Abstract
During early embryogenesis in Drosophila melanogaster, extensive vesicle transport occurs to build cell boundaries for 6,000 nuclei. Here we show that this important process depends on a functional complex formed between the tumour suppressor and adaptor protein Discs-Large (Dlg)1 and the integral membrane protein Strabismus (Stbm)/Van Gogh (Vang)2,3. In support of this idea, embryos with mutations in either dlg or stbm displayed severe defects in plasma membrane formation. Conversely, overexpression of Dlg and Stbm synergistically induced excessive plasma membrane formation. In addition, ectopic co-expression of Stbm (which associated with post-Golgi vesicles) and the mammalian Dlg homologue SAP97/hDlg4,5 promoted translocation of SAP97 from the cytoplasm to both post-Golgi vesicles and the plasma membrane. This effect was dependent on the interaction between Stbm and SAP97. These findings suggest that the Dlg–Stbm complex recruits membrane-associated proteins and lipids from internal membranes to sites of new plasma membrane formation.
Formation of new plasma membranes is dependent on the delivery of post-Golgi vesicles to a pre-existing plasma membrane. An excellent model system to study this process is the cellularization that occurs during early Drosophila embryogenesis. During cellularization, the embryonic plasma membrane must rapidly grow inwards to segregate thousands of nuclei, generated by synchronized cycles of nuclear division without cytokinesis, into individual cells6. This process occurs through addition of post-Golgi vesicles to defined regions of lengthening plasma membranes and may be required for establishing proper cell polarity7. The Dlg tumour suppressor is essential both for establishing proper cell polarity and for assembling multiprotein complexes at specialized cell–cell junctions8. Accumulating evidence also indicates that Dlg associates with its targets at intracellular membrane sites before accumulation at the plasma membrane9–12. The integral membrane protein Stbm, however, is a regulator of planar tissue polarity in the fly2,3, and mouse Loop-tail (Lp) and zebrafish trilobite mutants that also have mutations in stbm homologues display defects in gastrulation and neurogenesis13,14.Here,we report that Dlg binds to Stbm and that this complex is required for formation of new plasma membranes during cellularization.
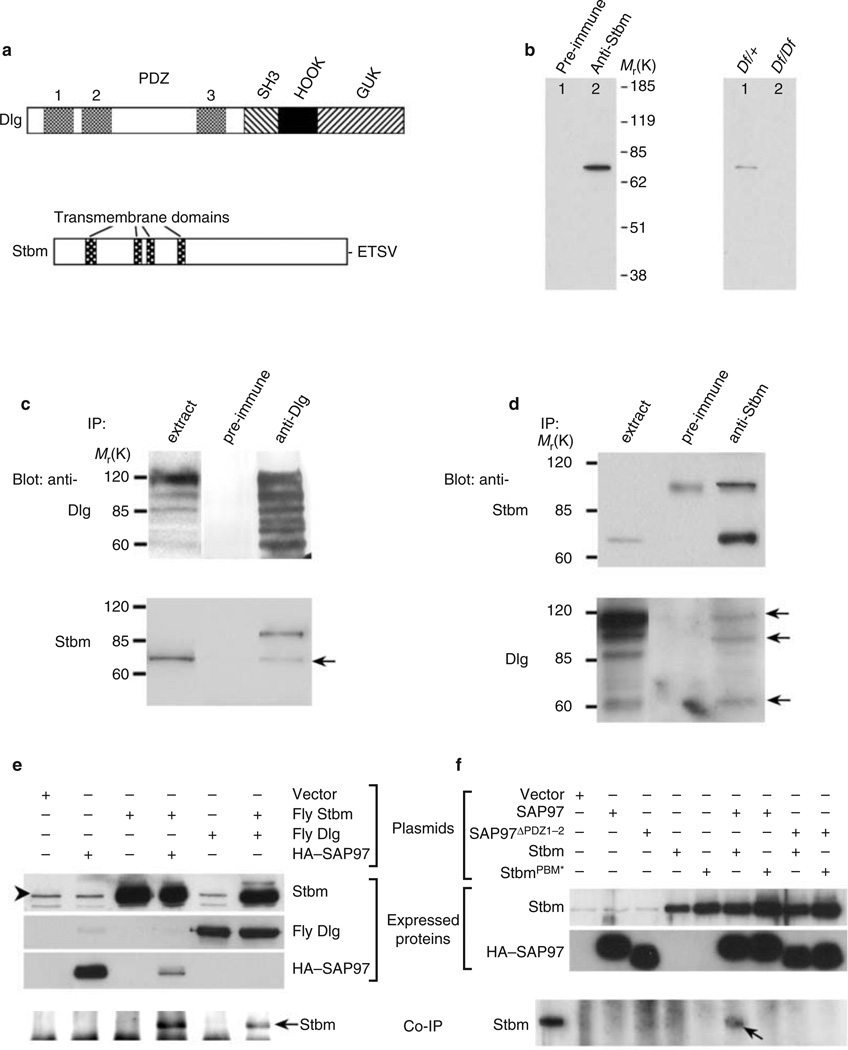
We identified Stbm as a binding partner of Dlg using the first and second Dlg PDZ domains (Dlg-PDZ1–2; Fig. 1a) as bait in the yeast two-hybrid system. Stbm contains four putative transmembrane domains and a consensus PDZ-domain-binding motif (PBM) at its extreme carboxyl terminus (Fig. 1a)2. In glutathione S -transferase (GST) pull-down assays, the 17 C-terminal residues of Stbm (GST–StbmPBM) were sufficient to mediate binding to Dlg-PDZ1–2 (see Supplementary Information, Fig. S1). Furthermore, changing - ETSV to -EASV (GST–StbmPBM*) abolished this interaction (see Supplementary Information, Fig. S1).
