Abstract
More than 900 cases of scarlet fever were recorded in Hong Kong during January–July, 2011. Six cases were complicated by toxic shock syndrome, of which 2 were fatal. Pulsed-field gel electrophoresis patterns suggested a multiclonal epidemic; emm12 was the predominant circulating type. We recommend genetic testing of and antimicrobial resistance monitoring for this reportable disease.
Keywords: Scarlet fever, epidemic, Streptococcus pyogenes, emm type, shock, septic, disease notification, antimicrobial resistance, streptococci, bacteria, Hong Kong
Scarlet fever is caused by infection with Streptococcus pyogenes and mainly affects children. An upsurge of scarlet fever occurred in Hong Kong, People’s Republic of China, in 2011, exceeding baseline annual incidence rates for the previous 2 decades. We investigated possible changes in clinical severity, transmissibility, and characteristics of the causative pathogen for this outbreak.
The Study
Scarlet fever is a statutory notifiable disease in Hong Kong. A clinical case is defined as illness in a person who has clinical features of scarlet fever (fever and fine, sandpaper rash of characteristic distribution that blanches on pressure, with or without strawberry tongue, desquamation, or sore throat). A confirmed case is defined as a clinical case with positive throat or wound culture for S. pyogenes or antistreptolysin titer >200.
Epidemiologic, clinical, and laboratory data were collected by standard questionnaire for every reported case. A cluster was defined as >2 cases in persons sharing the same residential or school address within the incubation period. We compared epidemiologic, clinical, and microbiological features of the scarlet fever cases from January–July 2011 (outbreak period) with features of those reported during 2008–2010 (baseline period). We used SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) for analyses; p<0.05 was considered significant.
For comparison, we performed a retrospective review of hospital discharge records kept by public hospitals. We extracted records of patients hospitalized during January 2008–July 2011 who had diagnoses that are known complications of scarlet fever, including toxic shock syndrome, acute rheumatic fever, and acute glomerulonephritis. These cases were reviewed to determine whether the complications were related to scarlet fever.
Bacterial culture of S. pyogenes was performed on diagnostic specimens in hospital laboratories and the Public Health Laboratory Centre of the Department of Health; the latter serves as the diagnostic and public health reference laboratory in Hong Kong. Antimicrobial drug susceptibility testing, emm typing, and detection of various virulence genes were performed at the Public Health Laboratory Centre on S. pyogenes isolates received during 2011 and archived during 2008–2010 (1). Pulsed-field gel electrophoresis (PFGE) was performed on the basis of the gram-positive protocol, and PFGE profiles were analyzed by using BioNumerics 5.0 software (Applied Maths, Sint-Martens-Latem, Belgium).
In June 2011, the Department of Microbiology of the University of Hong Kong announced the discovery of a unique 48-kb insertion sequence in the genome of S. pyogenes isolated from a blood specimen from a 7-year-old girl who died of scarlet fever (2). We tested for this insert in a sample of strains collected during 2008–2011 using the method provided by the University of Hong Kong.
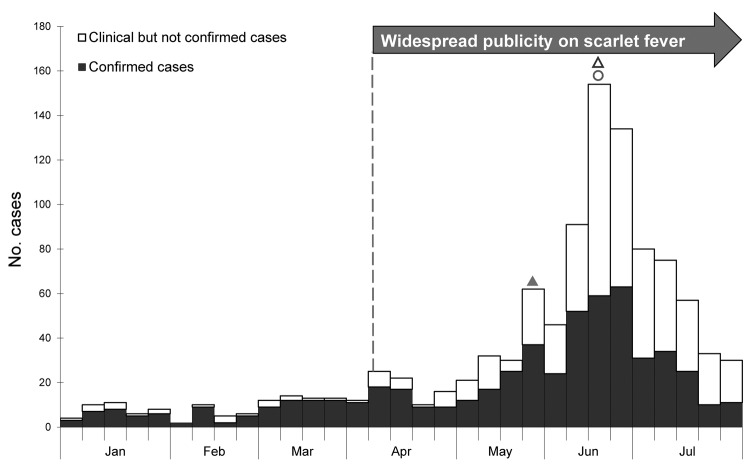
During January 1–July 31, 2011, a total of 996 cases of scarlet fever were reported, greatly exceeding the annual number of cases reported during 2008 (235), 2009 (187), and 2010 (128). Outbreak activity in 2011 peaked at week 26 (week ending June 25) (Figure 1). During the outbreak period (January–July 2011), the annualized incidence rate was 24.0/100,000 population, ≈9× higher than the average annualized incidence rate of 2.62/100,000 population during the baseline period of 2008–2010. During the previous 2 decades, baseline annual incidence rates ranged from 0.0351 to 3.37 cases/100,000 population.
