Background: Tetrandrine exhibits antitumor effects and causes liver cancer apoptosis.
Results: Tetrandrine induced hepatocellular carcinoma autophagy via ATG7 and ROS/ERK in vitro, in vivo, and in Caenorhabditis elegans muscle cells.
Conclusion: Tetrandrine is a potent autophagy agonist.
Significance: Tetrandrine may be a promising clinical chemotherapeutic agent for human hepatocellular carcinoma.
Keywords: Anticancer Drug, Autophagy, Cancer Therapy, ERK, Gene Regulation, Hepatocellular Carcinoma, Reactive Oxygen Species (ROS), Signal Transduction, Autophagy-related Gene 7 (ATG7), Tetrandrine
Abstract
Tetrandrine, a bisbenzylisoquinoline alkaloid isolated from the broadly used Chinese medicinal herb Stephaniae tetrandrae, exhibits potent antitumor effects and has the potential to be used as a cancer chemotherapeutic agent. We previously reported that high concentrations of tetrandrine induce apoptosis in liver cancer cells. Here, we found that in human hepatocellular carcinoma (HCC) cells, a low dose of tetrandrine (5 μm) induced the expression of LC3-II, resulted in the formation of acidic autophagolysosome vacuoles (AVOs), and caused a punctate fluorescence pattern with the GFP-LC3 protein, which all are markers for cellular autophagy. Tetrandrine induced the production of intracellular reactive oxygen species (ROS), and treatment with ROS scavengers significantly abrogated the tetrandrine-induced autophagy. These results suggest that the generation of ROS plays an important role in promoting tetrandrine-induced autophagy. Tetrandrine-induced mitochondrial dysfunction resulted in ROS accumulation and autophagy. ROS generation activated the ERK MAP kinase, and the ERK signaling pathway at least partially contributed to tetrandrine-induced autophagy in HCC cells. Moreover, we found that tetrandrine transcriptionally regulated the expression of autophagy related gene 7 (ATG7), which promoted tetrandrine-induced autophagy. In addition to in vitro studies, similar results were also observed in vivo, where tetrandrine caused the accumulation of ROS and induced cell autophagy in a tumor xenograft model. Interestingly, tetrandrine treatment also induced autophagy in a ROS-dependent manner in C. elegans muscle cells. Therefore, these findings suggest that tetrandrine is a potent autophagy agonist and may be a promising clinical chemotherapeutic agent.
Introduction
Human hepatocellular carcinoma (HCC)3 is the third leading cause of cancer-related lethality worldwide with over 600,000 deaths each year (1). Most early-stage HCC patients can be treated effectively with surgery, such as resection or liver transplantation, or radiotherapy. However, due to the poor prognosis and high recurrence of HCC after surgical resection, most advanced HCC cases result in fatality without effective treatment (2). In recent years, Chinese medicine has attracted interest for cancer therapeutics for their ability to effectively kill cancer cells and their low toxicity in normal cells.
Tetrandrine, a bisbenzylisoquinoline alkaloid, is a key ingredient of traditional Chinese medicine that is isolated from the roots of radix Stephaniae tetrandrae S. Moore (3, 4). For thousands of years, tetrandrine has been broadly used in China to treat patients with arthritis, hypertension, inflammation, and even silicosis. Studies have demonstrated that tetrandrine blocks Ca2+ channels (5), has anti-proliferative features and possesses immunosuppressive properties (6). In addition, tetrandrine regulates many intracellular signaling events, including cell cycle arrest, MAP kinase activation, IκB/NF-κB signaling, and the transforming growth factor-β signaling pathway (4, 7, 8). We and other researchers have demonstrated that tetrandrine effectively induces apoptosis in cancer cells, suggesting that tetrandrine may be a promising agent for the treatment of cancer (9, 10). Tetrandrine has been shown to induce cellular apoptosis by repressing AKT activity in HCC cells, activating the caspases and PKC-δ in U937 leukemia cells and stimulating the P38 MAPK signaling pathway in colon cancer cells (4, 11).
Although apoptosis type I, also known as programmed cell death, is the common mechanism for targeted chemotherapies (12), autophagy, also known as type II cell death, has recently received considerable attention in the oncogenesis field for its role in the response to anti-cancer therapies (13). Autophagy is a cellular process that involves protein and organelle degradation in the lysosome and the recycling of cellular components to ensure cellular survival during starvation. This process can be activated in cells by external or internal stimuli (13). Autophagy contributes to maintaining intercellular homeostasis, serves as a temporary survival mechanism, and possesses a number of connections to human disease and physiology (14).
Recent studies have shown that enhanced autophagy may function as a tumor suppressing mechanism, and defects in autophagy can promote cancer (15). The mechanisms of autophagic cell death can be used as an effective method for cancer prevention and treatment (16). Some synthetic chemotherapeutic agents, such as tamoxifen, suberoylanilide hydroxamic acid, temozolomide, and rapamycin, induce autophagic cell death in a variety of cancer cells. Also, many natural anticancer products can induce cancer cell autophagy. Arsenic trioxide (As2O3), resveratrol, and the soybean B-group triterpenoid saponins induce autophagy in malignant glioma cells, ovarian cancer cells, and colon cancer cells, respectively (17).
Tetrandrine, a natural medicinal chemical, is a promising agent for the treatment of cancer. We have previously demonstrated that tetrandrine at high concentrations induces apoptosis in HCC cells (9); however, whether tetrandrine has the capability to induce autophagy was unknown. In this study, we found that a low concentration of tetrandrine induces autophagy, but not apoptosis, in HCC cells. Moreover, our in vitro and in vivo data show that the autophagy-inducing activity is at least partially dependent on the accumulation of intracellular ROS and the repression of ATG7. Thus, our results indicate that tetrandrine treatment results in multiple beneficial effects for the potential treatment of cancer.
EXPERIMENTAL PROCEDURES
Chemicals and Antibodies
Tetrandrine was purchased from Shanghai Ronghe Medical, Inc. (Shanghai, China) and dissolved in dimethyl sulfoxide. For the in vivo studies, tetrandrine was suspended in 0.5% (w/v) methylcellulose. DCFH-DA and MitoTracker Red were obtained from Invitrogen, and 3-methyladenine and N-acetyl-l-cysteine were purchased from Sigma. Cyclosporin A was purchased from Beyotime (Nantong, China), rapamycin and PD98059 was purchased from Cell Signaling Technology (Beverly, MA). The antibody against microtubule-associated protein 1 light chain 3 (LC3) was purchased from Sigma, and antibodies against CASPASE-3, CASPASE-8, poly(ADP-ribose) polymerase, phospho-AKT (Ser-473), total-ERK, phospho-ERK (Thr-202/Tyr-204), phospho-P38 MAPK (Thr-180/Tyr-182), phospho-JNK (Thr-183/Tyr-185), CYTOCHROME c, and ATG7 were purchased from Cell Signaling Technology. The antibody against BCL-2, BAX, and MCL-1 were purchased from Proteintech Group Inc. (Chicago, IL). The antibody against GAPDH was purchased from Beyotime.
Cell Lines and Cell Culture
Huh7, BEL7402, HepG2, and L02 cells were purchased from CCTCC (Wuhan, China). MEF wild-type and MEF Atg7 knock-out cells were a gift from Dr. Masaaki Komatsu (Tokyo Metropolitan Institute of Medical Science, Japan). All the cells were cultured in high-glucose DMEM (HyClone) supplemented with 10% fetal bovine serum (FBS, HyClone), penicillin (100 units/ml), and streptomycin (100 μg/ml) and incubated at 37 °C in a humidified atmosphere containing 5% CO2. Cell culture dishes and plates were obtained from Wuxi NEST Biotechnology. Co., Ltd.
