Abstract
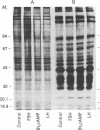
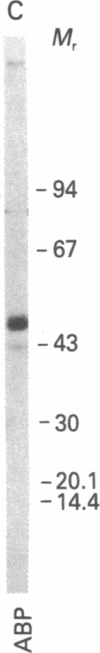
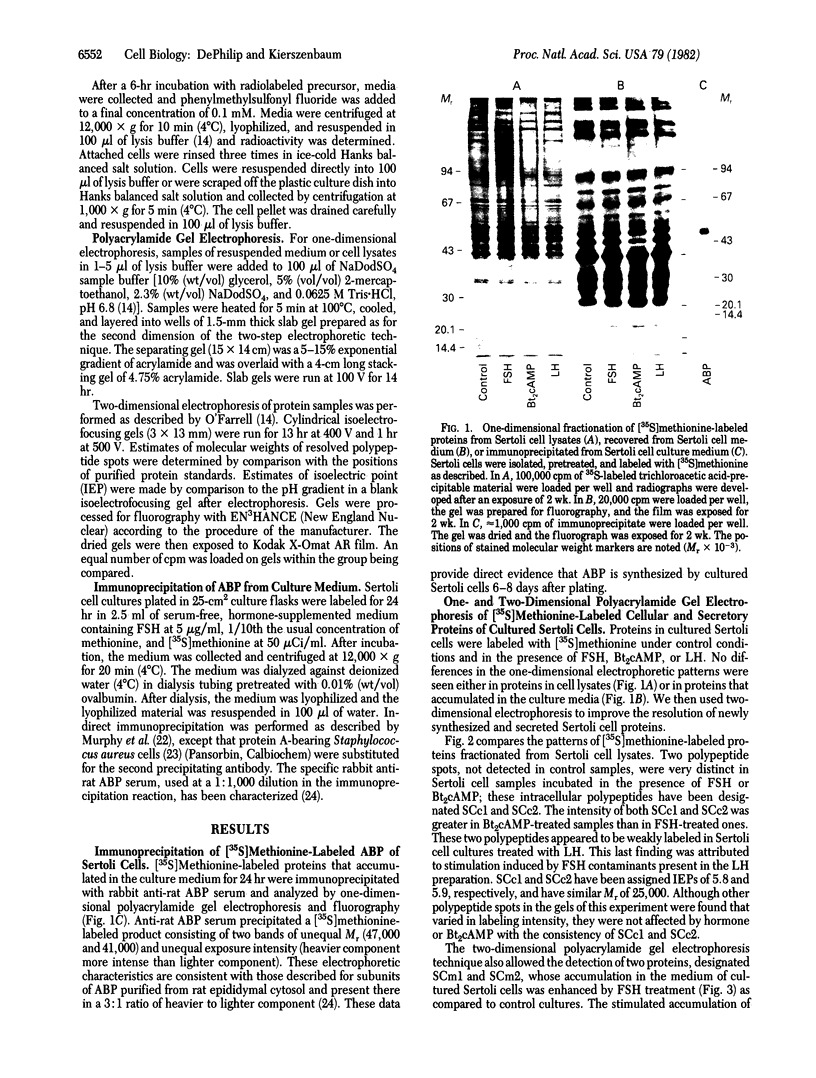
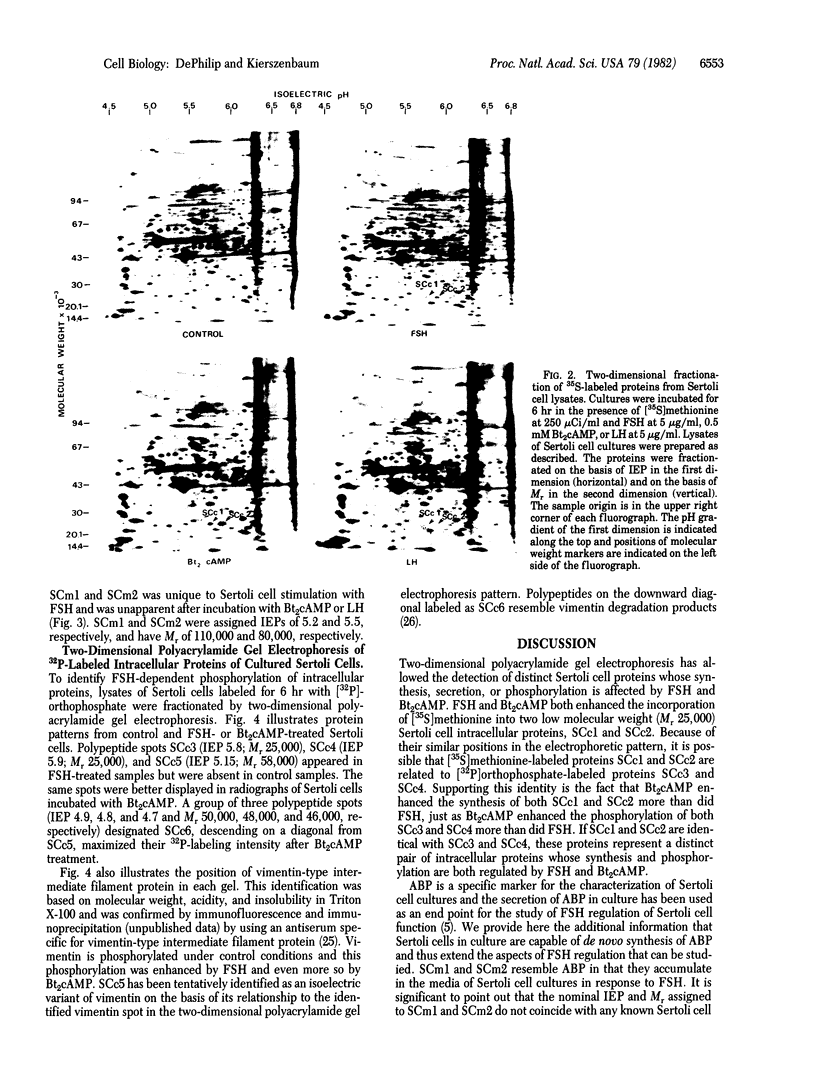
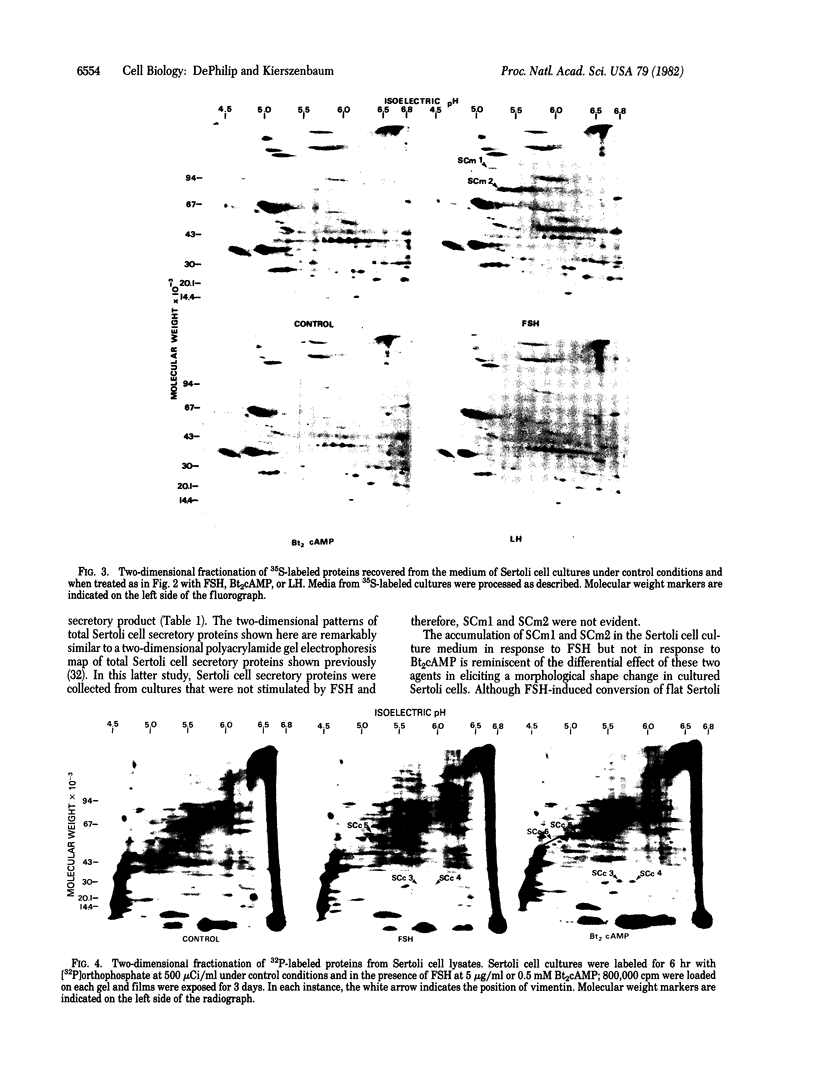
The accumulation of two polypeptides, SCm1 and SCm2, in the medium of Sertoli cell cultures is enhanced by follicle-stimulating hormone (FSH) but is unaffected by either the cAMP analog, N6,O2'-dibutyrl cAMP or luteinizing hormone. The assigned molecular weights of SCm1 and SCm2 differ from those of androgen-binding protein subunits or any other previously identified Sertoli cell secretory product. Incubation of Sertoli cell cultures with either FSH or N6,O2'-dibutyryl cAMP also stimulates the incorporation of [35S]methionine into two intracellular polypeptides, SCc1 and SCc2. In addition, the phosphorylation of three intracellular polypeptides, SCc3, SCc4, and SCc5, is intensified when Sertoli cell cultures are treated with either FSH or N6,O2'-dibutyryl cAMP. Based on these results and on previous work, we conclude that (i) SCm1 and SCm2 may, like androgen-binding protein, be secreted by Sertoli cells and function extracellularly while SCc1 and SCc2 are involved in FSH-dependent intracellular activity; (ii) SCc3, SCc4, and SCc5 are possible substrates for FSH-stimulated, cAMP-dependent protein kinase activity; and (iii) SCc5 is an isoelectric variant of vimentin-type intermediate filament protein presumably involved in FSH- and N6,O2'-dibutyryl cAMP-induced Sertoli cell shape changes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottenstein J., Hayashi I., Hutchings S., Masui H., Mather J., McClure D. B., Ohasa S., Rizzino A., Sato G., Serrero G. The growth of cells in serum-free hormone-supplemented media. Methods Enzymol. 1979;58:94–109. doi: 10.1016/s0076-6879(79)58127-0. [DOI] [PubMed] [Google Scholar]
- Browning E. T., Sanders M. M. Vimentin: a phosphoprotein under hormonal regulation. J Cell Biol. 1981 Sep;90(3):803–808. doi: 10.1083/jcb.90.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrington J. H., Fritz I. B. Cellular localization of 5alpha-reductase and 3alpha-hydroxysteroid dehydrogenase in the seminiferous tubule of the rat testis. Endocrinology. 1975 Apr;96(4):897–889. doi: 10.1210/endo-96-4-879. [DOI] [PubMed] [Google Scholar]
