Summary
The development of human cancers is frequently associated with a failure of epithelial cells to form tight junctions and to establish proper apicobasal polarity. Interestingly, the oncogenic potential of the adenovirus E4-ORF1 protein correlates with its binding to the cellular PDZ proteins MUPP1, MAGI-1, ZO-2 and SAP97, the first three of which assemble protein complexes at tight junctions. Given that E4-ORF1 sequesters these three PDZ proteins in the cytoplasm of fibroblasts, we postulated that E4-ORF1 would inhibit tight junction formation in epithelial cells. Providing further support for this idea, we identified MUPP1-related PATJ, a key component of the tight junction-associated CRB3-PALS1-PATJ polarity complex, as a new PDZ-protein target for both the E4-ORF1 and high-risk human papillomavirus type 18 E6 oncoproteins. Moreover, in epithelial cells, E4-ORF1 blocked the tight junction localization of PATJ and ZO-2, as well as their interacting partners, and disrupted both the tight junction barrier and apicobasal polarity. These significant findings expose a direct link between the tumorigenic potential of E4-ORF1 and inactivation of cellular PDZ proteins involved in tight junction assembly and polarity establishment.
Keywords: Tight junction, Polarity, PATJ, ZO-2, E4-ORF1, E6
Introduction
Oncoproteins encoded by tumorigenic viruses are powerful tools for elucidating mechanisms responsible for the development of cancer (Butel, 2000). Human adenovirus type 9 (Ad9) generates exclusively mammary tumors in experimental animals (Javier et al., 1991), and the E4 region-encoded ORF1 (E4-ORF1) protein represents the primary oncogenic determinant of this virus (Javier, 1994). Three discrete regions (I, II and III) of the 125 amino-acid residue E4-ORF1 protein are required for its tumorigenic potential (Weiss et al., 1997a). Regions I and II define a single domain that binds to an undetermined cellular phosphoprotein (S. Chung and R.T.J., unpublished data), whereas carboxyl-terminal region III is a type I PDZ-domain-binding motif (PBM) having the consensus sequence -X-(S/T)-X-(V/I/L)-COOH (X = any amino acid residue). The PBM of E4-ORF1 has been shown to mediate interactions with a select group of cellular PDZ-domain-containing proteins, including MUPP1 (Lee et al., 2000), MAGI-1 (Glaunsinger et al., 2000), ZO-2 (Glaunsinger et al., 2001) and DLG/SAP97 (Lee et al., 1997).
Providing plausible links for these PDZ proteins to cancer, MUPP1, ZO-2 and its interacting partner ZO-1, and SAP97 are suspected tumor suppressor proteins (Fuja et al., 2004; Gonzalez-Mariscal et al., 2003; Martin et al., 2004; Watson et al., 2002). Moreover, MUPP1 and ZO-2, as well as MAGI-1, are aberrantly sequestered by E4-ORF1 in the cytoplasm of transformed fibroblasts (Glaunsinger et al., 2000; Glaunsinger et al., 2001; Lee et al., 2000). Also noteworthy is that the E6 oncoproteins encoded by high-risk human papillomaviruses (HPVs), the causative agents of cervical carcinomas in women, possess a type I PBM required for binding to select PDZ proteins, including MUPP1, MAGI-1 and SAP97, and for targeting them for degradation in cells (Gardiol et al., 1999; Glaunsinger et al., 2000; Lee et al., 2000). These interactions also may contribute to HPV-induced tumorigenesis, as functional disruption of the E6 PBM abolishes E6-mediated transformation and abnormal proliferation of cells (Kiyono et al., 1997; Nguyen et al., 2003a; Nguyen et al., 2003b). Taken together, these findings suggest that inactivation of certain PDZ proteins in cells may provoke the development of cancer.
In epithelial cells of vertebrates, E4-ORF1-interacting PDZ proteins localize to specialized membrane structures present at sites of cell-cell contact, where SAP97 associates with the adherens junction (AJ) (Reuver and Garner, 1998) and MUPP1, MAGI-1 and ZO-2 associate with the tight junction (TJ) (Gumbiner et al., 1991; Hamazaki et al., 2002; Ide et al., 1999). While the AJ is responsible for cell-cell adhesion (Gumbiner, 1996), the TJ acts as an impermeable barrier that divides epithelial cells into functionally distinct apical and basolateral membrane domains, and also serves as a paracellular barrier to separate the external environment from internal tissue compartments (Yeaman et al., 1999). Notably, TJ disruption and loss of apicobasal polarity are common features of epithelial-derived cancer cells (Cochand-Priollet et al., 1998; Soler et al., 1999), and accumulating evidence suggests that such defects directly contribute to carcinogenesis by deregulating normal proliferation and differentiation programs in epithelial cells (Matter and Balda, 2003).
The TJ consists of several integral membrane proteins, including occludin, claudins and JAMs, as well as a number of peripheral membrane proteins, many of which are PDZ proteins (Gonzalez-Mariscal et al., 2003). Considering that PDZ proteins generally function as scaffolding proteins to organize supramolecular complexes at specialized sites of the plasma membrane (Harris and Lim, 2001), the E4-ORF1-interacting PDZ proteins MUPP1, MAGI-1 and ZO-2 represent attractive candidates for regulators of TJ assembly. Consistent with this possibility, ZO-2 has been proposed to stabilize TJs by linking TJ proteins to the actin cytoskeleton (Gonzalez-Mariscal et al., 2003) and to compensate partially for TJ defects in cells unable to express the closely related protein ZO-1 (Umeda et al., 2004). Additionally, MAGI-1 can enhance JAM4-mediated cell adhesion and sealing activities in epithelial cells, possibly through recruitment of ZO-1 and occludin to JAM4-mediated membrane adhesion sites (Hirabayashi et al., 2003). Finally, MUPP1 is a paralogue of PATJ, a key component of the evolutionarily conserved CRB3-PALS1-PATJ polarity complex required for TJ establishment and proper apicobasal polarity in epithelial cells (Shin et al., 2005). The fact that, like PATJ, MUPP1 also binds PALS1 hints that PATJ and MUPP1 may share common functions (Roh and Margolis, 2003).
Given that E4-ORF1 sequesters MUPP1, MAGI-1 and ZO-2 in the cytoplasm of fibroblasts, we hypothesized that this viral oncoprotein would inhibit TJ formation and apicobasal polarity establishment in epithelial cells. Supporting this assertion, we identified the PATJ polarity protein as a new cellular TJ target for not only E4-ORF1 but also for the high-risk HPV type 18 (HPV-18) E6 oncoprotein. We further showed that, in addition to preventing TJ localization of PATJ and ZO-2, and their associated proteins, E4-ORF1 also functionally disrupts TJs and impairs establishment of proper apicobasal polarity in epithelial cells. These findings reveal an intriguing link between the tumorigenic potential of adenovirus E4-ORF1 and its capacity to functionally inactivate important cellular TJ-associated PDZ proteins.
Materials and Methods
Plasmids
Plasmid pRK5-myc encoding wild type (wt), ΔPDZ6, or ΔPDZ8 human PATJ cDNA (Roh et al., 2002a; Roh et al., 2002b) and plasmids pCMVBam3-Neo, pGEX-2T, pGEX-2TK and pGW1 encoding wt or mutant Ad9 E4-ORF1 cDNA (Frese et al., 2003; Lee et al., 2000; Weiss et al., 1997a; Weiss and Javier, 1997; Weiss et al., 1997b) have been described. pGEX-2T plasmids encoding HPV types 11 and 18 E6 were generous gifts from P. Howley. HA epitope-tagged wt PKB was subcloned from pCDNA3-HA-PKB (a gift from J. Woodgett) into pGW1 to create pGW1-HA-PKB.
Cell lines and transfections
Cells were maintained in DME medium (Life Technologies, Inc.) supplemented with antibiotics and 10% fetal bovine serum (FBS) (Gemini Bioproducts).
COS7 and MDCK II cells were transfected with Lipofectamine (Invitrogen Life) or Fugene 6 (Roche Molecular Biochemicals), respectively, according to manufacturer’s recommendations. In transient transfection assays, analyses were conducted 48 hours post-transfection.
MDCK II cell lines or pools stably expressing wt or mutant E4-ORF1 proteins were generated by transfection with the corresponding pCMVBam3-Neo-E4-ORF1 plasmid followed by selection in medium containing 600 μg/ml G418 (Life Technologies, Inc.). Cell lines were derived by isolation of individual G418-resistant colonies. Cell lines and cell pools were propagated in medium containing 300 μg/ml G418.
