Abstract
We report a fully defined synthetic polymer coating, poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] (PMEDSAH), which sustains long-term human embryonic stem (hES) cell growth in several different culture media, including commercially available defined media. The development of a standardized, controllable and sustainable culture matrix for hES cells is an essential step in elucidating mechanisms that control hES cell behavior and in optimizing conditions for biomedical applications of hES cells.
Considerable progress has been made in the development of defined hES cell media1,2; however, long-term culture still requires use of recombinant extracellular matrix proteins3 or animal-derived matrices4, which are sources of variability5 and xenogeneic contamination6. A compositionally defined matrix that supports hES cell expansion in defined media is critical for determining factors that regulate stem cell growth and differentiation, expanding the use of hES cells in biotechnologies and enabling potential clinical applications. Thus far, synthetic polymer matrices have sustained only short-term hES cell propagation7–10. Here, we synthesized six polymer coatings by surface-initiated graft polymerization and tested their ability to promote attachment and proliferation of undifferentiated hES cells (Fig. 1a and Supplementary Methods).
Figure 1.
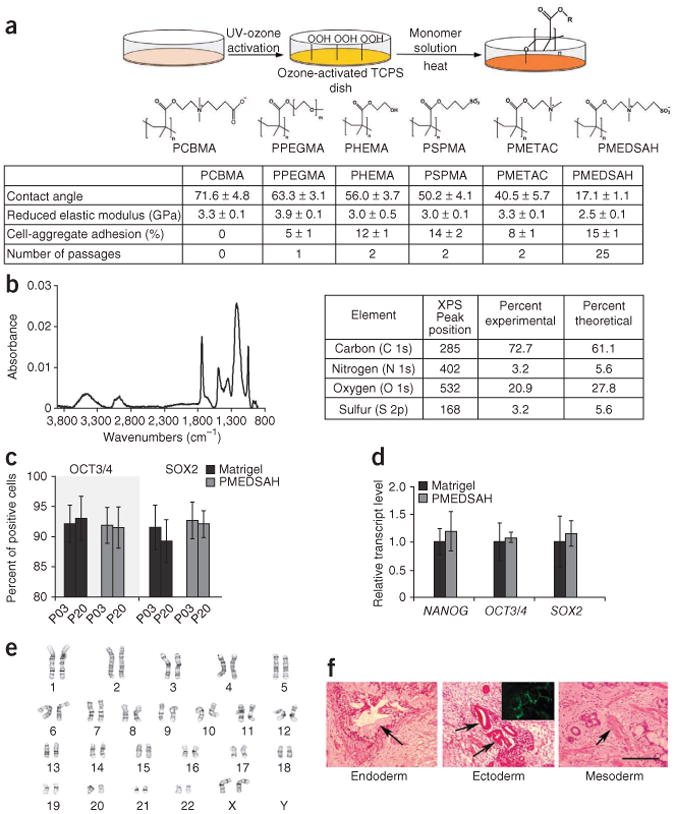
Long-term culture of H9 hES cells on PMEDSAH with MEF-conditioned media. (a) Schematic diagram showing graft-polymerization used to synthesize the polymer coatings and their chemical structures. Tissue culture polystyrene dishes were first activated by UV ozone and then methacrylate-based monomers were polymerized from the surface. Table lists contact angle, reduced elastic modulus (GPa) (mean ± s.d.), initial hES cel aggregate adhesion (mean ± standard error) and number of passages achieved on each polymer coating. (b) Fourier transform infrared spectrum of PMEDSAH coating showing distinct bands at 1,732.9 cm−1 and 1,208.4 cm−1, which indicated presence of carbonyl and sulfonate groups, respectively. Table lists elemental composition of PMEDSAH obtained using X-ray photoelectron spectroscopy. Relative composition of these elements was in agreement with the expected chemical composition of PMEDSAH. (c) Percentage (mean ± standard error) of hES cells expressing OCT3/4 and SOX2 at passages 3 (P03) and 20 (P20). (d) Relative transcript levels of NANOG, OCT3/4 and SOX2 from hES cells cultured on PMEDSAH and Matrigel. (e,f) After 25 passages, hES cells cultured on PMEDSAH and Matrigel (Supplementary Fig. 3) (e) maintained a normal karyotype and (f) retained pluripotency as demonstrated by teratoma formation in mmunosuppressed mice. H&E-stained paraffn sections indicating endoderm (goblet-like cells at arrow), ectoderm (neuroepithelial aggregates at arrow; and cells expressing neuron-restricted protein β-III tubulin in inset) and mesodermal derivatives (cartilage, connective tissue and muscle at arrow). Scale bar, 200 μm.