- Fakunding J. L., Means A. R. Characterization and follicle stimulating hormone activation of Sertoli cell cyclic AMP-dependent protein kinases. Endocrinology. 1977 Nov;101(5):1358–1368. doi: 10.1210/endo-101-5-1358. [DOI] [PubMed] [Google Scholar]
- Feldman M., Lea O. A., Petrusz P., Tres L. L., Kierszenbaum A. L., French F. S. Androgen-binding protein. Purification from rat epididymis, characterization, and immunocytochemical localization. J Biol Chem. 1981 May 25;256(10):5170–5175. [PubMed] [Google Scholar]
- Franke W. W., Grund C., Schmid E. Intermediate-sized filaments present in Sertoli cells are of the vimentin type. Eur J Cell Biol. 1979 Aug;19(3):269–275. [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz I. B., Rommerts F. G., Louis B. G., Dorrington J. H. Regulation by FSH and dibutyryl cyclic AMP of the formation of androgen-binding protein in Sertoli cell-enriched cultures. J Reprod Fertil. 1976 Jan;46(1):17–24. doi: 10.1530/jrf.0.0460017. [DOI] [PubMed] [Google Scholar]
- Gordon W. E., 3rd, Bushnell A., Burridge K. Characterization of the intermediate (10 nm) filaments of cultured cells using an autoimmune rabbit antiserum. Cell. 1978 Feb;13(2):249–261. doi: 10.1016/0092-8674(78)90194-0. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell. 1979 Dec;18(4):1053–1063. doi: 10.1016/0092-8674(79)90218-6. [DOI] [PubMed] [Google Scholar]
- Hansson V. Further characterization of the 5 -dihydrotestosterone binding protein in the epididymal cytosol fraction. In vitro studies. Steroids. 1972 Nov;20(5):575–596. doi: 10.1016/0039-128x(72)90016-5. [DOI] [PubMed] [Google Scholar]
