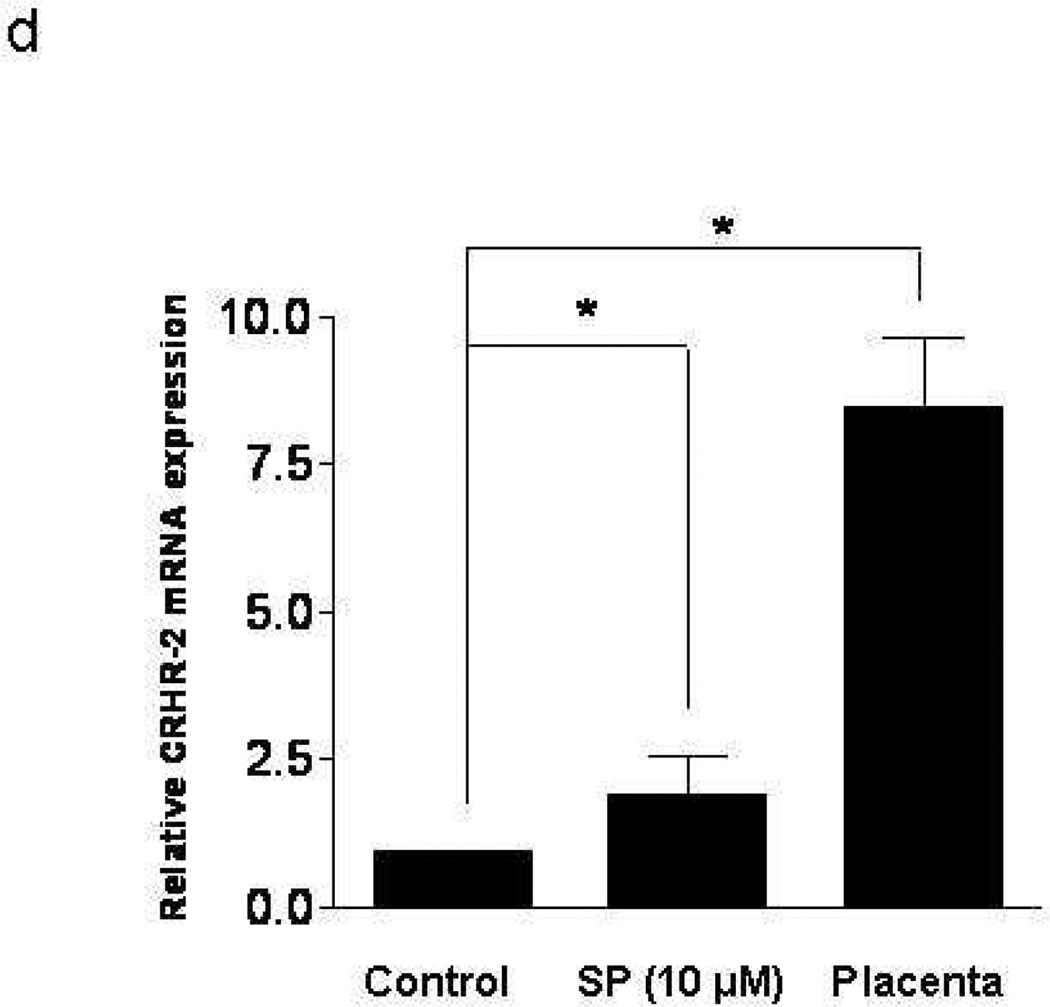
LAD2 mast cells show weak gene expression of CRHR-1, but CRHR-2 gene expression was bellow detection by the method used. Addition of SP (0.5, 1, 2 µM) to LAD2 cells for 6 hr leads to statistically significant increase in CRHR-1 mRNA (Fig. 1a). FACS analysis shows that 48 hr following treatment with SP (0.5, 1 µM) expression of CRHR-1 protein is also increased (Fig. 1b). In order to investigate if these findings are limited to the leukemic nature of LAD2 mast cells, we repeated some of the experiments using ten week-old hCBMCs. (Gene expression of CRHR-1> CRHR-2 is significantly induced (Fig. 1c,d) following 6 hr treatment with SP 10 µM, with human placenta used as a positive control because it expresses both CRHR-1 and CRHR-2). In contrast, human basophils do not express any CRHR (results not shown).
Figure 1. Effect of SP on CRHR-1 mRNA expression in human mast cells.
LAD2 cell expression of CRHR-1 (a) mRNA following treatment with SP (0.5, 1, 2 µM) for 6 hr and (b) protein following treatment with SP (0.5, 1 µM) for 48 hr; y-axis indicates “counts” of cells, while the x-axis indicates log fluorescence intensity (n=3, p<0.05). (c,d) Ten week-old hCBMCs expression of CRHR-1 and CRHR-2 mRNA, before and after 6 hr incubation with SP (10 µM) at 37°C. Human placenta was used as a positive control for CRHR-1 and CRHR-2. Gene expression was analyzed by RT-PCR and expression is indicated relative to control (n=3, p<0.05).