Plasmids and RNA Interference
The pEGFP-LC3 plasmid was a gift from Dr. Tamotsu Yoshimori (National Institute of Genetics, Mishima, Japan); the pSUPER puro plasmid was purchased from Oligoengine (Seattle, WA). The human helper plasmid was kindly provided by Dr. Zan Huang (College of Life Sciences, Wuhan University, China). The BAX plasmid was kindly provided by Dr. Xiaodong Zhang (College of Life Sciences, Wuhan University, China). The BCL-2 plasmid was kindly provided by Dr. Hong-bing Shu (College of Life Sciences, Wuhan University, China). shRNA ATG7 sequences for the sense strand (5′-GCCTGCTGAGGAGCTCTCCAT-3′) and the antisense strand (5′-AAGGAAGAGCTGTGACTCC-3′) were designed against the ATG7 gene sequence.
Western Blot Analysis
The cells were harvested and lysed in 1% SDS on ice. Then, the cell lysates were heated at 95 °C for 20 min and centrifuged at 12,000 × g for 10 min. The supernatant was collected, and the protein concentration was determined by the Pierce BCA Protein Assay Kit (Thermo Scientific). Equivalent amounts of protein (20 μg) from each sample were loaded and run on SDS-PAGE gels (Amresco) and transferred to PVDF membranes (Millipore). After blocking the membranes with 5% nonfat milk (Bio-Rad) in Tris-buffered saline with 0.1% Tween 20 (TBST) at room temperature for 1 h, the membranes were incubated with specific primary antibodies at 4 °C overnight, washed with TBST, and incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. After washing with TBST, the immunoblots were visualized by chemiluminescence using a HRP substrate (Millipore). GAPDH was probed to ensure equal protein loading.
Transient Transfection and MitoTracker Red Labeling
To detect autophagy in Huh7 and BEL7402 cells, the cells were seeded on coverslips in 12-well plates, grown for 16 h, and then transiently transfected with the pEGFP-LC3 plasmid using FuGENETM HD (Roche Applied Science) according to the manufacturer's protocol. The cells were then incubated for 24 h before treatment with the indicated drugs. After treatment, the cells were observed with a fluorescent microscope (Olympus BX51). The percentage of cells with more than five GFP-LC3 dots, which were considered to be autophagic, was quantified. To detect mitophagy in tetrandrine-treated Huh7, MitoTracker Red dye was added into the cell culture and incubated at 37 °C for one-half hour. Then cells were observed with a confocal fluorescent microscope.
The Measurement of ROS Accumulation
The intracellular ROS levels were detected using the DCFH-DA probe (Sigma) by flow cytometry. Briefly, the cells were cultured and treated with 5 μm tetrandrine for the indicated time intervals. Then, the cells were harvested, washed twice with PBS, incubated with DCFH-DA (1 μm) in serum-free DMEM at 37 °C in a 5% CO2 incubator for 20 min, washed twice with PBS, and analyzed by flow cytometry. The data were processed using the FCS Express V3 program (DeNovo, Los Angeles, CA).
The Measurement of Mitochondrial Membrane Potential (Δψm)
Tetrandrine-induced changes in Δψm were measured using the JC-1 probe (Multisciences Biotech) by flow cytometry. The cells were processed for flow cytometry as described above, except the JC-1 probe (2 μm) was used in place of DCFH-DA. The red to green fluorescence ratio was calculated using the FCS Express V3 program (DeNovo, Los Angeles, CA).
The Isolation of CYTOCHROME c
Huh7 cells were seeded in six-well culture plates at 40–50% confluence. The next day, the cells were incubated with 5 μm tetrandrine for various time intervals. Then, the cells were trypsinized, washed twice with ice-cold PBS, and CYTOCHROME c was isolated using the Cell Mitochondria Isolation Kit (Beyotime, Nantong, China) according to the manufacturer's protocol.
CASPASE 3 Activity Analysis
Huh7 cells were planted in six-well culture plates and treated with 5 μm tetrandrine for the indicated time. CASPASE 3 activity was measured using the CASPASE 3 Activity Assay Kit (Beyotime) according to the manufacturer's protocol.
Tumor Xenograft
Five-week-old male BALB/c nude mice were obtained from the Disease Prevention Center of Hubei Province (Wuhan, Hubei, China). The experimental protocols were approved by the Experimental Animal Center of Wuhan University. The Huh7 cell numbers were counted, and 2 × 107 cells were implanted in the right flank of each mouse. When the tumor volume reached 200–300 mm3, the mice were randomly distributed into control and treatment groups (n = 7) and gavaged. The control group received treatment with the vehicle, which consisted of 0.5% (w/v) methylcellulose and 0.1% (v/v) Tween 80 in sterile water. The treatment group was given tetrandrine at 25 or 50 mg/kg of body weight every other day for 20 days. During the treatment, the mice were weighed, and the tumor volumes were measured. The tumor volumes were calculated by the following equation: V = L × W2/2 (L = large diameter; W = small diameter).
The TUNEL Assay and Transmission Electron Microscopy
The TUNEL assay identifies DNA-strand breaks labeled with fluorescein, which is a marker for apoptosis. For this assay, the tumor tissue samples were incised and treated according to the manufacturer's directions (Roche Applied Science). The tumor sections were inspected under a fluorescent microscope (Olympus) to examine blue DAPI staining at 460 nm and green fluorescence (apoptotic cells) at 520 nm.
For transmission electron microscopy, the tumor tissues were incised immediately after tumor extraction into ∼1 mm3 pieces. The tumor pieces were soaked in 2.5% glutaraldehyde for tissue fixation, washed three times in 0.1 m PBS, and fixed with 1% OsO4. Next, the tissues were dehydrated using a range of alcohol concentrations for 15 min. The tumor tissues were then embedded into paraffin and sliced using a LKB-V ultramicrotome (BROMMA, Sweden). For TEM inspection, the prepared sections were examined under an H-600 transmission electron microscope (Hitechi, Japan).
Tissue Protein Isolation from the Tumor Xenograft, MDA Assay, and Serum ALT Activity Analysis
The tumor tissue samples were homogenized and sonicated in RIPA buffer (Beyotime, Nantong, China) on ice. Then, the tissue lysates were centrifuged at 12,000 × g for 15 min at 4 °C, and the supernatant was collected for Western blotting analysis, as described previously. The tumor tissue samples were subjected to MDA assay as described in the Lipid Peroxidation MDA assay kit (Beyotime, Nantong, China). The MDA levels were detected by Multi-Mode Microplate Readers (SpectramMax M5) at 532 nm. The blood samples from nude mice were subjected to serum ALT activity analysis as described in the serum ALT assay kit (Nanjing Jiancheng Bioengineering Institute, Nangjing, China). The ALT levels were detected by Multimode Microplate Readers (SpectraMax M5) at 510 nm.
The Assessment of Autophagy in C. elegans
The DA2123 strain (adIs2122[lgg-1:GFP rol-6(df)]) was obtained from the Caenorhabditis Genetics Center (CGC) at the University of Minnesota. Synchronized worms were raised at 20 °C and treated after hatching with either tetrandrine (25, 50, or 100 μm) or dimethyl sulfoxide as a control. L3 larvae worms were immobilized with 5 mm sodium azide on a 2% agarose pad and photographed using an Olympus upright microscope at a ×40 magnification. GFP::LGG-1 positive puncta were counted in the seam cells (lateral epidermal) of L3 transgenic worms. At least 3–10 seam cells were examined in 10–25 animals in two independent experiments, and the results were averaged. The statistical analysis was performed using an unpaired, two-tailed t test.
Statistical Analyses
All the experiments were performed at least three times. Student's t test was used for all the statistical analyses, and the differences were considered significant if the p value was less than 0.05.