- Karl A. F., Griswold M. D. Prolonged ABP synthesis by Sertoli cells cultured in defined medium. Cell Biol Int Rep. 1980 Jul;4(7):669–674. doi: 10.1016/0309-1651(80)90206-4. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kierszenbaum A. L., Feldman M., Lea O., Spruill W. A., Tres L. L., Petrusz P., French F. S. Localization of androgen-binding protein in proliferating Sertoli cells in culture. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5322–5326. doi: 10.1073/pnas.77.9.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix M., Smith F. E., Fritz I. B. Secretion of plasminogen activator by Sertoli cell enriched cultures. Mol Cell Endocrinol. 1977 Dec;9(2):227–236. doi: 10.1016/0303-7207(77)90124-1. [DOI] [PubMed] [Google Scholar]
- McCullagh D. R. DUAL ENDOCRINE ACTIVITY OF THE TESTES. Science. 1932 Jul 1;76(1957):19–20. doi: 10.1126/science.76.1957.19. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Kopchick J. J., Watson K. F., Arlinghaus R. B. Cell-free synthesis of a precursor polyprotein containing both gag and pol gene products by Rauscher murine leukemia virus 35S RNA. Cell. 1978 Feb;13(2):359–369. doi: 10.1016/0092-8674(78)90204-0. [DOI] [PubMed] [Google Scholar]
- Müller U., Siebers J. W., Zenzes M. T., Wolf U. The testis as a secretory organ for H-Y antigen. Hum Genet. 1978 Dec 18;45(2):209–213. doi: 10.1007/BF00286965. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., Balzer D. R., Jr, Lazarides E. Phosphorylation of subunit proteins of intermediate filaments from chicken muscle and nonmuscle cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):819–823. doi: 10.1073/pnas.76.2.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C. M., Gard D. L., Lazarides E. Phosphorylation of intermediate filament proteins by cAMP-dependent protein kinases. Cell. 1981 Jan;23(1):135–143. doi: 10.1016/0092-8674(81)90278-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Picard J. Y., Tran D., Josso N. Biosynthesis of labelled anti-müllerian hormone by fetal testes: evidence for the glycoprotein nature of the hormone and for its disulfide-bonded structure. Mol Cell Endocrinol. 1978 Oct;12(1):17–30. doi: 10.1016/0303-7207(78)90098-9. [DOI] [PubMed] [Google Scholar]
