Abstract
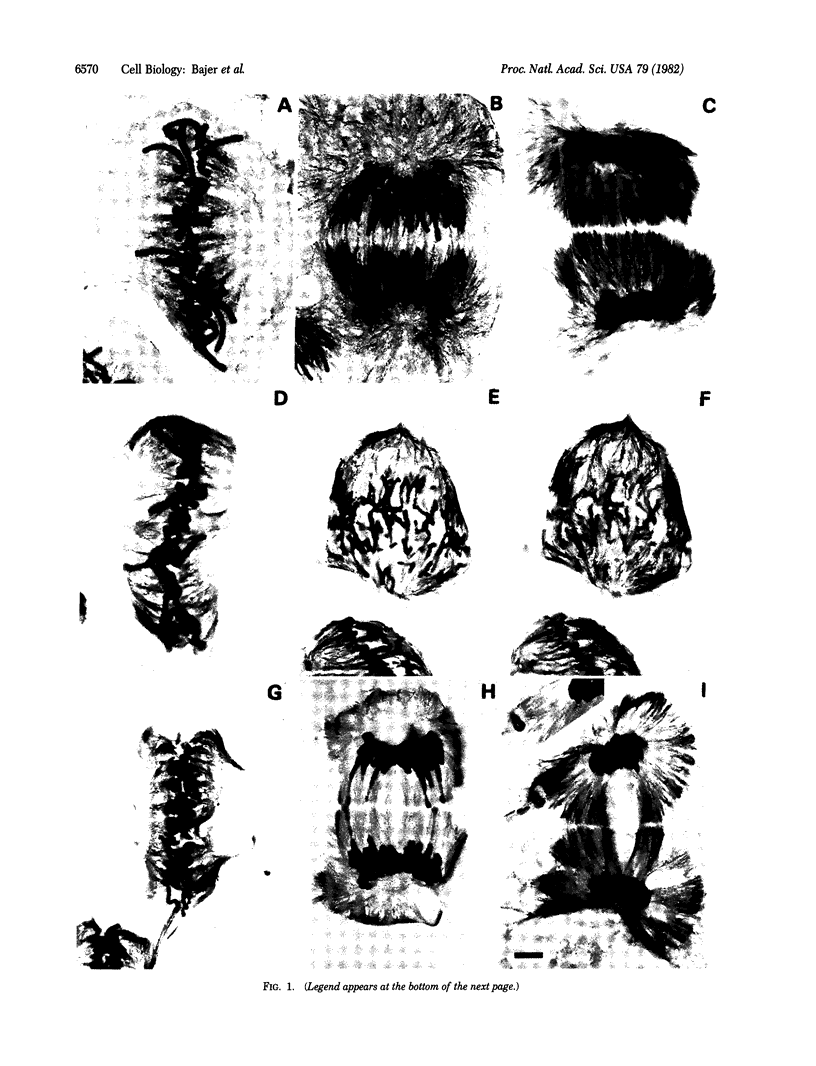
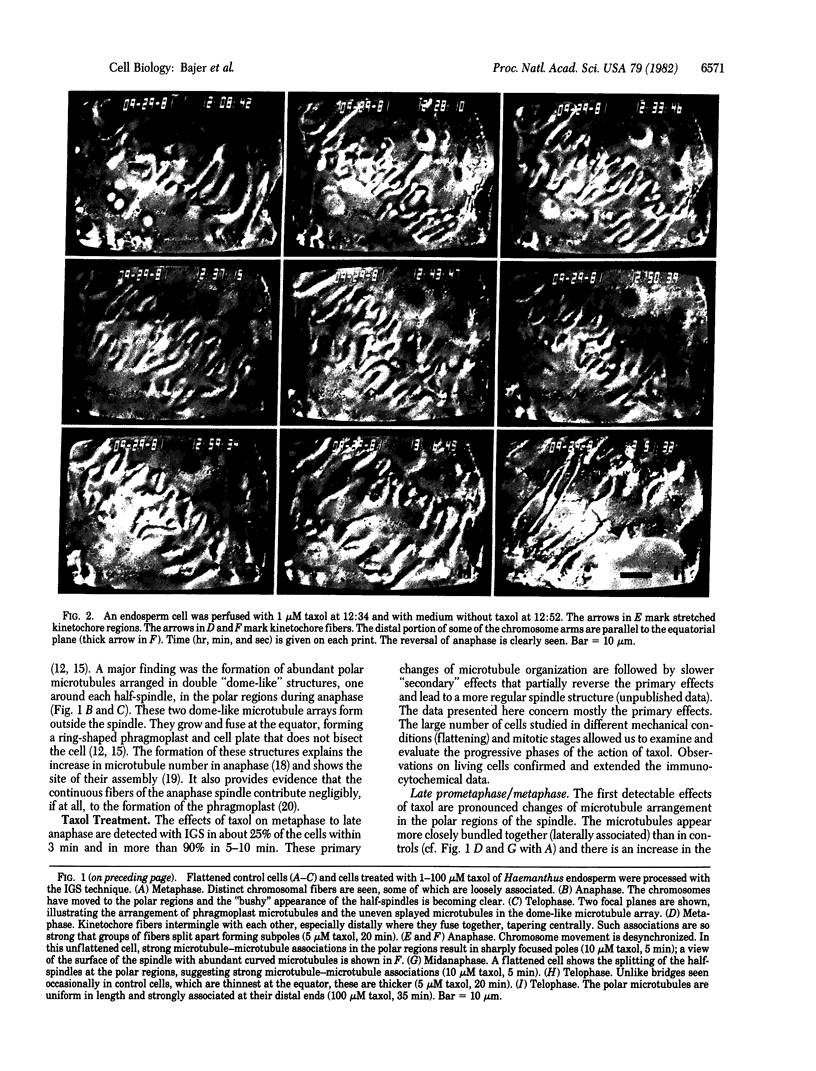
The effects of taxol on mitosis in Haemanthus endosperm were studied. Immuno-gold staining was used to visualize microtubules; observations on microtubule arrangements were correlated with studies in vivo. Mitosis is slowed down, but not arrested, by taxol over a wide range of concentrations. Taxol promotes the formation of abundant new microtubules and lateral association within and between microtubule arrays (spindle fibers). This leads to a pronounced reorganization of the spindle, especially at the polar regions. Chromosome arms may be pushed toward the equator in metaphase. Anaphase chromosomes, with their kinetochores still pointing to the poles, move backward before resuming their poleward migration. During anaphase, the interzone is depleted of microtubules and trailing chromosome arms are stretched and often torn apart by rapidly elongating polar microtubules. Fragments are transported away from the poles, apparently "riding" on the tips of microtubules. This provides evidence of "pushing" by elongating microtubules. The desynchronization of anaphase, often observed as one of the first effects of taxol, indicates that the anchorage of different kinetochore fibers varies. The data draw attention to modifications of spindle structure due to increased microtubule lateral associations and to the role of this process in spindle integrity and chromosome movement.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajer A. S. Functional autonomy of monopolar spindle and evidence for oscillatory movement in mitosis. J Cell Biol. 1982 Apr;93(1):33–48. doi: 10.1083/jcb.93.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajer A. S. Interaction of microtubules and the mechanism of chromosome movement (zipper hypothesis). 1. General principle. Cytobios. 1973 Nov;8(31):139–160. [PubMed] [Google Scholar]
- Baum S. G., Wittner M., Nadler J. P., Horwitz S. B., Dennis J. E., Schiff P. B., Tanowitz H. B. Taxol, a microtubule stabilizing agent, blocks the replication of Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4571–4575. doi: 10.1073/pnas.78.7.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Abraham I., Gottesman M. M. Isolation of a taxol-resistant Chinese hamster ovary cell mutant that has an alteration in alpha-tubulin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4388–4391. doi: 10.1073/pnas.78.7.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Willebrords R., De Mey J. Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5608–5612. doi: 10.1073/pnas.78.9.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J., Lambert A. M., Bajer A. S., Moeremans M., De Brabander M. Visualization of microtubules in interphase and mitotic plant cells of Haemanthus endosperm with the immuno-gold staining method. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1898–1902. doi: 10.1073/pnas.79.6.