RESULTS
Tetrandrine Induces Autophagy in Human Hepatocellular Carcinoma Cells
Previously, we reported that tetrandrine induced apoptosis in HCC cells at relatively high concentrations (>20 μm) (9). To determine whether tetrandrine could induce autophagy in HCC cells, Huh7, BEL7402, and HepG2 cells were treated with a low concentration of tetrandrine (≤5 μm) for 24 h. We found that poly(ADP-ribose) polymerase, CASPASE-8, and CASPASE-3, which are all markers for apoptosis, were not activated at this concentration of tetrandrine treatment. However, the expression of LC3-II, a membrane-bound form of LC3 and an established marker of cell autophagy, gradually increased in a dose- and time-dependent manner with tetrandrine treatment (Fig. 1, A and B). Rapamycin, a well known inducer of autophagy was used as a positive treatment control (supplemental Fig. S1). Moreover, we examined the effects of tetrandrine on normal liver cell line (L02) and found no appearance of autophagy (Fig. 1B). Staining Huh7 and BEL7402 cells with acridine orange resulted in considerable red fluorescence in tetrandrine-treated cells, which suggested the formation of numerous acidic autophagolysosome vacuoles (AVOs) (supplemental Fig. S2). The formation of puncta with the GFP-LC3 fusion protein is a well characterized method for visualizing autophagosomes (18, 19). To further confirm that tetrandrine induces autophagy in HCC cells, Huh7 and BEL7402 cells were transfected with the LC3-GFP plasmid and then treated with tetrandrine for 24 h. Microscopic examination showed the characteristic punctate fluorescent pattern of LC3-GFP, indicating autophagosome formation and the occurrence of autophagy (Fig. 1C). Treatment with a common specific inhibitor of autophagic/lysosomal protein degradation, 3-methyladenine, significantly blocked the formation of the LC3-GFP fluorescent puncta (Fig. 1D). The LC3-II protein level was consistent with the cell fluorescence results (supplemental Fig. S3). These data suggest that tetrandrine-induced HCC cancer cells to undergo autophagy, but not apoptosis, at low treatment concentrations.
FIGURE 1.
Tetrandrine treatment induced autophagy in HCC cells. A, Huh7, BEL7402, and HepG2 cells were treated with the indicated concentrations of tetrandrine for 24 h. Then, the cell lysates were subjected to Western blotting using antibodies against pro-poly(ADP-ribose) polymerase, pro-CASPASE-8, pro-CASPASE-3, and LC3. A GAPDH antibody was used as a loading control. The molecular weights of bands are shown on the right. B, after exposure to 5 μm tetrandrine for the indicated time intervals in HCC cell lines (Huh7 and BEL7402) and normal liver cell line L02, the cells were lysed and immunoblotted with an anti-LC3 antibody. C, Huh7 and BEL7402 cells were transiently transfected with the GFP-LC3 plasmid for 24 h, subsequently treated with or without 5 μm tetrandrine for 8 or 24 h and observed with a fluorescence microscope. Representative images are shown to indicate the cellular localization patterns of the GFP-LC3 fusion protein (×40 magnification). The percentage of cells with GFP-LC3 puncta was used to quantify the percentage of autophagic cells. The data represent an average ± S.D. of at least three independent experiments. At least 100 GFP-LC3-transfected cells were counted in each experiment. D, GFP-LC3-transfected cells were pretreated with or without 2 mm 3-methyladenine (MA) for 1 h. The cells were then exposed to 5 μm tetrandrine for 24 h, and the localization of GFP-LC3 was observed using a fluorescent microscope (×40 magnification). The percentage of GFP-LC3-transfected cells with punctate fluorescence is shown. At least 100 cells from each treatment group were examined (* = p < 0.05).
Intracellular Reactive Oxygen Species (ROS) Are Involved in Tetrandrine-induced Autophagy in HCC Cells
Many studies have shown that some chemotherapeutic agents activate the production of intracellular ROS and then induce cellular apoptosis and/or autophagy in certain types of cancer cells (20–22). Moreover, ROS are essential for autophagosome formation under starvation conditions (23, 24). Therefore, we next investigated whether tetrandrine-induced autophagy was related to the production of ROS in HCC cells. Intracellular ROS levels were measured by flow cytometry using the specific ROS-detecting fluorescent dye H2DCFDA. We found that exposure of Huh7 and BEL7402 cells to tetrandrine for 6 h dramatically increased the DCF fluorescence intensity, indicating an increase in ROS species (Fig. 2A). However, when the cells were pretreated with the free radical scavenger N-acetyl-l-cysteine (NAC) 1 h prior to the administration of tetrandrine, the fluorescence intensity of DCF decreased compared with the levels found in cells without NAC pretreatment (Fig. 2B). To determine whether ROS production plays a role in tetrandrine-induced autophagy, we examined the levels of autophagy in Huh7 and BEL7402 cells transfected with the LC3-GFP plasmid by observing the fluorescent LC3-GFP pattern and examining the LC3-II levels. As shown in Fig. 2, C and D, pretreatment with NAC for 1 h significantly decreased the LC3-II protein level and abrogated the formation of LC3-GFP puncta induced by tetrandrine treatment. These results therefore suggest that NAC treatment can rescue cells form tetrandrine-induced autophagy and that tetrandrine-induced autophagy in HCC cells is mediated by the production of intracellular ROS.
FIGURE 2.
Intracellular ROS are involved in tetrandrine-induced autophagy in HCC cells. A, Huh7 and BEL7402 cells were treated with 5 μm tetrandrine for the indicated times and subsequently subjected to DCF fluorescence analysis to evaluate the levels of intracellular ROS (* = p < 0.05). B, Huh7 and BEL7402 cells were either incubated for 6 h with 5 μm tetrandrine or pretreated for 1 h with 20 mm NAC and then co-incubated with 20 mm NAC and 5 μm tetrandrine for 6 h. The ROS levels were measured by flow cytometry. C, the protein lysates were subjected to a Western blot analysis of LC3 in cells pretreated with NAC. D, an analysis of the percentage of cells undergoing autophagy as detected by the GFP-LC3 assay with a fluorescent microscope in cells pretreated with NAC. The columns indicate the mean percentage of autophagic cells, and the bars indicate the S.D. (* = p < 0.05).
The Increase in Intracellular ROS follows Mitochondrial Depolarization during Tetrandrine-induced Autophagy
Mitochondrial homeostasis is critical in regulating cell physiology and is the predominant source of ROS produced in response to cellular stresses (25, 26). Mitochondrial membrane depolarization leads to a reduction in the mitochondrial membrane potential (Δψm) and an increase in the permeability of the outer membrane. This results in the release of proapoptotic proteins such as CYTOCHROME c (27, 28). Therefore, we next examined whether mitochondrial events were associated with tetrandrine-induced autophagy in HCC cells. We detected changes in the Δψm by FACS analysis. As shown in Fig. 3A, a decrease in Δψm was observed in Huh7 cells following tetrandrine treatment. CYTOCHROME c was also gradually released into the cytoplasm from the mitochondria (Fig. 3B). However, when the cells were preincubated with cyclosporine A (CsA), a mitochondrial membrane potential stabilizer, the tetrandrine-induced autophagy in Huh7 cells was at least partially abrogated (Fig. 3, C and D). Next, we determined the relationship between mitochondrial depolarization and ROS levels. We found that CsA treatment blocked the production of tetrandrine-induced intracellular ROS; however, NAC treatment failed to prevent a loss in Δψm, which suggests that mitochondrial depolarization precedes the increase in intracellular ROS during tetrandrine-induced autophagy (Fig. 3, E and F). These observations suggest that the mitochondria play a regulatory role in controlling membrane potential and ROS production during tetrandrine-induced autophagy.
FIGURE 3.
The increase in intracellular ROS follows mitochondrial depolarization during tetrandrine-induced autophagy. A, Huh7 cells were treated with 5 μm tetrandrine for various lengths of time, and the mitochondrial membrane potential (Δψm) was measured by the JC-1 fluorescence intensity using flow cytometry. B, a Western blot of CYTOCHROME c from cytoplasmic protein lysates. C, the protein lysates were subjected to a Western blot analysis of LC3 in cells pretreated with 2 μm cyclosporin A (CsA) for 1 h. D, autophagic cells were detected by the GFP-LC3 fluorescence assay in cells pretreated with 2 μm CsA for 1 h. The columns indicate the mean percentage of autophagic cells, and the bars indicate the S.D. (* = p < 0.05). E, Δψm was measured in Huh7 cells pretreated with 20 mm NAC for 1 h and subsequently treated with 5 μm tetrandrine for 6 h. The bars indicate the S.D., and # indicates no significant difference between the conditions. F, the ROS levels were measured after the Huh7 cells were pretreated with 2 μm CsA for 1 h and subsequently treated with 5 μm tetrandrine for 6 h. G, the mitophagy was detected in GFP-LC3 expressing Huh7 cells treated with 5 μm tetrandrine for 24 h by labeling with MitoTracker Red dye and observing under a confocal microscope.