CRH stimulates LAD2 cell secretion of VEGF, TNF, and IL-8
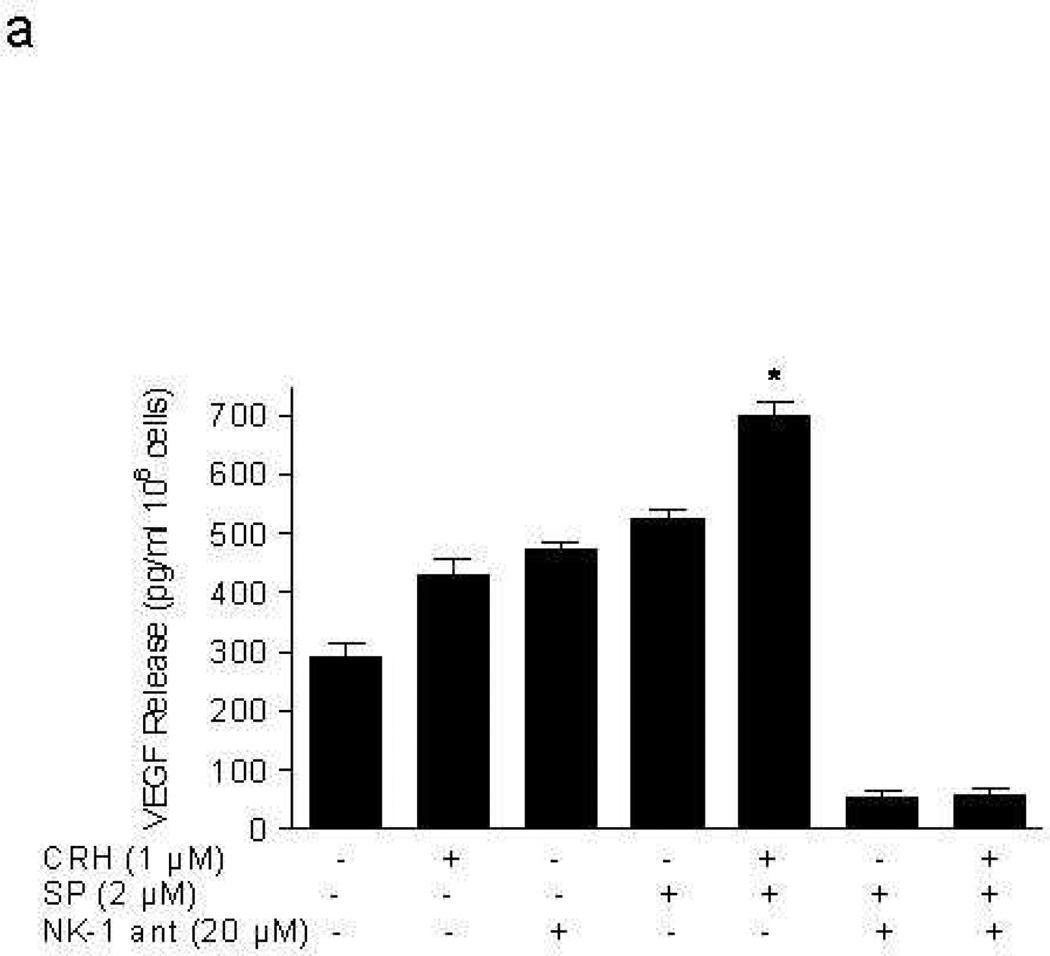
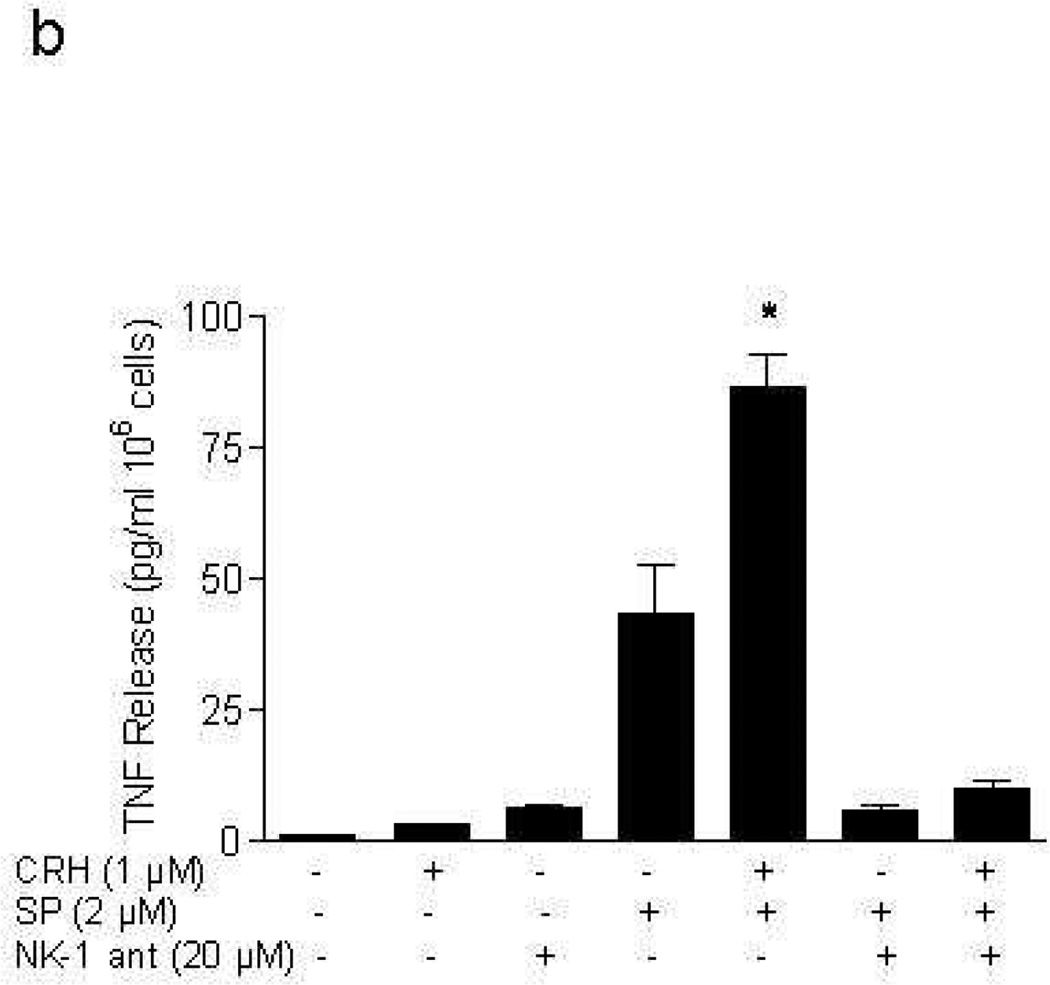
Addition of CRH (1 µM; lower concentration gave inconsistent results) for 24 hr to LAD2 cells “primed” with SP (2 µM) for 48 hr leads to statistically significant (p<0.05) more VEGF release (429.4±47 pg/106 cells by CRH alone to 700.2±37 pg/106 cells in SP primed cells). This increase could not be due to any residual effect of the original SP stimulation, because parallel tubes with cells treated with SP alone washed at 48 hr and then incubated for another 24 hr without CRH had much less VEGF release compared to the samples to which CRH was added (Fig. 2a). The effect of SP on VEGF release is blocked by an NK-1 antagonist added 30 min before SP (Fig. 2a). Moreover, CRH (1 µM) increases TNF (Fig. 2b) and IL-8 (Fig. 2c) release from LAD2 cells (2.9±0.14 pg/106 cells and 0 pg/106 cells to 