First, we examined growth using mouse embryonic fibroblast (MEF)-conditioned media (CM). H9 hES cells were mechanically harvested from cultures grown on MEFs and placed onto dishes coated with polymer or Matrigel. Matrigel supported adhesion and colony formation of >90% of hES cell aggregates, but no attachment was observed on poly[carboxybetaine methacrylate]. hES cells adhered, but spontaneously differentiated, during the first two passages on poly[[2-(methacryloyloxy)ethyl]trimethylammonium chloride], poly[3-sulfopropyl methacrylate], poly[2-hydroxyethyl methacrylate], and poly[poly(ethylene glycol) methyl ether methacrylate] (Fig. 1a). However, PMEDSAH (Fig. 1b for polymer characterization) supported attachment and proliferation of two hES cell lines (BG01 and H9), long-term growth, as well as normal genetic, proteomic and differentiation potential. Throughout 25 passages, H9 hES cells seeded on PMEDSAH expressed characteristic hES cell markers, displayed a normal karyotype and retained pluripotency (Fig. 1c–f and Supplementary Fig. 1). At passage 20, expression levels of the hES cell markers OCT3/4 (91 ± 3%) and SOX2 (92 ± 2%) were similar to levels expressed by cells cultured on Matrigel (Fig. 1c). Microarray analysis revealed similar expression levels of hES cell–specific genes in cells grown on PMEDSAH or Matrigel (Supplementary Table 1). Further validation using qPCR revealed similar RNA expression levels of NANOG, OCT3/4 (also known as POU5F1) and SOX2 (Fig. 1d; primers listed in Supplementary Table 2). The pluripotency of H9 hES cells was confirmed at passage 25 by tri-lineage differentiation in teratomas (Fig. 1f). Taken together, these results demonstrate the ability of PMEDSAH to support long-term culture of undifferentiated, pluripotent hES cells.
Next, we grew BG01 and H9 cells on PMEDSAH-coated dishes in the presence of a xeno-free (free of nonhuman animal products) commercially available human cell–conditioned medium (Supplementary Methods). Throughout 15 passages, BG01 and H9 cells showed similar cell population–doubling times (38 ± 6 h and 37 ± 4 h, respectively) that compared well to values reported for Matrigel11, expressed hES cell markers, retained normal karyotypes and remained pluripotent (Supplementary Fig. 2). On PMEDSAH, a significantly higher H9 hES cell–aggregate adhesion was observed for all passages using human cell–conditioned medium (86 ± 6%) compared to MEF-conditioned media (15 ± 1%; Fig. 2a). Cell-aggregate adhesion was also significantly higher for H9 cells in human cell–conditioned medium than for BG01 cells cultured under the same conditions (47 ± 5%; Supplementary Fig. 2), suggesting that there may be important biological differences between cell lines in their expression of adhesion receptors.
Figure 2.
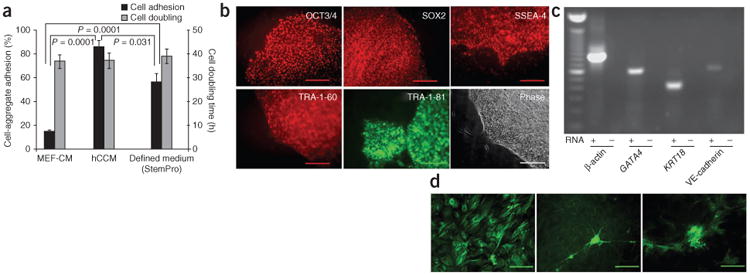
PMEDSAH supports culture of hES cells in defined medium. (a) Percentage (mean ± standard error) of cell aggregate adhesion (number of aggregates attached with respect to total aggregates passaged) and population doubling time (twofold increase in colony area) for H9 hES cells cultured on PMEDSAH in MEF-conditioned medium, human cell–conditioned medium and defned medium. P-values calculated using unpaired t-test. (b) Fluorescence micrographs of colonies of H9 cells cultured on PMEDSAH in StemPro medium showing expression of hES cell markers: OCT3/4, SOX2, SSEA-4, TRA-1-60 and TRA-1-81; and a phase-contrast image. (c) RT-PCR analysis of RNA from embryoid bodies showing expression of endoderm (GATA4), ectoderm (KRT18) and mesoderm derivatives (VE-cadherin; also known as CDH5). β-Actin (also known as ACTB) was used as positive control, and for each primer set tested, a reaction lacking RNA was assessed in parallel as a negative control. Scale bars, 200 μm, except for SOX2, which is 100 μm (d) Micrographs showing immunoreactivity for α-fetoprotein (endoderm), β-III tubulin (ectoderm) and smooth muscle actin (mesoderm) demonstrating the pluripotent state of H9 cells cultured on PMEDSAH in StemPro medium. Scale bars, 200 μm.