- Price J. M. The secretion of Mullerian inhibiting substance by cultured isolated Sertoli cells of the neonatal calf. Am J Anat. 1979 Sep;156(1):147–157. doi: 10.1002/aja.1001560116. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Dryburgh H., Millership A., Matsuda Y., Inglis A., Phillips J., Edwards K., Maggs J. The synthesis and secretion of rat transferrin. J Biol Chem. 1979 Dec 10;254(23):12013–12019. [PubMed] [Google Scholar]
- Sharpe R. M., Fraser H. M., Cooper I., Rommerts F. F. Sertoli-Leydig cell communication via an LHRH-like factor. Nature. 1981 Apr 30;290(5809):785–787. doi: 10.1038/290785a0. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Sertoli cells synthesize and secrete transferrin-like protein. J Biol Chem. 1980 Oct 25;255(20):9523–9525. [PubMed] [Google Scholar]
- Spruill W. A., White M. G., Steiner A. L., Tres L. L., Kierszenbaum A. L. Temporal sequence of cell shape changes in cultured rat sertoli cells after experimental elevation of intracellular cAMP. Exp Cell Res. 1981 Jan;131(1):131–148. doi: 10.1016/0014-4827(81)90414-6. [DOI] [PubMed] [Google Scholar]
- Steinberger A., Heindel J. J., Lindsey J. N., Elkington J. S., Sanborn B. M., Steinberger E. Isolation and culture of FSH responsive Sertoli cells. Endocr Res Commun. 1975;2(3):261–272. doi: 10.3109/07435807509053853. [DOI] [PubMed] [Google Scholar]
- Steinberger A., Steinberger E. Secretion of an FSH-inhibiting factor by cultured Sertoli cells. Endocrinology. 1976 Sep;99(3):918–921. doi: 10.1210/endo-99-3-918. [DOI] [PubMed] [Google Scholar]
- Strickland S., Beers W. H. Studies on the role of plasminogen activator in ovulation. In vitro response of granulosa cells to gonadotropins, cyclic nucleotides, and prostaglandins. J Biol Chem. 1976 Sep 25;251(18):5694–5702. [PubMed] [Google Scholar]
- Welsh M. J., Wiebe J. P. Rat sertoli cells: a rapid method for obtaining viable cells. Endocrinology. 1975 Mar;96(3):618–624. doi: 10.1210/endo-96-3-618. [DOI] [PubMed] [Google Scholar]
- Wright W. W., Musto N. A., Mather J. P., Bardin C. W. Sertoli cells secrete both testis-specific and serum proteins. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7565–7569. doi: 10.1073/pnas.78.12.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]