86.4±11.1 pg/106 cells, 41.7±5.1 pg/106 cells in SP (2 µM) primed cells, respectively), and these effects are also blocked by the NK-1 antagonist (Fig. 2b, 2c).
Figure 2. CRH stimulates LAD2 cell secretion of VEGF, TNF, and IL-8.
Release of (a) VEGF, (b) TNF, and (c) IL-8 following stimulation with CRH (1 µM) for 24 hr after pretreatment with SP (2 µM) for 48 hr; in certain experiments mast cells were pretreated with NK-1 antagonist (ant, 20 µM for 30 min prior to SP) (n=3, p<0.05).
CRH stimulates truncated NK-1 gene expression
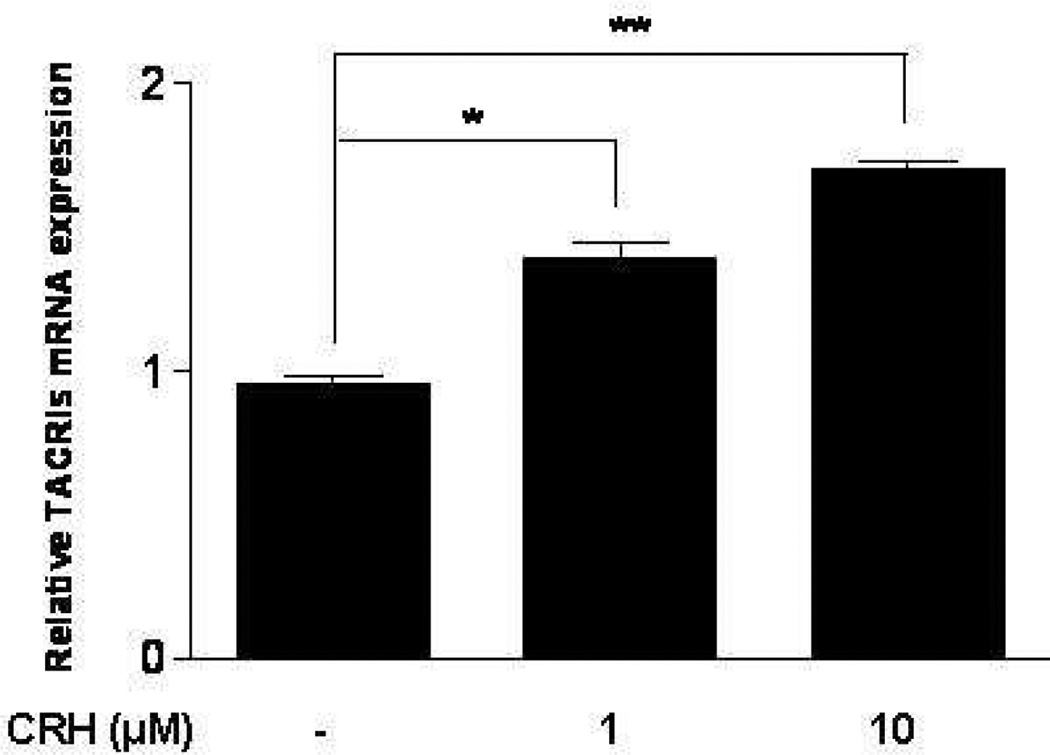
CRH and SP may be released together in inflammatory diseases that worsen by stress. We therefore, investigated if CRH could affect NK-1 gene expression. Addition of CRH (1, 10 µM) to LAD2 cells for 6 hr results in statistically significant increase in gene expression of only truncated NK-1 mRNA (Fig. 3). The full length NK-1 was not expressed (data not shown).