Many drugs like carbonyl cyanide m-chlorophenylhydrazone (29) induce a mitochondrial rupture that leads to the activation of mitophagy to eliminate the portion of the mitochondrial population that is damaged. To test whether tetrandrine-induced autophagy is mitophagy, we explored it by staining GFP-LC3-overexpressing Huh7 with MitoTracker Red dye and found that the tetrandrine-induced cell autophagy is indeed a mitophagy, because green light points of GFP-LC3 and red light points of MitoTracker had been co-located, which was observed under a confocal microscopy (Fig. 3G).
BCL-2 Family Proteins Have Not Been Involved in Tetrandrine-induced Autophagy
Many studies showed involvement of BCL-2 family proteins in autophagy (19, 30). To determine whether BCL-2 family proteins played roles in tetrandrine-induced autophagy in HCC cell, we detected BCL-2, BAX, and MCL-1 expression levels by Western blotting. As shown in Fig. 4A, no significant changes were found in tetrandrine-induced autophagy. Further investigation indicated that these proteins were overexpressed (Fig. 4B) and autophagy were measured by LC3 blotting (Fig. 4B), GFP-LC3 assay (Fig. 4C), and AVO analysis (Fig. 4D). However, results showed that these BCL-2 family proteins were not involved in tetrandrine-induced autophagy.
FIGURE 4.
BCL-2 family proteins have not been involved in tetrandrine-induced autophagy. A, Huh7 cells were treated with the indicated concentrations of tetrandrine for 24 h. Then, the cell lysates were subjected to Western blotting using BCL-2, BAX, and MCL-1 antibodies. GAPDH was used as a loading control. B, Huh7 cells were transfected with BCL-2, BAX, and MCL-1 overexpression plasmids, or their empty vectors for 24 h. After that, cells were treated with 5 μm tetrandrine for another 24 h, and then collected and subjected to Western blotting for detecting BCL-2, BAX, MCL-1, and LC3. C, Huh7 cells were planted in 12-well plates and transiently transfected with GFP-LC3 and BCL-2/BAX/MCL-1 plasmids, or their empty vectors for 24 h. Then cells were incubated within 5 μm tetrandrine for 24 h. Autophagic cells were detected by the GFP-LC3 fluorescence assay under a fluorescent microscope. D, Huh7 cells were planted in 12-well plates and transiently transfected with BCL-2, BAX, and MCL-1 overexpression plasmids, or their empty vectors for 24 h. After that, cells were incubated with 5 μm tetrandrine for 24 h, and then stained with acridine orange (1 μg/ml), and examined by a fluorescence microscope. Left, the ratio of cells containing AVOs. Columns, mean percentage of autophagy cells; bars, S.D. At least 300 cells from each treatment group were observed. OE, overexpression of BCL-2/BAX/MCL-1.
Tetrandrine-induced Autophagy in HCC Cells Is Dependent on Activating ERK
Both MAPKs, including ERK, P38 MAPK, and JNK, as well as AKT, are critical kinases that regulate a variety of biological processes, such as survival, proliferation, and differentiation, through downstream signal transduction cascades (31, 32). To determine the role of these kinases in tetrandrine-induced autophagy, we evaluated the levels and phosphorylation statuses of the MAPKs and AKT-related proteins after tetrandrine treatment. The results showed that the levels of phosphorylated ERK1/2 dramatically increased after tetrandrine treatment, but no significant changes were observed in the levels of phospho-P38 MAPK, JNK, or AKT (Fig. 5A). The protein expression levels of total ERK after tetrandrine treatment did not alter in vitro (Fig. 5A) and in vivo (Fig. 7F). Therefore, the activation of ERK was neither due to the increased level of phospho-ERK, nor to the total ones. To determine whether activation of ERK plays a role in tetrandrine-induced cell autophagy, we pretreated Huh7 and BEL7402 cells with PD98059, a common ERK inhibitor, before exposing the cells to tetrandrine. The results showed that PD98059 not only inhibited ERK activity but also decreased the levels of LC3-II and GFP-LC3 fluorescent puncta (Fig. 5, B and C).
FIGURE 5.
Tetrandrine-induced autophagy in HCC cells is dependent on ERK activation. A, Huh7 and BEL7402 cells were exposed to varying doses of tetrandrine for 24 h and subsequently blotted for the phosphorylation status of MAP kinase, AKT, and total ERK. B, Huh7 and BEL7402 cells were preincubated with PD98059 for 1 h and then treated with 5 μm tetrandrine for 24 h. A Western blot for phospho-ERK and LC3 is shown. C, autophagic cells were detected by the GFP-LC3 fluorescence assay in cells pretreated with PD98059 for 1 h and subsequently treated with 5 μm tetrandrine for 24 h. The columns indicate the mean percentage of autophagic cells, and the bars indicate the S.D. (* = p < 0.05). D, Huh7 and BEL7402 cells were treated for 24 h with 5 μm tetrandrine alone or in combination with 200 mm NAC after a 1-h NAC pretreatment. The expression of phospho-ERK was detected by Western blotting. E, the ROS levels were measured in Huh7 and BEL7402 cells pretreated with PD98059 for 1 h and subsequently treated with 5 μm tetrandrine for 6 h.
FIGURE 7.
Tetrandrine induces autophagy and suppresses Huh7 xenograft growth in vivo. Nude mice bearing Huh7 cell tumor xenografts were randomly distributed into two groups (n = 7) and administered either the vehicle only (Vehicle), 25 or 50 mg/kg of body weight tetrandrine by gavage for 20 days. A, the tumor volumes were measured every 2 days. B, after 20 days, all the mice were sacrificed, and the tumors were removed and subjected to the TUNEL assay. C, transmission electron microscopy pictures of autophagosomes. The tumor tissues were fixed with glutaraldehyde and prepared as previously described. Ultramicroscopic structures were examined using H-600 transmission electron microscopy (Hitechi, Japan). The arrows indicate autophagosomes. N means nucleus. D, a Western blot showing LC3-II levels from cellular lysates isolated from tetrandrine-treated or vehicle-treated Huh7 cell xenografts. E, the ROS levels detected by the MDA assay in tumor tissues treated with either the vehicle or 25 mg/kg of body weight tetrandrine. The values represent the mean ± S.D. (n = 7 and * = p < 0.05). F, a Western blot showing the phospho- and total ERK levels found in the lysates from the tetrandrine-treated or vehicle-treated Huh7 cell xenografts. G, the serum ALT activities were detected from the indicated dose of tetrandrine-treated nude mice.
The activation of ERK and the levels of intracellular ROS have been shown to be related in the induction of apoptosis by a variety of mechanisms (33, 34). To address if this was also the case in tetrandrine-induced autophagy, Huh7 and BEL7402 cells were treated with tetrandrine alone or pretreated with NAC for 1 h, and the phosphorylation of ERK was subsequently analyzed by Western blotting. The results indicated that the removal of the intracellular ROS by NAC (Fig. 2B) corresponded with a decreased phospho-ERK protein level after tetrandrine treatment (Fig. 5D). In contrast, inhibiting ERK activity with PD98059 did not reduce the production of tetrandrine-induced intracellular ROS (Fig. 5, B and E). Taken together, these results demonstrate that tetrandrine-induced autophagy in HCC cells is dependent on ERK activation, and the induction of intracellular ROS occurs upstream of this event.
