Abstract
Neurorestorative therapy targets multiple types of parenchymal cells in the intact tissue of the injured brain tissue to increase neurogenesis, angiogenesis, oligodendrogenesis, and axonal remodeling during recovery from neurological injury. In our laboratory, we tested thymosin β4 (Tβ4) as a neurorestorative agent to treat models of neurological injury. This review discusses our results demonstrating that Tβ4 improves neurological functional outcome in a rat model of embolic stroke, a mouse model of multiple sclerosis, and a rat model of traumatic brain injury. Tβ4 is a pleiotropic peptide exhibiting many actions in several different types of tissues. One mechanism associated with improvement of neurological improvement from Tβ4 treatment is oligodendrogenesis involving the differentiation of oligodendrocyte progenitor cells to mature myelin-secreting oligodendrocytes. Moreover, our preclinical data provide a basis for movement of Tβ4 into clinical trials for treatment of these devastating neurological diseases and injuries.
Keywords: thymosin β4, stroke, multiple sclerosis, traumatic brain injury, rat
Treatment of neurological injury remains an elusive goal in health care. Despite billions of dollars invested in basic neuroscience and clinical trials, meaningful treatment of many neurological diseases remains a difficult task. As the population ages, certain neurological illnesses such as stroke and dementia will increasingly cause severe neurologic disability [1, 2]. At an individual level, the overall quality of life of those stricken with these diseases as well as those who care for them is severely affected both financially and emotionally. At the national level, the projected rate of health care spending will be severely affected unless meaningful treatments are discovered and implemented [3].
Neurorestorative therapy is a concept that is just beginning to be recognized in both the basic science and clinical arena. Neurorestorative therapy treats the intact tissue and not the lesion to invoke a repair of damage tissues from any type of neurological injury [4]. Neurorestorative therapy acts on intact parenchymal cells, specifically neuroprogenitor (adult neural stem cells), oligodendrocyte progenitor cells, astroglial cells, and cerebral endothelial cells, to promote neurogenesis, oligodendrogenesis, axonal sprouting, synaptogenesis and angiogenesis, in the injured brain. These restorative processes are associated with improvement in neurological functional outcome. Administration of neurorestorative agents involve treatment of stroke at subacute (>24 h) time points resulting in greater availability for treatment for all patients rather than the few, that is, approximately 5% of patients who are treated with the time limited thrombolytic agent rt-PA (<4.5 h)[5]. Preclinical data demonstrating neurorestoration is growing robustly with many agents poised to be tested in the clinical trials.
Tβ4 is a pleiotropic peptide exhibiting many actions in several different types of tissues [6]. Tβ4 has anti-inflammatory activity and promotes angiogenesis in dermal wound healing and cardiac ischemia models. Tβ4’s fundamental property is sequestration of G-actin monomers, which promote cell migration by inhibiting actin-cytoskeletal organization [7]. Because of its G-actin binding properties, Tβ4 promotes cardiomyocyte migration in models of cardiac infarction and keratinoctye migration in wound healing models [8] [9]. These properties support our hypothesis that Tβ4 is a neurorestorative agent. For example, after focal cerebral ischemia, the injured brain attempts to repair and remodel itself [10]. An important, well-documented regenerative response is the proliferation of neural progenitor cells (NPC) in the subventricular zone (SVZ) of the lateral ventricle after stroke [11, 12]. The SVZ of the rat contains neural stem cells that produce neuroblasts that regularly migrate to the olfactory bulb via the rostral migratory stream and differentiate into granule neurons [12, 13]. The replacement of olfactory neurons is critical to the survival of the rat. However, after experimental focal cerebral ischemia, the NPCs migrate out of the SVZ to the ischemic boundary regions to promote a process of repair [13]. Since actin dynamics play a critical role in cell migration and synaptogenesis, we propose that administration of Tβ4 may enhance this migration of progenitor cells to accelerate and potentiate recovery after stroke or other models of neurological injury.
