Abstract
Aim of the study
Bamboo leaves are used as a component in traditional Chinese medicine for the anti-inflammatory function. Our previous studies have demonstrated that an ethanol/water extract from Phyllostachys edulis ameliorated obesity-associated chronic systemic inflammation in mice, and therefore relieving the symptoms of type 2 diabetes. The aim of this project was to further investigate the effects of this bamboo extract on hepatic biotransformation enzymes in both lean and obese mice, as an initial step in the toxicological evaluation of using this traditional medicine in obese/diabetic population.
Materials and methods
Male C57BL/6J mice were randomized to 4 groups and fed standard (10% kcal from fat) diet with or without bamboo extract supplementation at a dose of 10 gram per kilogram diet (n = 10 and n = 9, respectively), or high fat (45% kcal from fat) diet with or without bamboo extract (n = 8 and N = 7, respectively). The dietary treatment lasted for 6 months. Subsequently, the activities and expression of the major Phase I and II hepatic biotransformation enzymes were assessed in subcellular fractions from murine livers.
Results
Three groups of mice, lean bamboo extract-supplemented, obese/diabetic, and bamboo extract-supplemented obese/diabetic, showed greater activities of cytochromes P450 1a2 and 3a11 compared to control but no changes in the expression level of these proteins. For Phase II enzymes, bamboo extract supplementation in lean mice caused decreased glutathione-S-transferase activity (−12%) and greater uridine diphosphate glucuronosyltransferase activity (+46%), but had no effect on sulfotransferase activity. Conversely, the obese/diabetic condition itself increased glutathione-S-transferase and uridine diphosphate glucuronosyltransferase activities, but decreased total sulfotransferase activity and sulfotransferase 2a1 expression.
Conclusions
Bamboo extract and obesity/diabetes show significant independent effects on hepatic bio-transformation as well as interaction effects in mice. These changes may alter the clearance of endo-and xenobiotics, including bamboo extract itself, hence this effect should be carefully considered in the medicinal application of bamboo extract as it has potential to alter its own metabolism and that of other medications concurrently administered to obese diabetic patients.
Keywords: Bamboo extract, Obesity, Diabetes, Phase I enzyme, Phase II enzyme, Liver
1. Introduction
1.1. Traditional use and modern application of bamboo extracts in China
Bamboo is a rich natural resource in China. In traditional Chinese medicine, bamboo leaves are used as a component to reduce the energy of “fire” (an element usually related to inflammation), and treat hypertension, arteriosclerosis, and cardiovascular disease (Yuan, 1983). Besides the medicinal application, bamboo leaves and stems have been traditionally used in family kitchens to enhance the flavor and color of food, especially in the rural areas surrounded by “Bamboo Seas” in southern China. Correlations between the use of bamboo components in cooking processes and generally improved health status of the residents have been observed in some of the so called “bamboo villages”, but solid scientific examinations are yet to be conducted.
Growing from the root of tradition into the industrialized era, China sees the continuous usage of bamboo in modernized Chinese medicine, such as “Zhu Li Kou Fu Ye”, an extract of bamboo leaves inhibiting inflammation in the throat. Moreover, for the purpose of benefiting health and preserving the products from oxidation, bamboo extracts are also used in the beverage and food industries in China.
1.2. Potential anti-diabetic functions of bamboo extract
During the last few years, an ethanol/water extract of bamboo Phyllostachys edulis produced in China has been introduced in the market of the United States as a dietary supplement. Investigations carried out in our laboratory have demonstrated a potent anti-lipotoxicity function of this bamboo extract (BEX) using in vitro models (Panee et al., 2008). Subsequent in vivo studies further revealed that, when used as a dietary supplement in a high fat diet, BEX significantly improved glucose tolerance, inhibited hyperinsulinemia, lowered hepatic fat content and decreased circulating levels of tumor necrosis alpha (a major pro-inflammatory cytokine) in obese C57BL/6J mice (manuscript under preparation). These results indicate that BEX inhibits obesity-associated chronic systemic inflammation and ameliorates the symptoms of type 2 diabetes, suggesting a potential application of this natural product as an anti-diabetic nutraceutical.
1.3. Role of xenobiotic biotransformation enzymes in the safety of nutraceuticals
Xenobiotic biotransformation, mainly carried out in the liver, is a biological process that transforms lipophilic endo- or xenobiotics into hydrophilic substances that can be eliminated from the body, thus avoiding potentially harmful accumulation of the lipophilics (Klaassen, 2001). Phase I enzymes catalyze reactions that usually result in a small increase in hydrophilicity and involve hydrolysis, reduction, and oxidation to expose or introduce a functional group. Of these, the Cytochromes P450 (CYP) are most important (Klaassen, 2001). While many CYPs exist, the main enzymes involved in xenobiotic biotransformation belong to the families CYP1, CYP2, and CYP3 (Klaassen, 2001). Phase II enzymes often use the substrates produced by Phase I reactions and further increase the size and popularity of these compounds for ready excretion from the body. The major Phase II enzymes are uridine diphosphate glucuronosyltransferases (UGT), sulfotransferases (SULT) and glutathione-S-transferases (GST) (Klaassen, 2001).
Phases I and II enzymes can be induced or inhibited by nutraceuticals, with a net result that globalized enzyme induction usually results in greater clearance and elimination while general enzyme inhibition often causes harmful accumulation of chemicals (Martignoni and Groothuis, 2006). The effect of nutraceuticals on hepatic biotransforming reactions should be carefully considered during the development of such products.
1.4. Influences of obesity, and diabetes on xenobiotic biotransformation
Physiological factors such as obesity and diabetes can alter the hepatic biotransformation processes and change the metabolism of nutraceuticals and drugs (Ioannides and Barnett, 1999). In humans, obesity is associated with increased CYP2E1 but decreased CYP3A4 activities in the liver (Kotlyar and Carson, 1999; Burton et al., 2005), and with increased Phase II GST, UGT, and SULT activities (Abernethy and Greenblatt, 1986; Blouin et al., 1987; Barnett et al., 1993; Roe et al., 1999). In rodent models of obesity-induced diabetes, decreased hepatic GST activity has been reported (Barnett et al., 1992; Roe et al., 1999). Therefore, special attention is needed when administering nutraceuticals to obese and/or diabetic animals since changes in Phases I and II enzymes can result in altered efficacy and safety of the endogenous compounds (Roe et al., 1999). Additionally, there is further need for defining baseline levels of biotransformation enzymes as well as the independent effects of any compound (e.g. BEX) or disease state (e.g. obesity or type 2 diabetes).