Figure 1.
Weekly number of scarlet fever cases, by onset date, Hong Kong, January–July 2011. White bars indicate clinically diagnosed but not laboratory-confirmed cases; solid bars indicate laboratory-confirmed cases. Solid triangle indicates May 30 dissemination of press release about first fatal case (in a 7-year-old girl); open triangle indicates June 21 dissemination of press release about second fatal case (in a 5-year-old boy); circle indicates June 23 launch of health education campaign.
Table 1 compares the epidemiologic features, clinical features, and laboratory results for scarlet fever cases reported during 2011 and 2008–2010. Highest incidence (547 cases/100,000 population) was reported for children 4–7 years of age (Table 1). Clinical features, complications, and case-fatality rate for cases reported in 2011 were largely comparable to those reported during the baseline period. The proportion of case-patients requiring hospitalization during 2011 was lower, and mean duration of hospital stay was ≈0.5 days shorter than for the baseline period. Details of the 9 complicated cases are shown in Table 2.
Table 1. Epidemiologic characteristics, clinical features, and laboratory results for scarlet fever cases reported in Hong Kong during January–July 2011 compared with cases reported during 2008–2010.
| Characteristic* | 2008–2010, n = 550 | January 1–July 31, 2011, n = 996 | p value |
|---|---|---|---|
| Epidemiology | |||
| Sex ratio, M:F | 1.6:1 | 1.5:1 | 0.50 |
| Age range (median) | 9 mo–40 y (5 y) | 1 mo–51 y (6 y) | 0.40 |
| Local cases | 98.0 (539/550) | 97.4 (970/996) | 0.56 |
| Clustering | |||
| Cases in a cluster | 5.45 (30/550) | 14.4 (143/996) | <0.0001† |
| Cases in home clusters | 3.3 (18/550); 9 clusters | 6.5 (65/996); 31 clusters | |
| Cases in each home cluster, range (median) | 2 (2) | 2–3 (2) | 0.34 |
| Cases in school clusters | 2.2 (12/550); 4 clusters | 7.8 (78/996); 28 clusters | |
| Persons affected in each school cluster, no. (median) | 2–4 (3) | 2–7 (2) | 0.42 |
| Clinical features | |||
| Fever | 95.6 (526/548) | 93.2 (928/996) | 0.065 |
| Sandpaper rash | 97.4 (534/548) | 95.4 (950/996) | 0.13 |
| Strawberry tongue | 45.1 (248/550) | 51.4 (512/996) | 0.020‡ |
| Sore throat | 74.4 (409/550) | 78.5 (782/996) | 0.073 |
| Desquamation | 27.8 (153/550) | 23.7 (236/996) | 0.084 |
| Hospitalization | 63.9 (351/549) | 56.6 (561/991) | 0.005§ |
| Duration of hospitalization, d (mean) | 1– 25 (3.8) | 1–33 (3.3) | 0.005¶ |
| Concomitant chickenpox | 5.5 (30/550) | 1.9 (19/996) | 0.0002# |
| Complications** | 0.73 (4/550) | 0.90 (9/996) | 0.79 |
| Toxic shock syndrome | 0.18 (1/550) | 0.60 (6/996) | 0.43 |
| Case-fatality rate | 0 | 0.20 (2/996) | 0.54 |
| Laboratory results | |||
| Laboratory confirmation | 46.0 (253/550) | 51.8 (533/996) | 0.0055†† |
| Positive throat or wound culture | 95.3 (241/253) | 97.2 (521/533) | 0.094 |
| Antistreptolysin O titer >200 IU/mL | 4.74 (12/253) | 4.37 (12/533) | 0.094 |
*Values are % (no./total no.) unless otherwise indicated. Lower denominators indicate data missing or not applicable. †χ2 = 27. ‡χ2 = 5.40. §χ2 = 7.85. ¶t = –2.8 (95% CI of difference 0.15–0.83 d). #χ2 = 14. **Complications include toxic shock syndrome, septicaemia, parapharyngeal abscess, rheumatic fever, quinsy and hepatitis. ††χ2 = 7.7.
