Abstract
Adenosine is an inhibitor of neuronal activity in the brain. The local release of adenosine from grafted cells was evaluated as an ex vivo gene therapy approach to suppress synchronous discharges and epileptic seizures. Fibroblasts were engineered to release adenosine by inactivating the adenosine-metabolizing enzymes adenosine kinase and adenosine deaminase. After encapsulation into semipermeable polymers, the cells were grafted into the brain ventricles of electrically kindled rats, a model of partial epilepsy. Grafted rats provided a nearly complete protection from behavioral seizures and a near-complete suppression of afterdischarges in electroencephalogram recordings, whereas the full tonic–clonic convulsions in control rats remained unaltered. Thus, the local release of adenosine resulting in adenosine concentrations <25 nM at the site of action is sufficient to suppress seizure activity and, therefore, provides a potential therapeutic principle for the treatment of drug-resistant partial epilepsies.
Epilepsy is one of the most common neurological disorders that affects ≈1% of the population, of whom as many as 40% may have complex partial epilepsy, in particular, temporal lobe epilepsy (1). Despite optimal drug treatment, seizures persist in ≈35% of patients with partial epilepsy (2). Although patients with drug-resistant forms of epilepsy can undergo resective surgery, many patients cannot be treated surgically because of unacceptable risks for loss of brain functions (3). In the search for new therapeutic approaches, the local release of adenosine from intracerebrally grafted cells was evaluated.
Adenosine inhibits neuronal activity (4–6) through activation of high-affinity adenosine receptors (A1 and A2A) (7) and receptors with lower affinity (A2B and A3) (8, 9). The release of various neurotransmitters, in particular, of excitatory amino acids, is inhibited via presynaptic A1 receptors (10, 11), which are linked via G proteins to Ca2+ and K+ ion channels (12). Postsynaptically, activation of A1 receptors leads to a stabilization of the membrane potential by modulation of Ca2+ and K+ fluxes (13). A1 receptors are believed to be activated by adenosine released from cells, whereas A2A receptors seem to be activated by adenosine, which is formed by the extracellular cleavage of adenine nucleotides by the enzyme ecto-5′-nucleotidase (14).
Adenosine and its analogues have powerful antiseizure activities in various models of epilepsy. However, when administered systemically, adenosine and its analogues cause strong, adverse effects ranging from sedation and hypothermia to the suppression of cardiovascular functions and an almost complete cessation of spontaneous motor activity (15), preventing its therapeutic use. The effectiveness of intracerebroventricular adenosine was assessed recently by the intraventricular implantation of an adenosine-releasing synthetic polymer (16). Adenosine released in extremely low concentrations (in the range of 20 to 50 ng per day) was found to protect against electrically induced seizures without any overt side effects (16). These results demonstrated that the amounts of adenosine required locally to suppress seizure activity were in the range achievable for adenosine released from biological sources.
The minimally effective concentration of adenosine at its site of antiseizure activity was expected to be in the range of the Kd value of rat brain A1 receptors (Kd = 10 nM at 37°C) (17). In this concentration range, adenosine transporters (18, 19) in brain tissue with a Kd of 25 μM (20) would not be expected to limit the therapeutic effectiveness. In the present investigation, an attempt therefore was made (i) to develop cellular delivery systems for adenosine, (ii) to test whether amounts of adenosine sufficient for seizure suppression could be obtained, and (iii) to assess whether undue side effects could be avoided under a local mode of adenosine delivery.
Physiologically, adenosine is formed from adenine nucleotides by dephosphorylation and from S-adenosylhomocysteine by hydrolysis. It is degraded by the enzymes adenosine deaminase (ADA) and adenosine kinase (ADK), of which ADK is considered to be the key enzyme for adenosine metabolism because of its lower Km value (21). In our study, a cell-encapsulation technology was used to immunoisolate the cells (22). Its safety had been demonstrated in clinical trials for the treatment of amyotrophic lateral sclerosis (23) and chronic pain (24). Here, we report on the experimental suppression of seizures after intraventricular grafting of adenosine-releasing encapsulated cells as tested in the rat kindling model of epilepsy. Encapsulated adenosine-releasing cells were capable of seizure suppression without any overt side effects.
Materials and Methods
Drug Treatment.
DPCPX (8-cyclopentyl-1,3-dipropylxanthine) was purchased from Research Biochemicals and dissolved in DMSO (1 mg/ml). Phenytoin (5,5-diphenylhydantoin) was purchased from Sigma and dissolved in 50% polyethyleneglycol 400 (30 mg/ml). DPCPX (1 mg/kg, i.p.) or phenytoin (60 mg/kg, i.p.) was injected into kindled rats that contained adenosine-releasing cell capsules.
Assay for Adenosine.