Figure 1.
Dlg interacts with Stbm. (a) Domain structures of Dlg and Stbm. (b) Stbm antiserum, but not pre-immune serum, recognizes a 75K band in embryonic extracts (left). Western blot of two individual embryos (stage 16) derived from Df(2R)NP4/CyO parents (right). All control CS embryos expressed the 75K protein (data not shown). (c, d) Stbm and Dlg (arrow) co-immunprecipitate from embryo extracts. (e, f) Stbm and Dlg also interact in COS-7 cells. Combinations of transfected expression plasmids are indicated. Stbm recovered from the immunocomplex, as shown in e and f, or expressed endogenously in COS-7 cells, as shown in e, is indicated by arrows or an arrowhead, respectively. The size of fly Stbm expressed in the COS-7 cells (asterisk in f) is shown for comparison.
We used GST–StbmWT as an antigen to generate mouse polyclonal serum that specifically recognizes Stbm (Fig. 1b; also see Supplementary Information, Fig. S2). In embryonic extracts, the anti-Stbm, but not pre-immune, serum recognized a protein that migrates with a relative molecular mass (Mr) of 75K (Fig. 1b, left panel), slightly larger than the predicted 66K mass of Stbm. This 75K band was absent in 27% (8/30) of stage-16 embryos individually collected from the Df(2R)NP4/CyO adults, concordant with the predicted 25% inheritance frequency for a homozygous stbm deficiency (Fig. 1b, right). In addition, 75K Stbm co-immunoprecipitated with Dlg from fly embryonic extracts (Fig. 1c).Multiple alternative splicing isoforms of fly Dlg have recently been identified15, and in a reciprocal co-immunoprecipitation assay, three isoforms (110, 95 and 60K) co-immunoprecipitated with Stbm (Fig. 1d).
Similarly, fly Stbm co-immunoprecipitated with SAP97 from extracts of transfected COS-7 cells (Fig. 1e). This interaction is probably conserved as a result of high sequence similarity (60–70% identity) between the PDZ domains of fly and mammalian Dlg4,5, as well as between Stbm family proteins2,13,14. In contrast, Stbm with a disrupted PBM domain (StbmPBM*) or SAP97 missing the first and second PDZ domains (SAP97ΔPDZ1–2) failed to bind to SAP97WT or StbmWT, respectively (Fig. 1f), indicating that the interaction is mediated by the Stbm-PBM and Dlg-PDZ1–2 domains.
Consistent with reports that Stbm functions during early embryogenesis2, we detected expression of Stbm throughout fly embryogenesis, including the 0–3-h stage (data not shown), indicating that Stbm is maternally contributed to fly eggs. We also found that 100% of stbm15/stbm15 or stbm6 /stbm6 embryos, 94% (268/284) of stbm7–6/stbm7–6 embryos, 81% (281/318) of stbm7-6 /stbm153 embryos and 20% (92/471) of stbm153 /stbm153 embryos obtained from stbm homozygous parents failed to hatch, and their phenotype was similar to unfertilized eggs (data not shown). The fact that only 1% of wild-type Canton-S (CS) embryos (5/390) displayed this defect indicates that parental Stbm is important during gametogenesis, fertilization or possibly both.
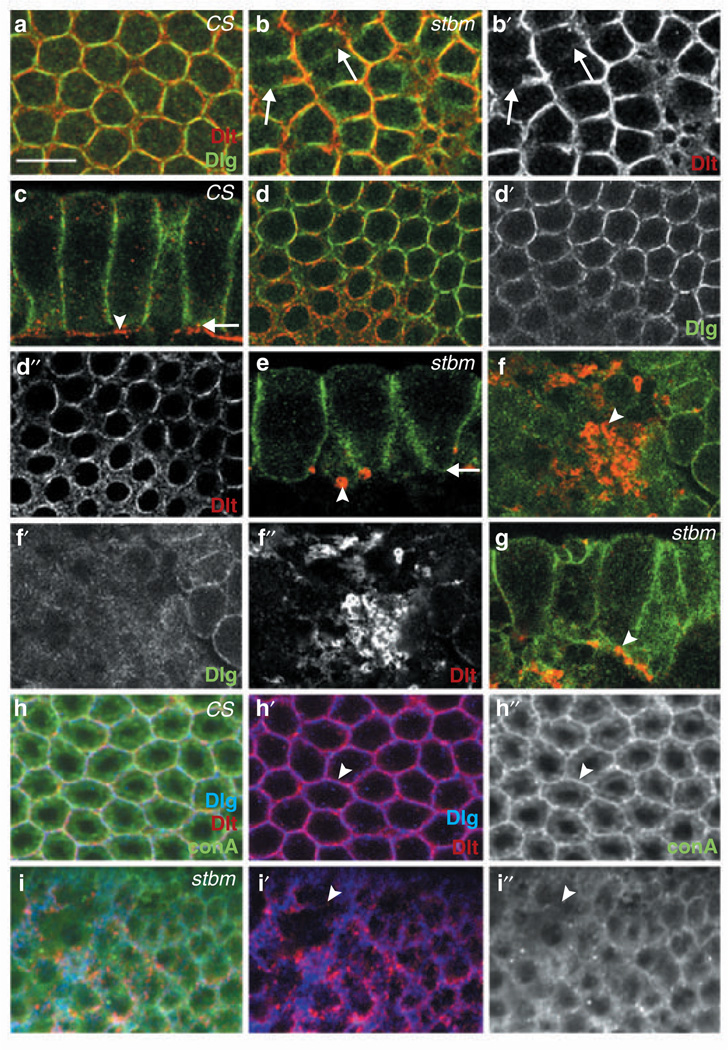
To evaluate stbm mutant embryos for hypomorphic phenotypes during early-stage embryogenesis, we crossed homozygous stbm adults and looked for defects in resulting stbm7–6 /stbm7–6 or stbm7-6/stbm153 embryos that were capable of reaching the 2–5-h stage of development (~10% and ~23% of embryos, respectively). In such stbm mutant embryos, we found that Dlg and Discs Lost (Dlt), which are maternally provided to eggs and show dynamic expression patterns during early embryogenesis16, failed to localize properly to the plasma membrane during the early cellularization stage (Fig. 2b). In contrast, control wild-type CS embryos exhibited regular honeycomb-like staining patterns for Dlg and Dlt at the plasma membrane (Fig. 2a). At later stages of cellularization, mutant embryos displayed increasingly severe abnormalities, particularly speckled Dlt and diffused Dlg staining (Fig. 2e–g). In control CS embryos, however, Dlg was distributed continuously along the plasma membrane at regions of cell–cell contact, and Dlt was enriched at invaginating cleavage furrows (Fig. 2c, d). Therefore, Stbm is required for proper localization of Dlg and Dlt during cellularization.