Tβ4 is an agent that has been tested in three different models of neurological injury in our laboratory, and its small peptide properties (43 amino acids) make it an ideal candidate for neurorestorative therapy [14–16]. The first model is a rat embolic stroke model, which involves carefully placing a fibrin-rich blood clot into the middle cerebral artery (MCA) to induce stroke-like symptoms [17]. This model has the advantages of reproducibility, and more importantly, it is a clinically relevant model of human stroke. The second model is an experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis, which is generated by immunization with myelin proteolipid protein (PLP) to induce neurological symptoms of paralysis, ataxia, and poor muscle tone [15]. Finally, our laboratory has a rat model of traumatic brain injury (TBI) in which injury is delivered to the anesthetized rat by impacting the cortex with a pneumatic piston [16]. All three models have the advantages of producing neurological deficits that can be measured over a long period of time. In this review, we describe observations of neurological improvement in each model of neurological injury as well as data supporting our hypothesis that Tβ4 is a neurorestorative agent, specifically promoting differentiation of oligodendrocyte progenitor cells (OPCs) into myelin-secreting oligodendrocytes (OLs), a process known as oligodendrogeneisis.
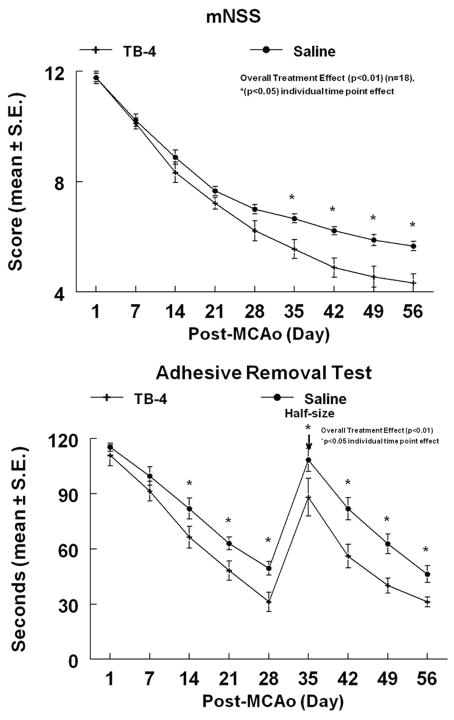
Embolic stroke model
The MCA of male Wistar rats (320 to 380 g, n = 18) was occluded by placement of an embolus at the origin of the MCA, as previously described [17]. Twenty-four hours after stroke, Tβ4 was administered intraperitonally (IP) and then every threedays (6 mg/kg, IP) for four additional doses (n = 9). A battery of behavioral tests, including the adhesive-removal test (ART) and the modified Neurological Severity Score (mNSS) was performed before MCA occlusion and at 1, 7, 14, 21, 28, 35, 42, 49, and 56 days after MCAo by an investigator who was blinded to the experimental groups [18]. The ART measures the time it takes for the animal to remove sticky tabs from its paws and the mNSS measures motor, sensory, proprioception, and balance. Ischemic rats treated with saline (n = 9) were used as a control group. Animals were sacrificed after 56 days. Results (Fig. 1) from this experiment demonstrated that Tβ4 treated rats a showed a 24.2% and a 29.9% overall improvement in the ART and mNSS scores (at time of sacrifice), respectively, when compared to controls (overall treatment effect, P < 0.01). Functional improvements persisted for at least 56 days after MCA occlusion. There were no significant differences of ischemic lesion volumes between the rats treated with Tβ4 (35.2% ± 6.7%) and with saline (33.1 % ± 7.8%, P > 0.05), indicating that a neuroprotective mechanism was not responsible for the improvement. We, therefore, tested whether Tβ4 promotes axonal remodeling after stroke. Brain sections were stained using the Bielshowsky and Luxol fast blue staining to detect myelinated axons. Figure 2A demonstrates significant increases in staining area of myelinated axons in the striatal (white matter) ischemic boundary in the Tβ4 treatment group (215.3 ± 29.9%) when compared to the control group (115.2 ± 9.0%) (P < 0.05). The increase of remylination, which was associated with functional improvement, would suggest that cells that produce myelin, OLs and its precursors, and OPCs would be increased. We measured markers of OPCs, NG-2 (chondroitin sulfate proteoglycan), and OLs, CNPase (2”, 3”-cyclic nucleotide 3’-phosphodiesterase). Figure 2B and 2C demonstrate the expression of these two markers. When compared to controls, Tβ4 treatment significantly increased the density (cells/mm2) of NG-2 positive cells in the SVZ (396.6 ± 19.6 vs. 209.1 ± 42.7) and striatum (130 ± 15.3 vs. 61.0 ± 7.6) (P < 0.05). NG-2 immunoreactivity was also increased in the corpus collosum (166.8 ± 26.0 vs. 78.3 ± 12.2, P < 0.05). CNPase area of increased staining was increased in the striatum (149.1% ± 9.4% vs. 115.2% ± 7.1%, P < 0.05). The association of improvement of neurological outcome and oligodendrogenesis supports our hypothesis of neurorestoration by Tβ4.