1.5. Aims of this study
The aims of the present study were to assess the effects of BEX dietary supplement and obesity-induced type 2 diabetes on hepatic Phases I and II enzymes, and the potential interaction between the two factors. This is an initial step toward the safety evaluation of BEX in its future nutraceutical application.
2. Material and methods
2.1. Dietary treatments
The following diets were manufactured at Research Diets (New Brunswick, NJ): Lean control diet (10% kcal from fat, cat# D12450B), abbreviated as Lean Ctl; lean diet with BEX supplementation, abbreviated as Lean BEX; high fat diet (45% kcal from fat, cat# D12451), labeled as Obese Ctl; and high fat diet with BEX supplementation, labeled as Obese BEX. The dose of BEX supplementation in both diets was 10 g dry mass per kg of diet.
2.2. Animals
Thirty-four three-week-old male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, MN) and housed at the University of Hawaii Laboratory Animal Services facility. The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Hawaii. After one week of acclimation with standard laboratory rodent chow, 9 mice were fed Lean Ctl diet (referred to as the Lean Ctl group), 10 mice were fed Lean BEX diet (Lean BEX group), 7 mice were fed Obese Ctl diet (Obese Ctl group), and 8 mice were fed Obese BEX diet (Obese BEX group). The behavior and appearance of all mice appeared normal and there was no observed adverse effect from BEX supplementation. The dietary treatment lasted for 6 months before the mice were sacrificed by CO2 asphyxiation. The livers were excised, snap frozen in liquid nitrogen, and then stored at −80 °C until the time of assay.
2.3. Preparation of liver microsomal and cytosolic fractions
Liver samples were homogenized in a tissue grinder with buffer (0.1 M potassium phosphate, pH 6.5) at a 1:4 (weight:volume) ratio and then centrifuged at 20,000 × g at 4 °C for 20 min. The clear liquid fraction was aliquoted to a clean tube and centrifuged at 100,000 × g at 4 °C for 1 h. The clear cytosolic fraction was aliquoted out and the microsome pellets were resuspended in buffer. Fractions were stored at −80 °C.
2.4. Bicinchoninic acid assay for protein concentration
The protein concentration of microsomal and cytosolic liver fractions were assessed at 1:200 dilution using copper II sulfate solution in bicinchoninic acid with absorbance measured in a spectrophotometer at 562 nm (SpectraMax 340, Molecular Devices, Sunnyvale, CA) as previously described (Smith et al., 1985). Concentration was assigned by comparison to a standard curve of bovine serum albumin fraction V.
2.5. Enzyme activity assays
2.5.1. CYP1a2 (Dutton and Parkinson, 1989)
Microsomes (0.2 mg protein/mL final concentration) were incubated in black 96-well plates in the spectrophotometer at 37 °C for 2 min with 1 μL of 1 mM ethoxyresorufin substrate (in methanol) and 79 μL buffer (0.05 M Tris 25 mM MgCl2, pH 7.4). Then, 10 μL of 2.5 mM NADPH tetrasodium salt was added and fluorescence measured every 20 s for 10 min at 530/585 nm. All reactions were carried out in triplicate. The activity was determined using a standard curve of resorufin (0–1000 nM). For calculations, production of resorufin was within 10% of ethoxyresorufin concentration.
2.5.2. CYP3a11 (Price et al., 2000)
Microsomes (0.2 mg protein/mL final concentration) were prepared with and without the inhibitor, ketoconazole. Samples were incubated in black 96-well plates in a fluorometer (SpectraMax Gemini XS, Molecular Devices) at 37 °C for 2 min with or without 1 μL of 20 μM ketoconazole, 1 μL of 10 mM 7-benzyloxy-4-trifluoromethylcoumarin (BFC), and 78 μL buffer (0.1 M potassium phosphate 5 mM MgSO4, pH 7.4). An aliquot (10 μL) of 10 mM NADPH was added to all wells and the plate was read fluorescently at 37 °C for 25 min. All reactions were carried out in triplicate. Fluorescence was read at 410/510 nm, and high sensitivity. The activity was determined using a standard curve of 7-ethoxy-4-trifluoromethylcoumarin (HFC) (0–5 μM). For calculations, production of HFC was within 10% of BFC concentration. The final activity of CYP3a11 was calculated by subtracting the rate of activity with inhibitor from the rate of activity without inhibitor.
2.5.3. Total GST (Habig et al., 1974)
Cytosols (0.2 mg protein/mL final concentration) were incubated in optically clear 96-well plates in the spectrophotometer at 37 °C for 2 min with 1 μL of 50 mM 1-chloro 2,4-dinitrobenzene (CDNB) substrate (in dimethyl sulfoxide, DMSO) and 79 μL of buffer (0.1 M potassium phosphate, pH 6.5). Then, 10 μL of 10 mM reduced L-glutathione cofactor was added to a final volume of 100 μL. The absorbance at 340 nm was recorded every 10 s for 10 min. All reactions were carried out in triplicate. Specific activity was calculated using an extinction coefficient factor of 9.6 mM/cm.
2.5.4. Total UGT (Collier et al., 2000)
Microsomes (0.1 mg protein/mL final concentration) were incubated in black 96-well plates in the fluorometer at 37 °C for 2 min with 1 μL of Alamethicin (50 μg/mg protein in DMSO), 10 μL of 1 mM 4-methyl umbelliferone sodium salt (4 MU) substrate, and 74 μL of buffer (0.1 M Tris–HCl 5 mM MgCl2, pH 7.4). Then, 50 mM uridine 5′-diphosphoglucuronic acid triammonium salt (UDPGA) was added to reach a final volume of 100 μL. Fluorescent intensity was recorded for 10 min at 15 s intervals at 355/460 nm 37 °C. All reactions were carried out in triplicate. Activity of samples was determined by comparison to a standard curve of 4 MU (0–200 μM). For calculations, 4 MU depletion was within 10% of starting 4 MU concentration.