Figure 2.
Epithelial cells of stbm mutant embryos exhibit defects in membrane formation. The source of specimen and visualized proteins are indicated at the upper or lower right corners, respectively. All panels are single confocal images, except a and b, which are composites from multiple confocal sections spanning the vertical cell boundary. (a, b) Cross-section showing membrane pattern of CS and stbm7-6 embryos during cellularization. Arrows indicate absence of Dlg and Dlt. (c-f) Tangential sections (c, e) and cross-sections (d, f) of CS (c, d) and stbm7-6 (e, f) embryos. Arrows in c and e mark the cleavage furrow where confocal sections in d and f were taken, respectively. Arrowheads indicate Dlt pattern. (g) stbm7-6 /stbm153 embryo with similar phenotype as stbm7–6 in e. Abnormal Dlt pattern is marked with an arrowhead. (h, i) Visualization of membrane structure with conA in CS (h) and stbm7–6 (i) embryos. Arrowheads in h and i indicate the presence and absence, respectively, of Dlg, Dlt and conA. Scale bar represents 10 µm.
The absence of Dlg and Dlt in newly forming membranes of stbm mutant embryos (Fig. 2b) has two different possible interpretations: first, Dlg and Dlt fail to localize to plasma membranes, second, plasma membranes do not form. To distinguish between these two possibilities, we visualized plasma membranes in embryos by staining with fluorescein-conjugated concanavalin A (conA)17,which binds membrane glycoproteins and glycolipids. In wild-type CS embryos, Dlg and Dlt staining uniformly coincided with conA staining at the plasma membrane (100%, n = 20; Fig. 2h). In contrast, 60% of stbm embryos (n = 20) displayed regions lacking both Dlg and Dlt staining and, significantly, these regions invariably also lacked conA staining (Fig. 2i). This finding indicates that the formation of new plasma membranes is impaired in stbm mutant embryos.
To ascertain whether zygotic Stbm is involved in proper formation of plasma membranes, we analysed embryos obtained from stbm15/CyO-GFP parents. stbm15 (or stbm6) homozygous embryos, which contain maternal Stbm, but not zygotic wild-type Stbm, lacked detectable Dlg and Dlt staining in some cells, whereas this defect was absent in control embryos containing both wild-type maternal and zygotic Stbm (see Supplementary Information, Fig. S3). Therefore, we conclude that zygotic Stbm is required for membrane formation. A similarly important role for maternal Stbm in membrane formation was demonstrated in experiments showing that during cellularization, embryos derived from homozygous stbm7-6 females and wild-type CS males (and thereby containing zygotic but not maternal wild-type Stbm) also exhibited cells lacking detectable Dlg staining (data not shown).
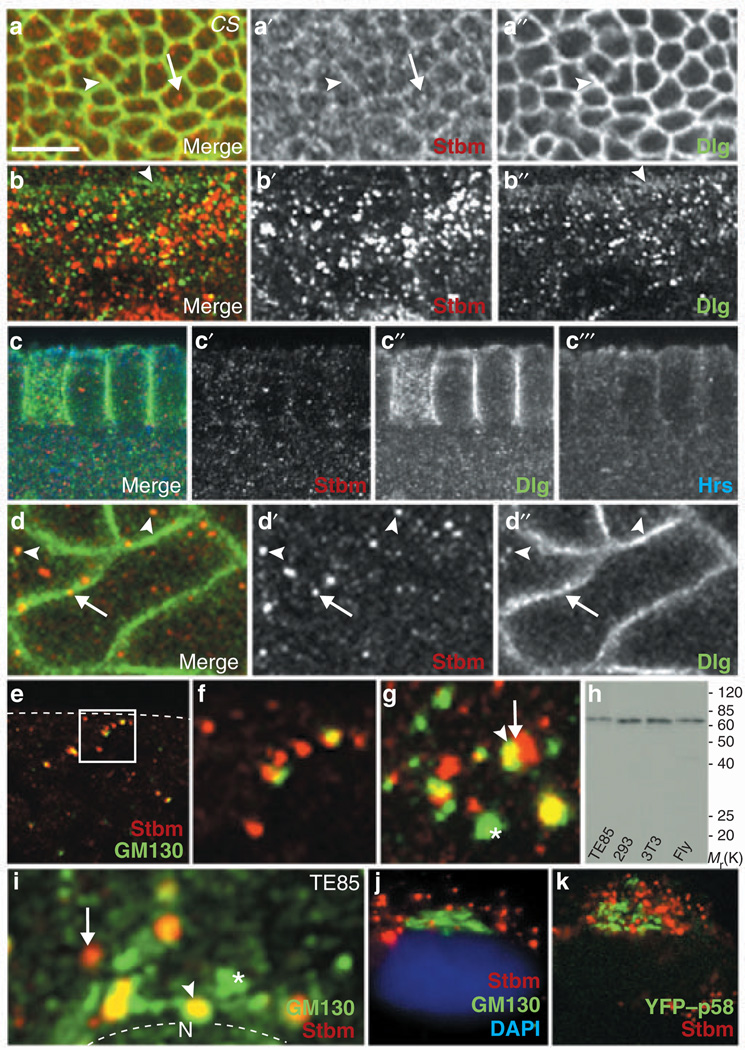
Next, we determined the sub-cellular location of Stbm. In epithelial cells of larval imaginal discs, Stbm colocalized with Dlg at membrane cell–cell junctions (Fig. 3a) and was also detected in association with cytoplasmic vesicular structures (Fig. 3a’). Similar vesicular structures containing either Dlg, Stbm or both were also abundant within the cytoplasm of salivary gland duct cells (Fig. 3b), suggesting that Stbm may associate with endosomes or Golgi vesicles. In this regard, mammalian Dlg and Hrs (an endosomal protein that regulates protein sorting in yeast, fly and mammalian cells) have been reported to form an endosome-associated complex18. We