Activation of ATG7 Contributes to Tetrandrine-induced Autophagy
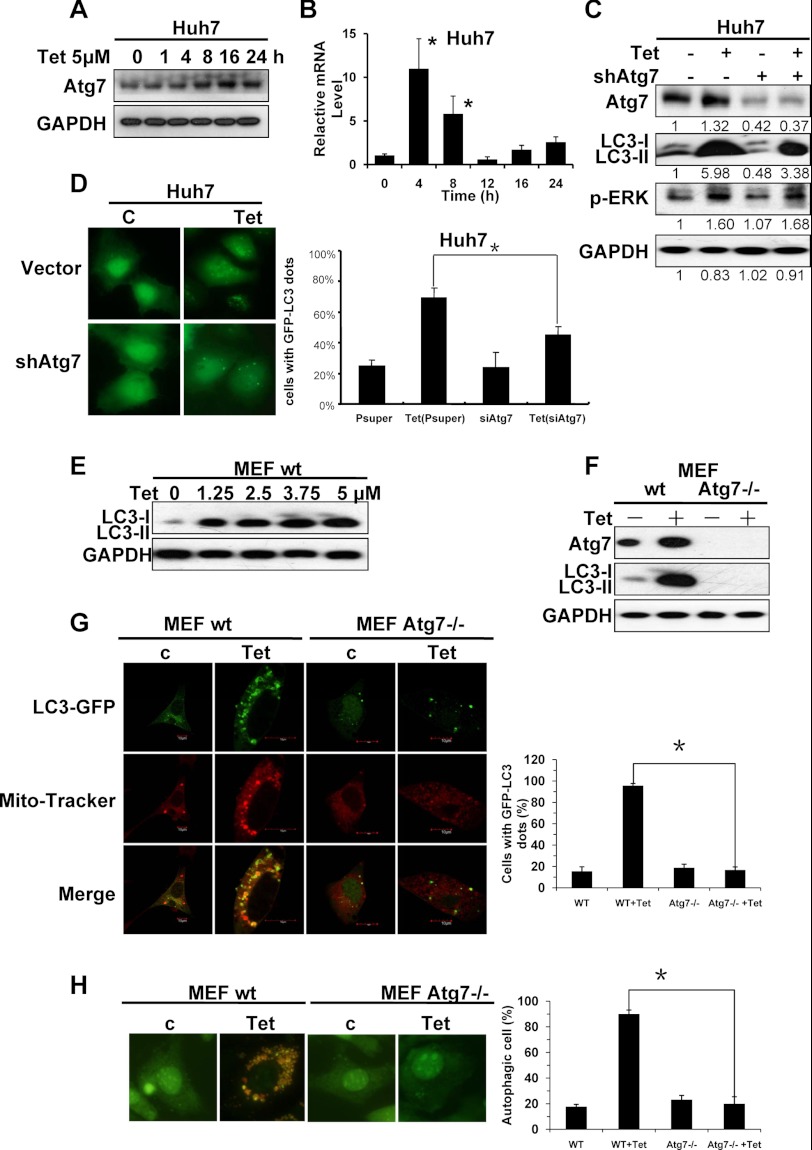
ATG7 is reported to be a core regulator of autophagy, which is required for membrane trafficking and turnover in neuronal axons. ATG7 deficiency leads to multiple cellular abnormalities (35, 36). Therefore, we examined whether tetrandrine treatment increased ATG7 protein levels in Huh7 cells. As shown in Fig. 6A, treatment with tetrandrine enhanced the protein expression level of ATG7 in a time-dependent manner. Results from real time PCR analysis indicated that ATG7 mRNA levels increased dramatically (about 10-fold) after a 4-h tetrandrine treatment, and then decreased to the non-treated level after 12 h (Fig. 6B), when the protein level of ATG7 was increased instead (Fig. 6A). It is a common view that the mRNA changes several hours earlier than the protein changes. The regulation of ATG7 after tetrandrine treatment corresponded with this point, which suggests that tetrandrine transcriptionally regulates the expression of ATG7. However, the silencing of ATG7 with shRNA reduced tetrandrine-induced LC3-II protein levels, indicating that ATG7 is also involved in facilitating tetrandrine-induced autophagy (Fig. 6C). Moreover, silencing of ATG7 also prevented accumulation of the membrane-bound form of GFP-LC3-II on autophagic vesicles (Fig. 6D). These results indicate that tetrandrine-induced autophagy in Huh7 cells is dependent on the up-regulation of ATG7 expression.
FIGURE 6.
The activation of ATG7 contributes to the tetrandrine-induced autophagy. A, Western blot of ATG7 in cells treated with 5 μm tetrandrine for the indicated times. B, real time-PCR analysis of the expression of ATG7. C, Western blotting showed the successful reduction of ATG7 after shRNA-ATG7 treatment, and the repression of LC3 and phospho-ERK levels. The Western blot densitometry analysis was performed by TotalLab Quant. D, autophagic cells were detected by the GFP-LC3 fluorescence assay in cells treated with ATG7 shRNA in the presence of 5 μm tetrandrine treatment for 24 h. The columns indicate the mean percentage of autophagic cells, and the bars indicate the S.D. (* = p < 0.05). E, LC3 expression levels were detected in MEF cells after the indicated concentrations of tetrandrine treatment for 24 h by Western blot analysis. F, MEF wild-type cells and MEF Atg7−/− knock-out cells were treated with 5 μm tetrandrine for 24 h, respectively. Then Atg7, LC3, and GAPDH expression levels were tested by Western blotting. G, the mitophagy was detected in GFP-LC3 expressing MEF wild-type and MEF Atg7−/− cells treated with 5 μm tetrandrine for 24 h by labeling with MitoTracker Red dye and observing under a confocal microscope. And autophagic cells were detected by the GFP-LC3 fluorescence assay as well. The columns indicate the mean percentage of autophagic cells, and the bars indicate the S.D. (* = p < 0.05). H, MEF wild-type cells and MEF Atg7−/− cells were treated with 5 μm tetrandrine or not for 24 h, and then stained with acridine orange (1 μg/ml), and examined by a fluorescence microscope. The ratios of cells containing AVOs were measured.
Additionally, we detected phospho-ERK after the expression level of ATG7 was knocked down by siRNA, and found no significant reduction of ERK activation (Fig. 6C). Thus, we believed that ATG7 and ERK activation are two independent pathways in tetrandrine-induced autophagy (Fig. 8E).
FIGURE 8.
Tetrandrine treatment causes ROS-dependent autophagy in C. elegans. GFP::LGG-1 positive puncta in the seam cells were counted in worms either treated with tetrandrine or dimethyl sulfoxide. The arrows shows representative GFP::LGG-1 positive puncta, which indicate preautophagosomal and autophagosomal structures. * = p < 0.05. A, images of the GFP::LGG-1 expression in the seam cells of L3 larvae worms at the indicated magnification. B, images of GFP::LGG-1 expression in the seam cells of L3 larvae worms treated with different concentrations of tetrandrine. C, the quantification of GFP::LGG-1 positive puncta in the seam cells of worms treated with tetrandrine or dimethyl sulfoxide. D, NAC treatment inhibits tetrandrine-induced autophagy in worm cells. E, putative mechanisms of tetrandrine-induced autophagy in HCC.
To further confirm that ATG7 was essential in tetrandrine-induced autophagy, we use Atg7 knock-out MEF and tested its responses to tetrandrine. As shown in Fig. 6, E and F, the protein expression levels of LC3-II and Atg7 in MEF wild-type cells were increased after tetrandrine treatment, suggesting autophagy was activated in MEF cells. However, in MEF Atg7−/− knock-out cells, LC3 were hardly detected, even after exposure for 24 h to 5 μm tetrandrine (Fig. 6F). Further experiments, including GFP-LC3 fluorescence assay, mitophagy detection (Fig. 6G), and acridine orange staining analysis (Fig. 6H), confirmed that no autophagy occurred in tetrandrine-treated MEF Atg7−/− cells. Therefore, these results indicated that Atg7 expression was essential for tetrandrine-induced autophagy.
Tetrandrine Induces Autophagy and Suppresses Huh7 Xenograft Growth in Vivo
Given the dramatic differences in physiological conditions between cancer cells in culture and cancer cells in vivo, we assessed the antitumor potential of tetrandrine in HCC cell xenografts. Nude mice bearing established Huh7 tumor xenografts were gavaged with tetrandrine (25 or 50 mg/kg of body weight) or vehicle every other day for 20 days. We found the tumor growth was inhibited by 44% with tetrandrine treatment (Fig. 7A).