2.5.5. Total SULT (Mulder and Van Doorn, 1975)
Cytosols (2 mg/mL) were incubated for 50 min in a water bath at 37 °C with 210 μL buffer (50 mM potassium phosphate 5 mM MgCl2, pH 6.5), 30 μL of 4 mM 4-nitrophenol (4NP) and 30 μL of 600 μM adenosine 3′-phosphate 5′-phosphosulfate tetralithium salt (PAPS). The reaction was terminated by adding 300 μL of 1 M sodium hydroxide. Then, for each sample, 100 μL was aliquoted in triplicate in clear 96-well plates and absorbance at 400 nm was measured in the spectrophotometer. Substrate depletion was determined using a standard curve of 4NP (0–800 μM). Incubation time was determined using a range-finding curve (20–120 min). For calculations, 4NP depletion was within 10% of starting 4NP concentration.
2.6. Western blotting
Four samples were randomly chosen from each group for western blotting. Sixteen (16) samples from the four experimental groups were loaded in the same Criterion precast 18-well 10% polyacrylamide gel (Biorad, Hercules, CA) for each electrophoresis. Amersham Rainbow Full-Range Marker (GE Healthcare, Piscat-away, NJ) was used for molecular weight determination. PVDF membrane (0.45 μM pore size, Millipore Corp., Billerica, MA) was used for protein transfer. The SULT 1a1 mouse monoclonal antibody was purchase from Abnova Corp. (Walnut, CA). SULT 2a1 rabbit polyclonal antibody was purchased from Abcam Inc. (Cambridge, MA). CYP1a2 rabbit polyclonal antibody, CYP3a11 goat polyclonal antibody, UGT1a rabbit polyclonal antibody, and UGT2b goat polyclonal antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies, biotin–streptavidin peroxidase-conjugated AffiniPure donkey (anti-goat, anti-mouse, anti-rabbit) IgG, and normal donkey serum were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Gove, PA). The detection of the specific binding was accomplished using the 3,3′,5,5′-tetramethylbenzidine (TMB) liquid substrate system. Incubation time ranged from 15 to 60 min depending on speed of the color development.
As a loading control, after the TMB incubation, the PVDF membranes were incubated with Amido Black Stain for 20–35 min for total protein staining, and then with destaining solution for 1.5–2 h to remove the background color. The total protein on the gels was also stained in Coomassie Stain for 40–45 min and then soaked in destaining solution overnight.
Image J software was used to determine the density of the bands of specific binding. The loading control was obtained from the average density of three or four bands of different molecular weights from the total protein staining in the membranes or gels. The protein expression was quantified by the ratio between the density of the target protein band and that of the loading control.
2.7. Statistical analysis
Multiple Student’s t-tests or two-way ANOVA were used to determine probability values when appropriate. Data were considered statistically significant when p ≤ 0.05.
3. Results
3.1. Body weight
At the end of the experiment, the body weights of the four groups of mice were: Lean Ctl 28.0 ± 2.8 g, Lean BEX 28.9 ± 3.0 g, Obese Ctl 36.3 ± 3.7 g, and Obese BEX 33.4 ± 4.4 g. BEX supplementation did not significantly affect the body weight at this time point in either the obese or lean mice. However, the high fat diet resulted in a 22% increase of the body weight in comparison to the low fat diet (p < 0.001).
3.2. Phase I enzymes
3.2.1. Protein expression and enzymatic activity of CYP1a2
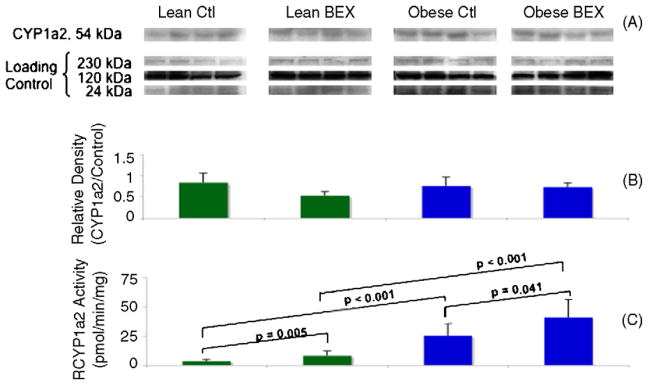
Western blotting of the protein expression of hepatic CYP1a2 did not show obvious changes among the four experimental groups (Fig. 1A and B). On the other hand, BEX supplementation significantly increased the enzymatic activity of hepatic CYP1a2 by 130% in lean mice and by 61% in obese–diabetic mice (Fig. 1C). Moreover, overall higher CYP1a2 activity was seen in obese–diabetic mice in comparison to the lean animals. The Obese Ctl group showed 620% more activity than the Lean Ctl group, and the Obese BEX group had 403% more activity than the Lean BEX group. These results indicate that both dietary BEX exposure and obesity–diabetes may have contributed to post-translational increase in CYP1a2 activity, and BEX and obesity–diabetes may have had a synergistic effect in increasing CYP1a2 activity, since the presence of both factors greatly increased CYP1a2 activity over either condition independently.
Fig. 1.
Western blot and enzymatic activity of CYP1a2 in mouse liver samples. Panel A shows the bands of CYP1a2 protein detected by a specific antibody and of the loading controls at three molecular weights derived from Amido Black staining of total proteins in the PVDF membrane. Panel B shows the quantification of relative density of the bands; n = 4 in each group. Panel C shows the enzymatic activity of CYP1a2; n = 9 for Lean Ctl group, n = 10 for Lean BEX group, n = 7 for Obese Ctl, and n = 8 for Obese BEX group. Average and SD are shown.