consistently found that Dlg partially colocalizes with vesicular Hrs (Fig. 3c′′, 3c′′′). Although Stbm also associated with vesicle-like structures in embryos during cellularization (Fig. 3c′), its staining pattern did not coincide with vesicular Hrs (Figs. 3c′, 3c′′′), suggesting that unlike Dlg, Stbm does not localize to endosomes. Dlg and Stbm instead colocalized on distinct vesicle-like structures in the cytoplasm and near the plasma membrane within apical regions of epithelial cells (Fig. 3d). This finding prompted us to explore whether Stbm associates with the Drosophila Golgi apparatus. Confocal microscopic analyses revealed that Stbm colocalizes with the Golgi-specific marker GM130 (refs 19, 20) in vesicular structures of CS embryos both before and during cellularization (Fig. 3e, f), albeit most prominently during cellularization (Fig. 3g). Thus, Stbm dynamically localizes on Golgi vesicles and at the plasma membrane in various cell types.
Figure 3.
Expression pattern of Stbm and Dlg in fly and mammalian cells. (a) Localization of Stbm in plasma membrane (arrowhead) and vesicle-like structures (arrow) from wing imaginal disc. (b) Expression of Dlg and Stbm in numerous vesicles of salivary gland duct cells. The basolateral plasma membrane slightly below the septate junction is marked with an arrowhead. (c) Localization of Stbm, Dlg and Hrs in cellularizing embryos. (d) Colocalization of Stbm and Dlg in vesicle-like structures (arrowheads) and patches of the plasma membrane (arrow). (e, f) Colocalization of Stbm and GM130 in vesicular structures in embryos before cellularization. Dashed line indicates surface of embryo. The region marked with a rectangle is magnified in f. (g) Vesicles in an cellularizing embryo containing Stbm (arrow), GM130 (asterisk), or both (arrowhead). (h) Detection of endogenous Stbm in human cell lines TE85 and 293, mouse cell line NIH3T3, and fly embryos. (i) Vesicles containing Stbm, GM130 or both in a TE85 cell lacking Golgi stack structure. Nucleus (N) is outlined. (j, k) TE85 cells with extensive Golgi stacks stained for endogenous GM130 and endogenous Stbm (j) or for exogenous YFP–p58 and endogenous Stbm (k). Nuclei were stained with DAPI. Scale bar represents 5 µm in a and b, 10 µm in c, 7 µm in d and e, 4 µm in f–I and 25 µm in j and k.
Whereas the mammalian Golgi apparatus has characteristic stacked structures, or cisternae, which fragment transiently during mitosis, the fly Golgi apparatus remains largely dispersed throughout the cell cycle21,22. Using Stbm antiserum that also reacts with mammalian Stbm in immunoblot assays (Fig. 3h), we showed that similarly to fly Stbm, mammalian Stbm localized predominantly on vesicles in TE85 and COS-7 cells. In TE85 cells that had just undergone mitosis (and therefore lacked defined Golgi stack structures) the majority of Stbm colocalized with GM130-containing vesicles (Fig. 3i). The size and pattern of vesicular structures containing Stbm, GM130, or both proteins, were similar in fly and mammalian cells (compare Fig. 3g with 3i). In TE85 cells displaying mature Golgi cisternae, however, Stbm was largely excluded from this structure, which was marked by endogenous GM130, a cis-Golgi marker19, or exogenous yellow fluorescent protein (YFP)–p58, a trans-Golgi marker23 (Fig. 3j, k). These observations demonstrate that Stbm associates with post-Golgi vesicles, but not with the mature Golgi apparatus, in mammalian cells.