Figure 3. CRH stimulates mRNA expression of NK-1 receptor.
Treatment of LAD2 cells with CRH (1, 10 µM) for 6 hr induces gene expression of truncated NK-1 (n=3, p<0.05).
Effect of repeated stimulation of LAD2 cells with CRH on CRHR-1 and truncated NK-1 gene expression
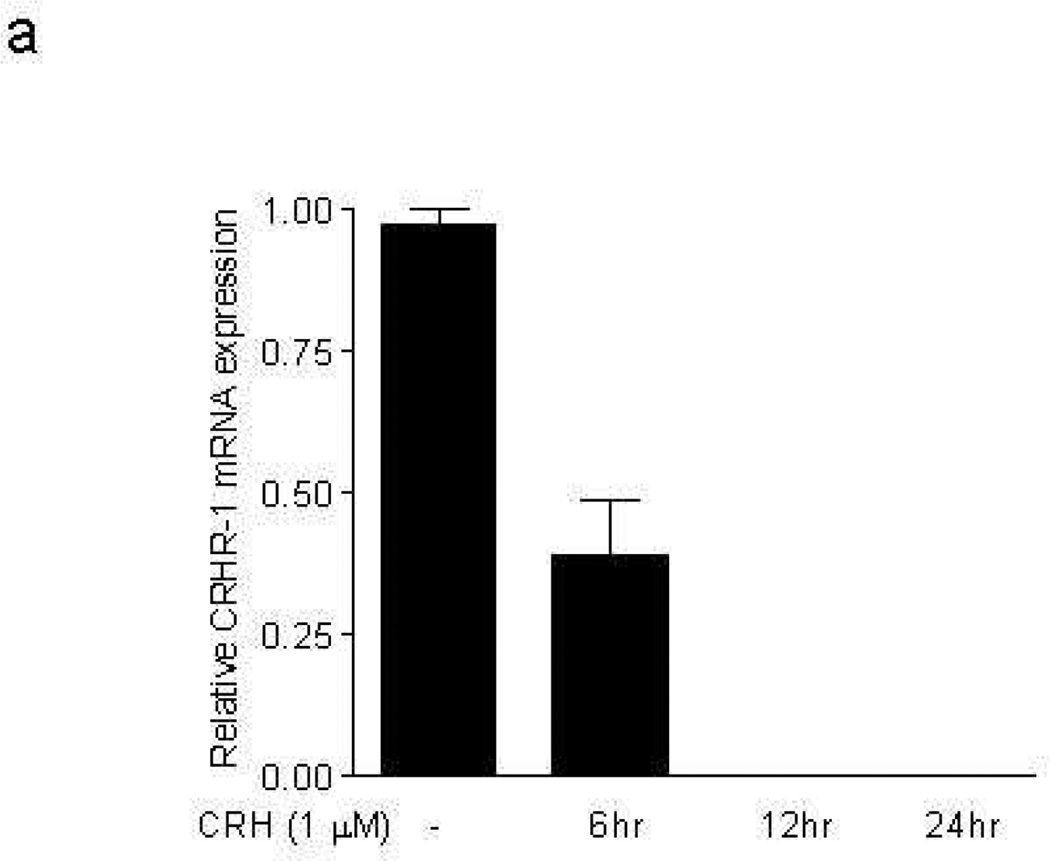
We then investigated if repeated stimulation with CRH would affect CRHR-1 or NK-1 gene expression. Repeated stimulation of mast cells every 6 hr up to 24 hr with CRH (1 µM) downregulates CRHR-1 mRNA expression (Fig. 4a), but upregulates NK-1 mRNA expression (Fig. 4b).
Figure 4. Effect of CRH on CRHR-1 and NK-1 receptor mRNA expression.
Repeated stimulation of mast cells with CRH every 6 hr up to 24 hr downregulates CRHR-1 (a), but upregulates truncated NK-1 receptor gene expression (b).
Discussion
This is the first report to our knowledge that SP can increase both mRNA and protein expression of CRHR-1 on human mast cells, activation of which leads to IL-8, TNF and VEGF secretion. This effect of SP could not be due to the leukemic nature of LAD2 cells because it was also present in hCBMC cells. Moreover, the effect of SP could not be nonspecific because contrary to our findings, SP was previously reported to decrease FcεRI expression on human mast cells (McCary et al., 2010). SP had previously been shown to induce CRHR-1 expression in human astrocytoma 4373 MG cells (Hamke et al., 2006).
CRH also induced mRNA expression of only the truncated NK-1 on LAD2 cells which is known to be at least ten times less sensitive than the full length receptor (Tuluc et al., 2009) and was recently shown to be associated with pathological states (Tuluc et al., 2009). These findings could be relevant for the pathogenesis of skin diseases that worsen by stress (Slominski, 2009), (Slominski et al., 2007), such as psoriasis, where both SP (Remröd et al., 2007) and CRH (Tagen et al., 2007), (Slominski et al., 2000) have been implicated.