Antibodies
Rabbit polyclonal antibodies specific for Ad9 E4-ORF1 (Javier, 1994), MUPP1 (Lee et al., 2000), PATJ and PALS1 (Roh et al., 2002b) and ZO-2 (Glaunsinger et al., 2001) were employed. Polyclonal antibodies to phosphoSer473 PKB and Erk1/2 (Cell Signaling Technologies), Par3 (Upstate), as well as monoclonal antibodies to E-cadherin (Sigma), HA (Covance), Myc (Novus Biologicals), ZO-1 (Zymed Laboratories), SAP97 (Stressgen Biotechnologies), and β-catenin (BD Transduction Laboratories) were used according to the manufacturers’ instructions. Anti-gp135 antibody was a gift from George Ojakian. Horseradish peroxidase-conjugated (Southern Biotechnology Associates) and fluorochrome-conjugated (Jackson ImmunoResearch Laboratories or Molecular Probes) secondary antibodies were utilized.
Cell extracts and fractionations
Preparation of cell extracts was carried out as described previously (Lee et al., 2000). Fractionation assays were performed by centrifugation (10,000 g, for 5 minutes at 4°C) of extracts from cells lysed in RIPA buffer (50 mM Tris-HCl pH 8.0, 150 mm NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) to generate a detergent-soluble supernatant and a detergent-insoluble pellet; the pellet was then resuspended in a volume of sample buffer [0.065 mM Tris-HCl (pH 6.8), 2% (wt/vol) sodium dodecyl sulfate (SDS), 10% (vol/vol) β-mercaptoethanol, 0.005% bromophenol blue] equal to that of the detergent-soluble supernatant. An equal volume of each fraction was immunoblotted with either PATJ or E4-ORF1 antibodies. Protein concentrations in cell extracts were determined by the Bradford method (Bradford, 1976).
GST pulldown and protein blotting assays
GST fusion proteins were expressed in bacteria and affinity purified by standard methods, and GST pull-downs were conducted as described previously (Lee et al., 1997). Protein gels were stained with Coomassie Blue to verify that an equivalent amount of each GST fusion protein was utilized in each reaction. Methods for preparing the [32P]GST-E4-ORF1 protein probe and performing protein blotting assays were detailed elsewhere (Weiss and Javier, 1997).
Immunoprecipitations and immunoblots
Immunoprecipitation and immunoblot assays were conducted as described previously (Lee et al., 1997; Weiss et al., 1996).
Immunofluorescence assays, confocal microscopy, and TER measurements
MDCK II cells, lines, or pools were seeded to confluence onto 24-mm Transwell polyester filters (Corning Inc.) in low-calcium (5 μM) medium, incubated overnight, and then switched to normal medium. Cell monolayers and cysts were processed and immunostained as described previously (Straight et al., 2004). All images were obtained using a Zeiss LSM 510 Axiovert 100M inverted confocal microscope and manipulated with Zeiss LSM 5 Image Browser, Adobe Photoshop 5.5 and Canvas 8 softwares. TER assays were carried out as described previously (Straight et al., 2004).
Virus infections
Methods for infecting A549 cells with Ad9 were detailed previously (Javier et al., 1991). Cells taken at the indicated times postinfection were lysed in sample buffer, and the protein concentration of each cell extract, diluted tenfold in water, was determined by a Bradford protein assay (Bradford, 1976). An equal amount of total cell protein (80 μg) from each sample was separated by SDS-PAGE and subjected to an immunoblot analysis with E4-ORF1 antibodies.
Results
E4-ORF1 binds an unidentified PDZ protein expressed in MDCK cells
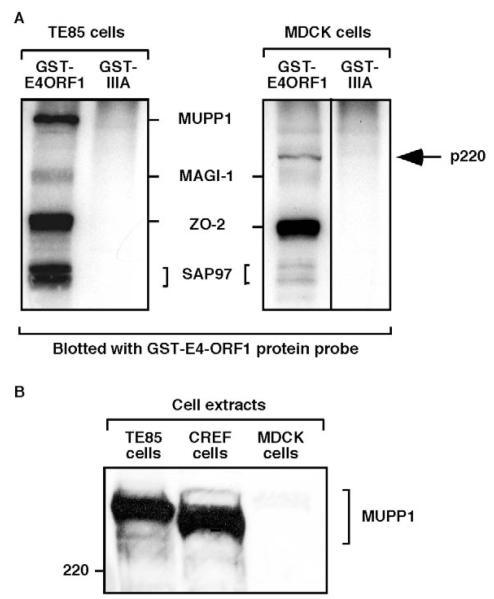
To study the potential consequences of E4-ORF1 on TJs in polarized epithelial cells, we employed MDCK cells, which are reported to express the E4-ORF1-interacting PDZ proteins MUPP1, MAGI-1, ZO-2 and SAP97 (Gumbiner et al., 1991; Hamazaki et al., 2002; Ide et al., 1999). Direct binding of E4-ORF1 to these MDCK cell-derived proteins was evaluated by subjecting MDCK cell extracts to a coupled GST pulldown and protein blotting assay with E4-ORF1 (Weiss and Javier, 1997). The results suggested that wild-type (wt) E4-ORF1, but not a negative-control E4-ORF1 mutant protein (IIIA), specifically lacking a functional PBM (Lee et al., 1997), interacts with MDCK cell-derived MAGI-1, ZO-2 and SAP97 (Fig. 1A). This conclusion was corroborated by immunoblotting the proteins recovered in a pulldown assay with antibodies to the PDZ proteins (our unpublished data). The fact that MUPP1 was not detected in the latter assay or in MDCK cell extracts immunoblotted with MUPP1-specific antibodies (Fig. 1B) indicates that the MDCK cells do not express this PDZ protein. Nonetheless, in the coupled GST pulldown and protein blotting assay shown in Fig. 1A, we instead detected a novel 220 kDa E4-ORF1-interacting cellular protein (p220) which, based on its interaction with wt E4-ORF1 but not mutant IIIA, represents an unidentified PDZ protein.
Fig. 1.
E4-ORF1 directly binds to PDZ proteins expressed in MDCK cells. (A) E4-ORF1 directly binds to MDCK cell-derived PDZ proteins having gel mobilities similar to the known E4-ORF1-interacting PDZ proteins MAGI-1, ZO-2 and SAP97, but not MUPP1, expressed in TE85 cells. The detection of an unknown 220 kDa protein (p220) is indicated. Extracts (1.2 mg protein) of RIPA buffer-lysed TE85 and MDCK cells were subjected to pulldown reactions with the indicated GST fusion proteins, and recovered proteins, immobilized on a membrane, were blotted with a radiolabeled GST-E4-ORF1 protein probe. Proteins were detected by autoradiography. A vertical line indicates boundary between spliced lanes from the same gel. (B) MDCK cells lack detectable MUPP1 expression. Extracts (100 μg protein) of RIPA buffer-lysed TE85, CREF and MDCK cells were immunoblotted with MUPP1 antiserum. TE85 and CREF cells represented positive controls for MUPP1 expression in this experiment.
The PATJ polarity protein is a new target for E4-ORF1
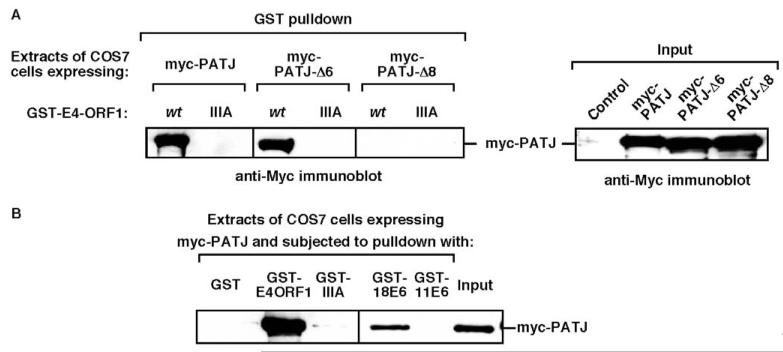
A search for known PDZ proteins similar in size to p220 led to two cell polarity proteins, Scribble (Nakagawa and Huibregtse, 2000) and MUPP1-related PATJ. Whereas human Scribble failed to interact with E4-ORF1 (our unpublished data), human PATJ bound to wt E4-ORF1 but not to mutant IIIA in GST pulldown assays and, additionally, the eighth PDZ domain of PATJ mediated this interaction (Fig. 2A). Like its paralogue MUPP1 (Lee et al., 2000), human PATJ also bound to the high-risk HPV-18 E6 oncoprotein but not to the low-risk HPV-11 E6 protein in GST pulldown assays (Fig. 2B). These findings importantly identified PATJ as a new cellular target for the adenovirus E4-ORF1 and HPV-18 E6 oncoproteins.