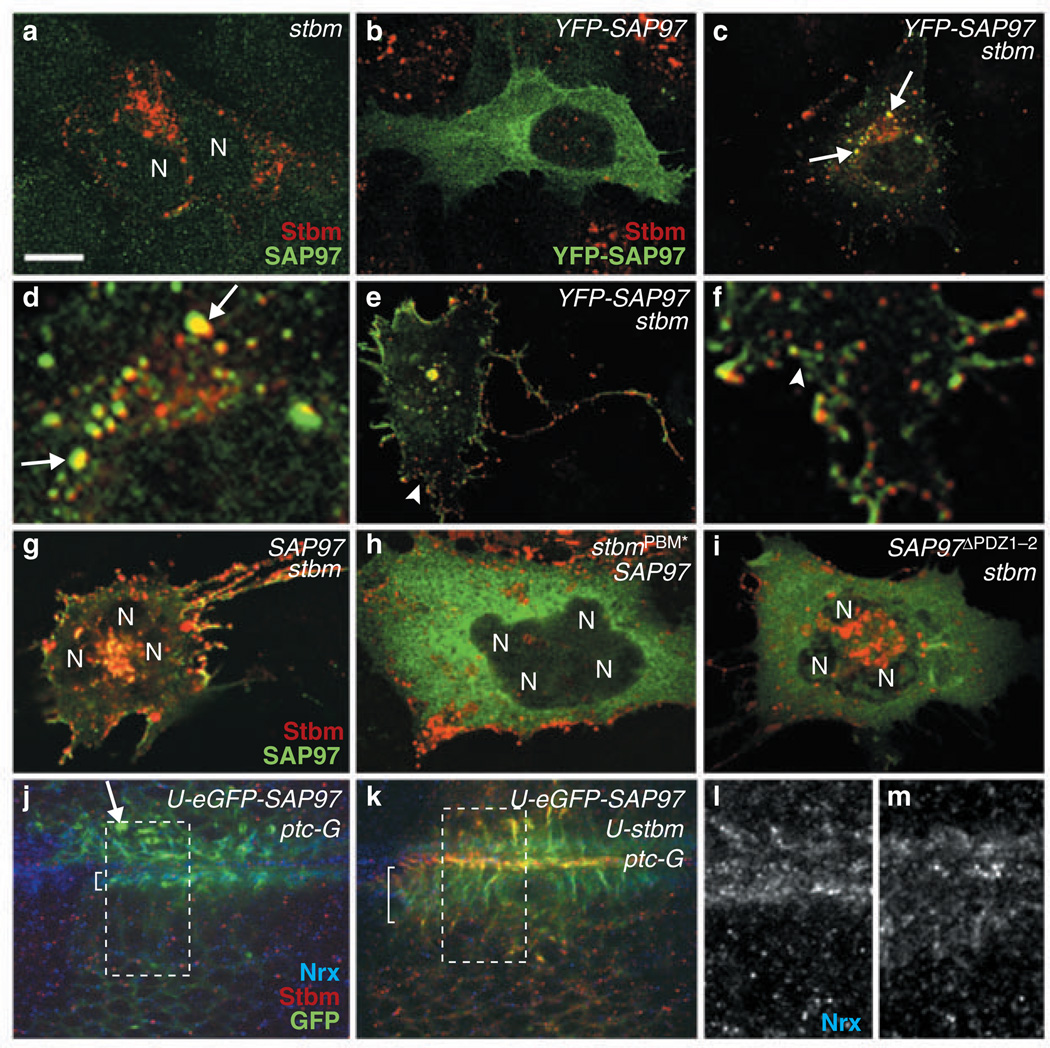
We next investigated whether the interaction between Dlg and Stbm may localize one or both proteins to post-Golgi vesicles or to the plasma membrane. In TE85 cells, which express low endogenous levels of Stbm and SAP97, exogenously expressed fly Stbm localized to cytoplasmic vesicles (Fig. 4a), similarly to endogenous mammalian Stbm (Fig. 3i–k). Exogenously expressed YFP–SAP97 remained diffuse in the cytoplasm, with small amounts also detected at plasma membrane (Fig. 4b). In cells ectopically co-expressing Stbm and YFP-SAP97, however, YFP–SAP97 instead associated with Stbm-containing vesicles located proximal either to the nucleus or to the plasma membrane (27/30 cells; Fig. 4c–f). This suggests that an interaction between Stbm and SAP97 is required for mobilization of SAP97, because YFP–SAP97 and SAP97 were efficiently recruited to vesicles by wild-type Stbm (Fig. 4c, e, g), but not by StbmPBM* (27/32 cells; Fig. 4h). Similarly, we failed to detect vesicle mobilization of SAP97 in cells ectopically co-expressing either YFP–SAP97 and StbmPBM* (data not shown), or SAP97ΔPDZ1–2 and wild-type Stbm (Fig. 4i). In addition, wild-type Stbm or StbmPBM* expressed alone localized to vesicle-like structures, suggesting that Dlg does not function reciprocally to recruit Stbm to vesicles (Fig. 4a; data not shown). It should also be noted that cells expressing both SAP97 and Stbm exhibited extensive membrane ruffling and filopodial formation (Fig. 4e, g), suggesting that this complex may function to regulate plasma membrane growth and maintenance.
Figure 4.
Dlg and Stbm must interact to colocalize and promote membrane formation. Various cDNAs transfected into TE85 cells are indicated at upper right corners. Nuclei (N) were visualized with DAPI (data not shown). (a) Vesicular localization of ectopically expressed fly Stbm. (b) Cytoplasmic localization of ectopically expressed YFP–SAP97. (c–f) Pattern of YFP–SAP97 in cells cotransfected with YFP–SAP97 and stbm. The regions marked with arrows in c and arrowhead in e are enlarged in d and f, respectively. Vesicles containing both YFP–SAP97 and fly Stbm are marked with arrows. (g–i) Colocalization of SAP97 and Stbm in cells transfected with fly stbm and SAP97 (g), but not with fly stbmPBM and SAP97 (h) or fly stbm and SAP97ΔPDZ1–2 (i). (j–m) Tangential views of the epithelial cells from wing disc expressing GFP–SAP97 alone (j) or together with Stbm (k). The UAS and Gal4 lines used to drive overexpression of Stbm or GFP–SAP97 are indicated at the upper right corners. The regions marked with rectangles in j and k are magnified and shown with the Nrx pattern in l and m, respectively. The extent of basolateral membrane visualized by Nrx is marked by brackets (l, m). One example of aggregated Dlg structures is indicated by an arrow (j). Scale bar represents 10 µm in a–c, e and g–k, 2.5 µm in d and f, and 5 µm in l and m.
