SUMMARY
Over the past 13 years, numerous crystal structures of complexes of the common γ-chain (γc) cytokine receptors and their cytokines have been solved. Even with the remarkable progress in the structural biology of γc receptors and their cytokines or interleukins, there are valuable lessons to be learned from the structural and biophysical studies of interleukin-7 (IL-7) and its α-receptor (IL-7Rα) and comparisons to other γc family members. The structure of the IL-7/IL-7Rα complex teaches that interfaces between the γc interleukins and their receptors can vary in size, polarity, and specificity, and that significant conformational changes might be necessary for complexes of interleukins and their receptors to bind the shared, activating γc receptor. Binding, kinetic, and thermodynamic studies of IL-7 and IL-7Rα show that glycosylation and electrostatics can be important to interactions between ILs and their receptor, even where the glycans and charged residues are distant from the interface. The structure of the IL-7Rα homodimer is a reminder that often-ignored non-activating complexes likely perform roles just as important to signaling as activating complexes. And last but not least, the structural and biophysical studies help explain and potentially treat the diseases caused by aberrant IL-7 signaling.
Keywords: interleukin-7, interleukin-7 receptor, common γ-chain family, structures, cancer
Introduction
Cells communicate with one another through extracellular signaling proteins known as cytokines. Each cytokine binds to the extracellular domain(s) of either one or two matching cell-surface receptors denoted as α- and β-chain receptors (Fig. 1). Certain cytokines and their matching receptors also bind to another membrane-bound receptor, the common γ-chain (γc) (CD132). The γc family of cytokines or interleukins includes interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21 (reviewed in 1, 2). The interaction of the cytokine, its matching receptor(s), and γc enables Janus kinase (JAK) and signal transducer and activator of transcription (STAT) proteins to interact with the intracellular domains of the cytokine receptors and trigger the classical JAK/STAT signaling pathway, which ultimately activates transcription of target genes (2). Careful regulation of the signaling cascade initiated by the interactions among γc interleukins and their receptors is fundamental to development, proliferation, and homeostasis of B, T, and natural killer (NK) cells of the immune system (2).
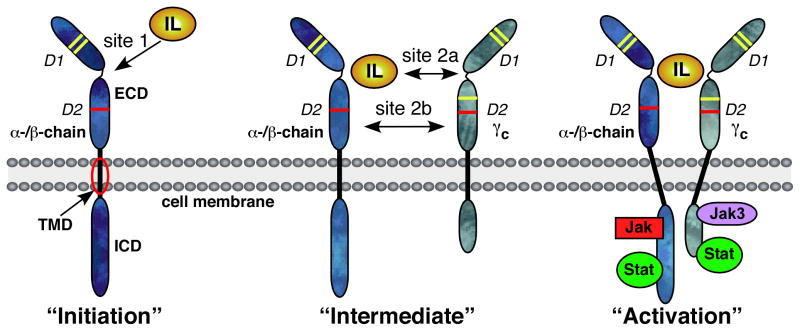
Fig. 1. Schematic representation of cytokine-induced receptor heterodimerization signaling mechanism.
The cytokine receptor is a transmembrane protein comprising an extracellular ligand binding domain (ECD), a single pass transmembrane domain (TMD), and an intracellular domain (ICD). The ECD folds into two fibronectin type III (FNIII) domains, D1 and D2. The γc receptors are members of the cytokine receptor homology class I (CRH) family that include receptors for growth hormone, erythropoietin, prolactin, and various interleukins (53, 54). The defining features of the CRH I family include conserved cysteine residues (depicted by yellow bars) involved in disulfide bonds in the D1 domain and a WSXWS primary sequence motif (depicted by red bars) in the D2 domain. During the initiation step, the cytokine or interleukin (IL) in this case interacts with the ECD of its α-/β-chain receptor forming the site 1 interface. This leads to the intermediate step where the 1:1 complex can associate with the shared common gamma-chain (γc) receptor. The binding of the γc receptor involves an interface between the interleukin and γc called site 2a and an interface between D2 regions of the α-/β-chain and γc receptors called site 2b. The bringing together of the α-/β-chain and γc receptors by the interleukin activates the classical janus kinase and signal transducers and activators of transcription (JAK/STAT) pathway and other signaling pathways.
IL-7 is in some ways a model example of a γc interleukin that induces signaling through receptor heterodimerization. IL-7 binds to its α-receptor, IL-7Rα (CD127), and γc through their extracellular domains (ECDs) to form a ternary complex that activates the JAK/STAT, phosphoinositol 3-kinase (PI3K)/Akt, or SRC pathways (reviewed in 1). IL-7 and IL-7Rα exhibit structural features similar to the other γc interleukins and their receptors, and IL-7 interacts with IL-7Rα using the same secondary structures used by other γc interleukins and their receptors. Both IL-7 and IL-7Rα, like the other γc interleukins and their receptors, are glycoproteins comprised of numerous asparagines that can be attached to N-linked glycans, or serines/threorines attached to O-linked glycans, or the first tryptophan of the WSXWS sequence motif attached to a C-mannose (3). Also similar to other γc IL-specific receptors (4–7), IL-7Rα self-associates to form homodimers incapable of signaling (8). Lastly, mutations in IL-7Rα, as seen for other mutated γc IL-specific receptors, can result in disease, such as autoimmune conditions (e.g. multiple sclerosis, type 1 diabetes, and colitis) (reviewed in 9), severe combined immunodeficiency (SCID) (reviewed in 10), and cancers (e.g. breast, leukemias, and lymphomas) (11–13).
IL-7 is in other ways unique and potentially creates a new paradigm for cytokine-induced receptor heterodimerization signaling. The interface between IL-7 and IL-7Rα is relatively small, more apolar, less charged, and less specific than the interfaces between other γc interleukins and their receptors, which may be important for IL-7Rα’s ability to bind partners besides IL-7. The α-helices of IL-7 and the angular geometries of IL-7Rα differ so much from the other γc interleukins and their receptors that either IL-7 and IL-7Rα undergo conformational changes to bind γc, or γc binds in a different conformation (14, 15). Glycosylation, although generally thought to be unimportant for γc interleukin/receptor interactions, significantly influences the binding affinity of IL-7Rα for IL-7 (14). Receptor-receptor association, also generally underappreciated among γc IL-specific receptors, likely regulates IL-7 signaling by sequestering the IL-7 binding surface and requiring dissociation and re-orientation of IL-7Rα and γc to bind IL-7. Lastly, the IL-7Rα homodimer and IL-7/IL-7Rα structures suggest rationales for the mutations causing B-cell acute lymphoblastic leukemia (B-ALL) and T-cell acute lymphoblastic leukemia (T-ALL) and SCID and potential diagnostic and therapeutic strategies to treat these diseases (15).
A comparison of the IL-7/IL-7Rα structure to other γc family members
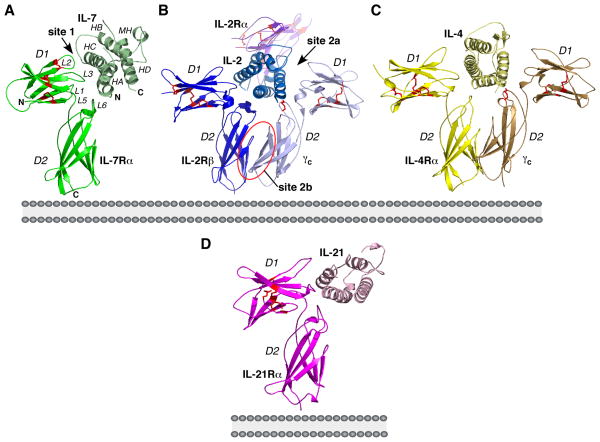
Complex structures of the γc interleukins and their receptors have been solved: IL-2 bound to its α-receptor, IL-2Rα, its β-receptor, IL-2Rβ, and γc (16, 17); IL-4 bound to its α-receptor (IL-4Rα) (18); IL-4 bound to IL-4Rα and γc (19); IL-7 bound its α-receptor, IL-7Rα (14); IL-15 bound to its α-receptor, IL-15Rα (20, 21); and IL-21 bound to its α-receptor, IL-21Rα (3) (Fig. 2). IL-2Rα and IL-15Rα adopt sushi domain structures and are not homologous to any component of the IL-7 signaling pathway and therefore are not covered in this review. These complex structures comprise the receptors’ ECDs. Furthermore, there are several unbound structures of IL-2 (22, 23), IL-4 (24–27), and IL-21 (28).
Fig. 2. Side views relative to the cell membrane of γc complex structures.