The samples were prepared as follows: 105 baby hamster kidney (BHK)-AK2, ADA-O, BHK-wild type (WT), and ADA-WT cells each were plated and grown for 16 h in 5 ml of DMEM including 10% FCS (Life Technologies, Gaithersburg, MD). Before collection of supernatants, the cells were preincubated in serum-free QBSF-56 (Sigma) for 1 h. The medium then was replaced by 5 ml of fresh QBSF-56. Aliquots of 0.5 ml were taken from the cultured cells after 1, 2, 4, 8, and 24 h to determine their respective adenosine concentrations. These aliquots were analyzed in an enzyme-coupled fluorescent assay as described (16).
Generation of BHK-AK2 Cells.
A BHK cell monolayer at about 50% confluence was mutagenized during a treatment of 18 h in complete DMEM containing 200 μg/ml ethyl methanesulfonate and 10% FCS (EMS, Sigma). The cells then were harvested and plated at a density of 1 × 105 cells per 90-mm culture dish. After 3 days, one-third of the cells were replated in complete DMEM containing 10% horse serum and 50 μM adenine 9-β-d-arabinofuranoside (araA; Sigma). In the following, each time the cells reached confluence, one-third of the cells was replated on a new plate and the concentration of araA was increased in steps of 50 μM at each round of replating. When the cells were resistant to 200 μM araA, they were trypsinized and replated at a density of 1 × 105 cells per dish and incubated in DMEM containing 10% horse serum, 10 μM erythro-9-[3-(2-hydroxynonyl)]adenine, and 100 μM araA. The cell line BHK-AK2 was isolated and released up to 19 ng of adenosine per 105 cells per h.
Generation of Conditionally Immortalized Fibroblast Cell Lines.
Heterozygous ADA knockout mice ADA+/− (25) were bred with Immortomice tsA58+/+ (26) to establish the new mouse line ADA+/−tsA58+/+. For the generation of cell lines, these mice then were bred to ADA+/− mice. From this intercross, embryos were isolated at embryonic day 14.5 and processed individually. Head, intestine, heart, liver, lung, and spleen were removed and frozen for later PCR analysis to determine those embryos with an ADA−/−tsA58+/− genotype. The remnant tissue from ADA−/−tsA58+/− embryos was used to harvest conditionally immortalized fibroblasts as follows. The tissue was cut, washed with DMEM, and centrifuged for 5 min at 1,000 rpm. The sediments were incubated for 10 min in 2 ml of PBS containing 0.1% (wt/vol) trypsin and 1 mM EDTA at room temperature and then passaged ≈20 times through a Pasteur pipette until a fine suspension resulted. This suspension was centrifuged for 5 min at 1,000 rpm, and the resulting sediment of cells (approximately 107 cells) was resuspended in 5 ml of complete DMEM, including 10 units/ml recombinant mouse IFN-γ (Life Technologies). After an incubation of 5 min, large clumps of cells or tissue had sedimented. The resulting supernatant then was seeded on two 90-mm culture dishes and incubated with 10 ml of complete DMEM including 10 units/ml IFN-γ at 33°C at 5% CO2. Under these conditions, the cells continued to proliferate at 33°C. The resulting fibroblast cell line was termed ADA-O (ADA−/−tsA58+/−). As a control, the cell line ADA-WT (ADA+/+tsA58+/−) was generated from littermates that were homozygous for the WT Ada allele, accordingly.
Manufacturing and Characterization of Implantation Devices.
As a cellular implantation device, ADA-O, ADA-WT, BHK-AK2, and BHK-WT cells were encapsulated into polyethersulfone (PES) polymer hollow-fiber capsules (7-mm long, 0.5-mm inner diameter, wall thickness of 50 μm) as described (22). The capsules were sterilized with ethylene oxide and antiseptically filled with 2 × 105 cells resuspended in a collagen matrix. Before implantation, the capsules were kept at 37°C for 1 week in complete DMEM to allow for the attachment and differentiation of the encapsulated cells. Individual ADA-O and BHK-AK2 capsules were placed in 1 ml of QBSF-56 medium. After 1, 2, 4, 8, and 24 h, 100-μl aliquots were removed and assayed for their adenosine content.
Kindling Model.
All experimentation with animals was performed according to the guidelines of the local animal welfare authorities. Male Oncins France strain A rats (Laboratory for Animal Research, University of Zurich) being housed in pairs under 12-h light/12-h dark conditions with ad libitum access to water and food and weighing 300 g at the time of surgery were used. Under general pentobarbital anesthesia (50 mg/kg i.p.), rats were placed in a Kopf stereotactic frame, and bipolar, coated, stainless-steel electrodes (0.20 mm in diameter; Bilaney Consultants, Düsseldorf, Germany) were implanted bilaterally into the hippocampus and fixed with a pedestal of dental acrylate. Coordinates for hippocampal electrodes were (toothbar at 0): 5.0 mm caudal to bregma, 4.8 mm lateral to midline, and 7.3 mm ventral to dura. One week after electrode implantation, the animals were stimulated unilaterally up to 12 times daily with a Grass S-88 stimulator (1-ms square-wave pulses of 50 V at 10-Hz frequency for 10 s, 5-min interval between stimulations). Stimulations were maintained for a total period of up to 15 days until all animals reacted reproducibly with a grade 5 seizure after the first daily test stimulus. The current intensity was just above the threshold for eliciting epileptiform activity (afterdischarges and/or wet dog shakes). The electroencephalogram (EEG) was recorded for periods of 1 min before and 5 min after application of the stimulating pulse by using a Human Scoring registration/software unit (B. Geehring, Institute of Pharmacology, University of Zurich). Behavioral seizures were scored according to the scale of Racine (27).