Fig. 2.
PATJ is a common cellular target for the adenovirus E4-ORF1 and high-risk HPV type 18 E6 oncoproteins. (A) PATJ PDZ8 mediates binding to E4-ORF1. COS7 cells on 6 cm dishes were lipofected with pRK5-myc-PATJ-wt, -ΔPDZ6, or -ΔPDZ8 (3 μg). Extracts (200 μg protein) of RIPA buffer-lysed cells were subjected to pulldown reactions with the indicated GST-E4-ORF1 fusion protein, and recovered proteins were immunoblotted with Myc antibody (left panel). An immunoblot assay of cell extracts with Myc antibody shows 3/40th of the input protein for each reaction (right panel). The control lane represents extracts from cells lipofected with pRK5-myc plasmid (3 μg). Vertical lines indicate boundary between spliced lanes from the same gel. (B) PATJ binds E4-ORF1 and the high-risk HPV-18 E6 oncoprotein. COS7 cells on 6 cm dishes were lipofected with pRK5-myc-PATJ (3 μg). Extracts (160 μg protein) of RIPA buffer-lysed cells were subjected to pulldown reactions with the indicated GST fusion proteins, and recovered proteins were immunoblotted with Myc antibody. An immunoblot assay of cell extracts with the Myc antibody shows 1/16 of the input protein for each reaction (rightmost lane). 18E6, HPV-18 E6; 11E6, HPV-11 E6.
E4-ORF1 binds and sequesters endogenous PATJ in MDCK cells
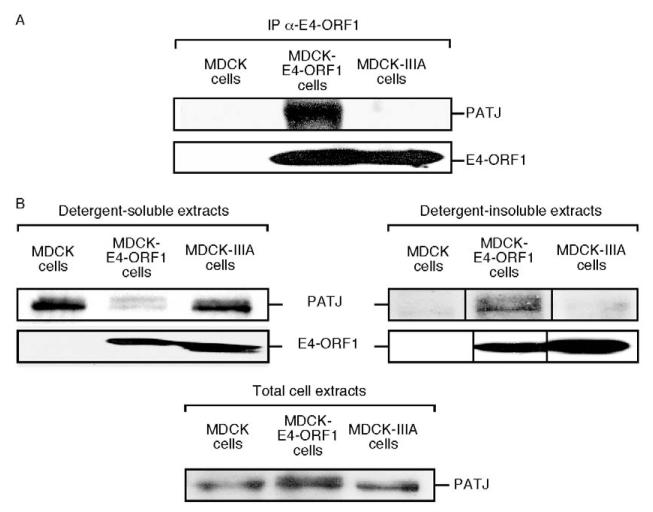
We showed that endogenous PATJ in MDCK cells stably expressing wt E4-ORF1 (MDCK-E4-ORF1 cells) also co-immunoprecipitates with this viral protein (Fig. 3A). This result is specific because endogenous PATJ in MDCK cells stably expressing mutant IIIA (MDCK-IIIA cells) failed to co-immunoprecipitate with this E4-ORF1 mutant (Fig. 3A). It is important to point out that cell lines stably expressing E4-ORF1 represent a relevant system to study Ad9-induced tumorigenesis as this process is initiated not by permissively infected cells but rather by abortively infected cells that stably express E4-ORF1 from viral DNA integrated by non-homologous recombination events into a host chromosome (Javier et al., 1991).
Fig. 3.
E4-ORF1 binds endogenous PATJ and redistributes it into detergent-insoluble complexes in MDCK cells. (A) Endogenous PATJ co-immunoprecipitates (IP) with E4-ORF1 in MDCK cells. Extracts (900 μg protein) of RIPA buffer-lysed MDCK cells, MDCK-E4-ORF1 cells and MDCK-IIIA cells were immunoprecipitated with E4-ORF1 antibodies, and recovered proteins were immunoblotted with PATJ and E4-ORF1 antibodies. (B) E4-ORF1 causes PATJ to redistribute into detergent-insoluble complexes in MDCK cells. Detergent-soluble or detergent-insoluble fractions of MDCK cells, MDCK-E4-ORF1 cells and MDCK-IIIA cells were prepared as described in the Materials and Methods, and an equivalent amount of each fraction was immunoblotted with PATJ or E4-ORF1 antibodies. An immunoblot assay of total cell extracts with PATJ antibody shows equivalent amounts of PATJ in the three lines (bottom panel). Vertical lines indicate the boundary between spliced lanes from the same gel.
Because E4-ORF1 redistributes MUPP1, MAGI-1 and ZO-2 from the detergent-soluble fraction to the detergent-insoluble fraction of fibroblasts (Glaunsinger et al., 2000; Glaunsinger et al., 2001; Lee et al., 2000), we investigated whether E4-ORF1 has a similar effect on PATJ in MDCK cells. In extracts of MDCK cells and MDCK-IIIA cells, PATJ was found primarily in the detergent-soluble fraction but, in extracts of MDCK-E4-ORF1 cells, approximately 80% of PATJ was redistributed to the detergent-insoluble fraction (Fig. 3B). Reminiscent of results reported previously for MUPP1, MAGI-1 and ZO-2, the latter cells also accumulated a form of PATJ with reduced gel mobility (Fig. 3B), an effect possibly associated with increased phosphorylation.
E4-ORF1 prevents TJ accumulation of PDZ proteins in MDCK cells
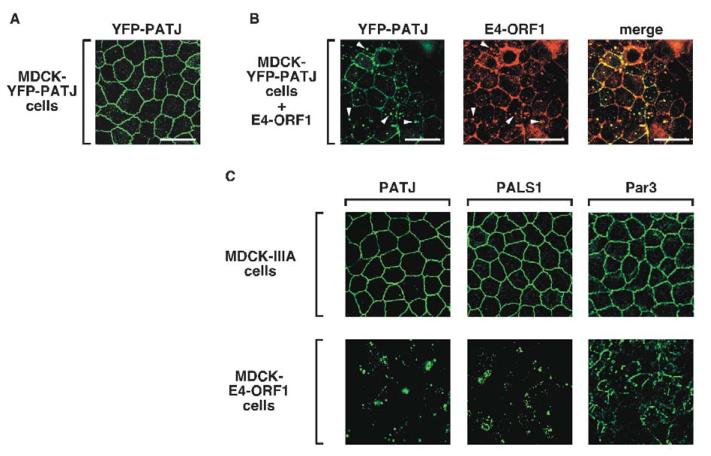
We next examined whether E4-ORF1 affects proper TJ accumulation of PATJ in polarized MDCK cells. For this purpose, we initially exploited an MDCK line stably expressing YFP-tagged PATJ (YFP-PATJ) (Roh et al., 2002a), which was verified to associate properly with TJs (Fig. 4A). Transient expression of wt E4-ORF1 in these cells, however, substantially reduced the amount of TJ-associated YFP-PATJ and caused concomitant accumulation of this fusion protein within punctate bodies, where it co-localized with E4-ORF1 (Fig. 4B).
Fig. 4.
E4-ORF1 blocks proper TJ localization of PATJ, PALS1 and Par3 in MDCK cells. (A) YFP-PATJ localizes at TJs of polarized MDCK-YFP-PATJ cells. Cells were fixed, permeabilized, and mounted on slides as described in the Materials and Methods. YFP-PATJ was directly visualized by fluorescence microscopy. (B) Transient expression of wt E4-ORF1 ablates proper TJ localization of YFP-PATJ, and the two proteins co-localize in punctate bodies. MDCK-YFP-PATJ cells were transfected with pCMVBam3-Neo-E4-ORF1 (5 μg). Each of the three panels represents the same field showing either YFP-PATJ staining, E4-ORF1 immunostaining, or their combined images (merge). Arrowheads indicate punctate bodies where YFP-PATJ and E4-ORF1 co-localize. (C) Endogenous PATJ, PALS1 and Par3 lack proper TJ localization in MDCK-E4-ORF1 cells. MDCK-E4-ORF1 cells and MDCK-IIIA cells were immunostained with PATJ, PALS1 or Par3 antibodies. Scale bars: 20 μm.