Ribbon diagrams of the (A) IL-7/IL-7Rα complex (3di3.pdb) (14), (B) IL-2/IL-2Rα/IL-2Rβ/γc complex (2bfi.pdb) (16), (C) IL-4/IL-4Rα/γc (3bpl.pdb) (19), and (D) IL-21/IL-21Rα (3tgx.pdb) (3). There is also a crystal structure of the IL-4/IL-4Rα binary complex (1iar.pdb) (18), but it is highly similar to the IL-4 ternary complex above. The different chains of each structure are colored with different shades of green (IL-7 proteins), blue (IL-2 proteins), yellow (IL-4 proteins), and magenta (IL-21 proteins). Red sticks in the cytokine receptors are the disulfide bonds. The six loop regions, L1-L6, which constitutes the cytokine binding epitopes on the receptors are labeled for the IL-7Rα structure. The interleukins adopt a 4-helix bundle topology and are labeled HA-HD for IL-7. MH denotes a turn of an α-helix in the first cross-over loop of IL-7. The site 1 interaction involves HA and HC of the interleukins to their α-/β-receptors. The site 2a interaction involves HA and HD of the interleukins to γc and the site 2b interaction involves the D2 domains of the α-/β-receptors and γc. PyMOL was used to generate the structural pictures (55).
The structure of the IL-7/IL-7Rα complex resembles the structures of IL-2/IL-2Rβ as seen in the quaternary complex, IL-4/IL-4Rα as seen in the binary and ternary complexes, and IL-21/IL-21Rα (Fig. 2). Similar to other γc interleukins and their receptors, IL-7 adopts an up-up-down-down four-helix bundle topology with two cross-over loops, and IL-7Rα forms an L-shaped architecture with each arm of the ‘L’ comprised of a fibronectin type III (FNIII) domain, denoted as D1 and D2, connected by a 310- helical linker. The D1 domain of IL-7Rα contains conserved cysteine residues across the receptors forming disulfide bonds and the D2 domain of IL-7Rα contains the highly conserved WSXWS sequence motif as well. Also similar to other γc interleukin/receptor complexes, IL-7 sits at the elbow region of the D1 and D2 domains of IL-7Rα and interacts with these domains at site 1 through residues on helices A and C. Yet, the global similarities of these complexes give way to meaningful differences at the interface between IL-7 and IL-7Rα and the potential interfaces between IL-7/IL-7Rα and γc.
The IL-7/IL-7Rα interface
The IL-7/IL-7Rα interface is more apolar and less specific than the interfaces of the other γc interleukin/receptor interfaces (Table 1). The IL-7/IL-7Rα interface comprises on average 47% apolar residues, 34% polar residues, and 5 hydrogen bonds. In contrast, the IL-2/IL-2Rβ interface comprises 24% apolar residues, 39% polar residues, and 8 hydrogen bonds. The IL-4/IL-4Rα interface comprises on average 27% apolar residues, 43% polar residues, and 15 hydrogen bonds, and the IL-21/IL-21Rα interface comprises 32% apolar residues, 30% polar residues, and 17 hydrogen bonds. The IL-7/IL-7Rα interface predominantly involves non-specific hydrophobic and van der Waals contacts with a shape complementarity (Sc) value on average of 0.67 (Sc value gives how well packed an interface is with values ranging from 0, poorly packed, to 1, perfectly packed)(29). Unlike the IL-7/IL-7Rα interface, the IL-2/IL-2Rβ interface involves a mixture of hydrophobic and hydrophilic residues with a Sc of 0.74. The IL-4/IL-4Rα interface is dominated by side chain-specific hydrophilic contacts with a Sc of on average 0.72, and the IL-21/IL-21Rα interface includes both hydrophobic and hydrophilic residues with a Sc of 0.70.
Table 1.
Summary of the binding interfaces of Type 1 γc complexesa
| Interface | b BSA (Å2) | H-bonds | c Sc | % Polar | % Apolar | % Charged |
|---|---|---|---|---|---|---|
| IL-7/unglyco IL-7Rαd | 728 | 5 | 0.69 | 32 | 50 | 18 |
| IL7/glyco IL-7Rα | 705 | 5 | 0.65 | 36 | 43 | 21 |
| IL-7Rα/IL-7Rα | 584 | 1 | 0.60 | 17 | 56 | 27 |
| IL-2/IL-2Rβ | 670 | 8 | 0.74 | 39 | 24 | 37 |
| IL-2/γc | 497 | 5 | 0.84 | 58 | 29 | 13 |
| IL-2Rβ/γc | 874 | 14 | 0.64 | 41 | 33 | 26 |
| IL-4/IL-4Rα binary | 835 | 15 | 0.74 | 40 | 31 | 29 |
| IL-4/IL-4Rα ternary | 778 | 14 | 0.70 | 46 | 22 | 32 |
| IL-4/γc | 536 | 10 | 0.82 | 59 | 18 | 23 |
| IL-4Rα/γc | 675 | 21 | 0.49 | 57 | 24 | 19 |
| IL-21/IL-21Rα | 1,000 | 17 | 0.70 | 30 | 32 | 38 |
Values calculated from PISA, CCP4, and the protein-protein interaction server at http://www.bioinformatics.sussex.ac.uk/protorp/index.html. The % type of residues is defined according to Thornton and coworkers (56). Polar = N, C, Q, H, S, T, W, Y. Apolar = A, G, I, L, M, F, P, V. Charged = D, R, K, E.
Averaged buried surface area between the cytokine/receptor and receptor at the interface.
Shape complementarity of the interface.
Averaged numbers for the two IL-7/unglycosylated IL-7Rα complexes in the asymmetric unit.
Of all the observed characteristics of the γc interleukin/receptor interfaces, the size of the interface correlates best with the binding affinities of these complexes (Table 2). The obvious trend is that the larger the buried surface area (BSA), the stronger the equilibrium binding dissociation constant (Kd): IL-21/IL-21Rα (BSA = 1000 Å2, Kd = 70 pM) > IL-4/IL-4Rα (BSA = 807 Å2, Kd = 150 pM) > IL-7/IL-7Rα (BSA = 717 Å2, Kd = 59 nM) > IL-2/IL-2Rβ (BSA = 670 Å2, Kd = 530 nM). There do not appear to be any trends correlating the strength of the binding affinities with the other measured parameters, such as the composition of residues at the interfaces.
Table 2.
Binding affinities of γc family members
The IL-7/IL-7Rα interface consists of the fewest charged residues of the γc interleukin/receptor complexes, but electrostatics still significantly influence association (30). The IL-7/IL-7Rα interface comprises only 20% charged residues, whereas the IL-4/IL-4Rα, IL-2/IL-2Rβ, and IL-21/IL-21Rα interfaces comprise 30%, 37%, and 38% charged residues, respectively. Yet, the binding affinity of IL-7 and IL-7Rα decreased significantly as a function of increasing sodium chloride concentrations, as measured by surface plasmon resonance spectroscopy (30). Fitting these data using an ion linkage mechanism (31), the number of ions consumed or released upon binding (2.0–2.8 ions) was much greater than that measured for the electrostatically driven IL-4/IL-4Rα interaction (0.8 ions) (32) and was within the range of ions typically associated with highly charged nucleic acid-protein interactions (31, 33). Given the lack of complementary charged residues in the IL-7/IL-7Rα interface, global long-range electrostatic charges are likely responsible for these impressive electrostatic parameters. By primary sequence analysis, IL-7 is a basic protein with an isoelectric point (pI) of 8.5, and the IL-7Rα ECD is an acidic protein with a pI of 5.4. The negative charge and field potential of IL-7Rα is distributed throughout the molecule, but mainly concentrated on the backside of the receptor away from the binding face of IL-7 (30). The positive charge and field potential of IL-7 is localized to a few residues at the amino-terminus and to a patch on the top part of the molecule (30).
The atypical interface between IL-7 and IL-7Rα demonstrates that the interactions between γc interleukins and their receptors can vary widely in size, polarity, charge, and specificity. Diverse characteristics at the γc IL/receptor interface is desirable, because they allow these molecules to bind to multiple partners, not unlike γc. For instance, both IL-4Rα and IL-7Rα participate in type I and type II interactions. In type I interactions, the α-receptor is the interleukin-specific receptor; in type II interactions, it is the activating receptor, playing the role that γc performs in the type I interactions. Thus, IL-4Rα binds not only IL-4 in a type I interaction but also IL-13/IL-13Rα in a type II interaction (19), and IL-7Rα binds not only IL-7 in a type I interaction, but also thymic stromal lymphopoietin (TSLP) and thymic stromal lymphopoietin receptor (TSLPR) in a type II interaction (34). The adaptive nature of the interactions between γc interleukins and receptors that are involved in type I and type II complexes is exemplified by the increase in size, polarity, and specificity of the IL-4/IL-4Rα interface as compared to the IL-13/IL-4Rα interface in the IL-13 ternary complex (19). It remains to be seen how the IL-7/IL-7Rα interface will compare to the TLSP/IL-7Rα interface. Nevertheless, for the γc interleukins and interleukin-specific receptors to bind different partners, they must be flexible and able to interact through interfaces with distinguishing attributes.