We also report that overstimulation with CRH leads to downregulation of CRHR-1 on LAD2 cells. This finding may explain the fact that CRHR-1 gene expression is decreased in lesional psoriatic skin, possibly due to chronic overstimulation (Tagen et al., 2008). Human skin expresses primarily CRHR-1, while CRHR-2 protein is found only in hair follicles and eccrine glands (Slominski et al., 2004). It was previously shown that IL-1, IL-4, and LPS could increase CRHR-2 expression on hCBMCs (Papadopoulou et al., 2005b). These results suggest that activation of CRHR-1 and CRHR-2 may have different functions on the same or different cells or tissues. For instance, activation of these receptors was shown to have different actions on keratinocytes (Zbytek et al., 2005;Zbytek et al., 2004) and macrophages (Tsatsanis et al., 2007;Agelaki et al., 2002). Moreover, CRHR-1 was responsible for skin (Agren et al., 1976), while CRHR-2 for bladder (Boucher et al., 2010) and cardiac inflammation (Huang et al., 2002). Moreover, while skin CRHR-1 was overexpressed in chronic urticaria (Papadopoulou et al., 2005a), skin CRHR-2 was overexpressed in affected areas of alopecia areata (Katsarou-Katsari et al., 2001). It is, therefore, interesting that hair root progenitor cells were recently shown to develop into mature mast cells in response to CRH (Paus et al., 1994).
CRH secreted outside the brain has pro-inflammatory actions (Karalis et al., 1991). Such pro-inflammatory activities could mediate the action of lipopolysaccharide (Zbytek and Slominski, 2007), or through mast cell activation leading to increased vascular permeability (Theoharides et al., 1998). CRH stimulates CRHR-1-dependent selective release of VEGF without histamine from human mast cells (Cao et al., 2005). Mast cell stimulation by SP and CRH, would result in secretion of pro-inflammatory cytokines that would recruit and activate local immune accessory cells that would also stimulate peripheral nerves (Theoharides et al., 2004). Some of these cytokines may have synergistic action with either SP and / or CRH. For instance, we recently reported that the ability of SP to stimulate VEGF release from human mast cells is augmented by IL-33 (Theoharides et al., 2010a). Mast cells had been previously shown to release VEGF in response to IgE (Boesiger et al., 1998;Grutzkau et al., 1998), and CRH (Cao et al., 2005).
SP could be involved in the pathogenesis of inflammatory skin disorders, such as psoriasis (Remröd et al., 2007;Farber et al., 1986), which worsens by acute stress (Fortune et al., 2005;Harvima et al., 1996). SP-positive nerve fibers are more dense in psoriatic lesions compared to normal skin and have an increased number of contacts with mast cells (Jiang et al., 1998;Chan et al., 1997;Al'Abadie et al., 1995;Naukkarinen et al., 1996), which are also increased in lesional psoriatic skin (Harvima et al., 1993;Özdamar et al., 1996). SP-positive nerve fibers making contact with mast cells were increased by acute stress in mice, (Peters et al., 2005) leading to dermal mast cell degranulation (Kawana et al., 2006;Fortune et al., 2005;Paus et al., 1995).
The ability of CRH and SP to induce VEGF release from mast cells is also relevant to psoriasis. Psoriatic plaques contain increased levels of VEGF compared to normal skin (Heidenreich et al., 2009;Yalcin et al., 2007;Simonetti et al., 2006), and the VEGF 121 isoform also causes vascular permeability (Zhang et al., 2005;Creamer et al., 2002). Several VEGF polymorphisms are associated with an increased risk of developing psoriasis (Detmar, 2004;Barile et al., 2006) and epidermal overexpression of VEGF in transgenic mice led to a phenotype nearly identical to that of psoriasis (Xia et al., 2003).
Mast cells, CRH, and SP have been implicated in a brain-skin connection (Paus et al., 2006), and SP was shown to be involved in stress-induced hair loss in mice (Arck et al., 2005). In addition, to the role of CRH in a local skin “hypothalamic-pituitary-adrenal axis” (Slominski et al., 2000), the present results indicate that CRH and SP have proinflammatory actions not only on their own, but also by augmenting the expression of their respective receptors on human mast cells.
Materials and methods
Recombinant human stem cell factor (rhSCF) was kindly donated by Biovitrum AB (Stockholm, Sweden). SP, CRH were obtained from Sigma-Aldrich (St. Louis, MO). Anti-human CRHR-1-Allophycocyanin (APC) for FACS analysis and Enzyme-Linked Immunosorbent Assay (ELISA) kits were from R&D Systems (Minneapolis, MN).
Human mast cell culture
LAD2 human mast cells (obtained from NIH) were cultured in serum-free media (StemPro-34; Gibco, Grand Island, NY) supplemented with 2 mM L-glutamine, 100 IU/ml penicillin, 50 µg/ml streptomycin, and 100 ng/ml rhSCF. For optimal cell growth, LAD2 cell density was maintained between 0.5×106 and 1×106 cells/ml.