Endogenous PATJ also completely failed to associate with TJs in MDCK-E4-ORF1 cells yet retained a normal prominent TJ staining pattern in MDCK-IIIA cells (Fig. 4C), despite the fact that both MDCK lines express comparable levels of E4-ORF1 protein (Fig. 3). Notably, the localization of PATJ in MDCK-IIIA cells was indistinguishable from that in MDCK cells, as was the case for all TJ- and AJ-associated proteins examined throughout this study (our unpublished results). We also examined PALS1, an additional component of the CRB3-PALS1-PATJ polarity complex. Significantly, the prominent TJ staining pattern of endogenous PALS1 in MDCK-IIIA cells was likewise entirely eliminated in MDCK-E4-ORF1 cells(Fig. 4C). Given that TJ localization by PALS1 depends on its interaction with PATJ (Roh et al., 2002b) and that E4-ORF1 fails to bind PALS1 (our unpublished data), we conclude that PALS1 mislocalization in MDCK-E4-ORF1 cells represents an indirect consequence of PATJ sequestration.
The CRB3-PALS1-PATJ complex has been shown to regulate TJ formation and apicobasal polarity establishment in epithelial cells through an interaction with the evolutionarily-conserved TJ-associated Par3-Par6-aPKC polarity complex (Hurd et al., 2003). Despite an inability of E4-ORF1 to bind the PDZ proteins Par3 and Par6 in this complex (our unpublished data), Par3 could not properly localize to TJs in MDCK-E4-ORF1 cells but could in control MDCK-IIIA cells (Fig. 4C). As the Par3-Par6-aPKC complex requires the CRB3-PALS1-PATJ complex to localize to the TJ (Hurd et al., 2003), Par3 mislocalization in MDCK-E4-ORF1 cells probably results indirectly from inactivation of the latter complex by E4-ORF1. Taken together, our findings demonstrate that E4-ORF1 blocks key components of two conserved polarity complexes from localizing to the TJ, suggesting that the complexes are functionally inactivated.
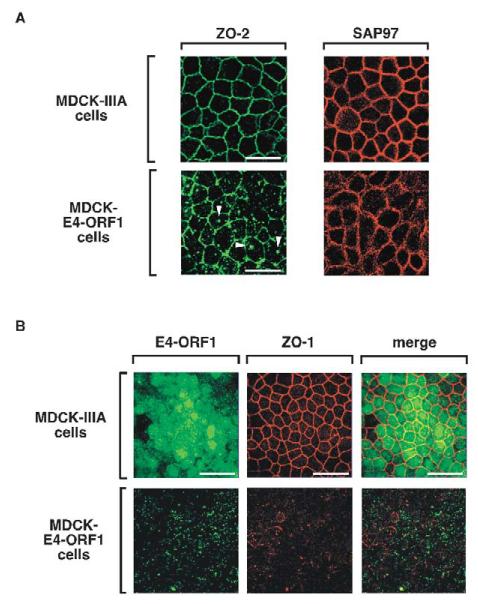
ZO-2 is also a direct target of E4-ORF1 (Fig. 1A) (Glaunsinger et al., 2001), so we next investigated whether E4-ORF1 likewise prevents this PDZ protein from properly associating with TJs. Our results indicated that, whereas ZO-2 prominently localized to TJs in MDCK-IIIA cells, it displayed a substantially reduced capacity to do so in MDCK-E4-ORF1 cells (Fig. 5A). In the latter cells, the related TJ-associated PDZ protein ZO-1, which interacts with ZO-2 (Wittchen et al., 1999) but not with E4-ORF1 (Glaunsinger et al., 2000), also specifically failed to localize to TJs (Fig. 5B). Unlike PATJ (Fig. 4B), however, ZO-1 lacked appreciable co-localization with E4-ORF1 in MDCK-E4-ORF1 cells (Fig. 5B), suggesting that E4-ORF1 does not sequester ZO-1-ZO-2 complexes in the cells. As available antibodies did not recognize MDCK cell-derived MAGI-1 in immunofluorescence assays, we could not evaluate the effects of E4-ORF1 on this PDZ protein.
Fig. 5.
E4-ORF1 disrupts proper TJ localization of ZO-1 and ZO-2 in MDCK cells. (A) ZO-2 localization to TJs is impaired in MDCK-E4-ORF1 cells. Control MDCK-IIIA cells and MDCK-E4-ORF1 cells were immunostained with ZO-2 or SAP97 antibodies. Arrowheads indicate representative punctate bodies where ZO-2 becomes aberrantly sequestered in MDCK-E4-ORF1 cells. (B) Endogenous ZO-1 fails to associate with TJs in MDCK-E4-ORF1 cells. Control MDCK-IIIA cells and MDCK-E4-ORF1 cells were immunostained with ZO-1 (middle panels) and E4-ORF1 (left panels) antibodies. The combined images are shown in the right panels (merge). Scale bars: 20 μm (A); 40 μm (B).
In contrast to results described thus far for TJ proteins, the E4-ORF1-interacting PDZ protein SAP97 properly localized to AJs of both MDCK-IIIA cells and MDCK-E4-ORF1 cells (Fig. 5A). The slightly more diffuse AJ staining pattern of SAP97 in MDCK-E4-ORF1 cells probably reflects morphological transformation induced by E4-ORF1, as has been described for E4-ORF1-expressing fibroblasts (Weiss et al., 1996). Additionally, the mislocalization of TJ proteins but not AJ-associated SAP97 in MDCK-E4-ORF1 cells agrees with previous results showing that MUPP1 (Lee et al., 2000), MAGI-1 (Glaunsinger et al., 2000) and ZO-2 (Glaunsinger et al., 2001), but not SAP97 (K.K.F., I.J.L. and R.T.J., unpublished data), become aberrantly sequestered within detergent-insoluble complexes in the cytoplasm of wt E4-ORF1-expressing fibroblasts.
E4-ORF1-induced phosphatidylinositol 3-kinase activation is not required to block TJ accumulation of PDZ proteins in MDCK cells
The transforming and tumorigenic potentials of E4-ORF1 depend in part on its ability to stimulate phosphatidylinositol 3-kinase (PI 3-kinase) in a PDZ-protein-dependent manner (Frese et al., 2003). Because PI 3-kinase can influence TJ assembly (Sheth et al., 2003; Woo et al., 1999), it was important to evaluate a potential requirement for E4-ORF1-induced PI 3-kinase activation in mislocalization of TJ proteins. The inability of E4-ORF1 mutant IIIA to mislocalize TJ proteins in MDCK cells (see Figs 4, 5) could stem from its failure either (i) to activate PI 3-kinase (Frese et al., 2003) (Fig. 6), (ii) to bind cellular PDZ proteins (Glaunsinger et al., 2000; Glaunsinger et al., 2001; Lee et al., 2000; Lee et al., 1997), or (iii) both.
Fig. 6.
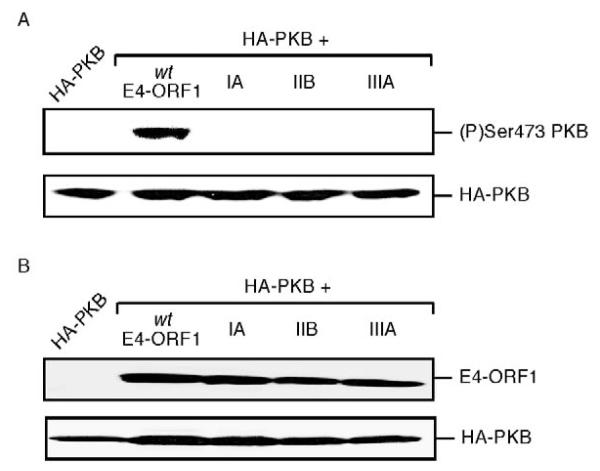
Wt E4-ORF1, but not mutant IIB or IIIA E4-ORF1, activates the PI 3-kinase effector PKB in MDCK cells. (A) PKB activation by E4-ORF1 in MDCK cells. MDCK cells on 6 cm dishes were lipofected with pGW1-HA-PKB (1.0 μg) in combination with pGW1-wt-E4-ORF1, -IIB-E4-ORF1 or -IIIA-E4-ORF1 (50 ng). Extracts (120 μg protein) of RIPA buffer-lysed, serum-starved cells were immunoblotted with (P)Ser473PKB or HA antibodies. (B) Wt and mutant E4-ORF1 proteins are expressed at comparable levels in MDCK cells. MDCK cells on 6 cm dishes were lipofected with pGW1-HA-PKB (0.5 μg) in combination with pGW1-wt-E4-ORF1, -IIB-E4-ORF1 or -IIIA-E4-ORF1 (2.5 μg). Extracts (100 μg protein) of sample buffer-lysed, serum-starved cells were immunoblotted with E4-ORF1, or HA antibodies.