The potential IL-7/IL-7Rα interface with γc
The structures of the IL-2 quaternary complex and IL-4 ternary complex demonstrate that γc interacts with both the interleukins and interleukin-specific receptors (Fig. 2). The contacts between IL-2 and IL-4 with γc at site 2a are rather small, polar, and specific (Table 1). The IL-2/γc interface buries 497 Å2 of surface area, comprises 58% polar and 29% apolar residues, and exhibits a Sc of 0.84. The IL-4/γc interface buries 536 Å2 of surface area, comprises 59% polar and 18% apolar residues, and exhibits a Sc of 0.82. The site 2a interfaces involve residues from helices A and D in IL-2 and IL-4 and various elbow loop residues of γc. The surfaces of IL-2 and IL-4 that bind to γc, are characterized as apolar ‘canyons’ surrounded by specific peripheral polar interactions, and the surface of γc that binds to the interleukins is described as ‘rigid’ and ‘flat’ (19).
The contacts between IL-2Rβ and IL-4Rα with γc at site 2b are more extensive than those between the interleukins and γc at site 2a. The BSAs of the IL-2Rβ/γc and IL-4Rα/γc interfaces are 874 and 675 Å2, respectively. Both interfaces are more polar than apolar: the IL-2Rβ/γc interface comprises 41% polar and 33% apolar residues, and the IL-4Rα/γc interface comprises 57% polar and 24% apolar residues. Yet, the IL-2Rβ/γc interface exhibits much higher shape complementarity (Sc of 0.64) than the IL-4Rα/γc interface (Sc of 0.49), but the IL-4Rα/γc interface displays more hydrogen bonds than the IL-2Rβ/γc interface (21 versus 14), which suggests that receptor/receptor contacts can enhance the specificity of complex formation. The two receptors interact through their D2 domains and are related by almost a twofold symmetry (19).
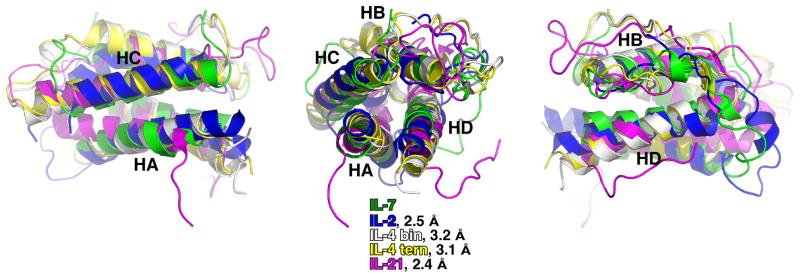
The structure of the IL-7/IL-7Rα complex shows that IL-7 is positioned to bind to γc via helices A and D, but not in the same orientation as IL-2 and IL-4. Backbone Cα superimpositions of the four-helix bundles of IL-2, IL-4, and IL-21 onto IL-7 result in root mean squared deviations (rmsds) of 2.5 Å, on average 3.2 Å, and 2.4 Å, respectively (Fig. 3). Helix D of IL-7 is approximately 7°–12° displaced from helix D of IL-2 and IL-21 and 14°–26° displaced from helix D of IL-4. The overall poor superimposition of the four-helix bundle and angular displacements of helix D in particular suggest that IL-7 presents a different orientation of helices to the flat, rigid surface of γc (Fig. 4A).
Fig. 3. Structural superimpositions of γc interleukins to IL-7.
Three different views of backbone superimpositions of the 4-helix bundle of each known interleukin structure onto IL-7. The colors for each interleukin are labeled along with the corresponding root mean squared deviations (rmsds) to IL-7 performed with PyMOL. The left figure highlights the site 1 interface (HA and HC) of the interleukins to their high affinity cytokine receptors (IL-7Rα, IL-2Rβ, IL-4Rα, and IL-21Rα) and the site 2a interface with γc (HA). The middle view is looking down the 4-helix bundles. The right view highlights the site 2a binding interface of HD of the interleukins to γc.
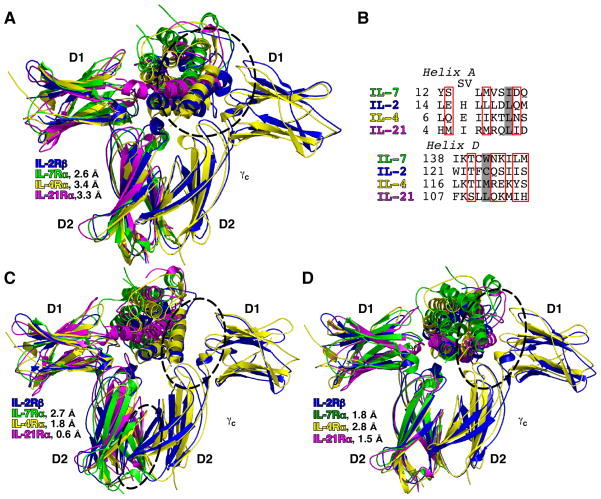
Fig. 4. Structural superimpositions of the γc receptors.
(A) Backbone superimpositions of the IL-7Rα, IL-4Rα, and IL-21Rα onto IL-2Rβ using both the D1 and D2 domains. (B) Primary sequence alignments based on the structure of IL-7 to IL-2 and IL-4. Red boxes indicate contact residues observed in the IL-2/IL-4/γc structures and predicted for IL-7 and IL-21. The shaded box highlights residues buried in the hydrophobic cores of the 4-helix bundles of the interleukins. (C, D) Further backbone superimpositions of the IL-7Rα, IL-4Rα, and IL-21Rα onto IL-2Rβ using either D1 domains (C) or D2 domains (D). The circles indicate steric clashes or residues being too distant in the potential interactions of IL-7 or IL-7Rα to γc for sites 2a or 2b.
In addition to using a different orientation to bind γc, IL-7 likely uses residues at the site 2a interface that were not predicted to interact with γc. The residues on IL-7 that were predicted to constitute a γc recognition motif include M17, I19, and L23 on helix A and W142, among others, on helix D (19, 35, 36). However, a turn of a π-helix in helix A that is unique to IL-7 changes the alignment of residues, such that S13, M17, and D23 of IL-7 align with the residues in IL-2 and IL-4 that comprise the predicted γc recognition motif (Fig. 4B). W142 of helix D also was reported to be critical to the interaction between IL-7 and γc, but W142 is buried into the hydrophobic core of the four-helix bundle and not interacting with γc. Accordingly, mutation of W142 likely causes a folding defect in helix D and/or in IL-7 that, in turn, causes the reported decreases in signaling (35, 36).
The differences in the structures of IL-7Rα, IL-2Rβ, IL-4Rα, and IL-21Rα indicate that conformational changes must occur before the IL-7/IL-7Rα complex interacts with γc. Backbone superimpositions of the D1, D2, or both domains of IL-7Rα onto IL-2Rβ result in rmsds from as low as 1.8 Å to has high as 2.7 Å (Fig. 4A,C,D). None of the receptors possesses angular geometries between the D1 and D2 domains similar to IL-7Rα (Table 3). Although IL-7Rα, IL-2Rβ, and IL-21Rα all share the same elbow (ε) angle between the D1 and D2 domains (Δε = 75°), the twist (τ) and swivel (σ) angles show that the D1 and D2 domains of IL-7Rα are rotated away from IL-2Rβ (Δτ =13° and Δσ = 24°) and IL-21Rα (Δτ =17° and Δσ = 23 °). The D1 and D2 domains of IL-7Rα and IL-4Rα have very similar twist angles (Δτ ~1°) but differ in their elbow angles (Δε = 12°) and dramatically in their swivel angles (Δσ = 33°). The poor superimposition and considerably different angular geometries of the IL-7Rα FNIII domains prevent the binding of γc onto the IL-7/IL-7Rα structure. Superimposing the D1, D2, or both D1/D2 domains of the IL-7/IL-7Rα structure onto the corresponding domains of the IL-2 quaternary or IL-4 ternary structures yields steric clashes between the D2 domains of IL7-Rα and γc (Fig. 4C), steric clashes between IL-7 and γc, (Fig. 4D), or IL-7 being too distant to contact γc (Fig. 4A, C).