Next, we wished to determine whether this reduction in tumor growth by tetrandrine treatment was facilitated by cellular apoptosis and/or autophagy in this xenograft model. The levels of apoptosis were determined by the TUNEL assay, and autophagic cells were inspected by transmission electron microscopy. As shown in Fig. 7, B and C, the levels of apoptotic cells were very low in both the vehicle and tetrandrine-treated tumor tissues. However, compared with the vehicle-treated group, autophagosomes were observed in large quantities in the tetrandrine-treated tumor cells. These observations suggest that tetrandrine treatment also induced tumor cell autophagy in vivo. Increased LC3-II protein levels were also detected by Western blotting, which further confirmed the occurrence of autophagy in tetrandrine-treated tumor tissues (Fig. 7D). Consistent with the in vitro results, tetrandrine treatment also increased the levels of phospho-ERK (Fig. 7F) and the lipid peroxidation product (MDA), which marks ROS-mediated injury in vivo (Fig. 7E). Moreover, the serum ALT activities could indicate normal (liver) cell toxicity in vivo (37). Here, we further tested serum ALT in nude mice after tetrandrine treatment, and found no ALT increase (Fig. 7G) and body weight lose (supplemental Fig. S5) after a low dose of tetrandrine treatment (25 mg/kg), which dramatically induced the cells autophagy in this concentration. Therefore, these results showed that tetrandrine had minimal damage to normal (liver) cells at low dose. Therefore, these data indicate that tetrandrine suppresses Huh7 xenograft growth in vivo by inducing autophagy in cancer cells.
Tetrandrine Treatment Causes ROS-dependent Autophagy in Caenorhabditis elegans
C. elegans has been extensively used as a model organism to address many biological questions, including screening anticancer agents. To determine whether tetrandrine could also induce autophagy in an intact organism, adult C. elegans were treated with 25, 50, or 100 μm tetrandrine for 24 h, and autophagy was detected by observing the cellular localization of the GFP::LGG-1 fusion protein expressed by the DA2123 strain. LGG-1 is a homologue of yeast atg8 and the mammalian LC3 proteins that are specific markers for autophagosomes. As shown in Fig. 8A, normal worms without any treatment resulted in only a few positive GFP::LGG-1 puncta in the seam cells. However, 24 h of treatment with tetrandrine resulted in the formation of punctate GFP::LGG-1 foci, which represent autophagosomal structures, in the worm seam cells (Fig. 8, B and C). Moreover, tetrandrine-induced autophagy in the worm cells was significantly inhibited when the worm was pretreated with the free radical scavenger NAC (Fig. 8D). These results in C. elegans provide further evidence for the in vivo induction of autophagy by tetrandrine treatment in an intact organism.
DISCUSSION
To follow our previously reported observation that tetrandrine treatment resulted in apoptosis in human hepatocellular carcinoma cells (9), here, we show that tetrandrine treatment also induces autophagy in HCC cells at a low concentration. Twenty-four hour tetrandrine treatment at a 30 μm concentration induced a considerable amount of apoptosis in HCC cells. Although 5 μm tetrandrine induced a very slight apoptosis (9), most cells underwent autophagy. At this low treatment concentration, cells underwent autophagy starting after 4 h of treatment (Fig. 1). Because apoptosis is the most common mechanism for targeted chemotherapies, the significance of autophagy in antitumor therapeutics has not been given considerable attention. Autophagy can be a double-edged sword in tumor oncogenesis and anticancer therapies (38, 39). When cells are subjected to adverse stress, such as nutrient starvation or chemotherapeutic agents, cancer cells may trigger the autophagic response that degrades unnecessary molecules to promote cellular survival (40, 41). On the other hand, treatment with many anticancer agents, such as rapamycin or ionizing radiation, has been shown to induce autophagic cancer cell death, suggesting that autophagy might be a crucial mechanism for cancer treatment (42–44). HCC cells treated with 5 μm tetrandrine for 5 days underwent autophagic death in vitro (data not show), and Huh7 xenograft growth was suppressed in vivo with tetrandrine treatment (Fig. 7). These results suggest that tetrandrine may prove useful as an anticancer therapy. Additionally, as different concentrations of tetrandrine induce apoptosis or autophagy in HCC cells, tetrandrine may work by multiple mechanisms to inhibit oncogenesis.
ROS are important mediators that regulate both cellular survival and cell death in response to different stimuli, such as starvation, ionizing radiation, senescence, or chemotherapeutic agents (45–47). In our study, tetrandrine treatment significantly induced intracellular ROS production, which has been suggested to be essential for both autophagy and apoptosis (Fig. 2). Moreover, tetrandrine-induced autophagy and apoptosis in HCC cells were facilitated by the mitochondria-dependent pathway. Loss of Δψm and the release of CYTOCHROME c into the cytoplasm demonstrated an increase in the permeability of the outer mitochondrial membrane and mitochondrial dysfunction (Fig. 3). CYTOCHROME c release from the mitochondria, which triggers caspase activation, has been reported to be an important event in cellular apoptosis induced by apoptotic stimuli such as chemotherapeutic agents (48–50). In the present study, we observed that tetrandrine induced the release of CYTOCHROME c but did not cause the activation of the CASPASEs or apoptosis (Figs. 1A, 3B, and supplemental Fig. S4). Considering the possibility of mitophagy (Fig. 3G), it is probable that a low dose of tetrandrine triggered induction of mitophagy and selective removal of damaged mitochondria. In the process of mitophagy, permeabilization of mitochondria could alter and release of CYTOCHROME c could occur, but cells had not undergone CASPASEs activation and apoptotic cell death. This mechanism above had been reported recently (51, 52).
The ERK signaling pathway can be activated in response to a diverse range of extracellular stimuli, including mitogens, growth factors, and cytokines, as well as immediate extracellular stresses, such as radiation or chemotherapeutic agents (53–55). We previously demonstrated that treatment with 30 μm tetrandrine for 24 h repressed ERK activation and led to apoptosis in cancer cells (Fig. 5). These results are consistent with reports demonstrating that the inhibition of ERK promoted autophagic cell death. In the present study, we found that treatment with 5 μm tetrandrine for 12 h induced ERK activation, and tetrandrine-induced autophagy in HCC cells was at least partially dependent on ERK activity. We are currently investigating the possibility that low-dose tetrandrine treatment activates ERK as a response to cellular stress, and ERK kinase activation may serve as a switch that regulates different cell death pathways induced by tetrandrine treatment.
Many evidences suggested that ROS contribute to ERK activation and the regulatory mechanism is multiple (56, 57). It has been reported that ROS regulated the Ras/Raf/ERK signaling pathway to modulate downstream AP-1 binding gene expression (58). Some researchers believe that ERK activation offers a survival advantage, but growing evidence suggests it also contributes to the other side, cell death. In our present study, ERK is activated after low dose tetrandrine treatment to trigger autophagy (probably mitophagy) to protect cells from apoptotic cell death and thus survive, because our previous study found high dose tetrandrine decreased phospho-ERK and induced apoptosis (9). Next, we will explore by which means ROS regulated ERK activation.
ATG5 and ATG7 were both essential for autophagy. In this research, we found that protein levels of both ATG5 (data not shown) and ATG7 (Fig. 6A) were increased after tetrandrine treatment. As shown in Fig. 6, tetrandrine-induced autophagy in Huh7 and MEF cells is dependent on the up-regulation of ATG7. However, the detailed mechanism of this transcriptional up-regulation will also require further investigation. We knocked down ATG5 by siRNA as well; however, no significant reduction of autophagy was found (data not shown).
In the Huh7 xenograft model, we administrated a relatively low dose of tetrandrine (25 or 50 mg/kg of body weight) to animals because a high dose of tetrandrine can induce apoptosis in vivo. We also administrated 5 mg/kg of body weight tetrandrine to nude mice, and the results indicated that this concentration of tetrandrine had almost no effect on tumor growth and cell autophagy (data not shown). However, tetrandrine treatment induced autophagy in a considerable number of worm muscle cells within the intact organism. Moreover, ROS were also identified to play a vital role in tetrandrine-induced autophagy in vivo and in C. elegans models.