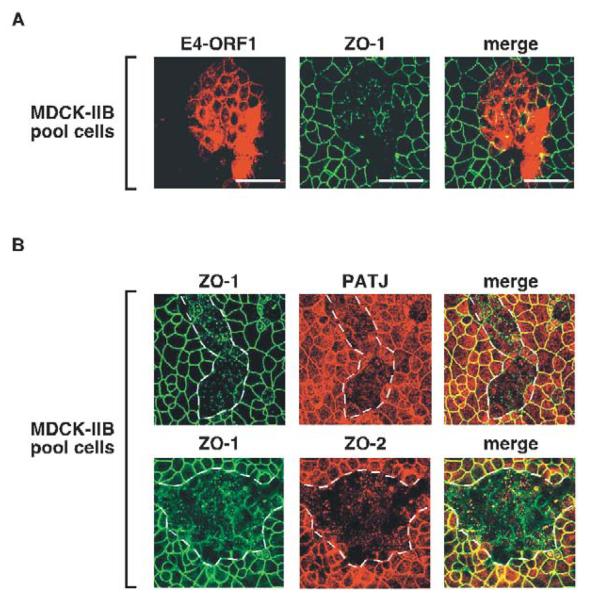
To distinguish between these possibilities, we utilized E4-ORF1 mutants IA and IIB, which carry mutations in the region I/II domain (Weiss et al., 1997a). While mutants IA, IIB and IIIA share a similar inability to activate PI 3-kinase and its downstream effector protein kinase B (PKB) (Frese et al., 2003) (Fig. 6), mutants IA and IIB differ from mutant IIIA in lacking any detectable defect in binding to PDZ proteins (Weiss and Javier, 1997). Upon examination of an MDCK cell pool composed of a mixture of mutant IIB-expressing and non-expressing MDCK cells (MDCK-IIB pool), we found that endogenous ZO-1, PATJ and ZO-2 failed to accumulate at TJs in the mutant IIB-expressing cells yet exhibited a normal TJ localization in adjacent non-expressing cells (Fig. 7). Mutant IA-expressing MDCK cells yielded identical results (our unpublished data). As mutants IA and IIB behaved similarly to wt E4-ORF1 in mislocalizing TJ proteins in MDCK cells, we conclude that this activity does not require E4-ORF1-induced PI 3-kinase activation and, instead, probably results solely from direct binding of E4-ORF1 to its TJ-associated PDZ-protein targets.
Fig. 7.
PI 3-kinase activation by E4-ORF1 is not required to block TJ localization of PDZ proteins in MDCK cells. (A) Endogenous ZO-1 fails to associate with TJs in MDCK cells expressing mutant IIB E4-ORF1. Cells of the MDCK-IIB pool were immunostained with ZO-1 (middle panel) or E4-ORF1 (left panel) antibodies. The combined images are shown in the right panel (merge). Scale bars: 40 μm. (B) Endogenous PATJ and ZO-2 also fail to associate with TJs in MDCK cells expressing mutant IIB E4-ORF1. Cells of the MDCK-IIB pool were immunostained with ZO-1, PATJ, and ZO-2 antibodies. The top three panels represent the same field of cells showing immunostaining of ZO-1, PATJ, or the merge of the latter two images (merge). The bottom three panels represent the same field of cells showing immunostaining of ZO-1, ZO-2, and the merged images (merge). Dashed lines outline groups of cells inferred to express E4-ORF1 based on the results shown in (A).
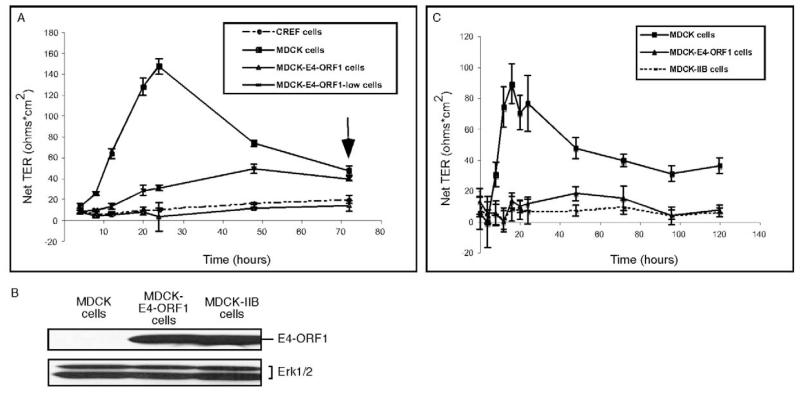
Dramatic TJ protein mislocalization depends on E4-ORF1 expression levels
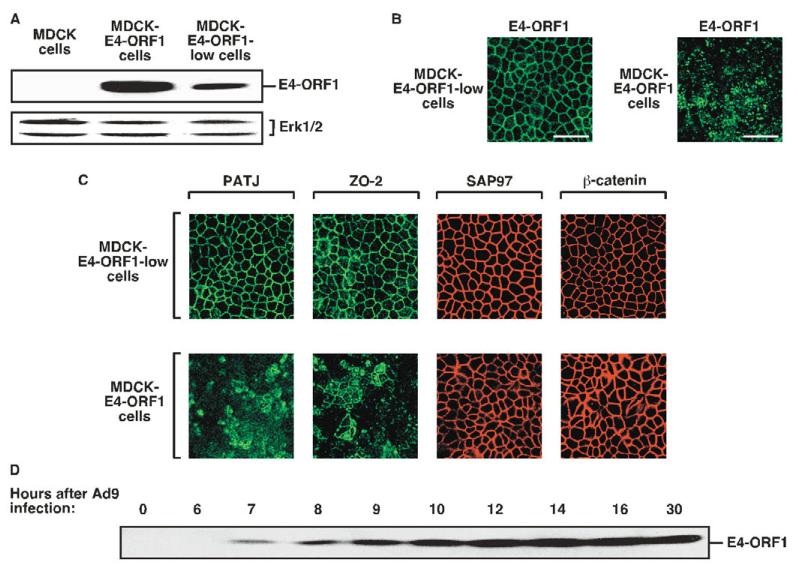
During the course of this work, we isolated two different MDCK lines that express either higher relative levels (MDCK-E4-ORF1 cells) or lower relative levels (MDCK-E4-ORF1-low cells) of wt E4-ORF1 (Fig. 8A). MDCK-E4-ORF1 cells were employed in experiments presented thus far, so MDCK-E4-ORF1-low cells were subjected to similar analyses. Contrary to the striking punctate cytoplasmic staining pattern of E4-ORF1 in MDCK-E4-ORF1 cells, nearly all of the E4-ORF1 protein in MDCK-E4-ORF1-low cells instead concentrated at sites of cell-cell contact (Fig. 8B). The fact that E4-ORF1 transiently expressed in MDCK-YFP-PATJ cells displayed both membrane and punctate cytoplasmic staining (see Fig. 4B) may indicate that these cells express E4-ORF1 at a level intermediate between that of MDCK-E4-ORF1 cells and MDCK-E4-ORF1-low cells. More importantly, endogenous PATJ and ZO-2 also remained properly localized to TJs of MDCK-E4-ORF1-low cells but not MDCK-E4-ORF1 cells and, as expected, SAP97 and β-catenin properly localized to AJs in both MDCK lines (Fig. 8C). These results suggest that a minimal threshold level of E4-ORF1 may be required for mislocalization of TJ proteins in MDCK cells.
Fig. 8.
Threshold levels of E4-ORF1 expression are required to block TJ localization of PDZ proteins in MDCK cells. (A) High or low levels of E4-ORF1 protein expression by two MDCK lines. Extracts of sample buffer-lysed MDCK cells, MDCK-E4-ORF1-low cells and MDCK-E4-ORF1 cells (180 μg protein) were immunoblotted with E4-ORF1 or Erk1/2 antibodies. Erk1/2 served as a loading control. (B) The localization pattern of E4-ORF1 depends on its expression level in MDCK cells. MDCK-E4-ORF1-low cells or MDCK-E4-ORF1 cells were immunostained with E4-ORF1 antibodies. Scale bar: 40 μm. (C) E4-ORF1 must be expressed at a minimal threshold level to block TJ localization of PDZ proteins in MDCK cells. MDCK-E4-ORF1-low cells and MDCK-E4-ORF1 cells were immunostained with PATJ, ZO-2, SAP97 or β-catenin antibodies. (D) E4-ORF1 protein levels progressively increase during an Ad9 infection of epithelial cells. A549 cells were infected with Ad9 virus for the indicated times, and extracts (80 μg protein) of sample buffer-lysed cells were immunoblotted with E4-ORF1 antibodies.