Table 3.
Receptor domain orientationsa
| receptor | elbow angle (ε,°) | twist angle (τ,°) | swivel angle (σ,°) |
|---|---|---|---|
| unglyco IL-7Rα | 75 | 159 | 112 |
| glyco IL-7Rα | 74 | 158 | 113 |
| IL-7Rα dimer A | 77 | 162 | 108 |
| IL-7Rα dimer B | 79 | 164 | 107 |
| IL-2Rβ | 75 | 172 | 88 |
| γc (IL-2) | 89 | 158 | 112 |
| IL-4Rα (binary) | 87 | 155 | 78 |
| IL-4Rα (ternary) | 84 | 160 | 79 |
| γc (IL-4) | 94 | 167 | 103 |
| IL-21Rα | 75 | 176 | 89 |
The angles relate the D2 domains relative to the D1 domains. The angular geometries of the FNIII domains were determined using methods described previously (58). The elbow angle defines the angle between the two domains forming the “L” shape architecture. The twist or roll angle defines the angle between the x axes of the D1 and D2 domains. The swivel or spin of the D2 domain in the x-z plane defines the swivel angle.
The structure of the IL-21/IL-21Rα complex is also not compatible with the γc conformation from the IL-2 and IL-4 structures but for different reasons. The angular geometries of IL-21Rα are nearly identical to those of IL-2Rβ (Δε = 0°, Δτ = 4°, and Δσ = 1°) (Table 3), and a superimposition of the IL-21/IL-21Rα structure onto the D2 domain of IL-2Rβ in the quaternary structure results in an rmsd of 0.6 Å (Fig. 4C). IL-21, however, is nowhere near γc, in the superimposition, most probably due to the extensive interface between IL-21 and IL-21Rα (BSA = 1000 Å2) drawing helix D away from γc. Therefore, either the IL-7/IL-7Rα and IL-21/IL-21Rα complexes undergo structural rearrangements or γc, uses a different conformation than the one observed in the IL-2 and IL-4 structures to form the IL-7 and IL-21 ternary complexes.
The influence of glycosylation on the interactions of γc family members
The γc interleukins and their receptors are all glycoproteins. Despite its universal presence in the γc family, glycosylation appears to play varying roles in the interactions between the interleukins and their receptors. At one end of the spectrum is IL-4 and its α-receptor. Glycosylation of either the IL-4 or IL-4Rα does not influence the binding affinity or their ability to signal (18, 32, 37, 38). Also at this end of the spectrum is IL-2. Glycosylation of the IL-2 does not influence the binding affinity to its receptors or its ability to signal (39, 40). There are no studies reporting the effects of glycosylation of the IL-2 receptors.
Towards the other end of the spectrum is IL-21 and its receptor. The structure of the IL-21/IL-21Rα reveals that the glycan chain of N-glycosylated N54 in the D1 domain of IL-21Rα forms a bridge to the mannose glycan attached to the sidechain of W195 (C-mannosylation), the first tryptophan in the highly conserved WSXWS motif in the D2 domain of IL-21Rα (3). Although there are no studies reporting the impact of glycosylation on binding or signaling, the glycan chain attached to N54 of IL-21Rα is required for proper production and secretion from HEK293 cells, and the structure indicates that the N- and C-linked glycan bridge may stabilize the two fibronectin domains (3).
Also at the other end of the spectrum is IL-7 and its α-receptor. Glycosylation of IL-7Rα clearly does influence its binding to IL-7. The binding affinity of IL-7 to unglycosylated IL-7Rα is 18 μM, whereas the binding affinity of IL-7 to glycosylated IL-7Rα is on average 59 nM (Table 2). This approximately 300-fold enhancement in binding affinity results from an at least 5200-fold increase in the association rate. An increase in the on-rate, especially of this magnitude, is highly unusual not just for glycosylated cytokine-receptor interactions but for glycosylated protein-protein interactions as a whole (reviewed in 33, 41). Moreover, the faster association rate is wholly accounted for by the proximal N-acetyl glucosamine groups attached to the asparagine residues of IL-7Rα, and thus is indifferent to the type of glycans and the extent of branching from expression from insect or mouse cell lines.
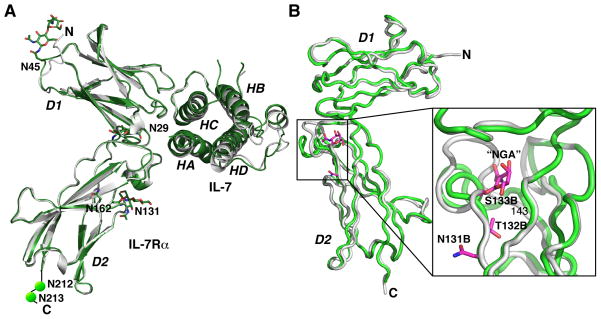
The structures of IL-7/IL-7Rα do not provide a clear structural basis for the impact of glycosylation on the binding kinetics and affinity (Fig. 5A). The global structural differences between the complexes of glycosylated and unglycosylated IL-7Rα with IL-7 are fairly small and subtle. These structures superimpose with rmsds of on average 0.59 Å. Locally, none of the six potential N-linked glycosylation sites (N29, N45, N131, N162, N212, and N213) on IL-7Rα are in the interface with IL-7. The glycans extend away from the structure and generally do not make contacts with residues besides their attached asparagines.
Fig. 5. Structural views of glycosylation of the IL-7Rα.
(A) Structural superimposition of the IL-7 complex with an unglycosylated IL-7Rα onto the IL-7 complex with a glycosylated IL-7Rα, indicating that N-linked glycosylation does not participate directly in the site 1 interface with IL-7 and does not induce large conformational changes between the two structures. The six asparagines of the IL-7Rα that can be N-linked glycosylated (N29, N45, N131, N162, N212, and N213) are labeled and two N-acetyl glucosamines attached to N29, N45, and N131 were experimentally observed. The secondary elements of the IL-7Rα molecules superimpose with an rmsd of 0.6 Å. (B) Experimental observation of an O-linked glycosylation of an N-acetyl galactosamine to S133 and the conformational changes induced by the glycan from residues 133-143 of the two IL-7Rα structures (15).
One possible way in which glycosylation of IL-7Rα could contribute to its interaction with IL-7 is through the overall electrostatic potential of IL-7Rα. A long-range electrostatic attraction drives the interaction between IL-7 (pI 8.4) and IL-7Rα (pI 5.3) through complementary overall charges of the molecules (30). Even though there are very few charged residues at the IL-7/IL-7Rα interface, the IL-7Rα residues interspersed throughout the protein but away from the interface are mainly acidic, and the IL-7 residues above and away from the interface are mainly basic and predicted to interact with negatively charged glycosaminoglycans, such as heparin (42). Glycosylation, in theory, could modulate those charges through the negatively charged sialic acid groups at the ends of complex N-glycans produced by vertebrates. This mechanism of action seems unlikely, however, because the degree of branching and the types of glycans typically determine to what extent glycosylation affects electrostatic potential and the IL-7/IL-7Rα interaction is insensitive to variations in branching and glycans (14).
A more likely way in which glycosylation of IL-7Rα may contribute to its interaction with IL-7 is by shifting the equilibrium of conformations sampled by unbound IL-7Rα. Unbound IL-7Rα is likely undergoing conformational exchange between at least two states: IL-7Rα unable and able to bind IL-7. The existence of these two states is consistent with the fact that the binding kinetics of IL-7 and IL-7Rα best fit to a two-state exchange mechanism and that the binding kinetics of IL-4 and IL-4Rα, which are unaffected by glycosylation, fit to a single-step reaction (also the other IL/receptor binding kinetics) (32, 40, 43, 44). Unpublished results from my laboratory demonstrate an increased thermodynamic stability of glycosylated IL-7Rα relative to unglycosylated IL-7Rα, which also supports glycosylation’s effect on the IL-7Rα states. We know through thermodynamic analysis the role of glycosylation of IL-7Rα is affecting the unbound states of IL-7Rα versus the bound state with IL-7 (unpublished results). By shifting the equilibrium toward the IL-7Rα state that is able to bind IL-7, glycosylation very well could increase the binding k1 on-rate and affinity measured for the IL-7/IL-7Rα interaction. Further unpublished results by my laboratory reveal extensive interplay among the N-glycans acting synergistically (positive binding cooperativity) to enhance its binding affinity to IL-7. The more difficult question of ‘how’ are the N-glycans on IL-7Rα enhancing its binding affinity to IL-7 are being tackled now.