Grafting.
After completion of kindling, capsules containing either adenosine-releasing cells, control cells, or matrix material only were implanted into the lateral ventricle ipsilateral to the site of stimulation under general pentobarbital anesthesia (50 mg/kg i.p.). Coordinates for capsule implantation were (toothbar at −8.3): 1.8 mm caudal to bregma, 1.4 mm lateral to midline, and 8.5 mm ventral to dura. Because of the construction of the pedestal, fixing the electrodes a retrieval and exchange of implanted capsules was not possible.
Histology.
Twenty-four days after capsule implantation, the animals were killed, their capsules were retrieved, and the brains were used for cryosectioning and Nissl staining to verify the locations of electrodes and capsules. The retrieved capsules were fixed in 4% paraformaldehyde, rinsed in PBS, and placed in sequentially in 1 ml of 70%, 80%, 95%, and 100% ethanol for 1 h at each concentration. For the embedding reaction, a commercial glycol methacrylate kit (Fluka) was used. After hardening of the embedded capsules at 60°C for 3 days, sections of 1.5-μm thickness (Supercut microtome 2065; Reichert) were mounted on glass slides and stained with 3% toluidine blue.
Quantification of Cells in Capsule Sections.
The number of viable cells within representative squares of 400 × 400 μm was determined visually under ×400 magnification of toluidine blue-stained capsule sections. Five counts were taken for each time point and averaged. Errors were calculated as ±SEM. The number of cells within each square at the time of capsule implantation was set as 100%. Cell counts at later time points are given as relative values in comparison to the original cell numbers.
Behavioral Analysis.
Blinded personnel observed the experimental rats (n =18 with adenosine implant and n = 28 with control implant) on a daily basis for 1 h. The following parameters were studied by gross examination of the animal's behavior: signs for sedation or ataxia were assessed as well as spontaneous and exploratory activity; locomotion and motor coordination were tested by placing the rat onto a grid to screen for any signs of imbalance; and social behavior was assessed by the observation of allogrooming and signs of aggression to cagemates. No differences were found among the rats treated with adenosine-releasing grafts compared with those that received control grafts and compared with untreated animals.
Results
Generation of Adenosine-Releasing Cells.
The inactivation of the adenosine-metabolizing enzymes ADK and ADA was expected to result in the intracellular accumulation and net efflux of adenosine. Two approaches focusing on the enzymes ADK and ADA were used to generate adenosine-releasing cells. (i) BHK cells were mutagenized chemically and selected for ADK deficiency with vidarabine (araA) that is transformed in the presence of ADK into toxic nucleotides. With this strategy, the araA-resistant clone BHK-AK2 was obtained, which was completely deficient in ADK as verified by Western blot analysis (not shown). (ii) Using transgenic mice that carried the conditionally immortalizing oncogene H-2KbtsA58 (26) in an ADA-deficient background (25), ADA-deficient fibroblasts (ADA-O) with a temperature-sensitive oncogene were generated.
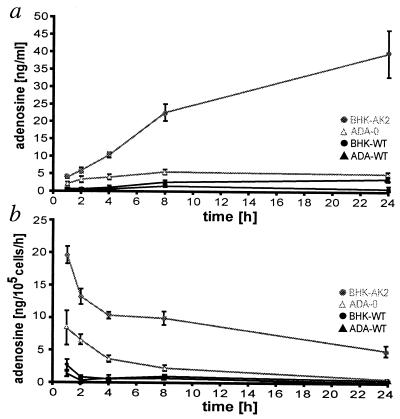
The amount of adenosine in the supernatants of the cultured BHK-AK2 and ADA-O cells was analyzed in an enzyme-coupled fluorescent assay. Adenosine concentrations of 39.7 ± 6.7 ng/ml were found after incubating 105 adherent BHK-AK2 cells for 24 h (Fig. 1a), a time at which the steady-state concentration in the supernatants had not yet been reached. When ADA-O cells were cultured under the same conditions, the adenosine concentration in the supernatant increased until it reached a steady state of 5.7 ± 1.9 ng/ml after ≈8 h of incubation (Fig. 1a). It can be roughly estimated that 105 BHK-AK2 cells released a total of 19.5 ± 1.3 ng adenosine during the first hour of incubation compared with 8.4 ± 2.6 ng released by ADA-O cells (Fig. 1b). Thus, BHK-AK2 cells released about 2.3 times more adenosine than ADA-O fibroblasts, which is in line with the higher efficiency of ADK in the removal of adenosine. In contrast, in supernatants of 105 BHK-WT and ADA-WT cells, 2.6 ± 1.8 ng and 1.4 ± 1.0 ng adenosine was found during the first hour, respectively (Fig. 1).