3.2.2. Protein expression and enzymatic activity of CYP3a11
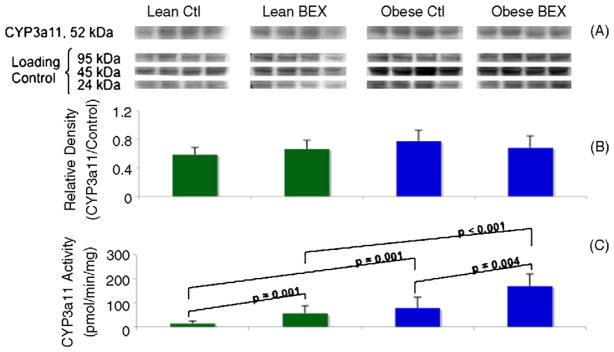
When CYP3a11 protein was detected by western blotting, no significant difference in protein expression was observed among the four groups (Fig. 2A and B). Similar to CYP1A2, overall higher CYP3a11 activity was seen in obese mice in comparison to the lean animals (Fig. 2C). The Obese Ctl group showed 446% more activity than the Lean Ctl group, and the Obese BEX group had 203% more activity than the Lean BEX group. BEX supplementation also significantly increased the enzymatic activity of CYP3a11 by 293% in lean mice and by 118% in obese mice. These results indicate that BEX exposure may have contributed to post-translational increase in CYP3a11 activity in both lean and obese–diabetic mice, and in the absence of BEX, obesity–diabetes also may have contributed to an increase in CYP3a11 activity at the post-translational level. Moreover, obesity–diabetes and BEX may have had a synergistic effect in increasing CYP3a11 activity.
Fig. 2.
Western blot and enzymatic activity of CYP3a11 in mouse liver samples. Panel A shows the bands of CYP3a11 protein detected by a specific antibody and of the loading controls at three molecular weights derived from Amido Black staining of total proteins in the PVDF membrane. Panel B shows the quantification of relative density of the bands; n = 4 in each group. Panel C shows the enzymatic activity of CYP3a11; n = 9 for Lean Ctl group, n = 10 for Lean BEX group, n = 6 for Obese Ctl, and n = 8 for Obese BEX group. Average and SD are shown.
3.3. Phase II enzymes
3.3.1. Enzymatic activity of total GST
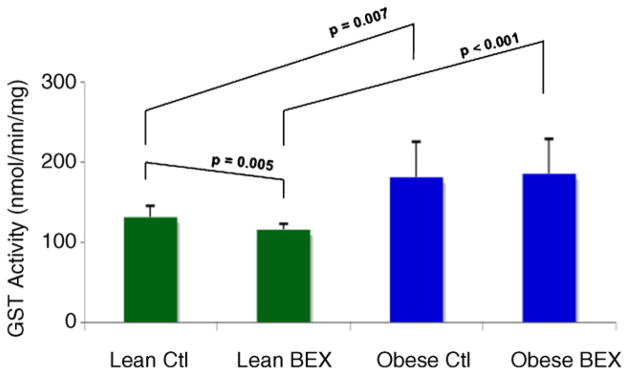
Overall higher total hepatic GST activity was detected in the obese–diabetic vs. lean mice (Fig. 3). The Obese Ctl group showed a 37% increase in activity compared to the Lean Ctl group, and the Obese BEX group showed 59% more activity than the Lean BEX group. Moreover, BEX supplementation led to a slight but significant 12% decrease in the GST activity in the lean mice, but did not affect the GST activity in the obese–diabetic mice. Due to the lack of suitable antibody, the result of western blotting on total GST was not obtained.
Fig. 3.
Enzymatic activity of GST in mouse liver samples. n = 9 for Lean Ctl group, n = 10 for Lean BEX group, n = 7 for Obese Ctl, and n = 8 for Obese BEX group. Average and SD are shown.
3.3.2. Protein expression of UGT1a and 2b and enzymatic activity of total UGT
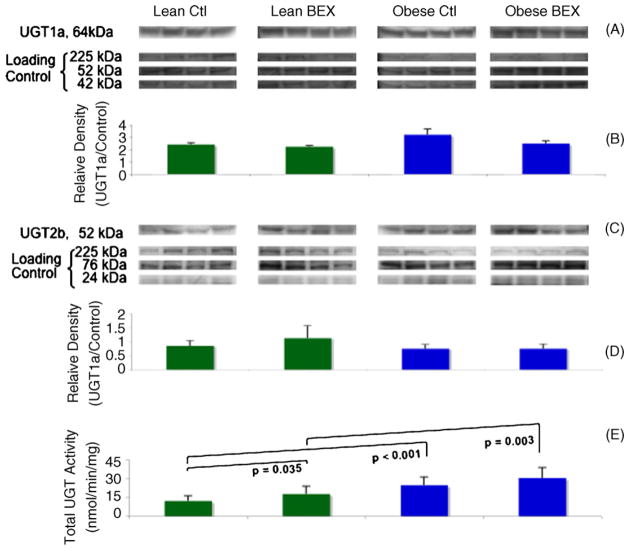
The protein expression of two sub-families of the UGT family, UGT1a (Fig. 4A and B) and UGT2b (Fig. 4C and D), was detected by western blotting. No obvious change in the protein levels was observed among the experimental groups.
Fig. 4.
Western blot of UGT1a and UGT2b and enzymatic activity of total UGT in mouse liver samples. Panels A and C show the bands of UGT1a and 2b proteins detected by specific antibodies and of the loading controls at three molecular weights derived from Amido Black staining of total proteins in the PVDF membranes. Panels B and D show the quantification of relative densities of the bands; n = 4 in each group. Panel E shows the enzymatic activity of total UGT; n = 9 for Lean Ctl group, n = 10 for Lean BEX group, and n = 7 for Obese Ctl and Obese BEX groups. Average and SD are shown.