The crystal structure of the unbound state of IL-7Rα ECD identified the first O-linked glycosylation site of this receptor (15). The unbound IL-7Rα structure comprised two receptor molecules in the asymmetric unit. Chain B of the IL-7Rα displayed clear difference electron density around the sidechain of S133 that fit well with a N-acetyl galactosamine (GalNAc) glycan. Mass spectrometry confirmed that S133 was glycosylated with a GalNAc. Mass spectrometry further identified that T132 also is O-glycosylated with a GalNAc. Chain A of IL-7Rα showed no signs of these O-glycans on these residues. The N-acetyl galactosamine of S133 forms numerous hydrogen bonds with receptor residues and water-mediated interactions. Backbone superimposition of the two receptor chains shows that the O-glycan changes the conformation from residues 133–143 to adopt two turns of an α-helix relative to chain A. The importance of O-linked glycosylation of the IL-7Rα is an area of active investigation.
The role of unliganded receptor/receptor complexes in γc signaling
Research on the γc family has focused primarily on the formation of the complexes involved in the stepwise cytokine-induced heterodimerization mechanism (Fig. 1). According to this mechanism, the complexes of interest for IL-7 signaling are IL-7/IL-7Rα and IL-7/IL-7Rα/γc. Yet, lurking in the research background have been observations of γc complexes not contemplated by this textbook mechanism. For instance, there have been reports of homodimers of IL-7Rα, IL-2Rβ, IL-4Rα, and IL-9Rα, and heterodimers of these receptors with γc (4–8, 45). None of these complexes has been reported to induce signaling, but their inability to signal should not lead to a conclusion that they are irrelevant or undeserving of attention. To the contrary, these unliganded receptor/receptor complexes likely perform important roles in γc family signaling, such as protecting the γc interleukin machinery from degradation, localizing the right γc family members to specific cellular niches, and modulating the timing and degree of signaling. They also may explain and serve as new targets for treating diseases implicating IL-7 signaling.
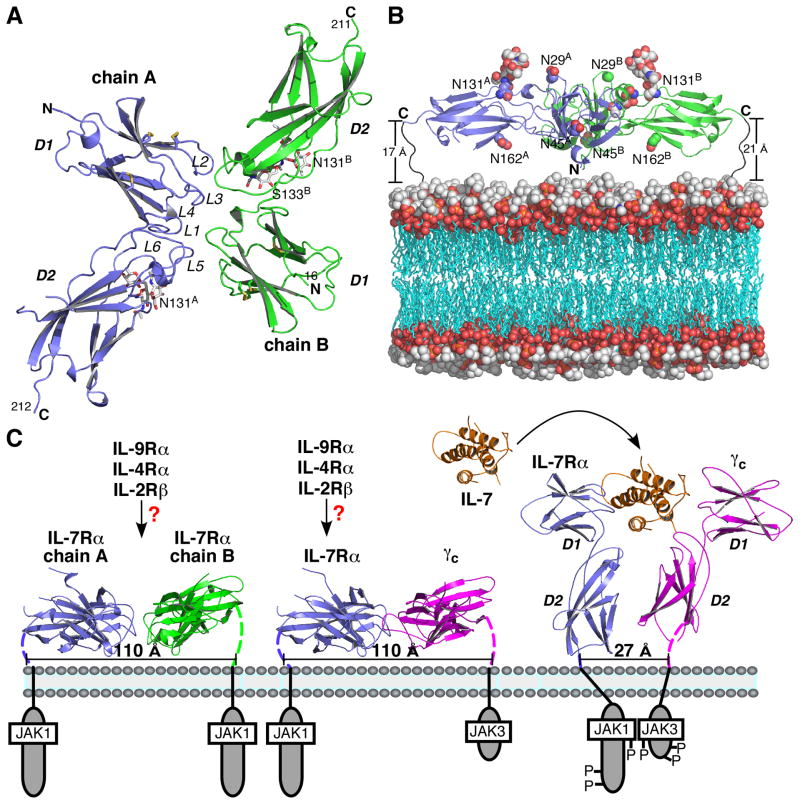
The only structure of a γc unliganded receptor/receptor complex available is the IL-7Rα homodimer, although there is a non-γc unliganded homodimer structure of erythropoietin receptor not discussed here (15, 46). The individual IL-7Rα molecules in the homodimer are very similar to the IL-7Rα structure seen in the IL-7/IL-7Rα complex (Fig. 6A). Backbone superimpositions give an average rmsd of less than 1 Å. IL-7Rα self-associates with a binding affinity of 610 μM, which is weaker than the IL-2Rβ homodimer (Kd = 3 μM) and the IL-7Rα/γc heterodimer (Kd = 3 μM) (5, 15). Consistent with these in vitro measurements, full-length IL-7Rα and IL-2Rβ bind more tightly to γc than to themselves in vivo as well (8, 45).
Fig. 6. Proposed signaling mechanism(s) of the IL-7 pathway and the γc family.
(A) Ribbon diagram of an IL-7Rα homodimer structure viewed as looking down onto the cell membrane (3up1.pdb) (15). (B) Side view of the IL-7Rα homodimer structure modeled on a lipid bilayer showing that there is plenty of room for the C-termini of both chains to reach the top of the cell membrane. (C) Proposed signaling mechanism(s) of the IL-7 and γc pathways of preassembly of the receptors before interleukin binding. The IL-7Rα/γc is a model generated by superimposing the γc structure onto chain B of the IL-7Rα homodimer structure. Mutagenesis data indicate that the elbow loop residues of IL-7Rα are important to its interaction with γc independent of IL-7 (unpublished results from my laboratory). The IL-7 ternary complex is also a structural model made by docking the γc structure close to the IL-7/IL-7Rα binary complex (14).
IL-7 binding is likely inhibited by the unliganded homodimer because all of the residues participating in the homodimer interface are also involved in IL-7 binding, including certain elbow loop residues. Because only a subset of residues are involved, the IL-7Rα homodimer interface buries less surface area, is more apolar, and less specific than the IL-7/IL-7Rα interface (Table 1). The homodimer buries on average 584 Å2; comprises 56% apolar residues, 17% polar residues, 27% charged residues, and 1 hydrogen bond; and exhibits a Sc of 0.60; whereas the IL-7/IL-7Rα complex buries on average 717 Å2; comprises on average 47% apolar residues, 34% polar residues, 20% charged residues, and 5 hydrogen bonds; and exhibits Sc of on average 0.67. The IL-7Rα homodimer interface is also smaller and includes many more apolar residues and much fewer hydrogen bonds than the IL-2Rβ/γc and IL-4Rα/γc interfaces. The IL-2Rβ/γc interface buries 874 Å2 of surface area and comprises 33% apolar residues and 14 hydrogen bonds, whereas the IL-4Rα/γc interface buries 675 Å2 of surface area and comprises 24% apolar residues and 21 hydrogen bonds.
The IL-7Rα homodimer structure as a whole is oriented entirely differently with respect to the membrane than any of the other γc complex structures (compare Figs. 2 and 6B). The N- and C- termini of the receptors are located on opposite ends such that the receptors form an ‘X’ when looking down onto the cell surface. The average distance between the C-terminal domains is 110 Å (Fig. 6C). In stark contrast, the average distance between the C-terminal domains of IL-2Rβ and IL-4Rα to γc is 27 Å. This 83 Å distance between the C-terminal domains of the non-activating IL-7Rα homodimer and activating IL-2Rβ and IL-4Rα complexes is likely the reason why IL-7Rα association does not activate the JAK/STAT pathway. The JAK1 molecules bound to the intracellular domains of IL-7Rα are physically kept apart in the IL-7Rα homodimer and can only be activated when brought together.
The binding constants of the IL-7Rα homodimer and IL-7Rα/γc heterodimer, the residues involved in their interfaces, and their orientation on the membrane lead to a proposed mechanism for IL-7 activation, and possibly γc family signaling generally, not envisioned by the stepwise, cytokine-induced heterodimerization mechanism (Fig. 6C). During inactivation, both the IL-7Rα homodimer and IL-7Rα/γc heterodimer are preformed at the membrane with their C-termini 110 Å apart, and the JAK1 and JAK3 molecules attached to the their respective intracellular domains are separated. IL-7 cannot bind to IL-7Rα in either the homodimer or heterodimer because the IL-7Rα loop residues that bind IL-7 also bind to IL-7Rα in the homodimer and heterodimer (author’s unpublished results). Accordingly, these complexes must dissociate to activate the pathway. Because IL-7Rα binds γc more tightly than itself and the elbow loop residues not involved in the IL-7Rα interface are involved in the IL-7Rα/γc interface, the IL-7Rα homodimer dissociation will require less energy than the IL-7Rα/γc dissociation. Upon dissociation, IL-7Rα and γc are able to bind IL-7 and rotate 90° away from the cell surface. This rotation brings the C-termini of IL-7Rα and γc within less than 30 Å from each other so that JAK1 and JAK3 attached to the intracellular domains are in close proximity to each other and may activate signal transduction.