Figure 1.
The accumulation of adenosine in the supernatants of cultured cells. (a) The amount of adenosine in the supernatants of BHK-AK2 (shaded circles), ADA-O (▵), and their WT counterparts, BHK-WT and ADA-WT cells (● and ▴, respectively) was measured over 24 h by using an enzyme-coupled fluorescent assay from 105 cells each. (b) The adenosine levels determined under a were recalculated to represent the amount of adenosine that accumulated within a time interval (ng of adenosine per 105 cells per h). Each release study was performed six times; bars were calculated as ±SEM.
Encapsulation of Adenosine-Releasing Cells.
To use ADA-O and BHK-AK2 cells as intraventricular delivery systems for adenosine, the cells were encapsulated into semipermeable hollow fibers. After 1 week in culture medium, a multilayer of viable BHK-AK2 cells was present adjacent to the capsule wall and the interior of the capsule contained fewer clusters of cells with matrix material apparent (Fig. 2 a and b). When capsules with BHK-AK2 cells (n = 8) were kept in culture medium, they retained the ability to release adenosine for at least 14 days; within 8 h of incubation, a steady-state concentration of up to 29 ng/ml adenosine was reached in the capsule supernatants each time the culture medium was changed. In comparison, adenosine steady-state concentrations in the supernatants of ADA-O capsules (n = 8) reached within 24 h ranged from 3 to 7 ng/ml. These values were largely constant over a period of 14 days when measured 24 h after each transfer into fresh medium. Thus, BHK-AK2 and ADA-O cells remained viable in the encapsulation matrix.
Figure 2.
Histology of encapsulated cells by toluidine blue staining. (a) BHK-AK2 cells before grafting. Viable cells were located mainly adjacent to the capsule walls (left and right stripes), with the interior of the capsule also showing the collagen matrix. The square indicated is enlarged in b. (b) BHK-AK2 cells from a at higher magnification. (c) BHK-AK2 cells in capsule retrieved after having been grafted for 2 weeks in the lateral ventricle of control rats; an area adjacent to the capsule wall is shown. Note the dense clusters of viable cells. (d) BHK-AK2 cells in capsules kept in culture for 2 weeks. (e) BHK-AK2 cells retrieved from rat brain ventricles 24 days after grafting; scattered viable cells are surrounded by cellular debris. (f) BHK-WT control cells retrieved from rat brain ventricles 24 days after grafting. [Bars, 20 μm except in a (200 μm).]
For BHK-AK2 cells, the viability was tested further by grafting capsules of BHK-AK2 cells into the lateral brain ventricle of naïve control rats (n = 7). Capsules retrieved from the brain ventricles up to 2 weeks after grafting contained large aggregates of viable cells as determined by toluidine blue staining of longitudinal sections and only infrequently showed small areas of cellular debris (Fig. 2c). The cell content in BHK-AK2 capsules retrieved from rat brain after 2 weeks was the same as that in BHK-AK2 capsules kept in culture for the same period (Fig. 2d).
Seizure Suppression by Adenosine-Releasing Cell Capsules.
Kindled rats were prepared by bilateral implantation of bipolar electrodes into the CA1–CA3 region of the ventral hippocampus of male Oncins France strain A rats. The implanted electrodes permit both the recording of intrahippocampal EEGs as well as the delivery of electrical stimuli. By repetitive unilateral stimulation with initially subconvulsive currents, rats gradually developed seizures accompanied by electrical afterdischarges that were recorded bilaterally in the EEG from the CA1–CA3 region.
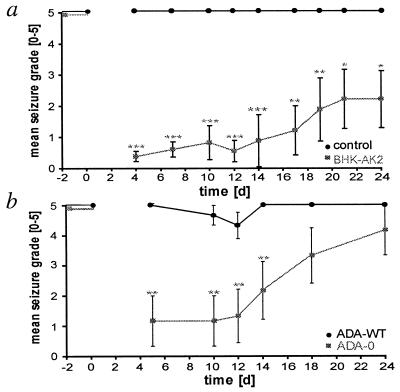
The anticonvulsant efficacy of encapsulated BHK-AK2 and encapsulated ADA-O cells was tested by stereotactic grafting of a single capsule into a lateral brain ventricle of kindled rats (n = 12 and n = 6, respectively). In addition, two types of control grafts were used: (i) control capsules containing matrix only (n = 12 grafted rats) and (ii) control capsules with WT fibroblasts ADA-WT (n = 9 grafted rats). Before implantation (day 0), the kindled animals responded to every test stimulus with a full tonic–clonic grade 5 seizure (Fig. 3).