In order to obtain human cord blood mast cells (hCBMCs), human cord blood was obtained from placenta during normal deliveries in accordance with established institutional guidelines (Kempuraj et al., 2004). Briefly, mononuclear cells were isolated by layering heparin-treated cord blood onto Lymphocyte Separation Medium (INC Biomedical, Aurora, OH). CD34+ progenitor cells were isolated from mononuclear cells by positive selection of AC133 (CD133+/CD34+) cells by magnetic cell sorting (Miltenyi Biotech, Auburn, CA). hCBMCs were derived by the culture of CD34+ progenitor cells with minor modifications. For the first six weeks, CD34+ cells were cultured in AIM medium (Gibco, Grand Island, NY) supplemented with 100 ng/ml rhSCF and after six weeks 50 ng/ml IL-6 (Chemicon) was added and cultured at 37°C in 5% CO2 balanced air. Mast cell viability was determined by trypan blue (0.3%) exclusion.
Human basophils were obtained from ATCC (Manassas, VA) and were cultured in Eagle's Minimum Essential Medium from ATCC.
Human placenta, while express both CRHR-1 and CRHR-2 (Florio et al., 2000;Grammatopoulos, 2008) was obtained from the division of maternal-fetal medicine Tufts medical center, under exemption #4 of H1RB.
IL-8, TNF, and VEGF assays
LAD2 cells were washed with DPBS and suspended in complete culture medium. LAD2 cells (2×105 cells/200µl/well) were plated in 96 well flat bottom Falcon cell culture plates from Becton Dickinson (Franklin Lakes, NJ), and then cells were incubated with the SP for 48 hr at 37°C in 5% CO2 incubator. CRH was added for 24 hr and the plates were centrifuged. IL-8 or VEGF were measured in the supernatant fluid by ELISA. The minimum detectable level of VEGF was 5 pg/ml. Control cells were treated with equal volume of only culture medium.
FACS analysis for CRH receptors
Mast cells were Fc-blocked by treatment with 1 µg of human IgG/105 cells for 15 min at room temperature prior to staining. APC-conjugated CRHR-1 reagent was added to the Fc-blocked cells and ware incubated for 30 min at 4°C. Following the incubation, unreacted CRHR-1 detection reagent we removed by washing. Finally, the cells were resuspend in PBS buffer for final flow cytometric analysis. As a control for analysis, cells in a separate tube were treated with APC-labeled mouse IgG2A antibody. Analysis was performed using the FACS analyzer Amnis Image Stream (Amnis, Seattle, WA).
Total RNA extraction and real time PCR analysis
Total RNA was extracted from LAD2 mast cells or hCBMCs (106) using the Qiagen RNeasy mini kit (Valencia, CA). RNA was then used to perform first-strand cDNA synthesis. The reaction (20 µl) was run at 25 °C for 10 min, 37°C for 50 min, and inactivated by heating at 70°C for 15 min. In order to measure CRHR-1, 2, and truncated NK-1 receptor gene expression, quantitative real time PCR was performed using Taqman gene expression assays. The following probes obtained from Applied Biosystems (Carlsbad, CA): CRHR-1 (Hs00366363_m1), CRHR-2 (Hs00266401_m1), NK-1 full length (Hs00185530_m1), NK-1 truncated (AIV13FD); the sequences are proprietary and not released by the company.
The cycling conditions consisted of 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min, 40 cycles at 95°C for 15 sec, 1 cycle at 60°C for 1 min, 1 cycle at 95°C for 15 sec, 1 cycle at 60°C for 30 sec and 1 cycle at 95°C for 15 sec. Relative mRNA quality was determined from standard curves run with each experiment, and the expression was normalized to GAPDH used as the endogenous control. Positive control included cDNA for CRH-R1 and 2 from hCBMCs and human placenta. Negative control included the sample with water instead of template to check for external contamination.
Statistical analysis
All conditions were performed in triplicate, and all experiments were repeated at least three times (n=3). Results are presented as mean±SD. Data from stimulated and control samples were compared using the unpaired 2-tailed, Student’s t-test. Significance of comparisons is denoted by p<0.05.
Acknowledgments
We thank Dr. Arnold S. Kirshenbaum and Dr. D. Metcalfe at NIH for providing LAD2 mast cells. We also thank Amgen, Inc. (Thousand Oaks, CA) for rhSCF. A.A and K-D.A were supported by postgraduate fellowships from the Hellenic State Scholarships Foundation (Athens, Greece).