In vitro, we examined the effects of tetrandrine on the normal liver cell line (L02) and found neither autophagy (Fig. 1B) nor apoptosis (data not show), and in vivo, we found no ALT increase (Fig. 7G) nor body weight lose (supplemental Fig. S5), after tetrandrine treatment at a low dose (25 mg/kg). These results indicated low toxicity of tetrandrine as a possible anti-tumor drug. Tumor cells usually had many different properties from normal cells and thus became sensitive to anti-tumor drugs, whose reason is very complex and is related with many issues. Whether tetrandrine acted on tumor cells only and the mechanism implicated were under further studied.
In conclusion, our results show that tetrandrine is a promising chemotherapeutic agent with a variety of anticancer effects. Tetrandrine treatment induced cancer cells to undergo autophagy at a low dose and apoptosis at a high dose. The production of intracellular ROS, activation of the MAP kinase ERK, and up-regulation of ATG7 were essential to the induction of tetrandrine-induced autophagy in HCC cells (Fig. 8E). These findings suggest that tetrandrine may be a potential clinical candidate for the treatment of HCC. This study also demonstrates that some anticancer agents can work by multiple mechanisms.
Supplementary Material
Acknowledgments
We thank Dr. Masaaki Komatsu (Tokyo Metropolitan Institute of Medical Science, Japan) for the gift of Atg7 knock-out MEF cells and Dr. Li Yu (Tsinghua University, China) for preparing these cells.
This work was supported by National Basic Research Program of China Grant 2010CB529800, National Nature Science Foundation of China Grant 81273540, The Chinese 111 project (B06018), and the major scientific and technological special project for “Significant Creation of New Drugs” Grants 2011ZX09102-001-32 and 2011ZX09401-302-4.

This article contains supplemental Figs. S1–S5.
- HCC
- human hepatocellular carcinoma
- LC3
- light chain 3
- AVO
- acidic autophagolysosome vacuoles
- ROS
- reactive oxygen species
- NAC
- N-acetyl-l-cysteine
- CsA
- cyclosporine A
- ALT
- alanine transaminase
- MDA
- malondialdehyde.
REFERENCES
- 1. Llovet J. M., Burroughs A., Bruix J. (2003) Hepatocellular carcinoma. Lancet 362, 1907–1917 [DOI] [PubMed] [Google Scholar]
- 2. Zender L., Spector M. S., Xue W., Flemming P., Cordon-Cardo C., Silke J., Fan S. T., Luk J. M., Wigler M., Hannon G. J., Mu D., Lucito R., Powers S., Lowe S. W. (2006) Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125, 1253–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L., Cao Y., Chen C., Zhang X., McNabola A., Wilkie D., Wilhelm S., Lynch M., Carter C. (2006) Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 66, 11851–11858 [DOI] [PubMed] [Google Scholar]
- 4. Wu J. M., Chen Y., Chen J. C., Lin T. Y., Tseng S. H. (2010) Tetrandrine induces apoptosis and growth suppression of colon cancer cells in mice. Cancer Lett. 287, 187–195 [DOI] [PubMed] [Google Scholar]
- 5. Shen Y. C., Chou C. J., Chiou W. F., Chen C. F. (2001) Anti-inflammatory effects of the partially purified extract of radix Stephaniae tetrandrae. Comparative studies of its active principles tetrandrine and fangchinoline on human polymorphonuclear leukocyte functions. Mol. Pharmacol. 60, 1083–1090 [PubMed] [Google Scholar]
- 6. Pang L., Hoult J. R. (1997) Cytotoxicity to macrophages of tetrandrine, an antisilicosis alkaloid, accompanied by an overproduction of prostaglandins. Biochem. Pharmacol. 53, 773–782 [DOI] [PubMed] [Google Scholar]
- 7. Lee J. H., Kang G. H., Kim K. C., Kim K. M., Park D. I., Choi B. T., Kang H. S., Lee Y. T., Choi Y. H. (2002) Tetrandrine-induced cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Int. J. Oncol. 21, 1239–1244 [PubMed] [Google Scholar]
- 8. Lin S. T., Wang Y., Xue Y., Feng D. C., Xu Y., Xu L. Y. (2008) Tetrandrine suppresses LPS-induced astrocyte activation via modulating IKKs-IκBα-NF-κB signaling pathway. Mol. Cell Biochem. 315, 41–49 [DOI] [PubMed] [Google Scholar]
- 9. Liu C., Gong K., Mao X., Li W. (2011) Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma. Int. J. Cancer 129, 1519–1531 [DOI] [PubMed] [Google Scholar]
- 10. Chen Y., Chen J. C., Tseng S. H. (2009) Tetrandrine suppresses tumor growth and angiogenesis of gliomas in rats. Int. J. Cancer 124, 2260–2269 [DOI] [PubMed] [Google Scholar]
- 11. Jang B. C., Lim K. J., Paik J. H., Cho J. W., Baek W. K., Suh M. H., Park J. B., Kwon T. K., Park J. W., Kim S. P., Shin D. H., Song D. K., Bae J. H., Mun K. C., Suh S. I. (2004) Tetrandrine-induced apoptosis is mediated by activation of caspases and PKC-δ in U937 cells. Biochem. Pharmacol. 67, 1819–1829 [DOI] [PubMed] [Google Scholar]
- 12. Ghobrial I. M., Witzig T. E., Adjei A. A. (2005) Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 55, 178–194 [DOI] [PubMed] [Google Scholar]
- 13. Klionsky D. J., Emr S. D. (2000) Autophagy as a regulated pathway of cellular degradation. Science 290, 1717–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mariño G., Salvador-Montoliu N., Fueyo A., Knecht E., Mizushima N., López-Otín C. (2007) Tissue-specific autophagy alterations and increased tumorigenesis in mice deficient in Atg4C/autophagin-3. J. Biol. Chem. 282, 18573–18583 [DOI] [PubMed] [Google Scholar]
- 15. Jiang M., Jerome W. G., Hayward S. W. (2010) Autophagy in nuclear receptor PPARγ-deficient mouse prostatic carcinogenesis. Autophagy 6, 175–176 [DOI] [PubMed] [Google Scholar]
- 16. Gozuacik D., Kimchi A. (2004) Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23, 2891–2906 [DOI] [PubMed] [Google Scholar]
- 17. Shao Y., Gao Z., Marks P. A., Jiang X. (2004) Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc. Natl. Acad. Sci. U.S.A. 101, 18030–18035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y., Azad M. B., Gibson S. B. (2009) Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 16, 1040–1052 [DOI] [PubMed] [Google Scholar]
- 19. Pattingre S., Tassa A., Qu X., Garuti R., Liang X. H., Mizushima N., Packer M., Schneider M. D., Levine B. (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122, 927–939 [DOI] [PubMed] [Google Scholar]
- 20. Jacobson M. D. (1996) Reactive oxygen species and programmed cell death. Trends Biochem. Sci. 21, 83–86 [PubMed] [Google Scholar]
- 21. Gong K., Li W. (2011) Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species. A potential new treatment for hepatocellular carcinoma. Free Radic. Biol. Med. 51, 2259–2271 [DOI] [PubMed] [Google Scholar]
- 22. Zhang M., Lee S. J., An C., Xu J. F., Joshi B., Nabi I. R., Choi A. M., Jin Y. (2011) Caveolin-1 mediates Fas-BID signaling in hyperoxia-induced apoptosis. Free Radic. Biol. Med. 50, 1252–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuo C. C., Liu T. W., Chen L. T., Shiah H. S., Wu C. M., Cheng Y. T., Pan W. Y., Liu J. F., Chen K. L., Yang Y. N., Chen S. N., Chang J. Y. (2011) Combination of arsenic trioxide and BCNU synergistically triggers redox-mediated autophagic cell death in human solid tumors. Free Radic. Biol. Med. 51, 2195–2209 [DOI] [PubMed] [Google Scholar]
- 24. Xie C. M., Chan W. Y., Yu S., Zhao J., Cheng C. H. (2011) Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic. Biol. Med. 51, 1365–1375 [DOI] [PubMed] [Google Scholar]