Diseases associated with IL-7 signaling
Gain-of-function mutations of the IL-7Rα
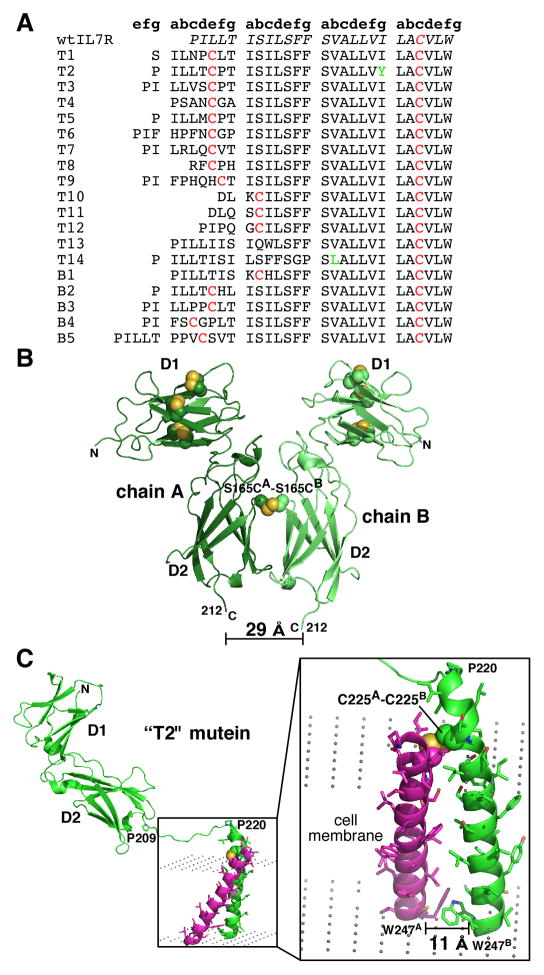
Gain-of-function mutations in the IL-7Rα have been identified from cancer patients with B-ALL and T-ALL by two independent research groups (12, 13). Given the importance of the IL-7 pathway in the survival of memory B and T cells (reviewed in 1, 47), it is not too surprising to finally isolate IL-7Rα sequences in leukemia patients. In one study, Barata, Durum, and coworkers (13) determined 14 T-ALL mutations and tested a couple of them for their in vitro and in vivo effects relative to wildtype IL-7Rα. These T-ALL mutations consist of insertions and/or deletions of residues at the N-terminal region of the IL-7Rα TMD (Fig. 7A). In another study, Izraeli and coworkers (12) determined BALL mutations that localize to either the ECD (S165-to-C165) or the TMD (multiple insertions and/or deletions) of the IL-7Rα. These B-ALL mutations were tested in in vitro experiments relative to wildtype IL-7Rα. These researchers also genotyped 30 different T-ALL mutations in the IL-7Rα TMD but did not test their properties (12). These B- and T-ALL mutations were different from the first study by Zenatti et al. (13). A common feature of the B-ALL and T-ALL mutations between the two studies involves the incorporation of an additional cysteine residue either in the IL-7Rα ECD or in the N-terminal region of the IL-7Rα TMD. Not all the T-ALL mutants contain an extra cysteine residue. Experimentally, a subset of the cysteine containing B- and T-ALL mutations displayed activation of the IL-7 pathway independent of IL-7 or γc (12, 13). Zenatti et al. (13) further demonstrated T-cell leukemogenesis in mouse models of a subset of the cysteine containing T-ALL mutations. The mechanism described above for the IL-7 signaling pathway can be applied to provide a structural rationale for the ALL mutations.
Fig. 7. Mutations identified in IL-7Rα from cancer patients.
(A) Sequence alignments of the mutations identified from patients with T-cell acute lymphocytic leukemia (T-ALL) (designated with a ‘T’) and B-ALL (designated with a ‘B’) in the N-terminal region of the juxtamembrane/transmembrane region of IL-7Rα (12, 13). (B) A model of the S165C mutation identified from patients with B-ALL. The S165C mutation induced homodimerization of the IL-7Rα ECDs and activates the IL-7 pathway independent IL-7 and γc. The C-termini of the two IL-7Rα chains are less than 30 Å apart. (C) A model generated of the homodimerized IL-7Rα with the ‘T2’ mutation that was demonstrated to activate the IL-7 pathway independent of IL-7 and γc and induced oncogenesis and death in mouse models. The C-termini of the two transmembrane domains are less than 30 Å apart.
I posit that the ALL mutations position either the ECDs or the TMDs of two or more copies of the IL-7Rα to a distance less than 30 Å between their C-termini and in a structural geometry allowing for the activation of the JAK1 molecules on the IL-7Rα ICDs in an IL-7- and γc-independent manner. Structural models have been constructed of the S165-to-C165 mutation in the IL-7Rα ECD in B-ALL and one of the cysteine containing insertion sequences near the IL-7Rα TMD in T-ALL (T2 mutation and also called JPSP7) to support this hypothesis. Fig. 7B displays a disulfide linked S165-to-C165 IL-7Rα ECDs. S165 is located on a solvent exposed loop formed by β-strands C’2 and E2 of the D2 domain and can easily be accessible to form a disulfide bond between two IL-7Rα molecules when mutated to a cysteine. The C-termini of the two IL-7Rα molecules in the structural model are separated by a distance of 29 Å, well within the predicted range to activate the signaling cascade independent of the α-receptors’ ligands.
Before I discuss a structural model of the T2 T-ALL mutation, further background information is presented to provide credence of our structural model and mechanism. First, biochemical treatment of the T1 and T2 sequences with reducing agents (dithiothreitol or β-mercaptoethanol) on the cell surface reduced the aggregation states of these sequences and the level of STAT5 phosphorylation, strongly indicating disulfide-bond formation between two IL-7Rα molecules (13). Second, mutation of the cysteine residues in the T1 and T2 sequences to either an alanine or serine abrogated STAT5 phosphorylation in cells (13). Third, unpublished results indicate mutation of the C-terminally positioned proline (P226) relative to the cysteine residue (C225) in T2 to either a serine or glycine abrogates cell survival (Dr. Scott Durum personal communication). These results suggest that it is more than just dimerization of this region, but there is also an important angular geometry at play across the bilayer, leading to activation of the pathway. Fourth, the crystal structures we have determined of the IL-7Rα ECD consist of residues 1-219 of the full-length receptor. We have only been able to observe electron density to build residues in the range of 209–212. The residues between 210 (or 213) to 219, termed the juxtamembrane region, are thus highly flexible and allow mobility of the α-receptor on the cell surface. Fifth, the IL-7Rα TMD is predicted to adopt a membrane-spanning α-helix. The T-ALL mutations and the wildtype IL-7Rα TMD were fed into a computational design algorithm developed by DeGrado and coworkers (48) to optimize packing geometries of α-helices in a lipid bilayer, based on known membrane crystal structures. It was clear from this analysis that the majority of T-ALL sequences (and the B-ALL sequences) cannot fit entirely within the lipid bilayer and the N-termini of these sequences will be solvent exposed on the extracellular side. With this current knowledge, a plausible structural model of the T2 T-ALL mutation was constructed.
Fig. 7C shows a structural model of the T2 T-ALL mutation. Residues of PILLTCPT of the T2 mutation were solvent exposed and I228 being the first residue in the lipid bilayer. Thus, it is reasonable for a disulfide bond to be formed between C225 of chains A and B. Disulfide bond formation of cysteine residues within the lipid bilayer does occur readily (e.g. the wildtype IL-7Rα TMD contains a cysteine at the C-terminal end of the sequence) (49). The juxtamembrane and transmembrane regions were built with a disulfide bond between C225 of chains A and B. The distance between the Cα atoms of the W247 residues in the TMD is 11 Å, well within the range to self-activate the JAK1 kinases independent of IL-7 and γc. It should be noted that not all the ALL mutations contained an unpaired cysteine residue at the N-terminal region. We are currently pursuing the crystal structures of several of the ALL mutations, the wildtype IL-7Rα TMD, and understanding their binding energetics with membrane environments. Therapeutically, it may be conceivable to isolate conformationally specific antibodies that can recognize a disulfide linked IL-7Rα T-ALL mutant over wildtype IL-7Rα on the cell surface. These experiments are underway between the Walsh and Durum laboratories.