Figure 1.
Embolic stroke rat model treated with Tβ4. The mNSS of embolic stroke rats treated with Tβ4 demonstrated a significant overall (treatment effect) improvement of neurological function (P < 0.01). The adhesive removal test of embolic stroke rats treated with Tβ4 also demonstrated a significant overall (treatment effect) improvement (P < 0.01). Significant effect (P < 0.05) at individual time points are indicated. Adhesive-backed paper dots were reduced in size by one-half at day 35 (arrow) to increase sensitivity. Reprinted from Ref. 14 with permission from Elsevier.
Figure 2.
Embolic stroke rat model treated with Tβ4. The staining by Bielshowsky and Luxol fast blue (A) shows the myelin and axons in the white matter bundles of the striatum of saline and Tβ4-treated rats (see arrows). There is a significantly increased density of Bielshowsky and Luxol fast blue staining in the Tβ4-treated rats compared to the demyelination of the saline control. LV = lateral ventricle and IC = ischemic core. NG-2 staining (B) is significantly increased in the ipsilateral SVZ and striatum adjacent to the ischemic core of Tβ4-treated rats when compared to saline control (see arrows). CNPase (C) is significantly increased in the striatum of Tβ4-treated rats when compared to saline control (see arrows). P < 0.05 for A, B and C. Reprinted from Ref. 14 with permission from Elsevier.
EAE model of multiple sclerosis
Our laboratory uses a standard mouse model of EAE [15]. EAE mice were administered saline (n = 11) or Tβ4 (n = 10) at a concentration of 6 mg/kg IP on the day of PLP immunization, and then every three days (6 mg/kg) for four additional doses. Neurological function was scored using a standard scoring scale of 0–5. Mice were scored daily for clinical symptoms of EAE, as follows: 0, healthy; 1, loss of tail tone; 2, ataxia and/or paresis of hindlimbs; 3, paralysis of hind limbs and/or paresis of forelimbs; 4, tetraparalysis; 5, moribund or dead [19]. The higher score the more severe the disease. Results from this study showed that Tβ4 treatment improved neurological outcome nearly 50% when compared to controls (Fig. 3). Improvement was observed beginning at day 11 and extended to time of sacrifice at day 30. Similar to the embolic stroke model an increase in NG2 OPCs (447.7 ± 41.9 vs. 195.2 ± 31 cells/mm2 in subventricular zone (SVZ), 75.1 ± 4.7 vs. 41.7 ± 3.2 cells/mm2 in white matter) and CNPase-positive mature OLs staining area (267.5 ± 10.3 vs. 141.4 ± 22.9/mm2) was also observed in the SVZ and white matter of the brain suggesting that oligodendrogenesis is occurring (Figs. 3B and 3C). Similar to the embolic stroke model, these results suggest an association of improvement of neurological outcome and oligodendrogenesis by Tβ4.
Figure 3.
EAE mouse model of multiple sclerosis treated with Tβ4. The neurological response of EAE mice treated with or without Tβ4. The significant therapeutic Tβ4 effects were detected as early as day 11 after EAE onset. Nearly 50% relative functional recovery was observed in the Tβ4 treated group, compared to the saline controls with P < 0.01 for either the median score or the cumulative score up to 30 days. NG-2 cells (B) and CNPase cells (C) were significantly increased at 30 days after EAE onset in the Tβ4 treatment group compared to that in the saline group (p<0.05). Reprinted from Ref. 15 with permission from Elsevier.