Table 2. Clinical characteristics of patients with scarlet fever who had medical complications, Hong Kong, January–July 2011*.
| Characteristic | Case-patient no. |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Patient age, y/sex | 14/M | M/11 | 8/F | 7/F | 5/M | 6/M | 3/M | 2/F | 12/M |
| Month of illness onset | April | April | April | May | June | June | July | July | July |
| Days from symptom onset to hospital admission | 1 | 4 | 7 | 7 | 4 | 10 | 1 | 1 | 0 |
| Complications | TSS | Parapharyngeal abscess | TSS | TSS | TSS | Septicemia | TSS | TSS | Septicemia |
| Intensive care unit admission | No | No | Yes | Yes | Yes | Yes | No | Yes | No |
| Concomitant chickenpox infection | No | No | No | No | Yes | No | Yes | No | Yes |
| Recovered | Yes | Yes | Yes | No (died) | No (died) | Yes | Yes | Yes | Yes |
| S. pyogenes isolates | Throat | Throat | None | Blood, lower limb blister fluid | Blood and pus | Blood | Throat | Throat | Blood and pus |
| emm type | NA | NA | NA | emm12 | emm1 | emm12 | NA | NA | emm12 |
| 48-kb insert | NA | NA | NA | + | + | + | NA | NA | – |
| Virulence geneprofile (speA¸speB, speC, speF, speH, ssa) | NA | NA | NA | −++−++ | ++++−+ | −+++++ | NA | NA | − + + + + − |
*All case-patients were healthy before infection. TSS, toxic shock syndrome; NA, not available; +, positive; –, negative.
Among the 996 scarlet fever cases reported during January–July 2011, S. pyogenes isolates from samples from 90 patients (mostly throat swab specimens) were characterized. Strains found belonged to the following emm types (number and percentage of strains): emm12 (70, 77.8%), emm1 (14, 15.6%), emm4 (2, 2.2%), emm22 (2, 2.2%), emm2 (1, 1.1%), and emm3 (1, 1.1%). All strains were susceptible to penicillin, but 77 (85.6%) strains were resistant to erythromycin. Of 59 strains tested for the 48-kb insert, 78.0% (46 strains) tested positive; 39 were emm12 strains and 7 emm1 strains. Antimicrobial drug susceptibility results were available for 39 strains positive for the 48-kb insert; 3 (7.70%) were susceptible to erythromycin. Conversely, among all erythromycin-resistant emm12 strains in 2011 tested for the 48-kb insert, 6/42 (14.3%) yielded a negative result.