Finally, we examined the ability of PMEDSAH to support hES cell cultures in two serum-free defined media1,2,4,12 (StemPro and mTeSR; Supplementary Methods). Cells grown in mTeSR could not be passaged, but StemPro medium was able to support ten passages of H9 hES cells (Fig. 2). BG01 hES cells could not be passaged beyond three passages in StemPro (Supplementary Fig. 4). Throughout ten passages of H9 cells in StemPro medium, cell population–doubling times, expression of hES cell markers and normal karyotypes were confirmed (Fig. 2a,b). Moreover, H9 cells maintained the ability to differentiate into endoderm, mesoderm and ectoderm (Fig. 2c,d).
The ability of PMEDSAH to support hES cell culture in defined media suggest the utility of this coating for commercial hES cell expansion. Additional studies will be required to determine contribution of the various physicochemical properties of the polymer, such as wettability, mechanical stiffness, surface topography and zeta potential. Compared to animal-derived matrices such as Matrigel,. PMEDSAH is chemically defined, can be synthesized reproducibly and has long-term stability. Unlike natural and recombinant matrices, PMEDSAH-coated dishes can be handled and stored with relative ease. This matrix therefore represents a significant step in the development of a fully defined, reproducible culture system for hES cell expansion.
Supplementary Material
Acknowledgments
H.N. acknowledges funding from the University of Michigan Rackham Predoctoral Fellowship. J.L. gratefully acknowledges support from the National Science Foundation (NSF) in the form of a CAREER grant and funding from the NSF under the MRI program. We would like to thank J.H. Elisseeff and N. Hwang for their insightful comments throughout this study. We further thank J. Garcia-Perez, M. Morell, L.S.D. Emmett, M. Dzaman, M. Bormann, C. Pacut, N.Leff and J. acDonald for their valuable assistance. We would also like to thank C. Smith, M. Yoshida and S. Brown for their invaluable comments on the manuscript. This research was supported by National Institutes of Health grants P20 GM-069985, R01 DE016530 and the National Institute of Dental and Craniofacial Research T32 Tissue Engineering and Regeneration Training Program.
Footnotes
Author Contributions: L.G.V.-D. and H.N. contributed equally to this work and were involved in experimental design, performing hES cell culture experiments, data analysis and manuscript preparation. L.G.V.-D. carried out cell analysis experiments, and H.N. fabricated the polymer coatings and performed surface analysis. J.D. was involved in immunocytochemistry and RT-PCR, while N.C.N.-d.S. conducted microarray analysis and qPCR. P.H.K. participated in manuscript preparation. K.S.O. participated in manuscript preparation and performed teratoma assays. J.L. and G.D.S. were involved in conceptual and experimental design, as well as in manuscript preparation.
Competing Financial Interests: The authors declare no competing financial interests.
Published online at http://www.nature.com/naturebiotechnology/.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
Note: Supplementary information is available on the Nature Biotechnology website.
References
- 1.Ludwig TE, et al. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, et al. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braam SR, et al. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 4.Brafman DA, et al. Stem Cells Dev. 2009;18:1141–1154. doi: 10.1089/scd.2008.0410. [DOI] [PubMed] [Google Scholar]
- 5.Mallon BS, et al. Int J Biochem Cell Biol. 2006;38:1063–1075. doi: 10.1016/j.biocel.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin MJ, et al. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 7.Li YJ, et al. J Biomed Mater Res A. 2006;79:1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 8.Derda R, et al. ACS Chem Biol. 2007;2:347–355. doi: 10.1021/cb700032u. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DG, et al. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 10.Hakala H, et al. Tissue Eng Part A. 2009;15:1775–1785. doi: 10.1089/ten.tea.2008.0316. [DOI] [PubMed] [Google Scholar]
- 11.Xu C, et al. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 12.Prowse AB, et al. Stem Cells Dev. 2009;18:1135–1140. doi: 10.1089/scd.2009.0080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