Abbreviations
- CRH
corticotropin-releasing hormone
- hCBMC
human umbilical cord blood-derived mast cell
- VEGF
vascular endothelial growth factor
- TNF
tumor necrosis factor
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Reference List
- Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agelaki S, Tsatsanis C, Gravanis A, et al. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 2002;70:6068–6074. doi: 10.1128/IAI.70.11.6068-6074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agren G, Milsson K, Ronquist G. A Mg2+ and Ca2+-stimulated ATPase at the outer surface of human peripheral leucocytes and hymatopoietic cell lines. Correlation between enzyme activity and immunoglobulin secretion. Acta Physiol Scand. 1976;98:67–73. doi: 10.1111/j.1748-1716.1976.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Al'Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis--an immunohistochemical study. Clin Exp Dermatol. 1995;20:384–389. doi: 10.1111/j.1365-2230.1995.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Kuhlmei A, et al. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med. 2005;83:386–396. doi: 10.1007/s00109-004-0627-z. [DOI] [PubMed] [Google Scholar]
- Barile S, Medda E, Nistico L, et al. Vascular endothelial growth factor gene polymorphisms increase the risk to develop psoriasis. Exp Dermatol. 2006;15:368–376. doi: 10.1111/j.0906-6705.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Boesiger J, Tsai M, Maurer M, et al. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fce receptor I expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher W, Kempuraj D, Michaelian M, et al. Corticotropin-releasing hormone-receptor 2 is required for acute stress-induced bladder vascular permeability and release of vascular endothelial growth factor. BJU Int. 2010 doi: 10.1111/j.1464-410X.2010.09237.x. [DOI] [PubMed] [Google Scholar]
- Cao J, Papadopoulou N, Kempuraj D, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- Chan J, Smoller BR, Raychauduri SP, et al. Intraepidermal nerve fiber expression of calcitonin gene-related peptide, vasoactive intestinal peptide and substance P in psoriasis. Arch Dermatol Res. 1997;289:611–616. doi: 10.1007/s004030050249. [DOI] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, et al. Expression cloning of a human corticotropin-releasing factor receptor. Proc Natl Acad Sci USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Creamer D, Allen M, Jaggar R, et al. Mediation of systemic vascular hyperpermeability in severe psoriasis by circulating vascular endothelial growth factor. Arch Dermatol. 2002;138:791–796. doi: 10.1001/archderm.138.6.791. [DOI] [PubMed] [Google Scholar]
- Detmar M. Evidence for vascular endothelial growth factor (VEGF) as a modifier gene in psoriasis. J Invest Dermatol. 2004;122:209–215. doi: 10.1046/j.0022-202X.2003.22140.x. [DOI] [PubMed] [Google Scholar]
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: Possible role of neuropeptides. J Am Acad Dermatol. 1986;14:305–311. doi: 10.1016/s0190-9622(86)70034-0. [DOI] [PubMed] [Google Scholar]
- Fewtrell CMS, Foreman JC, Jordan CC, et al. The effects of substance P on histamine and 5-hydroxytryptamine release in the rat. J Physiol. 1982;330:393–411. doi: 10.1113/jphysiol.1982.sp014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio P, Franchini A, Reis FM, et al. Human placenta, chorion, amnion and decidua express different variants of corticotropin-releasing factor receptor messenger RNA. Placenta. 2000;21:32–37. doi: 10.1053/plac.1999.0461. [DOI] [PubMed] [Google Scholar]
- Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681–694. doi: 10.1016/j.det.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK. Placental corticotrophin-releasing hormone and its receptors in human pregnancy and labour: still a scientific enigma. J Neuroendocrinol. 2008;20:432–438. doi: 10.1111/j.1365-2826.2008.01660.x. [DOI] [PubMed] [Google Scholar]
- Grutzkau A, Kruger-Krasagakes S, Baumeister H, et al. Synthesis, storage and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: Implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamke M, Herpfer I, Lieb K, et al. Substance P induces expression of the corticotropin-releasing factor receptor 1 by activation of the neurokinin-1 receptor. Brain Res. 2006;1102:135–144. doi: 10.1016/j.brainres.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168–176. doi: 10.1159/000288690. [DOI] [PubMed] [Google Scholar]
- Harvima RJ, Viinamäki H, Harvima IT, et al. Association of psychic stress with clinical severity and symptoms of psoriatic patients. Acta Derm Venereol (Stockh) 1996;76:467–471. doi: 10.2340/0001555576467471. [DOI] [PubMed] [Google Scholar]
- Heidenreich R, Rocken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol. 2009;90:232–248. doi: 10.1111/j.1365-2613.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Basu S, Theoharides TC. Urocortin induces corticotropin-releasing hormone receptor-2-mediated interleukin-6 production from neonatal rat cardiomyocytes through activation of MAP kinase and nuclear factor kappa B. 2002 [Google Scholar]
- Jiang WY, Raychaudhuri SP, Farber EM. Double-labeled immunofluorescence study of cutaneous nerves in psoriasis. Int J Dermatol. 1998;37:572–574. doi: 10.1046/j.1365-4362.1998.00533.x. [DOI] [PubMed] [Google Scholar]
- Karalis K, Sano H, Redwine J, et al. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254:421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- Katsarou-Katsari A, Singh LK, Theoharides TC. Alopecia areata and affected skin CRH receptor upregulation induced by acute emotional stress. Dermatology. 2001;203:157–161. doi: 10.1159/000051732. [DOI] [PubMed] [Google Scholar]