However, overall higher total UGT activity was seen in obese–diabetic mice in comparison to the lean animals (Fig. 4E). The Obese Ctl group showed 102% more activity than the Lean Ctl group, and the Obese BEX group had 71% more activity than the Lean BEX group. BEX supplementation caused a significant 46% increase of the total UGT activity in lean mice, and a non-significant 23% increase in the obese–diabetic mice. Base on substrate affinity, the total UGT activity is attributable mainly to UGT1a activity. Therefore, dietary BEX and obesity–diabetes seem to have contributed to post-translational increases in the activities of (but may not limited to) the UGT1a sub-family.
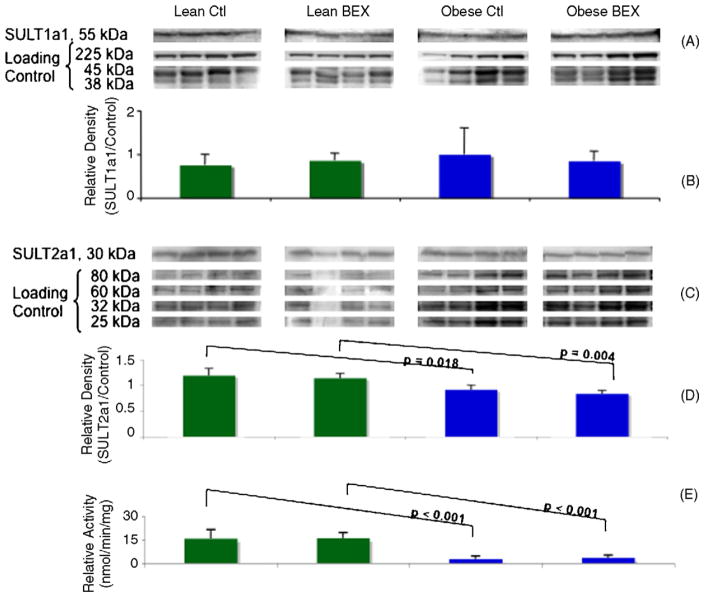
3.3.3. Protein expression of SULT1a1 and 2a1 and enzymatic activity of total SULT
Western blotting showed no differences at the protein level of SULT1a1 among the experimental groups (Fig. 5A and B). Interestingly, the protein expression of SULT2a1 was significantly inhibited in the obese–diabetic mice compared to the lean mice (Fig. 5C and D). Likewise, there was a significant decrease in the enzymatic activity of total hepatic SULT in the obese–diabetic mice (Fig. 5E). The Obese Ctl group showed an 83% decrease from the Lean Ctl group, and the Obese BEX group had 77% less activity than the Lean BEX group. Although it is difficult to estimate how much of the total SULT activity is attributable to the SULT2a1 isoform, it is likely that the down-regulation of SULT2a1 protein expression in the livers of obese–diabetic mice has at least partially contributed to the decreased total SULT activity. On the other hand, BEX supplementation showed no impact on the protein expression and enzymatic activities of the SULTs examined.
Fig. 5.
Western blot of SULT1a1 and SULT2a1 and enzymatic activity of total SULT in mouse liver samples. Panel A shows the bands of SULT1a1 protein detected by a specific antibody and of the loading controls at three molecular weights derived from Coomassie staining of total proteins in the SDS PAGE gel. Panel B shows the quantification of relative density of the bands; n = 4 in each group. Panel C shows the bands of SULT2a1 protein detected by a specific antibody and of the loading controls at four molecular weights derived from Amido Black staining of total proteins in the PVDF membrane. Panel D shows the quantification of relative density of the bands; n = 4 for Lean Ctl, Obese Ctl, and Obese BEX groups and n = 3 for Lean BEX group. Panel E shows the enzymatic activity of total SULT; n = 8 for Lean Ctl and Obese BEX groups, n = 9 for Lean BEX group, and n = 7 for Obese Ctl group. Average and SD are shown.
4. Discussion
Dietary components and pathophysiological conditions, such as obesity and diabetes, play important roles in modulating hepatic xenobiotic biotransformation enzymes. Any changes in hepatic Phases I and II enzymes may impact the metabolism of endo- and xenobiotics, which ultimately can affect the efficacy and safety of therapeutic and endogenous agents.
The current study demonstrated that BEX exposure and obesity–diabetes were separately associated with increased CYP1a2 and CYP3a11 activity. In addition, BEX and obesity–diabetes appear to have a synergistic effect in up-regulating both CYP1a2 and CYP3a11 since the presence of both factors showed a greater increase in CYP activity over either condition independently. Because activity increases were not accompanied by measurable increases in CYP protein, this up-regulation may be due to post-translational regulation and modifications.
Since mice in the present study were both obese and diabetic, it is likely that these two conditions interacted. This statement is strengthened by results presented herein that are consistent with previous studies reporting greater CYP3a activity in type 1 diabetic rats (Barnett et al., 1990a,b; Ioannides and Barnett, 1999), and another study reporting no changes in CYP1a2 or CYP3a protein expression in genetically obese Zucker rats or in normal Zucker rats fed high-fat diet (Khemawoot et al., 2007). Roe et al. (1999) reported that hepatic CYP1a activity was significantly decreased in genetically obese–diabetic mice (Roe et al., 1999). For the convenience of comparison, previous studies using rodent models investigating the influences of obesity and/or diabetes on hepatic Phases I and II enzymes are summarized in Table 1. Future studies should be designed to distinguish between the differential effects of diabetes and obesity on CYP enzyme, and to delineate their regulatory mechanisms.
Table 1.