- 25. Mailloux R. J., Harper M. E. (2011) Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 51, 1106–1115 [DOI] [PubMed] [Google Scholar]
- 26. Murphy M. P. (2009) How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grimm S., Brdiczka D. (2007) The permeability transition pore in cell death. Apoptosis 12, 841–855 [DOI] [PubMed] [Google Scholar]
- 28. Galluzzi L., Zamzami N., de La Motte Rouge T., Lemaire C., Brenner C., Kroemer G. (2007) Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis 12, 803–813 [DOI] [PubMed] [Google Scholar]
- 29. Narendra D., Tanaka A., Suen D. F., Youle R. J. (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tracy K., Dibling B. C., Spike B. T., Knabb J. R., Schumacker P., Macleod K. F. (2007) BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol. Cell Biol. 27, 6229–6242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang L., Karin M. (2001) Mammalian MAP kinase signalling cascades. Nature 410, 37–40 [DOI] [PubMed] [Google Scholar]
- 32. Ibáñez A., Río P., Casado J. A., Bueren J. A., Fernández-Luna J. L., Pipaón C. (2009) Elevated levels of IL-1β in Fanconi anaemia group A patients due to a constitutively active phosphoinositide 3-kinase-Akt pathway are capable of promoting tumor cell proliferation. Biochem. J. 422, 161–170 [DOI] [PubMed] [Google Scholar]
- 33. Park K. R., Nam D., Yun H. M., Lee S. G., Jang H. J., Sethi G., Cho S. K., Ahn K. S. (2011) β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 312, 178–188 [DOI] [PubMed] [Google Scholar]
- 34. Rosato R. R., Grant S. (2003) Histone deacetylase inhibitors in cancer therapy. Cancer Biol. Ther. 2, 30–37 [DOI] [PubMed] [Google Scholar]
- 35. Yue Z., Wang Q. J., Komatsu M. (2008) Neuronal autophagy. Going the distance to the axon. Autophagy 4, 94–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Komatsu M., Wang Q. J., Holstein G. R., Friedrich V. L., Jr., Iwata J., Kominami E., Chait B. T., Tanaka K., Yue Z. (2007) Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc. Natl. Acad. Sci. U.S.A. 104, 14489–14494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balagué C., Zhou J., Dai Y., Alemany R., Josephs S. F., Andreason G., Hariharan M., Sethi E., Prokopenko E., Jan H. Y., Lou Y. C., Hubert-Leslie D., Ruiz L., Zhang W. W. (2000) Sustained high-level expression of full-length human factor VIII and restoration of clotting activity in hemophilic mice using a minimal adenovirus vector. Blood 95, 820–828 [PubMed] [Google Scholar]
- 38. de Bruin E. C., Medema J. P. (2008) Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat. Rev. 34, 737–749 [DOI] [PubMed] [Google Scholar]
- 39. Kondo Y., Kanzawa T., Sawaya R., Kondo S. (2005) The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 5, 726–734 [DOI] [PubMed] [Google Scholar]
- 40. Bursch W., Karwan A., Mayer M., Dornetshuber J., Fröhwein U., Schulte-Hermann R., Fazi B., Di Sano F., Piredda L., Piacentini M., Petrovski G., Fésüs L., Gerner C. (2008) Cell death and autophagy. Cytokines, drugs, and nutritional factors. Toxicology 254, 147–157 [DOI] [PubMed] [Google Scholar]
- 41. Stipanuk M. H. (2009) Macroautophagy and its role in nutrient homeostasis. Nutr. Rev. 67, 677–689 [DOI] [PubMed] [Google Scholar]
- 42. Kimmelman A. C. (2011) The dynamic nature of autophagy in cancer. Genes Dev. 25, 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amelio I., Melino G., Knight R. A. (2011) Cell death pathology. Cross-talk with autophagy and its clinical implications. Biochem. Biophys. Res. Commun. 414, 277–281 [DOI] [PubMed] [Google Scholar]
- 44. Beugnet A., Tee A. R., Taylor P. M., Proud C. G. (2003) Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem. J. 372, 555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gutiérrez-Uzquiza ÁA., Arechederra M., Bragado P., Aguirre-Ghiso J. A., Porras A. (2012) p38α mediates cell survival in response to oxidative stress via induction of antioxidant genes. Effect on the p70S6K pathway. J. Biol. Chem. 287, 2632–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Z. Y., Yang Y., Ming M., Liu B. (2011) Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem. Biophys. Res. Commun. 414, 5–8 [DOI] [PubMed] [Google Scholar]
- 47. Scherz-Shouval R., Elazar Z. (2011) Regulation of autophagy by ROS:physiology and pathology. Trends Biochem. Sci. 36, 30–38 [DOI] [PubMed] [Google Scholar]
- 48. Allan L. A., Clarke P. R. (2009) Apoptosis and autophagy. Regulation of caspase-9 by phosphorylation. FEBS J. 276, 6063–6073 [DOI] [PubMed] [Google Scholar]
- 49. Caroppi P., Sinibaldi F., Fiorucci L., Santucci R. (2009) Apoptosis and human diseases. Mitochondrion damage and lethal role of released cytochrome c as proapoptotic protein. Curr. Med. Chem. 16, 4058–4065 [DOI] [PubMed] [Google Scholar]
- 50. Lin M. L., Lu Y. C., Chung J. G., Li Y. C., Wang S. G., Ng S., Wu C. Y., Su H. L., Chen S. S. (2010) Aloe-emodin induces apoptosis of human nasopharyngeal carcinoma cells via caspase-8-mediated activation of the mitochondrial death pathway. Cancer Lett. 291, 46–58 [DOI] [PubMed] [Google Scholar]
- 51. Quinsay M. N., Lee Y., Rikka S., Sayen M. R., Molkentin J. D., Gottlieb R. A., Gustafsson A. B. (2010) Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism. J. Mol. Cell Cardiol 48, 1146–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gottlieb R. A., Carreira R. S. (2010) Autophagy in health and disease. 5. Mitophagy as a way of life. Am. J. Physiol. Cell Physiol. 299, C203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kanwar J. R., Kamalapuram S. K., Kanwar R. K. (2011) Targeting survivin in cancer. The cell signaling perspective. Drug Discov. Today 16, 485–494 [DOI] [PubMed] [Google Scholar]
- 54. Gailhouste L., Ezan F., Bessard A., Frémin C., Rageul J., Langouët S., Baffet G. (2010) RNAi-mediated MEK1 knock-down prevents ERK1/2 activation and abolishes human hepatocarcinoma growth in vitro and in vivo. Int. J. Cancer 126, 1367–1377 [DOI] [PubMed] [Google Scholar]
- 55. Balmanno K., Chell S. D., Gillings A. S., Hayat S., Cook S. J. (2009) Intrinsic resistance to the MEK1/2 inhibitor AZD6244 (ARRY-142886) is associated with weak ERK1/2 signalling and/or strong PI3K signalling in colorectal cancer cell lines. Int. J. Cancer 125, 2332–2341 [DOI] [PubMed] [Google Scholar]
- 56. Sung B., Ravindran J., Prasad S., Pandey M. K., Aggarwal B. B. (2010) Gossypol induces death receptor-5 through activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer cells to TRAIL. J. Biol. Chem. 285, 35418–35427 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57. Rygiel T. P., Mertens A. E., Strumane K., van der Kammen R., Collard J. G. (2008) The Rac activator Tiam1 prevents keratinocyte apoptosis by controlling ROS-mediated ERK phosphorylation. J. Cell Sci. 121, 1183–1192 [DOI] [PubMed] [Google Scholar]
- 58. Cheng T. H., Shih N. L., Chen S. Y., Loh S. H., Cheng P. Y., Tsai C. S., Liu S. H., Wang D. L., Chen J. J. (2001) Reactive oxygen species mediate cyclic strain-induced endothelin-1 gene expression via Ras/Raf/extracellular signal-regulated kinase pathway in endothelial cells. J. Mol. Cell Cardiol 33, 1805–1814 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.