Human adenoviruses naturally infect epithelial cells lining the respiratory or gastrointestinal tract, or the eye (Horwitz, 2001). During the course of an Ad9 infection of human A549 lung epithelial cells, we found that E4-ORF1 protein levels progressively increased from undetectable at early times (0-6 hours), to low and moderate at intermediate times (6-10 hours), and finally to high at late times (10-30 hours) post-infection (Fig. 8D). These results interestingly hint that, as an adenovirus infection progresses from the early stage to the late stage of the viral life cycle in epithelial cells, E4-ORF1 may function to promote the gradual disassembly of TJs.
E4-ORF1 disrupts the TJ barrier in MDCK cells
To investigate whether E4-ORF1 functionally impairs TJs in MDCK cells, we conducted transepithelial resistance (TER) assays, which measure the capacity of a confluent epithelial cell monolayer to form an impermeable seal through assembly of TJs. In time-course analyses with MDCK cells plated to confluence, the TER rapidly increases, peaks, and then decreases to a stable TER value above that of cells unable to form TJs (Straight et al., 2004).
In TER assays with cells plated to confluence on Transwell filter membranes, CREF fibroblasts, which cannot form TJs, failed to achieve TER values above background at any time point, whereas MDCK cells attained a peak TER value at 24 hours post-plating and a stable TER value approximately twofold higher than that of CREF fibroblasts at 72 hours post-plating (Fig. 9A). More importantly, MDCK-E4-ORF1 cells behaved similarly to CREF fibroblasts in the TER assays (Fig. 9A), as did a MDCK line that stably and homogeneously expresses E4-ORF1 mutant IIB (MDCK-IIB cells) (Fig. 9B,C). Significantly, PATJ downregulation has similar effects in MDCK cells (Shin et al., 2005). These results indicate that TJ protein mislocalization by E4-ORF1 permanently disrupts the TJ barrier in MDCK cells. Compared to control MDCK cells, MDCK-E4-ORF1-low cells also yielded significantly lower TER values from 8 hours to 48 hours post-plating, although the latter cells eventually attained a stable TER value comparable to that of MDCK cells at 72 hours post-plating (Fig. 9A).
Fig. 9.
E4-ORF1 functionally disrupts TJs in MDCK cells. (A) Prevention or delay of TER establishment in MDCK-E4-ORF1 and MDCK-E4-ORF1-low monolayers, respectively. The arrow indicates the time when TER values became stabilized (72 hours post-plating). CREF fibroblasts were used as a negative control in the assay. (B) Comparable E4-ORF1 expression by MDCK-E4-ORF1 cells and MDCK-IIB cells. Extracts (150 μg protein) of the indicated sample buffer-lysed cells were immunoblotted with E4-ORF1 or Erk1/2 antibodies. Erk1/2 served as a loading control. (C) Prevention of TER establishment in MDCK-IIB monolayers. TER values were determined for fully confluent cells on Transwell filters at the indicated times post-plating.
E4-ORF1 causes apicobasal polarity defects in MDCK cells
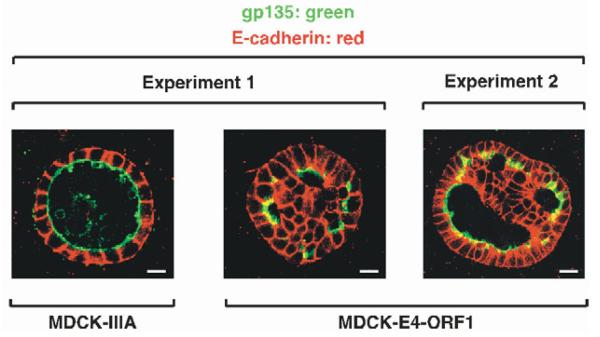
MDCK cells plated on filter membranes have predetermined basal attachment surfaces and free apical surfaces. By contrast, MDCK cells induced to form cysts by suspension in collagen represent a more stringent system to expose polarity defects because of the absence of initial polarity cues (O’Brien et al., 2002). Each cyst formed by normal MDCK cells appears microscopically as a hollow monolayer sphere of polarized cells where the internal apical surface encloses a single, large lumen and the external basal surface coated by a basement membrane faces the exterior. Importantly, either downregulation of PATJ or PALS1 in MDCK cells causes polarity defects, manifesting as cysts having their lumenal space partially or completely filled with abnormal cells that form mini-lumens or no lumen and that also show inappropriate intermixing of apical and basolateral membrane protein markers (Shin et al., 2005; Straight et al., 2004).
We next investigated whether E4-ORF1-induced mislocalization of TJ-associated PDZ proteins likewise causes defects in apicobasal polarity in MDCK cells. We initially verified that, from 7 days to 14 days post-suspension, the majority (≥85%) of MDCK-IIIA cells formed normal single-lumen cysts, where marker proteins gp135 and E-cadherin localized exclusively to apical membranes facing the lumen or to lateral membranes associated with AJs, respectively (Fig. 10) (O’Brien et al., 2001). In contrast, MDCK-E4-ORF1 cells displayed an eightfold reduction in cyst formation, similar to MDCK cells downregulated for PALS1 (S. Straight and B.M., unpublished data), whereas MDCK-IIB cells failed to form any cysts. These observations hint that severe TJ defects trigger anoikis of MDCK cells grown in suspension and that E4-ORF1-induced PI 3-kinase activation enhances survival of the cells. For cysts that did arise from MDCK-E4-ORF1 cells, however, the majority (~80%) displayed multiple mini-lumens where E-cadherin occasionally co-localized with gp135 in apical membranes and, conversely, gp135 occasionally co-localized with E-cadherin at basolateral membranes (Fig. 10). These results show that, like PATJ or PALS1 downregulation (Shin et al., 2005; Straight et al., 2004), E4-ORF1 also causes polarity defects in MDCK cells.
Fig. 10.
E4-ORF1 causes cell polarity defects in MDCK cells. Shown are cysts derived from MDCK-IIIA cells or MDCK-E4-ORF1 cells co-immunostained with gp135 and E-cadherin antibodies. Representative cysts from independent experiments 1 and 2 were photographed at 7 days (MDCK-IIIA cells) or 10 days and 14 days (MDCK-E4-ORF1 cells) post-suspension. Scale bars are 10 μm (experiment 1) and 20 μm (experiment 2).
Discussion
The development of human cancers is frequently associated with a failure of epithelial cells to form TJs and to establish proper apicobasal polarity. In this study, we investigated a possible relationship between these common defects of cancer cells and the known dependence of tumorigenesis induced by the adenovirus E4-ORF1 oncoprotein on its interactions with several different cellular PDZ proteins, most of which localize to the TJ of epithelial cells. Our results significantly revealed that such interactions block functional TJ formation and proper apicobasal polarity in MDCK epithelial cells (Figs 9, 10), supporting the hypothesis that such defects directly contribute to the oncogenic transformation of cells.
Cellular transformation induced by E4-ORF1 has been shown to depend on its ability to activate PI 3-kinase, an activity that requires both the PBM and the region I/II domain of E4-ORF1 (Frese et al., 2003). Nevertheless, expression of a constitutively activated PI 3-kinase mutant fails to recapitulate potent E4-ORF1-induced cellular transformation (Frese et al., 2003), implying the existence of one or more additional undetermined activities of E4-ORF1 required for its oncogenic potential. Findings presented in this paper suggest that one such activity is the functional inactivation of specific TJ-associated PDZ proteins, such as PATJ and ZO-2. Our results further indicate that PI 3-kinase activation and TJ protein inactivation represent two separable E4-ORF1 activities because TJ protein inactivation requires only the E4-ORF1 PBM whereas PI 3-kinase activation additionally requires the E4-ORF1 region I/II domain (Figs 4-7).
Our findings also showed that, in MDCK cells, E4-ORF1 directly binds and prevents TJ localization of PATJ and ZO-2 (Fig. 1A, Fig. 2A, Fig. 4C, Fig. 5A) and indirectly has similar effects on the localization of PALS1, Par3 and ZO-1 (Fig. 4C, Fig. 5B). By contrast, E4-ORF1 has no appreciable effect on the localization of the AJ-associated proteins SAP97 and β-catenin (Fig. 5A, Fig. 8C), indicating that this viral oncoprotein specifically mislocalizes TJ-associated proteins in the CRB3-PALS1-PATJ, Par3-Par6-aPKC and ZO-1-ZO-2 complexes.