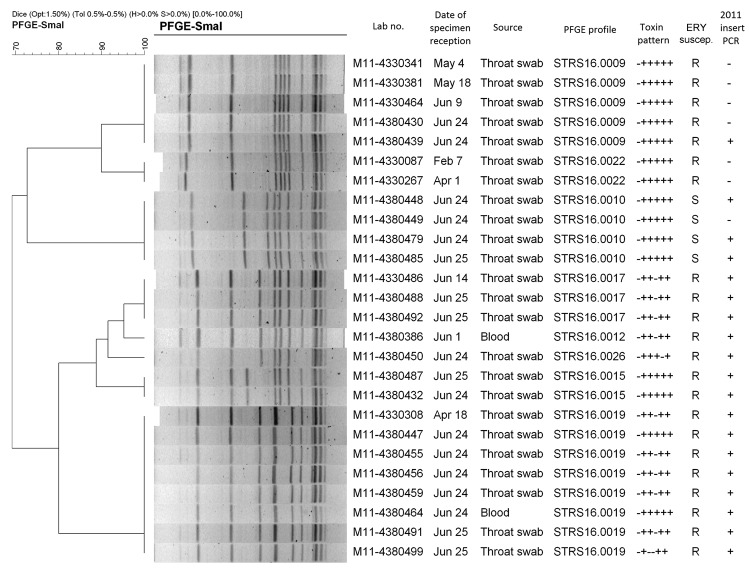
Forty-eight emm12 isolates during January–June 2011 that were subjected to virulence gene profiling showed 5 virulence gene profiles. No particular virulence gene profile was dominant among the 9 scarlet fever cases associated with medical complications (Table 2). Among 26 emm12 strains subjected to PFGE, 7 patterns were detected; the emm12 strain from 1 of the 2 fatal cases exhibited a unique PFGE pattern (Figure 2). For the other fatal case, an emm1 strain positive for speA was isolated.
Figure 2.
Pulsed-field gel electrophoresis patterns of 26 emm type 12.0 Streptococcus pyogenes strains, Hong Kong, 2011. Toxin profile results are shown as corresponding to the genes speA¸ speB, speC, speF, speH, and ssa. Strain M11–4380386 was from a fatal case. ERY suscep., erythromycin susceptibility result; R, resistant; S, susceptible. Scale bar indicates percent similarity.
Of the archived S. pyogenes strains collected during 2008–2010, few strains were from patients diagnosed with scarlet fever; therefore, we analyzed S. pyogenes strains isolated from throat and superficial wound specimens from outpatients <15 years of age. Among 28 such strains, emm28 was detected in 9 strains; emm4 in 4 strains; emm1 in 3 strains; emm12, 22, and 89 in 2 strains each; and 6 other emm types in 1 strain each. All strains were susceptible to penicillin; the erythromycin resistance rate was 10.7% (3/28). The 48-kb insert was found in 10 (35.7%) strains: 3 strains of emm28, and 1 strain each of emm types 1, 2, 4, 22, 44, 89, and stG485.
Conclusions
The 2011 S. pyogenes outbreak in Hong Kong attracted heightened media coverage, which might have increased reporting of cases; however, the higher proportion of laboratory-confirmed cases in 2011 than those during 2008–2010 suggests the upsurge was genuine. Overall clinical and epidemiologic profiles in 2011 did not differ from previous years. We found insufficient evidence that a particular emm type of virulence gene profile or presence of the 48-kb insert was associated with increased incidence or severity.
The reasons for the upsurge remain obscure. Laboratory findings showed diverse patterns of S. pyogenes strains, suggesting a multiclonal epidemic. The 48-kb insert identified in 2011 was found in S. pyogenes strains isolated in 2008–2010, albeit at lower rate (35.7% in 2008–2010 vs.78% in 2011). Thus, it is difficult to attribute the upsurge to the insert alone. A shift in prevailing emm type that occurred in 2011 might have contributed to fluctuations in the number of cases (3).
A higher rate of erythromycin resistance in S. pyogenes (>80%) was found in 2011 than in the reported previous years (20%–30%) (4). Because all erythromycin-resistant strains were also resistant to clindamycin (data not shown), we deduced the resistance mechanism to be resistance to macrolides, lincosamides, and streptogramins B system, as encoded by the erm genes (5).