SCID mutations
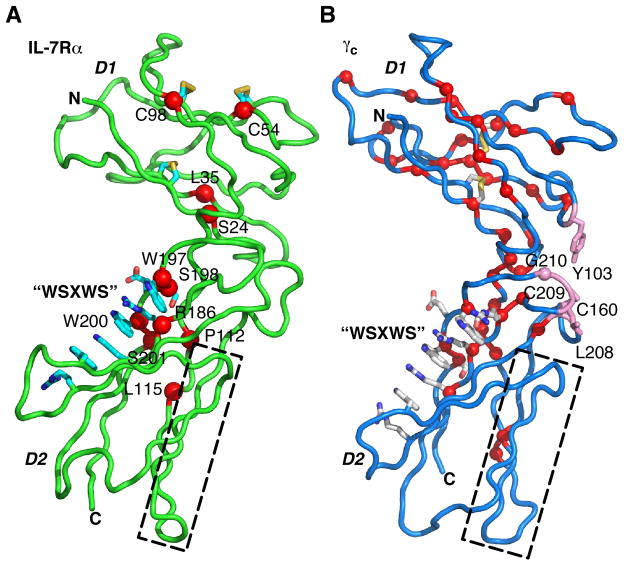
Mutations in the IL-7Rα ECD have been identified in patients suffering from SCID (Fig. 8A). The human phenotype of IL-7Rα SCID is T-B+NK+ (10, 50). No IL-7Rα SCID mutations have been identified in the TMD or ICD. Each of the IL-7Rα ECD mutations map onto residues outside the binding epitope with IL-7 and the predicted binding epitope with γc. Instead, these mutations map onto residues in the hydrophobic cores of the D1 and D2 domains, cysteines of disulfide bonds, or the highly conserved WSXWS sequence motif. Five of the SCID mutations (G8R, S24R, L35R, C54Y, and C98Y) are located in the D1 domain. The G8R mutation could not be mapped onto the IL-7Rα structure, because there was no electron density for this residue. The S24R mutation eliminates a hydrogen bond between S24 and L130 and likely requires movement to accommodate the bulky, polar arginine side chain, both of which may destabilize the linker connecting the D1 and D2 domains. The L35R mutation forces a bulky, polar side chain into the hydrophobic core of the D1 domain and presumably unfolds it. The two cysteine mutations, C54Y and C98Y, eliminate a disulfide bond and, in turn, disrupt the folding and/or stability of the D1 domain.
Fig. 8. IL-7Rα and γc severe combined immunodeficiency (SCID) mutations from patients.
(A) IL-7Rα SCID mutations are depicted as red spheres and labeled. (B) γc X-linked SCID mutations are shown as red spheres or pink sticks. The γc SCID mutations colored pink are involved in the site 2a interactions with the interleukins. For both figures, the dashed boxes represent the residues involved in the site 2b interaction between the D2 domains of γc and α-/β-receptors. The WSXWS sequence motifs of both structures that form extensive π-cation stacking interactions are displayed as sticks.
The remaining SCID mutations (P112H, P112S, L115R, R186stop, W197stop, W200C, S198N, W200C, S201I) are located in the D2 domain. The P112H, P112S, and L115R likely destabilize the hydrophobic core of the D2 domain. The R186 and W197 mutations convert these residues to stop codons, leading to premature termination of the mRNA. The S198N, W200C, and S201I mutations are in or near the WSXWS motif and potentially disrupt the extensive π-cation sidechain stacking interactions of this motif and its interactions with other with sidechains. Thus, all of the SCID mutations in IL-7Rα probably result in folding defects or destabilize the α-receptor, limiting its ability to interact with its ligands and signal.
To date, 344 mutations have been identified in the γc receptor in patients suffering from X-linked SCID (http://research.nhgri.nih.gov/scid/). The human phenotype of γc SCID is T−B+NK− (reviewed in 10). Unlike the IL-7Rα SCID mutations, the γc SCID mutations span the entire length of the receptor including the extracellular, transmembrane, and intracellular domains. Fig. 8B pinpoints the γc SCID mutations on the ECD. Similar to the IL-7Rα SCID mutations, the majority of the SCID mutations in the ECD localize to residues involved in hydrophobic cores of the domains, cysteines in disulfide bonds, and areas at or near the WSXWS sequence motif. These γc SCID mutations likely cause protein-folding defects, resulting in loss of binding activity and signal transduction. Unlike the IL-7Rα SCID mutations, a series of γc SCID mutations map to the elbow loop residues, Y103, C160, L208, C209, and G210, which interact directly with the interleukins at the site 2a interface. Mutagenesis studies have shown these residues to be important for γc interactions with all the interleukins (51, 52). For both IL-7Rα and γc SCIDs, the current treatment strategy is bone marrow transplantation.
Acknowledgments
I thank Dr. Julie Dohm, Esq. for critical reading and comments on the manuscript. The IL-7 research project is supported by NIH grant AI72142.
Footnotes
The author has no conflicts of interest to declare.
References
- 1.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 2.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamming OJ, et al. Crystal structure of interleukin-21 receptor (IL-21R) bound to IL-21 reveals that sugar chain interacting with WSXWS motif is integral part of IL-21R. J Biol Chem. 2012;287:9454–9460. doi: 10.1074/jbc.M111.311084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kammer W, et al. Homodimerization of interleukin-4 receptor alpha chain can induce intracellular signaling. J Biol Chem. 1996;271:23634–23637. doi: 10.1074/jbc.271.39.23634. [DOI] [PubMed] [Google Scholar]
- 5.Rose T, Moreau JL, Eckenberg R, Theze J. Structural analysis and modeling of a synthetic interleukin-2 mimetic and its interleukin-2Rbeta2 receptor. J Biol Chem. 2003;278:22868–22876. doi: 10.1074/jbc.M301757200. [DOI] [PubMed] [Google Scholar]
- 6.Malka Y, et al. Ligand-independent homomeric and heteromeric complexes between interleukin-2 or -9 receptor subunits and the gamma chain. J Biol Chem. 2008;283:33569–33577. doi: 10.1074/jbc.M803125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillet AH, et al. Human IL-Rbeta chains form IL-2 binding homodimers. Eur Cytokine Netw. 2008;19:49–59. doi: 10.1684/ecn.2008.0120. [DOI] [PubMed] [Google Scholar]
- 8.Rose T, et al. Interleukin-7 compartmentalizes its receptor signaling complex to initiate CD4 T lymphocyte response. J Biol Chem. 2010;285:14898–14908. doi: 10.1074/jbc.M110.104232. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Mazzucchelli RI, Riva A, Durum SK. The human IL-7 receptor gene: Deletions, polymorphisms and mutations. Semin Immunol. 2012;24:225–230. doi: 10.1016/j.smim.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 11.Barata JT, Cardoso AA, Nadler LM, Boussiotis VA. Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1) Blood. 2001;98:1524–1531. doi: 10.1182/blood.v98.5.1524. [DOI] [PubMed] [Google Scholar]
- 12.Shochat C, et al. Gain-of-function mutations in interleukin-7 receptor-alpha (IL7R) in childhood acute lymphoblastic leukemias. J Exp Med. 2011;208:901–908. doi: 10.1084/jem.20110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zenatti PP, et al. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat Genet. 2011;43:932–939. doi: 10.1038/ng.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McElroy CA, Dohm JA, Walsh ST. Structural and biophysical studies of the human IL-7/IL-7Ralpha complex. Structure. 2009;17:54–65. doi: 10.1016/j.str.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McElroy CA, et al. Structural reorganization of the interleukin-7 signaling complex. Proc Natl Acad Sci USA. 2012;109:2503–2508. doi: 10.1073/pnas.1116582109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005;310:1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 17.Stauber DJ, Debler EW, Horton PA, Smith KA, Wilson IA. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc Natl Acad Sci SUA. 2006;103:2788–2793. doi: 10.1073/pnas.0511161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hage T, Sebald W, Reinemer P. Crystal structure of the interleukin-4/receptor alpha chain complex reveals a mosaic binding interface. Cell. 1999;97:271–281. doi: 10.1016/s0092-8674(00)80736-9. [DOI] [PubMed] [Google Scholar]
- 19.LaPorte SL, et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–272. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen SK, et al. Crystal Structure of the interleukin-15. interleukin-15 receptor alpha complex: insights into trans and cis presentation. J Biol Chem. 2007;282:37191–37204. doi: 10.1074/jbc.M706150200. [DOI] [PubMed] [Google Scholar]
- 21.Chirifu M, et al. Crystal structure of the IL-15-IL-15Ralpha complex, a cytokine-receptor unit presented in trans. Nat Immunol. 2007;8:1001–1007. doi: 10.1038/ni1492. [DOI] [PubMed] [Google Scholar]
- 22.Brandhuber BJ, Boone T, Kenney WC, McKay DB. Three-dimensional structure of interleukin-2. Science. 1987;238:1707–1709. doi: 10.1126/science.3500515. [DOI] [PubMed] [Google Scholar]