Disruption of the ZO-1 gene, over-expression of Par6, or downregulation of PALS1 delays, but does not prevent, functional TJ assembly (Gao et al., 2002; Straight et al., 2004; Umeda et al., 2004). MDCK-E4-ORF1-low cells exhibit a similar modest functional defect in TJ assembly (Fig. 9A), presumably due to suboptimal levels of E4-ORF1 that only partially inactivate its PDZ-protein targets. In contrast, higher E4-ORF1 levels in MDCK-E4-ORF1 cells cause substantially more severe defects, manifesting as a permanent disruption of both the structure (Fig. 4C, Fig. 5, Fig. 8C) and the function of TJs (Fig. 9). The fact that the severe TJ functional defect of MDCK-E4-ORF1 cells closely resembles that of MDCK cells in which PATJ is downregulated (Shin et al., 2005) argues that PATJ sequestration by E4-ORF1 largely mediates this phenotype. Nonetheless, the TJ structural defect seen in MDCK-E4-ORF1 cells (Fig. 4C, Fig. 5, Fig. 8C) is more severe than that caused by PATJ downregulation (Shin et al., 2005), implying that direct sequestration of additional TJ-associated PDZ proteins, such as ZO-2, by E4-ORF1 may enhance this particular defect. As E4-ORF1 sequesters MAGI-1 in the cytoplasm of fibroblasts (Glaunsinger et al., 2000), it is feasible that E4-ORF1 binding to this PDZ protein (Fig. 1A) likewise enhances the TJ structural defects seen in MDCK-E4-ORF1 cells.
Although the mechanism whereby E4-ORF1 directly sequesters PATJ and ZO-2 in MDCK cells was not determined, the PATJ (Fig. 2A), MUPP1 (Lee et al., 2000), MAGI-1 (Glaunsinger et al., 2000) and ZO-2 (Glaunsinger et al., 2001) PDZ domains targeted by E4-ORF1 have been shown to mediate interactions with TJ transmembrane proteins (Hamazaki et al., 2002; Hirabayashi et al., 2003; Itoh et al., 1999; Roh et al., 2002a). Our results also suggest that E4-ORF1 promotes these PDZ proteins to become post-translationally modified and redistributed into detergent-insoluble complexes (Fig. 3B) (Glaunsinger et al., 2000; Glaunsinger et al., 2001; Lee et al., 2000), with the latter effect possibly reflecting an association with the actin cytoskeleton (Nunbhakdi-Craig et al., 2002; Stuart and Nigam, 1995). We therefore postulate that binding of E4-ORF1 to TJ-associated PDZ proteins not only competitively inhibits their normal interactions with TJ transmembrane proteins but also induces a modification(s) that promotes their strong sequestration with the actin cytoskeleton.
In experimental animals, Ad9 generates several distinct histological types of mammary tumors, including benign fibroadenomas and malignant cystosarcoma phyllodes and solid sarcomas (Javier et al., 1991). Tumor cells from cystosarcoma phyllodes and solid sarcomas express myoepithelial cell markers (Javier et al., 1991), implying an origin from progenitor mammary stem cells of the lumenal epithelial lineage that form functional TJs (Gudjonsson et al., 2002). Hence, we postulate that Ad9 infects such progenitor cells where two separate PDZ-protein-dependent E4-ORF1 activities, activation of PI 3-kinase and disruption of TJs and apicobasal polarity, cooperate to trigger malignant transformation. Contrary to Ad9-induced malignant mammary tumors, however, benign fibroadenomas originate from mammary fibroblasts that do not form TJs. Because E4-ORF1 binds and mislocalizes TJ-associated PDZ proteins expressed in both epithelial cells and fibroblasts, we additionally postulate that these cellular factors may function to regulate cellular growth and proliferation through both TJ-dependent and TJ-independent mechanisms. Consistent with this idea, transformation of fibroblasts by E4-ORF1 has been shown to depend in part on its ability to inactivate a growth-inhibitory activity of the suspected tumor suppressor protein ZO-2 (Glaunsinger et al., 2001).
The oncogenes of DNA tumor viruses evolved to promote quiescent cells to progress into S phase, providing an optimal environment for efficient viral DNA replication. Related to this concept, TJ disruption and loss of polarity in epithelial cells have been reported to cause activation of (i) a basolateral membrane growth factor receptor by permitting inappropriate intermixing with its apical membrane-localized growth factor (Vermeer et al., 2003) and (ii) certain growth-stimulatory transcription factors by triggering their release from TJs and their translocation into the nucleus (Balda et al., 2003; Betanzos et al., 2004). It will be important to test whether E4-ORF1-induced inhibition of TJ formation and cell polarity establishment has similar consequences in MDCK cells. Because the steady-state levels of E4-ORF1 were found to peak during the late stage of the viral life cycle (Fig. 8D), when virus release from cells is maximal, TJ disruption might also facilitate escape of progeny virions from infected epithelia by increasing intercellular permeability, as was recently reported for the adenovirus fiber protein (Walters et al., 2002).
Additional data presented in this paper demonstrated that PATJ is also a cellular target for the high-risk HPV-18 E6 oncoprotein (Fig. 2B), concordant with previous results showing that this viral protein likewise binds to MUPP1 (Lee et al., 2000). Thus, in addition to its reported capacity to disrupt AJs (Watson et al., 2003), HPV-18 E6 may also directly disrupt TJs in epithelial cells through its interactions with PATJ and possibly other TJ-associated PDZ proteins, including MUPP1, MAGI-1 and MAGI-3 (Glaunsinger et al., 2000; Lee et al., 2000; Thomas et al., 2002). The finding that two otherwise unrelated viral oncoproteins target the same TJ-associated cellular PDZ proteins also underscores the importance of TJ integrity in preventing malignant cellular transformation. Our failure to detect binding of either PATJ or MUPP1 to high-risk HPV-16 E6 (S. Lee and R.T.J., unpublished data) might also explain the observation that cervical carcinomas associated with HPV-18 are generally more aggressive and have an increased likelihood of recurrence (Schwartz et al., 2001) than those associated with HPV-16. Moreover, HPV-18 more efficiently transforms human keratinocytes, an activity that genetically maps to a region encompassing the E6 gene (Villa and Schlegel, 1991). Thus, an attractive hypothesis is that selective binding of HPV-18 E6 to MUPP1 and PATJ participates in conferring a heightened malignant phenotype to HPV-18-associated cervical cancers.
In summary, the findings presented in this paper suggest that studies of cellular TJ-associated PDZ proteins targeted by viral oncoproteins will facilitate elucidation of the mechanisms whereby TJ disruption and loss of polarity in epithelial cells contribute to the development of human cancers.
Acknowledgments
We are indebted to Sue Crawford and Mary Estes for providing advice and equipment to perform TER assays. I.J.L. and K.K.F. were recipients of predoctoral fellowships from the Research Training Program in Breast Cancer (DAMD17-99-1-9073) and the Viral Oncology Training Grant (DAMD17-94-J4204), respectively. M.H.R. was a recipient of the Medical Scientist Training Program Grant (T32GM-07863) and the NIH Predoctoral Training Program Grant in Genetics (T32GM-07544024) to the University of Michigan. This work was supported by grants from the National Cancer Institute (RO1 CA58541) to R.T.J. and from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-58208) to B.M.