The proposed role of Dlg and Stbm in plasma membrane formation was further investigated in flies by testing the effects of overexpressing SAP97 and Stbm on the distribution of Neurexin (Nrx), a transmembrane protein concentrated at septate junctions of fly cells24. Wing epithelial cells overexpressing SAP97 alone exhibited abnormal aggregations of SAP97 in the cytoplasm (Fig. 4j), and there was no noticeable change in the Nrx pattern at the basolateral membrane (Fig. 4l). However, wing epithelial cells expressing both SAP97 and Stbm showed abnormally extended basolateral membrane regions, where both SAP97 and Nrx were localized (Fig. 4k, m). A similar effect was observed in peripodial cells of eye-antenna discs overexpressing fly Dlg and Stbm (see Supplementary Information, Fig. S4). These findings suggest that elevated levels of Dlg–Stbm complexes in cells stimulate formation of plasma membranes, perhaps by increasing vesicle transport to these sites.
These observations suggest that similarly to Stbm, Dlg should be necessary for formation of plasma membranes. To test this hypothesis, we investigated whether temperature-sensitive dlgHF321 embryos cultured at the restrictive temperature showed defects in localization of Dlt. dlgHF321 embryos cultured at the restrictive temperature are known to exhibit developmental defects, such as dorsal closure and head involution25, but a possible role for dlg in embryonic cellularization has not yet been addressed. We found that Dlg in dlgHF321 embryos incubated at the restrictive temperature is present primarily in cytoplasmic vesicle-like structures, whereas Dlt localization is either diffuse or aggregated (Fig. 5a, b). These results are in marked contrast to those obtained in CS embryos, where Dlg and Dlt exhibited prominent localization at the plasma membrane (Fig. 2a). In dlgHF321 embryos, Stbm retained a normal vesicular staining pattern (Fig. 5c).
Figure 5.
Dlg is required for plasma membrane formation in both embryos and larvae. (a) Tangential view of a dlgHF321 embryo cultured at restrictive temperature lacks Dlg at the membrane. (b) Composite images of multiple cross-sections spanning the vertical cell boundary of the same embryo, revealing mis-localization of Dlg and Dlt. (c) A similar dlgHF321 embryo, showing cytoplasmic vesicle-like structures containing Stbm. Dlg localizes diffusely in the cytoplasm or in vesicle-like structures. (d, e) dlgm52 mutant clones are marked by the absence of Myc marker. Myc is expressed on the dlg+ chromosome. A mutant clone (dlg−/dlg−) and wild-type clones (twin spots; dlg+/dlg+) are marked by yellow and red lines, respectively. Unmarked cells are dlg+/dlg−. Localization of Nrx at the septate junction is more diffuse (arrowhead) or absent (arrow) in the dlg− clone. Note the higher level of Nrx in twin spot than in dlg+/dlg− cells (d). Membrane structure of the dlg− clone is visualized with Dlt (e), and a portion is magnified at the bottom left corner (e′). Scale bar represents 10 µm in a–c, and 20 µm in d and e.
If Dlg were essential for formation of plasma membranes, then this process should also be impaired in rapidly proliferating epithelial cells of dlg mutant imaginal discs. In small dlgm52 mutant clones where Dlg was not detected, Nrx levels were greatly reduced, resulting in disruption of septate junctions, whereas Nrx was not detected in larger mutant clones (Fig. 5d). As determined by Dlt staining, plasma membranes were also malformed and disrupted into vesicles in dlgm52 clones (Fig. 5e). Similar defects were evident in dlgm30 and dlgX1-2 mutant clones (data not shown). As expected, the staining pattern of Stbm remained vesicular in the dlg mutant clones, although the number of Stbm-containing vesicles located apically was somewhat reduced (data not shown). These results indicate that similarly to Stbm, Dlg is essential for plasma membrane formation in both embryos and larvae.
Proteins involved in establishing planar polarity, such as Stbm, Dishevelled (Dsh), Frizzled, Flamingo and Prickle (Pk), show dynamic changes in their localization between the plasma membrane and cytoplasmic vesicular structures26–28. The localization of these proteins most probably requires transport machinery capable of rapid activation at precise moments during development. Stbm was recently shown to bind directly to Dsh and Pk, and to induce their translocation to the plasma membrane28. Therefore, the processes of planar polarity and plasma membrane formation may depend on the ability of Stbm to mediate vesicle transport from the Golgi apparatus to the plasma membrane.Whether these tissue polarity proteins are simply mobilized by Stbm or actively participate in vesicle transport remains to be determined.