Figure 3.
Behavioral seizure suppression by grafts of encapsulated adenosine-releasing cells. (a) Adenosine-releasing BHK-AK2 cell capsules (shaded squares) and control capsules containing matrix only (●) were grafted into the lateral brain ventricles of kindled rats at day 0. The seizure activity was tested after the delivery of test stimuli at the indicated days. Before grafting (day −2, day 0) the stimulus invariably induced grade 5 seizures. After grafting (days 4–24), kindled rats containing control grafts (n = 12) consistently displayed tonic–clonic grade 5 seizures after the application of a test stimulus. During the same period for kindled rats containing BHK-AK2 grafts, tonic–clonic seizures (grades 3–5) were completely suppressed for at least 24 days (n = 9 until day 14; n = 6 from day 14 onward because three rats had lost their pedestal). (b) ADA-O cell capsules (shaded squares) and control capsules containing ADA-WT cells (●) were grafted into the lateral brain ventricles of kindled rats at day 0. Before grafting (day −2, day 0), the test stimulus invariably induced grade 5 seizures. After grafting (days 4–24), in animals that received ADA-O capsules (n = 6), seizure grades were reduced significantly for up to 14 days compared with the control group with ADA-WT capsules (n = 9). The statistics in a and b were performed by one-way ANOVA-repeated measurements on the same subjects: *, P < 0.05; **, P < 0.01; ***, P < 0.001 in the Student's t test. (Bars, ±SEM.)
After a postsurgical recovery period of 4–5 days, each rat was submitted to a single test stimulus at the indicated days (Fig. 3) and the behavioral seizure response was scored and averaged over the number of test animals. The seizure suppression in grafted animals was monitored for a period of 24 days, at which time the experiment was terminated. The tonic–clonic grade 5 seizure activity was not reduced significantly in kindled animals that had received either of the control capsules (Fig. 3). However, after the implantation of capsules with BHK-AK2 cells, behavioral seizure activity was almost as completely suppressed as when it was tested first at day 4 (Fig. 3a). Upon subsequent stimulations, the seizure activity remained drastically reduced during the first 14 days, with mean scores ranging between 0.3 and 0.8. This strong protection from seizures was highly significant compared with the continued grade 5 activity of the controls (P < 0.001). During the third week after grafting, seizure protection decreased slightly, with mean seizure scores upon stimulation of between 1.1 and 1.8 (P < 0.01). Thereafter, at day 21 and 24 after implantation, the animals were still completely protected from generalized tonic–clonic seizure activity, with mean seizure scores reaching a value of 2.2 ± 0.9 (P < 0.05).
The kindling process itself is known to potentially induce resistance to phenytoin, which is taken as an indication that such animals are refractory to seizure suppression (28). Therefore, at day 24, the BHK-AK2-treated group was analyzed for a potential phenytoin resistance to exclude that kindling-induced brain alterations may be responsible for an insensitivity to anticonvulsant treatment. Three animals of this group, which did not respond to the BHK-AK2 grafts, were found to be resistant to phenytoin (60 mg/kg, i.p.) and therefore were excluded from the analysis given above. To further exclude the possibility that the total number of test stimulations had an influence on the response to BHK-AK2 grafts, an additional group of kindled rats, which had received BHK-AK2 grafts (n = 8, data not shown), was stimulated only four times during the entire test period of 24 days. The seizure grades in this experimental group were comparable to the results described above for the BHK-AK2 group of kindled rats that was stimulated nine times during 24 days (Fig. 3a). Because at no time was there detected any difference in seizure grades between the two groups, the frequency of stimulation did not appear to impair the antiseizure activity of the grafts.
Behavioral seizure suppression also was observed in rats that received ADA-O grafts (Fig. 3b). Before grafting, all animals reacted consistently with tonic–clonic grade 5 seizures. However, during the first 12 days after grafting of ADA-O capsules, seizures were almost completely suppressed, with the average seizure grade upon stimulation below 1.4 (Fig. 3b). The suppression of seizure activity continued up to day 18. At this time, two of six animals were still completely protected from grade 2–5 seizures, resulting in an average seizure score of 2.2–3.3 compared with the grade 5 activity in all control animals that had received ADA-WT capsules. Even after 24 days, the seizure susceptibility had not yet reached preimplantation levels (Fig. 3b). In its initial 12-day period, this experiment demonstrates that the lower amount of adenosine released by ADA-O cells (8.4 ± 2.6 ng/h per 105 ADA-O cells compared with 19.5 ± 1.3 ng/h per 105 BHK-AK2 cells) is sufficient for seizure protection.
EEG Analysis of Treated Rats.