Summary of the studies on hepatic xenobiotic biotransformation enzymes using obese and/or diabetic rodent models.
| Animals
|
Method
|
Result | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Species | Gender | Age | Obesity induction | Diabetes induction | Measurements | ||
| Zucker rat | Male | 2–5 months | High fat diet or genetic | UGT activity Western blotting Real-time PCR |
UGT activity →a CYP1a2 and 3a protein → CYP1a2 and 3a mRNA → |
Khemawoot et al. (2007) | |
| Rat | Male | 6 months | Streptozotocin | CYP3a activity Western blotting |
CYP3a activity ↑ b CYP1a2 protein ↑ |
Barnett et al. (1990a,b) | |
| Ob/ob mouse | Male | 3–4 or 7–8 months | Genetic | CYP1a1/1a2, CYP3a, and GST activities ELISA |
CYP1a activity in older mice ↓ c GST activity ↓ CYP1a2 protein ↓ |
Roe et al. (1999) | |
| Ob/ob mouse | Male | 4 months | Genetic | GST activity | GST activity ↓ | Barnett et al. (1992) | |
| BioBreeding/Worcester rat | Male | 80 days | Spontaneous type 1 diabetes | UGT activity Western blotting |
UGT1a1 activity toward bilirubin ↑ UGT1a1 activity toward p-nitrophenol → UGT1a1 protein ↑ UGT2 protein → |
Braun et al. (1998) | |
| Sprague–Dawley rat | Male | 7–10 weeks | Streptozotocin | HS NDSTd activity Western blotting |
HS NDSTd activity ↓ HS NDSTd protein ↓ |
Williams et al. (2005) | |
| Sprague–Dawley rat | Female | 55–57 days | Streptozotocin | Western blotting | SULT2a1 protein ↓ | Runge-Morris and Vento (1995) | |
No change.
Increase.
Decrease.
Heparan sulfate N-deacetylase/N-sulfotransferase-1, a golgi apparatus enzyme of the SULT family.
Notably BEX upregulates the activities of hepatic CYP enzymes in lean and especially in obese mice. This effect will subsequently increase the metabolic rate of an array of endo- and xenobiotics, potentially including those of therapeutic drugs and BEX itself as a dietary supplement. As a result, during the course of treatment, the beneficial effect of BEX may be gradually compromised due to a faster reaction with the Phase I enzymes. The following approaches may be considered to address this problem in general: (i) increase the dose to compensate for a greater metabolic rate, but this may further stimulate the CYP activities forming a vicious circle; (ii) periodical administration, allow intervals to decrease the hepatic CYP activities; and (iii) remove impurities that may be responsible for the increase of the enzyme activities.
Furthermore, Phase I reactions usually result in reactive metabolites, which, if not conjugated by Phase II enzymes in time, can be carcinogenic. On the other hand, up-regulation of CYP1a2 may be beneficial by increasing the detoxification and elimination of harmful compounds, as has been shown for black tea which up-regulated hepatic CYP1a2 expression and activity leading to increased urinary excretion of mutagens (Yoxall et al., 2004). Thus the real result of the up-regulation of the Phase I enzyme activities depends on the nature of the substrates and the catalyzing capacity of the Phase II enzymes involved in the subsequent reactions.
The effects of obesity and diabetes on hepatic GST expression and activity are controversial (Kim et al., 2003). The present study showed that the total hepatic GST activity increased in the obese–diabetic mice, and this parallels reported increases in glutathione conjugation in obese humans (Abernethy and Greenblatt, 1986; Roe et al., 1999; Kim et al., 2003). However, other animal studies using spontaneous obese–diabetic male mice have decreased (Barnett et al., 1992; Roe et al., 1999) or unchanged GST activities (Rouer et al., 1981; Kim et al., 2003). The differences in GST activity reported throughout the literature is likely due to different methods of inducing obesity–diabetes, and the status of the disease in the animals.
The present study showed that, like GST, total hepatic UGT activity also increased under the obese–diabetic condition. Higher hepatic UGT activity has been reported in obese humans (Blouin et al., 1987; Roe et al., 1999), in streptozotocin-induced type 1 diabetic rats, and in spontaneous type 1 diabetic BioBreeding/Worcester (BB/Wor) rats (Tunon et al., 1991; Braun et al., 1998; Kim and Novak, 2007). In the latter experiment, BB/Wor diabetic rats were shown to have increased UGT1a1 activity and protein expression and the authors suggested that these results, in comparison to starved rats, indicate that glucuronidation is dependant on glycogen stores (Banhegyi et al., 1988; Braun et al., 1998). The present study measured total UGT activity, which included the activity of UGT1a1 (Collier et al., 2000). It is possible that the UGT activity increase observed in the obese mice in the present study was partially due to greater glycogen stores, which could have served as the UDP-glucose supply for the synthesis of co-factor UDPGA, the rate-limiting factor (Braun et al., 1997) for glucuronidation (Braun et al., 1998). Alternatively, obesity and diabetes (both independently and together) may have other effects on UGT regulation.
It has been reported that lower SULT activity may increase the availability of substrates for UGTs, and therefore greater UGT activity can be a compensatory mechanism for impaired sulfonylation (Braun et al., 1997). In the present study, significant increases in total UGT activity were coupled with significant deceases in total SULT activity in the obese mice, which correlates well with the previously published observations.
Significant decreases of SULT2a1 protein expression have been observed in obese mice in the present study, indicating that obesity and/or diabetes have contributed to translational down-regulation of this SULT isoform. Similarly, decreased SULT2a1 protein level was reported in rats with streptozotocin-induced type 1 diabetes (Runge-Morris and Vento, 1995), suggesting a potential regulatory role of diabetes on SULT2a1 expression.
BEX supplement showed less regulatory effects on Phase II enzymes in comparison to those on Phase I enzymes. BEX slightly inhibited GST activity, moderately enhanced UGT activity, and had no effect on SULT activity. It has been reported that several flavonoids induce both UGT and CYP enzymes (Felton and Malfatti, 2006), which is consistent with the previously reported high content of flavonoids in the BEX (Lin et al., 2008). Overall, UGT-catalyzed Phase II reactions may become an important detoxification pathway under BEX treatment, especially in the obese–diabetic mice when the activity of SULT is compromised.
5. Concluding remarks
This study investigated the influences of both BEX supplementation and obese–diabetic condition on the protein expression and enzymatic activities of hepatic xenobiotics biotransforming enzymes in male mice. It was found that obesity–diabetes upregulated the activities of CYP1a2, CYP3a11, GST, and UGT, but decrease that of SULT; BEX alone, and in synergy with obesity–diabetes, greatly enhanced the activities of Phase I enzymes, but had less effect on Phase II enzymes in general. Interestingly, the changes in activity observed were rarely correlated with changes in enzyme protein level, with SULT2a1 as an exception.