Traumatic brain injury model
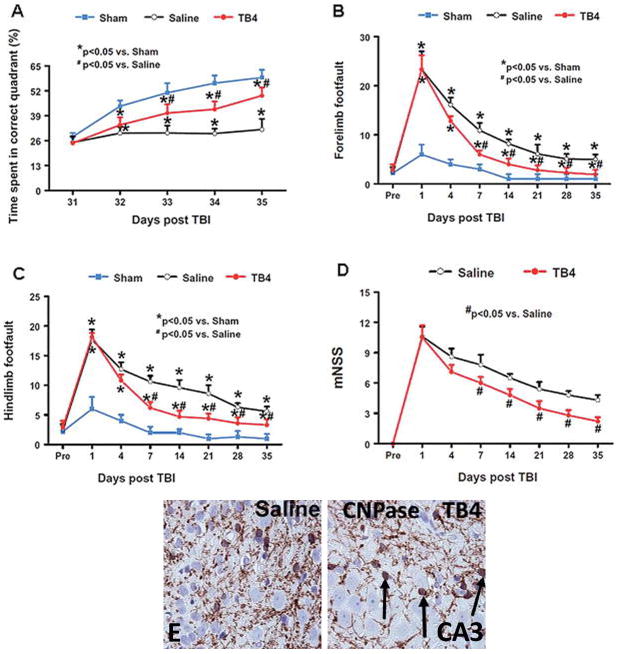
A controlled cortical impact model was used by our laboratory to model TBI in rats [20]. Young adult male Wistar rats (330 gm) were anesthetized with chloral hydrate and placed in a stereotactic frame. Two 10-mm diameter craniotomies were performed adjacent to the central suture. The contralateral craniotomy allowed for movement of cortical tissue laterally. The dura mater was kept intact over the cortex. Injury was delivered by impacting the left (ipsilateral) cortex with a pneumatic piston containing a 6-mm-diameter tip at a rate of 4 m/sec and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer. The animals were divided into three groups: 1) sham (surgery without TBI) group (n = 6); 2) surgery + TBI + saline group (n = 9); and 3) surgery+ TBI + Tβ4 group (n = 10). Tβ4 was administered at a dose of 6 mg/kg IP beginning at day 1 after injury, and every three days for 4 additional doses until sacrifice at day 35. Neurological outcome was measured using three standardized tests: the Morris water maze test, the foot fault test, and the mNSS test, as previously described. The Morris water maze test measures the animal’s spatial learning impairments while swimming in a shallow pool. The foot fault test measures sensorimotor function by allowing the rat to walk on a wired mesh, with each paw that slips between the wires counting as a misstep. The results are shown in Fig. 4A–D, Tβ4-treated rats showed reduced deficits in all three tests with a near 75% improvement in the Morris water maze test, and a 50% improvement in both the foot fault and mNSS.
Figure 4.
Rat model of traumatic brain injury treated with Tβ4. Tβ4 treatment improves spatial learning performance measured by the Morris water maze test at days 33–35 compared with the saline group (A). Tβ4 treatment significantly reduces forelimb foot faults at days 7–35 compared with the saline-treated group (B). Tβ4 treatment significantly reduces hindlimb foot faults at days 7–35 compared with the saline-treated group (C). Line graph showing the functional improvement detected on the mNSS (D). Treatment with Tβ4 significantly lowers mNSS at days 7–35 compared with the saline group. Pre = preinjury level. Treatment with Tβ4 significantly increases CNPase cells in the CA3 region of the hippocampus (E) (P < 0.05). Reprinted from Ref. 16 with permission from JNS Publishing Group.
Tβ4-mediated oligodendrogenesis was observed in the CA3 of the hippocampus (Fig. 4E). The hippocampus region participates in the organization of both short and long term memories and spatial orientation. This specific regional finding is associated with and may account for the reduced impairment of spatial learning test (Morris water maze). TBI by itself stimulates increased OPCs in all regions measured; however, TB4 only increased the more mature OL, as evidence by increased CNPase staining (120.5 ± 14 vs. 256 ± 24 cells/mm2) in the CA3 region of the hippocampus (P < 0.05). This measurement was performed at day 35, and future studies are needed to determine if Tβ4 promotes oligodendrogenesis at earlier time points in either the cortex or denate gyrus.
Clinical translation
The central nervous system has the ability to regenerate damaged axonal connections [4, 21]. The outgrowth of collateral sprouting resulting from plasticity of neurons creates new axonal connections and new circuitry in the injured brain [10]. Functional improvement occurs after stroke when surviving neurons undergo axonal sprouting and synaptogenesis [22]. After stroke, neuroblasts in the SVZ migrate toward the ischemic boundary regions, suggesting that the neurogenic response after stroke has the potential to be manipulated to increase the number of new migrating NPCs that possess the ability to differentiate into neurons and oligodendrocytes[13]. Increases in the proliferating progenitor cells in the ischemic brain may provide an opportunity to repair axonal connections.