Tumour suppressors, such as Dlg, Lethal-giant larvae (Lgl) and Scribble (Scrib), as well as other PDZ domain- or PBM-containing proteins such as Bazooka, Crumbs (Crb), Stardust and Dlt, have important roles in assembling cell–cell junctions and in establishing and maintaining cell polarity8,29,30. Interestingly, the yeast and mammalian Lgl homologues function in fusion of post-Golgi vesicles to the plasma membrane by interacting with Syntaxin31,32. Syntaxin, which controls membrane traffic in both neuronal and non-neuronal cells, has also been shown to be essential for embryonic cellularization17. In addition, stbm and scrib exhibit a genetic interaction in wild-type mice, and Lp and circletail mutant mice with mutations in stbm and scrib, respectively, develop neural tube defects33. Taken together, our results and those of others suggest that Dlg, Lgl, Scrib and Stbm functionally interact with components of an undetermined vesicular transport apparatus to promote formation of plasma membranes with proper apical–basal polarity. Interestingly, a recent report has shown that SAP102, a brain-enriched mammalian homologue of fly Dlg, regulates the trafficking of NMDA receptors through an interaction with Sec8, a component of the exocyst complex34. Components of the exocyst complex often concentrate at points of rapid membrane addition, and it is believed that this complex functions to direct intracellular membrane vesicles to their sites of fusion with the plasma membrane35. Determining whether the exocyst complex contributes to the vesicle transport pathway that we suggest is controlled by the Dlg–Stbm complex requires further investigation. In conclusion, dissecting molecular mechanisms for Stbm and Dlg may be key to understanding how vesicle transport is coupled to the formation of plasma membranes and cell–cell junctions, as well as to the establishment of cell polarity.
METHODS
Fly strains
Stbm mutants, dlg mutants and UAS-dlg, or UAS-eGFPSAP97 were kindly provided by T.Wolff, P. Bryant and U. Thomas, respectively. UAS-stbm flies were generated using a stbm cDNA obtained from T. Wolff2. Other fly strains were from the Bloomington Stock Center.
To evaluate dlg mutant phenotypes, dlgHF321 embryos were collected from homozygous mutant adults maintained at 25 °C for 5 h. dlg mutant clones in imaginal discs were generated by Flp-mediated mitotic recombination in late-first- to early-second-instar larval progeny obtained by crossing dlg FRT18A females to w, hs-FLP, P[mini-w+ hs-pM]5A, 10D, P[ry+;hs-neo;FRT]18A males.
Yeast two-hybrid screen
A cDNA encoding Dlg PDZ1 and PDZ2 (Dlg-PDZ1–2) was generated by PCR using larval poly(A)+ RNA as a template and cloned into the NdeI site of the pAS1 vector to generate pAS-dlg. A larval cDNA library constructed in the pACT vector was screened with the bait plasmid pAS-dlg, as described36.
Antibodies
The Dlg-PDZ1–2 cDNA was subcloned into the pGEX vector. The fusion protein expressed by this plasmid was affinity purified and used as an antigen to generate Dlg antiserum in rabbits. Stbm polyclonal antiserum raised to an affinity purified GST–Stbm lacking the amino-terminal 41 residues was produced by the Caltech Monoclonal facility, California Institute of Technology, Pasadena, CA (see Supplementary Information, Fig. S1).
Transfections
COS-7 or TE85 cells were maintained in DMEM supplemented with gentamicin and 10% foetal bovine serum. Transfections were performed with Fugene6 according to manufacturers’ recommendations (Roche, Indianapolis, IN). Experimental analyses were routinely conducted 48 h after transfection.All SAP97 expression constructs were derived from a GW1-SAP97 plasmid that expresses the full-length I2 isoform. The YFP cDNA present in the GW1-YFP-SAP97 plasmid was derived from the pEYFP-C1 vector (Clontech, Palo Alto, CA). The GW1-SAP97ΔPDZ1–2 plasmid contains a SAP97 cDNA lacking SAP97 amino-acid residues 193–336 (ref. 5).
Immunoprecipitation and immunoblot assays
For immunoblot analyses, fly embryos were homogenized in homogenization buffer (20 mM Hepes at pH 7.6, 70 mM potassium chloride, 2 mM dithiothreitol, 0.1% NP40, 8% glycerol, 1 mM phenyl methylsulphonyl fluoride, Complete protease inhibitors (Roche), EGTA and 1,10-phenanthroline).Homogenates were precleared by centrifugation for 5 min at 5,000g and equal amounts of protein (as determined by a Bradford assay (Bio-Rad, Hercules, CA)) were separated by SDS–PAGE and transferred to nitrocellulose membrane.Mouse anti-Stbm and rabbit anti-Dlg antisera were used at 1:1,000 and 1:10,000, respectively, for immunoblot analyses. For immunoprecipitation assays, fly embryonic extracts (10 mg of protein in 1 ml of homogenization buffer) were incubated overnight at 4 °C with 20 µl anti-Stbm or 6 µl anti-Dlg antibody, and 30 µg Sepharose 6MB (Amersham). Sepharose beads were then washed extensively with the homogenization buffer. Mammalian cell extracts were generated in NETN buffer (20 mM Tris-HCl at pH 8.0, 100 mM sodium chloride, 1 mM EDTA and 0.5% NP-40) supplemented with protease inhibitors and phosphatase inhibitors. Immunoprecipitation was performed as previously described37.
Immunocytochemistry
Embryos were fixed in 4% methanol-free formaldehyde (Polysciences, Warrington, PA). Samples were then stained with mouse antibodies to Stbm and rabbit antibodies to Dlg and GM130 (both at 1:1,000 dilution). TE85 or COS-7 cells were transfected on glass coverslips, fixed for 30 min in 4% paraformaldehyde (Polysciences) and then permeabilized for 5 min in 0.5% Triton X-100. Cells were blocked with Tris-buffered saline containing 10% goat serum, incubated with pre-immune serum or the indicated antiserum and then appropriate secondary antibodies. After extensive washes, cells were then fixed again for 10 min in 4% paraformaldehyde. Fluorescent images were captured using Zeiss LSM laser-scanning confocal microscope (Zeiss, Thornwood, NY) and presented using Adobe Photoshop.