The 48-kb insert provided a mechanism for macrolide resistance among S. pyogenes in Hong Kong, but our laboratory investigation found macrolide-resistant S. pyogenes strains and the macrolide-susceptible strains that bore them negative for this insert. Mutation of the PCR primer binding site might explain the former strains; further investigation is needed to explore this possibility.
The upsurge in scarlet fever cases in Hong Kong during 2011 likely reflects a regional phenomenon; a marked increase in cases was also observed in mainland China (6) and Macao (7) during this period. High resistance rates against macrolides were also observed for the outbreak in mainland China (8). We recommend close monitoring and surveillance of disease activity, genetic testing, antimicrobial susceptibility profiling, and maintaining scarlet fever’s statutory notifiable status.
Acknowledgments
We thank all members of the Surveillance and Epidemiology Branch and Public Health Laboratory Services Branch of Department of Health for their contributions to this work.
Biography
Dr Luk is a medical and health officer at the Centre for Health Protection, Department of Health, Hong Kong, People’s Republic of China. She was in the US Centers for Disease Control and Prevention’s Field Epidemiology Training Program during this study. Her research interests include studying the epidemiology and transmission of communicable disease.
Footnotes
Suggested citation for this article: Luk EYY, Lo JYC, Li AZL, Lau MCK, Cheung TKM, Wong AYM, et al. Scarlet fever epidemic, Hong Kong, 2011. Emerg Infect Dis [Internet]. 2012 Oct [date cited]. http://dx.doi.org/10.3201/eid1810.111900
References
- 1.Chan JC, Chu YW, Chu MY, Cheung TK, Lo JY. Epidemiological analysis of Streptococcus pyogenes infections in Hong Kong. Pathology. 2009;41:681–6. 10.3109/00313020903257723 [DOI] [PubMed] [Google Scholar]
- 2.University of Hong Kong. The University of Hong Kong finds genetic mutation in Streptococcus pyogenes possible cause for the recent community outbreak of scarlet fever [press release]. 2011. Jun 20 [cited 2011 July 27]. http://www.hku.hk/press/news_detail_6505.html
- 3.Chiou CS, Wang YW, Chen PL, Wang WL, Wu PF, Wei HL. Association of the shuffling of Streptococcus pyogenes clones and the fluctuation of scarlet fever cases between 2000 and 2006 in central Taiwan. BMC Microbiol. 2009;9:115. 10.1186/1471-2180-9-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho PL, Johnson DR, Yue AW, Tsang DN, Que TL, Beall B, et al. Epidemiologic analysis of invasive and noninvasive group a streptococcal isolates in Hong Kong. J Clin Microbiol. 2003;41:937–42. 10.1128/JCM.41.3.937-942.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livermore DM, Winstanley TG, Shannon KP. Interpretative reading: recognizing the unusual and inferring resistance mechanisms from resistance phenotypes. J Antimicrob Chemother. 2001;48(Suppl 1):87–102. 10.1093/jac/48.suppl_1.87 [DOI] [PubMed] [Google Scholar]
- 6.China Ministry of Health. China Ministry of Health notifiable disease statistics. 2011. Jul [cited 2011 Aug 19]. http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohjbyfkzj/s3578/201107/52298.htm
- 7.World Health Organization Western Pacific Region. Scarlet fever update, 14 July 2011. [cited 2011 Jul 27]. http://www.wpro.who.int/emerging_diseases/ScarletFever/en/index.html
- 8.Liang Y, Shen X, Huang G, Wang C, Shen Y, Yang Y. Characteristics of Streptococcus pyogenes strains isolated from Chinese children with scarlet fever. Acta Paediatr. 2008;97:1681–5. 10.1111/j.1651-2227.2008.00983.x [DOI] [PubMed] [Google Scholar]