The antiepileptic effect of BHK-AK2 and ADA-O cells was analyzed further by recording the afterdischarges in EEG measurements of all grafted and control animals at each stimulus event. One day before grafting, strong, electric afterdischarges that accompanied the stimulus-elicited grade 5 seizures were recorded (Fig. 4 a–c Upper). The afterdischarge developed first on the ipsilateral side before spreading to the contralateral side and the manifestation of a full tonic–clonic seizure (Fig. 4 a–c Upper). Control grafts consisting of capsules with matrix material only had no influence on the epileptic afterdischarge activity being comparable to the EEG recording before surgery (Fig. 4a). In contrast, after grafting BHK-AK2 and ADA-O implants, a strong suppression of afterdischarges at all test times was apparent after a test stimulus, compared with the pregrafting EEG activity (Fig. 4 b and c). The suppression of epileptic EEG activity correlated with a suppression of behavioral seizure activity during the same time period.
Figure 4.
Bilateral intrahippocampal EEGs recorded after test stimulations in kindled rats grafted with a control capsule containing matrix only (a), a capsule containing BHK-AK2 cells (b), and a capsule containing ADA-O cells (c). In each EEG, the upper trace represents the recording ipsilateral (i) to the side of stimulation and capsule location, whereas the bottom line represents the recording from the contralateral side (c). The vertical lines (arrow) mark the end of the 10-s electric test stimulus. The traces are representative recordings taken 1 day before and 4 or 7 days after grafting.
Reversal of Anticonvulsant Effect by DPCPX.
To validate that the antiepileptic efficacy of cellular implants was due to adenosine and not another agent released from the cells, an attempt was made to reverse the protective effect by the adenosine A1 receptor antagonist DPCPX. Three fully kindled rats received ADA-O capsules and were completely protected from seizures (mean seizure grade of 0.2 ± 0.3) as tested 1 day before the injection of DPCPX (1 mg/kg, i.p.). When the three animals were injected at days 5 and 10 after grafting with DPCPX, the seizure susceptibility was completely restored, with a mean seizure grade of 4.7 ± 0.3 as tested 20 min after drug injection (Fig. 5). The effect of DPCPX was transient, because 3 days after drug injection, the same animals again were protected from seizure induction (mean seizure grade of 0.9 ± 0.7). These findings indicate that the anticonvulsant effect of adenosine-releasing implants is a result of activation of A1 receptors. In addition, after DPCPX treatment, seizure protection was restored within 3 days. This finding indicates that the A1 receptors are not desensitized at least up to day 13.
Figure 5.
Inhibition of seizure protection by the A1-specific antagonist DPCPX. Grafting of ADA-O capsules into the lateral brain ventricles of kindled rats was followed by complete protection from seizures (mean seizure grade: 0.2 ± 0.3 as determined 4 and 9 days after grafting; n = 3 rats). Administration of DPCPX (1 mg/kg, i.p.) at days 5 and 10, respectively, resulted in a near-complete restoration of tonic–clonic seizure activity (grade 4.7 ± 0.3, mean value from both days) as tested 20 min after drug injection. The effect of DPCPX was transient, being strongly reduced 3 days after the injection (mean seizure grade: 0.9 ± 0.7). The values given are the means of the seizure grades measured at day 5 plus day 10 (n = 6). (Bars, ±SEM.)
Behavior.
It is important to note that rats protected from seizures by adenosine-releasing implants containing either BHK-AK2 (n = 12) or ADA-O (n = 6) cells did not show any overt behavioral deficits in the periods between stimulations as determined by an independent observer in daily observation sessions of 1 h. The spontaneous behavior in locomotion and exploratory activity was indistinguishable from sham-operated control animals (n = 12) and from animals containing implants with WT fibroblasts ADA-WT (n = 9). The social behavior of the animals likewise was unaltered. In particular, it was noteworthy that there were no signs of sedation or ataxia in the animals containing adenosine-releasing implants.
Cell Viability.
Twenty-four days after grafting, the animals were killed and the brains were removed and analyzed histologically. In all cases, the capsules were found to be placed correctly within the lateral ventricle. The tissue surrounding the implant site did not show any abnormalities, as demonstrated by Nissl staining. In particular, no evidence of any cell death surrounding the implant site was detected. Analysis of consecutive sections through the implant sites revealed that the electrodes were positioned correctly within the CA1–CA3 region of the ventral hippocampus.
The viability of the cells in the retrieved capsules was determined by toluidine blue staining. BHK-WT cells were found to survive for at least 24 days after grafting into a lateral rat brain ventricle (Fig. 2f) because there was no apparent change in cell number. In contrast, BHK-AK2 cells displayed prominent cell death (Fig. 2e). Prominent cell death also was observed in both ADA-O and ADA-WT capsules when retrieved from the lateral brain ventricles of kindled rats 24 days after grafting (data not shown).