Supplementary Material
ACKNOWLEDGEMENTS
We thank M. Kuroda, R. Kelley, S. Izaddoost, and K.-W. Choi for valuable discussions, T.Wolff for stbm mutants and stbm cDNA, P. Bryant for dlg mutants and guinea pig anti-Dlg antibody, S. Ou and P. Patterson for generating anti-Stbm antibody, M. Lowe for anti-GM130 antibody, S. Elledge for two-hybrid system reagents, H. Bellen for Hrs and Nrx antibodies, and the Bloomington Stock Center for fly strains. K.K.F. was the recipient of the Viral Oncology Training Grant predoctoral fellowship T32 CA009197. R.T.J. and K.-O.C. were supported by National Institutes of Health grants RO1 CA058541 and R29 NS35532, respectively.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
References
- 1.Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 2.Wolff T, Rubin G. strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- 3.Taylor J, Abramova N, Charlton J, Adler P. Van Gogh: A new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lue RA, Marfatia SM, Branton D, Chishti AH. Cloning and characterization of hDLG: the human homologue of the discs large tumor suppressor binds to protein 4.1. Proc. Natl Acad. Sci. USA. 1994;91:9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller BM, et al. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J. Neurosci. 1995;15:2354–2366. doi: 10.1523/JNEUROSCI.15-03-02354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foe VE, Odell GM, Edgar BA. In: The Development of Drosophila melanogaster. Bate M, Martinez-Ariaz A, editors. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1993. pp. 149–300. [Google Scholar]
- 7.Lecuit T, Wieschaus E. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryos. J. Cell Biol. 2000;150:849–860. doi: 10.1083/jcb.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilder D. PDZ proteins and polarity: functions from the fly. Trends Genet. 1991;17:511–519. doi: 10.1016/s0168-9525(01)02407-6. [DOI] [PubMed] [Google Scholar]
- 9.Rao A, Kim E, Sheng M, Craig AM. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J. Neurosci. 1998;18:1217–1229. doi: 10.1523/JNEUROSCI.18-04-01217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas U et al. Synaptic targeting and localization of discs-large is a stepwise process controlled by different domains of the protein. Curr. Biol. 2000;10:1108–1117. doi: 10.1016/s0960-9822(00)00696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Husseini AE, et al. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J. Cell Biol. 2000;148:159–172. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sans N, et al. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J. Neurosci. 2001;21:7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kibar Z, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nature Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 14.Jessen JR, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nature Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendoza C, et al. Novel isoforms of Dlg are fundamental for neuronal development in Drosophila. J. Neurosci. 2003;23:2093–2101. doi: 10.1523/JNEUROSCI.23-06-02093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhat MA, et al. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–845. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 17.Burgess RW, Deitcher DL, Schwarz TL. The synaptic protein Syntaxin1 is required for cellularization of Drosophila embryos. J. Cell Biol. 1997;138:861–875. doi: 10.1083/jcb.138.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chetkovich DM, et al. Postsynaptic targeting of alternative Postsynaptic density-95 isoforms by distinct mechanisms. J. Neurosci. 2002;22:6415–6425. doi: 10.1523/JNEUROSCI.22-15-06415.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura N, et al. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondylis V, Goulding SE, Dunne JC, Rabouille C. Biogenesis of Golgi stacks in imaginal discs of Drosphila melanogaster. Mol. Biol. Cell. 2001;12:2308–2327. doi: 10.1091/mbc.12.8.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanley H, Botas J, Malhotra V. The mechanism of Golgi segregation during mitosis is cell type-specific. Proc. Natl Acad. Sci. USA. 1997;94:14467–14470. doi: 10.1073/pnas.94.26.14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucocq JM, Warren G. Fragmentation and partitioning of the Golgi apparatus during mitosis in HeLa cells. EMBO J. 1987;6:3239–3246. doi: 10.1002/j.1460-2075.1987.tb02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole NB, et al. Golgi dispersal during a microtubule disruption-regeneration of Golgi stacks at peripheral endoplasmic-reticulum exit sites. Mol. Biol. Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumgartner S, et al. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- 25.Perrimon N. The maternal effect of lethal(1)discs-large-1 : a recessive oncogene of Drosophila melanogaster. Dev. Biol. 1988;127:382–407. doi: 10.1016/0012-1606(88)90326-0. [DOI] [PubMed] [Google Scholar]
- 26.Strutt D, Johnson R, Cooper K, Bray S. Asymmetric localization of frizzled and the determination of notch-dependent cell fate in the Drosophila eye. Curr. Biol. 2002;12:813–824. doi: 10.1016/s0960-9822(02)00841-2. [DOI] [PubMed] [Google Scholar]
- 27.Rawls AS, Wolff T. Strabismus requires Flamingo and Prickle function to regulate tissue polarity in the Drosophila eye. Development. 2003;130:1877–1887. doi: 10.1242/dev.00411. [DOI] [PubMed] [Google Scholar]
- 28.Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localized and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- 29.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 30.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2002;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 31.Fujita Y, et al. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 32.Lehman K, Rossi G, Adamo JE, Brenwald P. Yeast homologues of Tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, sec9. J. Cell Biol. 1999;146:125–140. doi: 10.1083/jcb.146.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murdoch JN, et al. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum. Mol. Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- 34.Sans N, et al. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nature Cell Biol. 2003;5:520–530. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- 35.Novick P, Guo W. Ras family therapy: Rab Rho and Ral talk to the exocyst. Trends Cell Biol. 2002;12:247–249. doi: 10.1016/s0962-8924(02)02293-6. [DOI] [PubMed] [Google Scholar]
- 36.Durfee T, et al. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 37.Weiss RS, McArthur MJ, Javier RT. Human adenovirus type 9 E4 open reading frame 1 encodes a cytoplasmic transforming protein capable of increasing the oncogenicity of CREF cells. J. Virol. 1996;70:862–872. doi: 10.1128/jvi.70.2.862-872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.