- 23.Arkin MR, et al. Binding of small molecules to an adaptive protein-protein interface. Proc Natl Acad Sci USA. 2003;100:1603–1608. doi: 10.1073/pnas.252756299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter MR, et al. Crystal structure of recombinant human interleukin-4. J Biol Chem. 1992;267:20371–20376. doi: 10.2210/pdb2int/pdb. [DOI] [PubMed] [Google Scholar]
- 25.Powers R, Garrett DS, March CJ, Frieden EA, Gronenborn AM, Clore GM. Three-dimensional solution structure of human interleukin-4 by multidimensional heteronuclear magnetic resonance spectroscopy. Science. 1992;256:1673–1677. doi: 10.1126/science.256.5064.1673. [DOI] [PubMed] [Google Scholar]
- 26.Smith LJ, et al. Human interleukin 4. The solution structure of a four-helix bundle protein. J Mol Biol. 1992;224:899–904. doi: 10.1016/0022-2836(92)90457-u. [DOI] [PubMed] [Google Scholar]
- 27.Wlodawer A, Pavlovsky A, Gustchina A. Crystal structure of human recombinant interleukin-4 at 2. 25 A resolution. FEBS Lett. 1992;309:59–64. doi: 10.1016/0014-5793(92)80739-4. [DOI] [PubMed] [Google Scholar]
- 28.Bondensgaard K, et al. The existence of multiple conformers of interleukin-21 directs engineering of a superpotent analogue. J Biol Chem. 2007;282:23326–23336. doi: 10.1074/jbc.M701313200. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence MC, Colman PM. Shape complementarity at protein/protein interfaces. J Mol Biol. 1993;234:946–950. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- 30.Walsh ST. A biosensor study indicating that entropy, electrostatics, and receptor glycosylation drive the binding interaction between interleukin-7 and its receptor. Biochemistry. 2010;49:8766–8778. doi: 10.1021/bi101050h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Record MT, Jr, Anderson CF, Lohman TM. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978;11:103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- 32.Shen BJ, Hage T, Sebald W. Global and local determinants for the kinetics of interleukin-4/interleukin-4 receptor alpha chain interaction. A biosensor study employing recombinant interleukin-4-binding protein. Eur J Biochem. 1996;240:252–261. doi: 10.1111/j.1432-1033.1996.0252h.x. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber G, Haran G, Zhou HX. Fundamental Aspects of Protein-Protein Association Kinetics. Chem Rev. 2009;109:839–860. doi: 10.1021/cr800373w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonard WJ. TSLP: finally in the limelight. Nat Immunol. 2002;3:605–607. doi: 10.1038/ni0702-605. [DOI] [PubMed] [Google Scholar]
- 35.Cosenza L, Rosenbach A, White JV, Murphy JR, Smith T. Comparative model building of interleukin-7 using interleukin-4 as a template: a structural hypothesis that displays atypical surface chemistry in helix D important for receptor activation. Protein Sci. 2000;9:916–926. doi: 10.1110/ps.9.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.vanderSpek JC, Sutherland JA, Gill BM, Gorgun G, Foss FM, Murphy JR. Structure function analysis of interleukin 7: requirement for an aromatic ring at position 143 of helix D. Cytokine. 2002;17:227–233. doi: 10.1006/cyto.2002.1004. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Shen BJ, Sebald W. A mixed-charge pair in human interleukin 4 dominates high-affinity interaction with the receptor alpha chain. Proc Natl Acad Sci USA. 1997;94:1657–1662. doi: 10.1073/pnas.94.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hage T, Reinemer P, Sebald W. Crystals of a 1:1 complex between human interleukin-4 and the extracellular domain of its receptor alpha chain. Eur J Biochem. 1998;258:831–836. doi: 10.1046/j.1432-1327.1998.2580831.x. [DOI] [PubMed] [Google Scholar]
- 39.Landgraf BE, et al. Recombinant interleukin-2 analogs. Dynamic probes for receptor structure. J Biol Chem. 1992;267:18511–18519. [PubMed] [Google Scholar]
- 40.Myszka DG, Arulanantham PR, Sana T, Wu Z, Morton TA, Ciardelli TL. Kinetic analysis of ligand binding to interleukin-2 receptor complexes created on an optical biosensor surface. Protein Sci. 1996;5:2468–2478. doi: 10.1002/pro.5560051209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schreiber G, Walter MR. Cytokine-receptor interactions as drug targets. Curr Opin Chem Biol. 2010;14:511–519. doi: 10.1016/j.cbpa.2010.06.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, et al. Biophysical characterization of glycosaminoglycan-IL-7 interactions using SPR. Biochimie. 2012;94:242–249. doi: 10.1016/j.biochi.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liparoto SF, Myszka DG, Wu Z, Goldstein B, Laue TM, Ciardelli TL. Analysis of the role of the interleukin-2 receptor gamma chain in ligand binding. Biochemistry. 2002;41:2543–2551. doi: 10.1021/bi011692m. [DOI] [PubMed] [Google Scholar]
- 44.Zhang JL, Foster D, Sebald W. Human IL-21 and IL-4 bind to partially overlapping epitopes of common gamma-chain. Biochem Biophys Res Commun. 2003;300:291–296. doi: 10.1016/s0006-291x(02)02836-x. [DOI] [PubMed] [Google Scholar]
- 45.Pillet AH, Lavergne V, Pasquier V, Gesbert F, Theze J, Rose T. IL-2 induces conformational changes in its preassembled receptor core, which then migrates in lipid raft and binds to the cytoskeleton meshwork. J Mol Biol. 2010;403:671–692. doi: 10.1016/j.jmb.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 46.Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283:987–990. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- 47.Khaled AR, Durum SK. Death and Baxes: mechanisms of lymphotrophic cytokines. Immunol Rev. 2003;193:48–57. doi: 10.1034/j.1600-065x.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 48.Senes A, Chadi DC, Law PB, Walters RF, Nanda V, Degrado WF. E(z), a depth-dependent potential for assessing the energies of insertion of amino acid side-chains into membranes: derivation and applications to determining the orientation of transmembrane and interfacial helices. J Mol Biol. 2007;366:436–448. doi: 10.1016/j.jmb.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 49.White SH, Wimley WC. Membrane protein folding and stability: physical principles. Annu Rev Biophys Biomol Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 50.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 51.Olosz F, Malek TR. Three loops of the common gamma chain ectodomain required for the binding of interleukin-2 and interleukin-7. J Biol Chem. 2000;275:30100–30105. doi: 10.1074/jbc.M004976200. [DOI] [PubMed] [Google Scholar]
- 52.Olosz F, Malek TR. Structural basis for binding multiple ligands by the common cytokine receptor gamma-chain. J Biol Chem. 2002;277:12047–12052. doi: 10.1074/jbc.M110520200. [DOI] [PubMed] [Google Scholar]
- 53.Kossiakoff AA, deVos AM. Structural basis for cytokine hormone-receptor recognition and receptor activation. Adv Protein Chem. 1998;52:67–108. doi: 10.1016/s0065-3233(08)60433-7. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Lupardus P, Laporte SL, Garcia KC. Structural biology of shared cytokine receptors. Annu Rev Immunol. 2009;27:29–60. doi: 10.1146/annurev.immunol.24.021605.090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeLano W. The PyMOL Molecular Graphics System. 2002. [Google Scholar]
- 56.Jones S, Thornton JM. Protein-protein interactions: a review of protein dimer structures. Prog Biophys Mol Biol. 1995;63:31–65. doi: 10.1016/0079-6107(94)00008-w. [DOI] [PubMed] [Google Scholar]
- 57.Letzelter F, Wang Y, Sebald W. The interleukin-4 site-2 epitope determining binding of the common receptor gamma chain. Eur J Biochem. 1998;257:11–20. doi: 10.1046/j.1432-1327.1998.2570011.x. [DOI] [PubMed] [Google Scholar]
- 58.Deivanayagam CC, et al. Novel fold and assembly of the repetitive B region of the Staphylococcus aureus collagen-binding surface protein. Structure. 2000;8:67–78. doi: 10.1016/s0969-2126(00)00081-2. [DOI] [PubMed] [Google Scholar]