The cell loss observed in BHK-AK2 capsules was quantified by counting viable cells in squares of 400 × 400 μm (n = 5) in toluidine blue-stained sections from capsules retrieved after 1 and 2 weeks from the lateral brain ventricles of naïve control rats and those retrieved after 24 days from the lateral brain ventricles of kindled rats (Fig. 2). We found that 63 ± 2.8% and 59 ± 15.8% of the encapsulated cells were still viable after 1 and 2 weeks, respectively. However, after being grafted in kindled rats for 24 days, the number of living cells dropped to 15.5 ± 5.2%. Thus, BHK-AK2 cells displayed a limited lifespan after grafting, which is in line with the time course of the loss of anticonvulsant efficacy of the implants. Thus, the loss of the antiseizure efficacy of the grafted cells over time is considered to be due to a decrease of cell viability over time and not to A1 receptor desensitization.
Discussion
Adenosine released locally from encapsulated cell grafts in the rat brain ventricle provided nearly complete protection from kindled seizures. The seizure protection was demonstrated for two different cell lines, ADA-O and BHK-AK2. They were engineered to release adenosine by disruption of the ADA and ADK genes, respectively. BHK-AK2 cells were slightly more efficient in seizure protection than ADA-O cells (Fig. 3), because they released ≈2.3 times as much adenosine (Fig. 1b). In vitro, a single, therapeutically active cell capsule (ADA-O, BHK-AK2) generated steady-state adenosine concentrations in 1 ml of culture medium of 25–100 nM adenosine (7 and 29 ng/ml, respectively). This concentration would be expected to be appreciably lower at the site of anticonvulsant action. Thus, concentrations of adenosine <25 nM at critical sites within the brain appear to be sufficient to provide for seizure suppression.
The antiepileptic activity of adenosine released from the grafts became evident by: (i) a highly significant reduction of seizure scores for a period of up to 24 days, which is most striking during an initial 12-day period; (ii) a reduction of epileptiform afterdischarges during the same period; (iii) the reversal of seizure protection after administration of the A1 receptor antagonist DPCPX in ADA-O-treated rats; and (iv) the apparent lack of A1 receptor desensitization. These results provide a proof of principle that adenosine-releasing cellular implants are effective in seizure suppression. This is of significance mainly for four reasons. (i) A sustained, local delivery of adenosine becomes a therapeutic option because it avoids the strong, peripheral side effects of systemically administered adenosine. (ii) The local delivery of adenosine does not appear to cause central side effects such as sedation or ataxia. (iii) Extracellular adenosine, in contrast to the local delivery of synthetic drugs, is not expected to accumulate, because it is taken up into cells by equilibrative transporters and then is metabolized. (iv) Clinically, local release of low concentrations of adenosine may become an option not only based on cellular delivery but also based on the release from synthetic polymers or the release from pumps, in particular, when the latter are coupled to an integrated system of seizure prediction (29).
The diminution of seizure protection from BHK-AK2 and ADA-O cells between days 12 and 24 was from a reduction of cell viability. Encapsulated WT BHK cells in control experiments survived for at least several weeks. Thus, it cannot be excluded that the chemical mutagenesis, which was used to generate the BHK-AK2 cells, may have led to a diminished viability of these cells. In this regard, a targeted disruption of the Adk gene in embryonic stem cells can be envisaged as a future strategy to develop ADK-deficient adenosine-releasing cells of different developmental lineages. For instance, adenosine-releasing myoblasts with a defined genetic origin might be generated and fused into myotubes, which have been demonstrated to survive for at least 6 months after encapsulation and grafting (30). Because ADA-WT cells display the same viability properties as ADA-O cells, cell survival after encapsulated cell grafting does not depend on the respective Ada allele. It is assumed, rather, that the conditional immortalization paradigm used to generate these cells prohibits the replenishment of cell loss within the capsules at the nonpermissive temperature of rat brain (39°C). As an alternative strategy for the generation of ADA-deficient cells with an enhanced viability, the introduction of survival-enhancing genes such as constitutive active immortalizing oncogenes or apoptosis inhibitors might be envisaged.
Acknowledgments
We thank M. Wakamiya for generously supplying us with a breeding stock of (Adam1/Adam1) mice and P. Jat for sharing the Immortomice. This work was supported by Grants 31-46965.96 and 31-59109.99 of the Swiss National Science Foundation and by the National Centre of Competence in Research on Neural Plasticity and Repair.