In summary, BEX as a dietary supplement had a minimal inhibitory effect on the enzymes examined, and therefore is not likely to inhibit the metabolism of itself or other endo- and xenobiotics in the liver. The remarkable increases of Phase I enzyme activities induced by BEX and obesity–diabetes may be useful if they correspond to greater detoxification of pro-carcinogens (Stedman et al., 2004; Yoxall et al., 2004), although they may also facilitate transformation of therapeutic drugs and BEX itself, thereby decreasing biological efficacy. Furthermore, increases of UGT and GST activities in the obese–diabetic mice contrast with lowered activity of SULT enzymes. The greater UGT activity noted may be particularly essential when BEX is introduced due to its slight inhibitory effect on GST activity in lean mice. However, variance caused by species and gender should be considered for any general application of these results (Tabrett and Coughtrie, 2003; Hayes et al., 2005; Yoshinari et al., 2006; Braeuning et al., 2009).
The changes in hepatic xenobiotic biotransformation observed due to BEX supplementation are known to occur with several clinically used drugs and other natural and non-natural products. Hence, examination of these properties is critical in the pre-clinical pharmacological and toxicological safety evaluation of new drugs and compounds, including BEX. Care needs to be taken for future pharmacokinetic and dosing studies of BEX to assess the effects of induced CYPs (particularly Cyp3a), however; similar to other clinically-used drugs such as Carbamazepine, enzyme induction by drugs and nutraceuticals does not absolutely preclude drug development and application.
Acknowledgments
This study was made possible by grant numbers R21 AT003874-02 (Panee) from the National Center for Complementary and Alternative Medicine (NCCAM), R21 AT005139-01 (Panee) from NCCAM and the Office of Research on Women’s Health (ORWH), G12RR003061 (RCMI Program) from NCRR, and 5P20 MD000173-08 from NCMHD. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ORWH, NCRR, NCMHD, or the National Institute of Health.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Abernethy DR, Greenblatt DJ. Drug disposition in obese humans. An update. Clinical Pharmacokinetics. 1986;11:199–213. doi: 10.2165/00003088-198611030-00002. [DOI] [PubMed] [Google Scholar]
- Banhegyi G, Garzo T, Antoni F, Mandl J. Glycogenolysis – and not gluconeo-genesis – is the source of UDP-glucuronic acid for glucuronidation. Biochimica et Biophysica Acta. 1988;967:429–435. doi: 10.1016/0304-4165(88)90106-7. [DOI] [PubMed] [Google Scholar]
- Barnett CR, Abbott RA, Bailey CJ, Flatt PR, Ioannides C. Cytochrome P-450-dependent mixed-function oxidase and glutathione S-transferase activities in spontaneous obesity–diabetes. Biochemical Pharmacology. 1992;43:1868–1871. doi: 10.1016/0006-2952(92)90724-w. [DOI] [PubMed] [Google Scholar]
- Barnett CR, Flatt PR, Ioannides C. Induction of hepatic microsomal P450 I and IIB proteins by hyperketonaemia. Biochemical Pharmacology. 1990a;40:393–397. doi: 10.1016/0006-2952(90)90708-s. [DOI] [PubMed] [Google Scholar]
- Barnett CR, Gibson GG, Wolf CR, Flatt PR, Ioannides C. Induction of cytochrome P450III and P450IV family proteins in streptozotocin-induced diabetes. The Biochemical Journal. 1990b;268:765–769. doi: 10.1042/bj2680765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett CR, Rudd S, Flatt PR, Ioannides C. Sex differences in the diabetes-induced modulation of rat hepatic cytochrome P450 proteins. Biochemical Pharmacology. 1993;45:313–319. doi: 10.1016/0006-2952(93)90066-6. [DOI] [PubMed] [Google Scholar]
- Blouin RA, Kolpek JH, Mann HJ. Influence of obesity on drug disposition. Clinical Pharmacy. 1987;6:706–714. [PubMed] [Google Scholar]
- Braeuning A, Sanna R, Huelsken J, Schwarz M. Inducibility of drug-metabolizing enzymes by xenobiotics in mice with liver-specific knockout of Ctnnb1. Drug Metabolism and Disposition. 2009;37:1138–1145. doi: 10.1124/dmd.108.026179. [DOI] [PubMed] [Google Scholar]
- Braun L, Coffey MJ, Puskas F, Kardon T, Nagy G, Conley AA, Burchell B, Mandl J. Molecular basis of bilirubin UDP-glucuronosyltransferase induction in spontaneously diabetic rats, acetone-treated rats and starved rats. The Biochemical Journal. 1998;336:587–592. doi: 10.1042/bj3360587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L, Kardon T, Puskas F, Csala M, Banhegyi G, Mandl J. Regulation of glucuronidation by glutathione redox state through the alteration of UDP-glucose supply originating from glycogen metabolism. Archives of Biochemistry and Biophysics. 1997;348:169–173. doi: 10.1006/abbi.1997.0379. [DOI] [PubMed] [Google Scholar]
- Burton MS, Shaw LM, Schentag JJ, Evans WE. Applied Pharmacokinetics and Pharmacodynamics. Lippincott Williams and Wilkins; 2005. pp. 235–236. [Google Scholar]
- Collier AC, Tingle MD, Keelan JA, Paxton JW, Mitchell MD. A highly sensitive fluorescent microplate method for the determination of UDP-glucuronosyl transferase activity in tissues and placental cell lines. Drug Metabolism and Disposition. 2000;28:1184–1186. [PubMed] [Google Scholar]
- Dutton DR, Parkinson A. Reduction of 7-alkoxyresorufins by NADPH-cytochrome P450 reductase and its differential effects on their O-dealkylation by rat liver microsomal cytochrome P450. Archives of Biochemistry and Biophysics. 1989;268:617–629. doi: 10.1016/0003-9861(89)90329-9. [DOI] [PubMed] [Google Scholar]