References
- Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp. Cell Res. 2004;292:51–66. doi: 10.1016/j.yexcr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butel JS. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis. 2000;21:405–426. doi: 10.1093/carcin/21.3.405. [DOI] [PubMed] [Google Scholar]
- Cochand-Priollet B, Raison D, Molinie V, Guillausseau PJ, Wassef M, Bouchaud C. Altered gap and tight junctions in human thyroid oncocytic tumors: a study of 8 cases by freeze-fracture. Ultrastruct. Pathol. 1998;22:413–420. doi: 10.3109/01913129809032276. [DOI] [PubMed] [Google Scholar]
- Frese KK, Lee SS, Thomas DL, Latorre IJ, Weiss RS, Glaunsinger BA, Javier RT. Selective PDZ protein-dependent stimulation of phosphatidylinositol 3-kinase by the adenovirus E4-ORF1 oncoprotein. Oncogene. 2003;22:710–721. doi: 10.1038/sj.onc.1206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuja TJ, Lin F, Osann KE, Bryant PJ. Somatic mutations and altered expression of the candidate tumor suppressors CSNK1 epsilon, DLG1, and EDD/hHYD in mammary ductal carcinoma. Cancer Res. 2004;64:942–951. doi: 10.1158/0008-5472.can-03-2100. [DOI] [PubMed] [Google Scholar]
- Gao L, Joberty G, Macara IG. Assembly of epithelial tight junctions is negatively regulated by Par6. Curr. Biol. 2002;12:221–225. doi: 10.1016/s0960-9822(01)00663-7. [DOI] [PubMed] [Google Scholar]
- Gardiol D, Kuhne C, Glaunsinger B, Lee SS, Javier R, Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene. 1999;18:5487–5496. doi: 10.1038/sj.onc.1202920. [DOI] [PubMed] [Google Scholar]
- Glaunsinger BA, Lee SS, Thomas M, Banks L, Javier R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene. 2000;19:5270–5280. doi: 10.1038/sj.onc.1203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaunsinger BA, Weiss RS, Lee SS, Javier R. Link of the unique oncogenic properties of adenovirus type 9 E4-ORF1 to a select interaction with the candidate tumor suppressor protein ZO-2. EMBO J. 2001;20:5578–5586. doi: 10.1093/emboj/20.20.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc. Natl. Acad. Sci. USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J. Biol. Chem. 2002;277:455–461. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- Harris BZ, Lim WA. Mechanism and role of PDZ domains in signaling complex assembly. J. Cell Sci. 2001;114:3219–3231. doi: 10.1242/jcs.114.18.3219. [DOI] [PubMed] [Google Scholar]
- Hirabayashi S, Tajima M, Yao I, Nishimura W, Mori H, Hata Y. JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol. Cell. Biol. 2003;23:4267–4282. doi: 10.1128/MCB.23.12.4267-4282.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MS. In: Adenoviruses. In Fields Virology. Knipe DM, Howley PM, editors. Vol. 2. Lippincott, Williams and Wilkins; Philadelphia: 2001. pp. 2301–2326. [Google Scholar]
- Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat. Cell Biol. 2003;5:137–142. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- Ide N, Hata Y, Nishioka H, Hirao K, Yao I, Deguchi M, Mizoguchi A, Nishimori H, Tokino T, Nakamura Y, et al. Localization of membrane-associated guanylate kinase (MAGI)-1/BAI-associated protein (BAP) 1 at tight junctions of epithelial cells. Oncogene. 1999;18:7810–7815. doi: 10.1038/sj.onc.1203153. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier RT. Adenovirus type 9 E4 open reading frame 1 encodes a transforming protein required for the production of mammary tumors in rats. J. Virol. 1994;68:3917–3924. doi: 10.1128/jvi.68.6.3917-3924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier R, Raska K, Jr, Macdonald GJ, Shenk T. Human adenovirus type 9-induced rat mammary tumors. J. Virol. 1991;65:3192–3202. doi: 10.1128/jvi.65.6.3192-3202.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Weiss RS, Javier RT. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA. 1997;94:6670–6675. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Glaunsinger B, Mantovani F, Banks L, Javier RT. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J. Virol. 2000;74:9680–9693. doi: 10.1128/jvi.74.20.9680-9693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur. J. Cancer. 2004;40:2717–2725. doi: 10.1016/j.ejca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Matter K, Balda MS. Signalling to and from tight junctions. Nat. Rev. Mol. Cell. Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell. Biol. 2000;20:8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6’s induction of epithelial hyperplasia in vivo. J. Virol. 2003a;77:6957–6964. doi: 10.1128/JVI.77.12.6957-6964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MM, Nguyen ML, Caruana G, Bernstein A, Lambert PF, Griep AE. Requirement of PDZ-containing proteins for cell cycle regulation and differentiation in the mouse lens epithelium. Mol. Cell. Biol. 2003b;23:8970–8981. doi: 10.1128/MCB.23.24.8970-8981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, 3rd, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat. Rev. Mol. Cell. Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Reuver SM, Garner CC. E-cadherin mediated cell adhesion recruits SAP97 into the cortical cytoskeleton. J. Cell Sci. 1998;111:1071–1080. doi: 10.1242/jcs.111.8.1071. [DOI] [PubMed] [Google Scholar]
- Roh MH, Margolis B. Composition and function of PDZ protein complexes during cell polarization. Am. J. Physiol. Renal Physiol. 2003;285:F377–F387. doi: 10.1152/ajprenal.00086.2003. [DOI] [PubMed] [Google Scholar]
- Roh MH, Liu CJ, Laurinec S, Margolis B. The carboxyl terminus of zona occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J. Biol. Chem. 2002a;277:27501–27509. doi: 10.1074/jbc.M201177200. [DOI] [PubMed] [Google Scholar]
- Roh MH, Makarova O, Liu CJ, Shin K, Lee S, Laurinec S, Goyal M, Wiggins R, Margolis B. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J. Cell Biol. 2002b;157:161–172. doi: 10.1083/jcb.200109010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SM, Daling JR, Shera KA, Madeleine MM, McKnight B, Galloway DA, Porter PL, McDougall JK. Human papillomavirus and prognosis of invasive cervical cancer: a population-based study. J. Clin. Oncol. 2001;19:1906–1915. doi: 10.1200/JCO.2001.19.7.1906. [DOI] [PubMed] [Google Scholar]
- Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J. Biol. Chem. 2003;278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- Shin K, Straight S, Margolis B. PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J. Cell Biol. 2005;168:705–711. doi: 10.1083/jcb.200408064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- Straight SW, Shin K, Fogg VC, Fan S, Liu CJ, Roh M, Margolis B. Loss of PALS1 expression leads to tight junction and polarity defects. Mol. Biol. Cell. 2004;15:1981–1990. doi: 10.1091/mbc.E03-08-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc. Natl. Acad. Sci. USA. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Laura R, Hepner K, Guccione E, Sawyers C, Lasky L, Banks L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21:5088–5096. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J. Biol. Chem. 2004;279:44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- Villa LL, Schlegel R. Differences in transformation activity between HPV-18 and HPV-16 map to the viral LCR-E6-E7 region. Virology. 1991;181:374–377. doi: 10.1016/0042-6822(91)90507-8. [DOI] [PubMed] [Google Scholar]
- Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110:789–799. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- Watson RA, Rollason TP, Reynolds GM, Murray PG, Banks L, Roberts S. Changes in expression of the human homologue of the Drosophila discs large tumour suppressor protein in high-grade premalignant cervical neoplasias. Carcinogenesis. 2002;23:1791–1796. doi: 10.1093/carcin/23.11.1791. [DOI] [PubMed] [Google Scholar]
- Watson RA, Thomas M, Banks L, Roberts S. Activity of the human papillomavirus E6 PDZ-binding motif correlates with an enhanced morphological transformation of immortalized human keratinocytes. J. Cell Sci. 2003;116:4925–4934. doi: 10.1242/jcs.00809. [DOI] [PubMed] [Google Scholar]
- Weiss RS, Javier RT. A carboxy-terminal region required by the adenovirus type 9 E4 ORF1 oncoprotein for transformation mediates direct binding to cellular polypeptides. J. Virol. 1997;71:7873–7880. doi: 10.1128/jvi.71.10.7873-7880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RS, McArthur MJ, Javier RT. Human adenovirus type 9 E4 open reading frame 1 encodes a cytoplasmic transforming protein capable of increasing the oncogenicity of CREF cells. J. Virol. 1996;70:862–872. doi: 10.1128/jvi.70.2.862-872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RS, Gold MO, Vogel H, Javier RT. Mutant adenovirus type 9 E4 ORF1 genes define three protein regions required for transformation of CREF cells. J. Virol. 1997a;71:4385–4394. doi: 10.1128/jvi.71.6.4385-4394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RS, Lee SS, Prasad BV, Javier RT. Human adenovirus early region 4 open reading frame 1 genes encode growth-transforming proteins that may be distantly related to dUTP pyrophosphatase enzymes. J. Virol. 1997b;71:1857–1870. doi: 10.1128/jvi.71.3.1857-1870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J. Biol. Chem. 1999;274:35179–35185. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- Woo PL, Ching D, Guan Y, Firestone GL. Requirement for Ras and phosphatidylinositol 3-kinase signaling uncouples the glucocorticoid-induced junctional organization and transepithelial electrical resistance in mammary tumor cells. J. Biol. Chem. 1999;274:32818–32828. doi: 10.1074/jbc.274.46.32818. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]