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J., Moeremans M., Geuens G., Nuydens R., De Brabander M. High resolution light and electron microscopic localization of tubulin with the IGS (immuno gold staining) method. Cell Biol Int Rep. 1981 Sep;5(9):889–899. doi: 10.1016/0309-1651(81)90204-6. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Simultaneous localization of myosin and tubulin in human tissue culture cells by double antibody staining. J Cell Biol. 1978 Apr;77(1):182–195. doi: 10.1083/jcb.77.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S. R., Gallas P. T. The effect of taxol on living eggs of Xenopus laevis. Dev Biol. 1980 Dec;80(2):489–494. doi: 10.1016/0012-1606(80)90421-2. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Kirschner M. W. Subunit treadmilling of microtubules or actin in the presence of cellular barriers: possible conversion of chemical free energy into mechanical work. Proc Natl Acad Sci U S A. 1982 Jan;79(2):490–494. doi: 10.1073/pnas.79.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Microfilament or microtubule assembly or disassembly against a force. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5613–5617. doi: 10.1073/pnas.78.9.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE S., BAJER A. Birefringence in endosperm mitosis. Chromosoma. 1961;12:48–63. doi: 10.1007/BF00328913. [DOI] [PubMed] [Google Scholar]
- Inoué S. Cell division and the mitotic spindle. J Cell Biol. 1981 Dec;91(3 Pt 2):131s–147s. doi: 10.1083/jcb.91.3.131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S., Ritter H., Jr Mitosis in Barbulanympha. II. Dynamics of a two-stage anaphase, nuclear morphogenesis, and cytokinesis. J Cell Biol. 1978 Jun;77(3):655–684. doi: 10.1083/jcb.77.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masurovsky E. B., Peterson E. R., Crain S. M., Horwitz S. B. Microtubule arrays in taxol-treated mouse dorsal root ganglion-spinal cord cultures. Brain Res. 1981 Aug 3;217(2):392–398. doi: 10.1016/0006-8993(81)90017-2. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. C., Wilson L., Purich D. L. Taxol induces microtubule assembly at low temperature. Cell Motil. 1981;1(4):445–454. doi: 10.1002/cm.970010405. [DOI] [PubMed] [Google Scholar]
- Van De Water L., 3rd, Guttman S. D., Gorovsky M. A., Olmsted J. B. Production of antisera and radioimmunoassays for tubulin. Methods Cell Biol. 1982;24:79–96. doi: 10.1016/s0091-679x(08)60649-4. [DOI] [PubMed] [Google Scholar]
- Wolf R. The cytaster, a colchicine-sensitive migration organelle of cleavage nuclei in an insect egg. Dev Biol. 1978 Feb;62(2):464–472. doi: 10.1016/0012-1606(78)90228-2. [DOI] [PubMed] [Google Scholar]
- Wolniak S. M., Hepler P. K., Jackson W. T. The coincident distribution of calcium-rich membranes and kinetochore fibers at metaphase in living endosperm cells of Haemanthus. Eur J Cell Biol. 1981 Aug;25(1):171–174. [PubMed] [Google Scholar]