The resulting improvement in neurological outcome after treatment with Tβ4 in our three models of neurological injury reflects a Tβ4-mediated neurorestorative process. A common observation in all three models is oligodendrogenesis and/or the production of mature myelin-secreting OL from OPC. Remyelination has been well studied in various adult animal models and involves the generation of new mature OLs [23, 24], which are derived from adult OPCs whose origins are from white matters and the SVZ. These mature OLs spread throughout the adult white matter. The general scientific consensus is that remyelination occurs only from OPCs and not from surviving OLs or from mature surviving OLs adjacent to the injured axons [23–25]. Mature OLs are, for the most part, unable to migrate or divide. OLs are highly vulnerable to focal cerebral ischemia [26] and other toxic factors resulting in neurological deficits. Our research suggests that Tβ4 is a potential candidate as a neurorestorative agent. Tβ4 was tested in a randomized, double-blind, placebo-controlled, dose-response phase 1A and 1B study of the safety and tolerability of the intravenous administration of Tβ4 and its pharmacokinetics after single doses in healthy volunteers (RegeneRx Biopharmaceuticals, Inc., Rockville, Maryland)[27]. The drug was found to be safe and well tolerated. Therefore, our preclinical results along with the clinical safety trial suggest that clinical trials using Tβ4 should be considered.
Conclusion
The three models of neurological injury treated with Tβ4 described in this review support our hypothesis that Tβ4 is a potential neurorestorative agent. Neurorestorative agents target intact parenchymal cells to promote brain remodeling or repair of damaged tissues. Improvement of neurological functional outcome observed in all three models is associated with oligodendrogenesis and/or the differentiation of OPC into mature OL. Tβ4 could, in theory, treat all three diseases, stroke, multiple sclerosis, and TBI. Presently, research is focusing on optimizing the dose and the time to administer the peptide after injury. These preclinical studies suggest that clinical trials are warranted for Tβ4 treatment of these debilitating diseases in humans.
Acknowledgments
This research was supported in part by NINDS and NIA Grants PO1 NS23393, RO1 NS062832, R01 NS075156, and R01 AG038648. Tβ4 was supplied by RegeneRx Biopharmaceuticals Inc. under a Material Transfer Agreement. A U.S. Provisional Patent 61/163,556 has been filed for use of Tβ4 in neurological disease.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Sharma JC, Fletcher S, Vassallo M. Strokes in the elderly - higher acute and 3-month mortality - an explanation. Cerebrovasc Dis. 1999;9:2–9. doi: 10.1159/000015889. [DOI] [PubMed] [Google Scholar]
- 2.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs VR. Three “inconvenient truths” about health care. N Engl J Med. 2008;359:1749–1751. doi: 10.1056/NEJMp0807432. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacke W, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein AL, Hannappel E, Kleinman HK. Thymosin β4: actin-sequestering protein moonlights to repair injured tissues. Trends Mol Med. 2005;11:421–429. doi: 10.1016/j.molmed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Huff T, et al. β-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–220. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 8.Smart N, et al. Thymosin β4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 9.Malinda KM, et al. Thymosin β4 accelerates wound healing. J Invest Dermatol. 1999;113:364–368. doi: 10.1046/j.1523-1747.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- 10.Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arvidsson A, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Morris DC, et al. Thymosin β4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience. 2010;169:674–682. doi: 10.1016/j.neuroscience.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, et al. Neurological functional recovery after thymosin β4 treatment in mice with experimental auto encephalomyelitis. Neuroscience. 2009;164:1887–1893. doi: 10.1016/j.neuroscience.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong Y, et al. Treatment of traumatic brain injury with thymosin β(4) in rats. J Neurosurg. 2010 doi: 10.3171/2010.4.JNS10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, et al. A mouse model of embolic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:1081–1088. doi: 10.1097/00004647-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, et al. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034:34–39. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 21.Hilliard MA. Axonal degeneration and regeneration: a mechanistic tug-of-war. J Neurochem. 2009;108:23–32. doi: 10.1111/j.1471-4159.2008.05754.x. [DOI] [PubMed] [Google Scholar]
- 22.Chopp M, Li Y, Zhang J. Plasticity and remodeling of brain. J Neurol Sci. 2008;265:97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 24.Nait-Oumesmar B, et al. The role of SVZ-derived neural precursors in demyelinating diseases: from animal models to multiple sclerosis. J Neurol Sci. 2008;265:26–31. doi: 10.1016/j.jns.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 26.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. discussion 1647. [DOI] [PubMed] [Google Scholar]
- 27.Ruff D, et al. A randomized, placebo-controlled, single and multiple dose study of intravenous thymosin β4 in healthy volunteers. Ann N Y Acad Sci. 2010;1194:223–229. doi: 10.1111/j.1749-6632.2010.05474.x. [DOI] [PubMed] [Google Scholar]