- Felton JS, Malfatti MA. What do diet-induced changes in phase I and II enzymes tell us about prevention from exposure to heterocyclic amines? The Journal of Nutrition. 2006;136:2683S–2684S. doi: 10.1093/jn/136.10.2683S. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases. The Journal of Biological Chemistry. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annual Review of Pharmacology and Toxicology. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Ioannides CF, Flatt PR, Barnett CR. The effects of experimental diabetes on the cytochrome P450 system and other metabolic pathways. In: McNeill JH, editor. Experimental Models of Diabetes. Informa Health Care; 1999. pp. 79–110. [Google Scholar]
- Khemawoot P, Yokogawa K, Shimada T, Miyamoto K. Obesity-induced increase of CYP2E1 activity and its effect on disposition kinetics of chlorzoxazone in Zucker rats. Biochemical Pharmacology. 2007;73:155–162. doi: 10.1016/j.bcp.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacology & Therapeutics. 2007;113:88–120. doi: 10.1016/j.pharmthera.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Woodcroft KJ, Novak RF. Insulin and glucagon regulation of glutathione S-transferase expression in primary cultured rat hepatocytes. The Journal of Pharmacology and Experimental Therapeutics. 2003;305:353–361. doi: 10.1124/jpet.102.045153. [DOI] [PubMed] [Google Scholar]
- Klaassen C. Biotransformation of xenobiotics. In: Klaassen C, editor. Casarett & Doull’s Toxicology: The Basic Science of Poisons. McGraw-Hill; New York, NY: 2001. pp. 134–225. [Google Scholar]
- Kotlyar M, Carson SW. Effects of obesity on the cytochrome P450 enzyme system. International Journal of Clinical Pharmacology and Therapeutics. 1999;37:8–19. [PubMed] [Google Scholar]
- Lin Y, Collier AC, Liu W, Berry MJ, Panee J. The inhibitory effect of bamboo extract on 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer. Phytotherapy Research. 2008;22:1440–1522. doi: 10.1002/ptr.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opinion on Drug Metabolism & Toxicology. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- Mulder GJ, Van Doorn ABD. A rapid NAD+-linked assay for microsomal uridine diphosphate glucuronosyl transferase of Rat liver and some observations on substrate specificity of the enzyme. Biochemical Journal. 1975;151:131–140. doi: 10.1042/bj1510131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panee J, Liu W, Lin Y, Gilman C, Berry MJ. A novel function of bamboo extract in relieving lipotoxicity. Phytotherapy Research. 2008;22:675–680. doi: 10.1002/ptr.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RJ, Surry D, Renwick AB, Meneses-Lorente G, Lak BG, Evans DC. CYP isoform induction screening in 96-well plates: use of 7-benzyloxy-4-trifluoromethylcoumarin as a substrate for studies with rat hepatocytes. Xenobiotica. 2000;30:781–795. doi: 10.1080/00498250050119844. [DOI] [PubMed] [Google Scholar]
- Roe AL, Howard G, Blouin R, Snawder JE. Characterization of cytochrome P450 and glutathione S-transferase activity and expression in male and female ob/ob mice. International Journal of Obesity and Related Metabolic Disorders. 1999;23:48–53. doi: 10.1038/sj.ijo.0800756. [DOI] [PubMed] [Google Scholar]
- Rouer E, Mahu JL, Dansette P, Leroux JP. UDP-glucuronosyltransferase, epoxide hydrolase and glutathione S-transferase activities in the liver of diabetic mice. Biochimica et Biophysica Acta. 1981;676:274–277. doi: 10.1016/0304-4165(81)90197-5. [DOI] [PubMed] [Google Scholar]
- Runge-Morris M, Vento C. Effects of streptozotocin-induced diabetes on rat liver sulfotransferase gene expression. Drug Metabolism and Disposition. 1995;23:455–459. [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Olson BJ, Klenk D. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stedman C, Robertson G, Coulter S, Liddle C. Feed-forward regulation of bile acid detoxification by CYP3A4: studies in humanized transgenic mice. The Journal of Biological Chemistry. 2004;279:11336–11343. doi: 10.1074/jbc.M310258200. [DOI] [PubMed] [Google Scholar]
- Tabrett CA, Coughtrie MW. Phenol sulfotransferase 1A1 activity in human liver: kinetic properties, interindividual variation and re-evaluation of the suitability of 4-nitrophenol as a probe substrate. Biochemical Pharmacology. 2003;66:2089–2097. doi: 10.1016/s0006-2952(03)00582-3. [DOI] [PubMed] [Google Scholar]
- Tunon MJ, Gonzalez P, Garcia-Pardo LA, Gonzalez J. Hepatic transport of bilirubin in rats with streptozotocin-induced diabetes. Journal of Hepatology. 1991;13:71–77. doi: 10.1016/0168-8278(91)90866-a. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Liu ML, Zhu Y, Xu X, Davidson WR, McCue P, Sharma K. Loss of heparan N-sulfotransferase in diabetic liver: role of angiotensin II. Diabetes. 2005;54:1116–1122. doi: 10.2337/diabetes.54.4.1116. [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Takagi S, Yoshimasa T, Sugatani J, Miwa M. Hepatic CYP3A expression is attenuated in obese mice fed a high-fat diet. Pharmaceutical Research. 2006;23:1188–1200. doi: 10.1007/s11095-006-0071-6. [DOI] [PubMed] [Google Scholar]
- Yoxall VR, Parker DA, Kentish PA, Ioannides C. Short-term black tea intake modulates the excretion of urinary mutagens in rats treated with 2-amino-3-methylimidazo-[4,5-f]quinoline (IQ): role of CYP1A2 upregulation. Archives of Toxicology. 2004;78:477–482. doi: 10.1007/s00204-004-0562-3. [DOI] [PubMed] [Google Scholar]
- Yuan JX. Research on the production and botanical origin of bamboo juice in Eastern China. Zhong Yao Tong Bao. 1983;8:10–12. [PubMed] [Google Scholar]