Abbreviations
- ADA
adenosine deaminase
- ADK
adenosine kinase
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- BHK
baby hamster kidney
- araA
adenine 9-β-d-arabinofuranoside
- EEG
electroencephalogram
- WT
wild type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Jallon P. Epilepsia. 1997;38:S37–S42. doi: 10.1111/j.1528-1157.1997.tb05203.x. [DOI] [PubMed] [Google Scholar]
- 2.Devinsky O. N Engl J Med. 1999;340:1565–1570. doi: 10.1056/NEJM199905203402008. [DOI] [PubMed] [Google Scholar]
- 3.Ojemann G A. Annu Rev Med. 1997;48:317–328. doi: 10.1146/annurev.med.48.1.317. [DOI] [PubMed] [Google Scholar]
- 4.Dunwiddie T V, Diao L. Neuroscience. 2000;95:81–88. doi: 10.1016/s0306-4522(99)00404-2. [DOI] [PubMed] [Google Scholar]
- 5.Fredholm B B. Int Rev Neurobiol. 1997;40:259–280. [PubMed] [Google Scholar]
- 6.Guieu R, Dussol B, Halimi G, Bechis G, Sampieri F, Berland Y, Sampol J, Couraud F, Rochat H. Gen Pharmacol. 1998;31:553–561. doi: 10.1016/s0306-3623(98)00071-8. [DOI] [PubMed] [Google Scholar]
- 7.Nyce J W. Trends Pharmacol Sci. 1999;20:79–83. doi: 10.1016/s0165-6147(99)01305-x. [DOI] [PubMed] [Google Scholar]
- 8.Feoktistov I, Biaggioni I. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- 9.Jacobson K A. Trends Pharmacol Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredholm B B. News Physiol Sci. 1995;10:122–128. [Google Scholar]
- 11.Palmer T M, Stiles G L. Neuropharmacology. 1995;34:683–694. doi: 10.1016/0028-3908(95)00044-7. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler D B, Randall A, Tsien R W. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 13.Rudolphi K A, Schubert P, Parkinson F E, Fredholm B B. Trends Pharmacol Sci. 1992;13:439–445. doi: 10.1016/0165-6147(92)90141-r. [DOI] [PubMed] [Google Scholar]
- 14.Sebastiao A M, Ribeiro J A. Trends Pharmacol Sci. 2000;21:341–346. doi: 10.1016/s0165-6147(00)01517-0. [DOI] [PubMed] [Google Scholar]
- 15.Dunwiddie T V. Adv Neurol. 1999;79:1001–1010. [PubMed] [Google Scholar]
- 16.Boison D, Scheurer L, Tseng J L, Aebischer P, Mohler H. Exp Neurol. 1999;160:164–174. doi: 10.1006/exnr.1999.7209. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson K A, van Rhee A M. In: Purinergic Approaches in Experimental Therapeutics. Jacobson K A, Jarvis M F, editors. New York: Wiley; 1997. pp. 101–128. [Google Scholar]
- 18.Anderson C M, Xiong W, Geiger J D, Young J D, Cass C E, Baldwin S A, Parkinson F E. J Neurochem. 1999;73:867–873. doi: 10.1046/j.1471-4159.1999.0730867.x. [DOI] [PubMed] [Google Scholar]
- 19.Cass C E, Young J D, Baldwin S A, Cabrita M A, Graham K A, Griffiths M, Jennings L L, Mackey J R, Ng A M, Ritzel M W, et al. Pharmacol Biotechnol. 1999;12:313–352. doi: 10.1007/0-306-46812-3_12. [DOI] [PubMed] [Google Scholar]
- 20.Thorn J A, Jarvis S M. Gen Pharmacol. 1996;27:613–620. doi: 10.1016/0306-3623(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 21.Geiger J, Nagy J I. In: Adenosine and Adenosine Receptors. Williams M, editor. Clifton, NJ: Humana; 1990. pp. 225–288. [Google Scholar]
- 22.Aebischer P, Tresco P A, Winn S R, Greene L A, Jaeger C B. Exp Neurol. 1991;111:269–275. doi: 10.1016/0014-4886(91)90093-r. [DOI] [PubMed] [Google Scholar]
- 23.Aebischer P, Schluep M, Déglon N, Joseph J M, Hirt L, Heyd B, Goddard M, Hammang J P, Zurn A D, Kato A C, et al. Nat Med. 1996;2:696–699. doi: 10.1038/nm0696-696. [DOI] [PubMed] [Google Scholar]
- 24.Buchser E, Goddard M, Heyd B, Joseph J M, Favre J, de Tribolet N, Lysaght M, Aebischer P. Anesthesiology. 1996;85:1005–1012. doi: 10.1097/00000542-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Wakamiya M, Blackburn M R, Jurecic R, McArthur M J, Geske R S, Cartwright J, Mitani K, Vaishnav S, Belmont J W, Kellems R E, et al. Proc Natl Acad Sci USA. 1995;92:3673–3677. doi: 10.1073/pnas.92.9.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jat P S, Noble M D, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Racine R. Neurosurgery. 1978;3:234–252. doi: 10.1227/00006123-197809000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Loscher W, Reissmuller E, Ebert U. Epilepsy Res. 2000;39:211–220. doi: 10.1016/s0920-1211(00)00100-5. [DOI] [PubMed] [Google Scholar]
- 29.Elger C E, Lehnertz K. Eur J Neurosci. 1998;10:786–789. doi: 10.1046/j.1460-9568.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- 30.Déglon N, Heyd B, Tan S A, Joseph J M, Zurn A D, Aebischer P. Hum Gene Ther. 1996;7:2135–2146. doi: 10.1089/hum.1996.7.17-2135. [DOI] [PubMed] [Google Scholar]