- Kawana S, Liang Z, Nagano M, et al. Role of substance P in stress-derived degranulation of dermal mast cells in mice. J Dermatol Sci. 2006;42:47–54. doi: 10.1016/j.jdermsci.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Papadopoulou NG, Lytinas M, et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- Liaw CW, Lovenberg TW, Barry G, et al. Cloning and characterization of the human corticotropin-releasing factor-2 receptor complementary deoxyribonucleic acid. Endocrinology. 1996;137:72–77. doi: 10.1210/endo.137.1.8536644. [DOI] [PubMed] [Google Scholar]
- McCary C, Tancowny BP, Catalli A, et al. Substance P downregulates expression of the high affinity IgE receptor (FcepsilonRI) by human mast cells. J Neuroimmunol. 2010;220:17–24. doi: 10.1016/j.jneuroim.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naukkarinen A, Jarvikallio A, Lakkakorpi J, et al. Quantitative histochemical analysis of mast cells and sensory nerves in psoriatic skin. J Pathol. 1996;180:200–205. doi: 10.1002/(SICI)1096-9896(199610)180:2<200::AID-PATH632>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- O'Connor TM, O'Connell J, O'Brien DI, et al. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- Özdamar SO, Seckin D, Kandemir B, et al. Mast cells in psoriasis. Dermatology. 1996;192:190. doi: 10.1159/000246359. [DOI] [PubMed] [Google Scholar]
- Papadopoulou N, Kalogeromitros D, Staurianeas NG, et al. Corticotroopin-releasing hormone receptor-1 and histidine decarboxylase expression in chronic urticaria. J Invest Dermatol. 2005a;125:952–955. doi: 10.1111/j.0022-202X.2005.23913.x. [DOI] [PubMed] [Google Scholar]
- Papadopoulou NG, Oleson L, Kempuraj D, et al. Regulation of corticotropin-releasing hormone receptor-2 expression in human cord blood-derived cultured mast cells. J Mol Endocrinol. 2005b;35:R1–R8. doi: 10.1677/jme.1.01833. [DOI] [PubMed] [Google Scholar]
- Paus R, Heinzelmann T, Robicsek S, et al. Substance P stimulates murine epidermal keratinocyte proliferation and dermal mast cell degranulation in situ. Arch Dermatol Res. 1995;287:500–502. doi: 10.1007/BF00373436. [DOI] [PubMed] [Google Scholar]
- Paus R, Maurer M, Slominski A, et al. Mast cell involvement in murine hair growth. Dev Biol. 1994;163:230–240. doi: 10.1006/dbio.1994.1139. [DOI] [PubMed] [Google Scholar]
- Paus R, Theoharides TC, Arck P. Neuroimmunoendocrine circuitry of the 'brain-skin connection'. Trends Immunol. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Peters EM, Kuhlmei A, Tobin DJ, et al. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–262. doi: 10.1016/j.bbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Remröd C, Lonne-Rahm S, Nordliond K. Study of substance P and its receptor neurokinin-1 in psoriasis and their relation to chronic stress and pruritus. Arch Dermatol Res. 2007;299:85–91. doi: 10.1007/s00403-007-0745-x. [DOI] [PubMed] [Google Scholar]
- Simonetti O, Lucarini G, Goteri G, et al. VEGF is likely a key factor in the link between inflammation and angiogenesis in psoriasis: results of an immunohistochemical study. Int J Immunopathol Pharmacol. 2006;19:751–760. doi: 10.1177/039463200601900405. [DOI] [PubMed] [Google Scholar]
- Slominski A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br J Dermatol. 2009;160:229–232. doi: 10.1111/j.1365-2133.2008.08958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Tobin DJ, et al. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Tuckey RC, et al. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagen M, Elorza A, Shirihai O, et al. Inhibition of mast cell degranulation by PPARa agonists is mediated through UCP2. 2008 in press. [Google Scholar]
- Tagen M, Stiles L, Kalogeromitros D, et al. Skin corticotropin-releasing hormone receptor expression in psoriasis. J Invest Dermatol. 2007;127:1789–1791. doi: 10.1038/sj.jid.5700757. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Donelan JM, Papadopoulou N, et al. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Kalogeromitros D. The critical role of mast cell in allergy and inflammation. Ann NY Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Singh LK, Boucher W, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its pro-inflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Zhang B, Kempuraj D, et al. IL-33 augments substance P-induced VEGFsecretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010a;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Zhang B, Kempuraj D, et al. IL-33 augments substance P-induced VEGFsecretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010b;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Dermitzaki E, et al. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-alpha release from macrophages via induction of COX-2 and PGE2. J Cell Physiol. 2007;210:774–783. doi: 10.1002/jcp.20900. [DOI] [PubMed] [Google Scholar]
- Tuluc F, Lai JP, Kilpatrick LE, et al. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009;30:271–276. doi: 10.1016/j.it.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Xia YP, Li B, Hylton D, et al. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- Yalcin B, Tezel GG, Arda N, et al. Vascular endothelial growth factor, vascular endothelial growth factor receptor-3 and cyclooxygenase-2 expression in psoriasis. Anal Quant Cytol Histol. 2007;29:358–364. [PubMed] [Google Scholar]
- Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone stimulates NF-kappaB in human epidermal keratinocytes. J Endocrinol. 2004;181:R1–R7. doi: 10.1677/joe.0.181r001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Pikula M, Slominski RM, et al. Corticotropin-releasing hormone triggers differentiation in HaCaT keratinocytes 1. Br J Dermatol. 2005;152:474–480. doi: 10.1111/J.1365-2133.2005.06217.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Slominski AT. CRH mediates inflammation induced by lipopolysaccharide in human adult epidermal keratinocytes. J Invest Dermatol. 2007;127:730–732. doi: 10.1038/sj.jid.5700607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Matsuo H, Morita E. Vascular endothelial growth factor 121 is the predominant isoform in psoriatic scales. Exp Dermatol. 2005;14:758–764. doi: 10.1111/j.1600-0625.2005.00356.x. [DOI] [PubMed] [Google Scholar]