Background: Cytosolic pH blocks actin disassembly in vitro.
Results: CAP actively participates in actin disassembly, augmenting cofilin activity across the physiological pH range.
Conclusion: CAP is an important actin disassembly factor necessary to drive actin turnover under physiological conditions.
Significance: We identify a new mechanism through which CAP affects fast actin dynamics even at acidic pH values found in cells.
Keywords: Actin, ATP, Cofilin, Cytoskeleton, pH Regulation, Actin, Cofilin, Cyclase-associated Protein (CAP), Cytoskeletal Dynamics, Intracellular pH
Abstract
Fast actin depolymerization is necessary for cells to rapidly reorganize actin filament networks. Utilizing a Listeria fluorescent actin comet tail assay to monitor actin disassembly rates, we observed that although a mixture of actin disassembly factors (cofilin, coronin, and actin-interacting protein 1 is sufficient to disassemble actin comet tails in the presence of physiological G-actin concentrations this mixture was insufficient to disassemble actin comet tails in the presence of physiological F-actin concentrations. Using biochemical complementation, we purified cyclase-associated protein (CAP) from thymus extracts as a factor that protects against the inhibition of excess F-actin. CAP has been shown to participate in actin dynamics but has been thought to act by liberating cofilin from ADP·G-actin monomers to restore cofilin activity. However, we found that CAP augments cofilin-mediated disassembly by accelerating the rate of cofilin-mediated severing. We also demonstrated that CAP acts directly on F-actin and severs actin filaments at acidic, but not neutral, pH. At the neutral pH characteristic of cytosol in most mammalian cells, we demonstrated that neither CAP nor cofilin are capable of severing actin filaments. However, the combination of CAP and cofilin rapidly severed actin at all pH values across the physiological range. Therefore, our results reveal a new function for CAP in accelerating cofilin-mediated actin filament severing and provide a mechanism through which cells can maintain high actin turnover rates without having to alkalinize cytosol, which would affect many biochemical reactions beyond actin depolymerization.
Introduction
Cells organize space, generate force, and respond to extracellular conditions through actin-dependent processes that necessitate both fast actin polymerization and fast depolymerization (1–3) with little spatial separation between assembly and disassembly (4). In vivo actin turnover is fast (5, 6) with a half-life on the order of tens of seconds (7, 8), much faster than has been typically reproduced in vitro (9). Actin-depolymerizing factor/cofilin, more specifically cofilin (10), has been shown to be necessary to disassemble physiological branched actin arrays in cell extracts (6, 11) and actin filaments in pure solution (12) but is insufficient to reconstitute physiological actin disassembly both qualitatively in terms of the ability to function in the presence of high concentrations of actin and quantitatively in terms of observed disassembly rate. Physiological actin disassembly is both fast and robust to high concentrations of monomeric (globular (G-)2) actin and polymeric (filamentous (F-)) actin.
The high concentrations of polymerizable G-actin maintained by the cell (5–20 μm (13)) help drive actin assembly, but this creates a barrier to actin disassembly as free filament ends will tend to grow and not shrink (9). Recently, coronin and actin-interacting protein 1 (AIP1) were identified as factors necessary for actin depolymerization in the presence of physiological concentrations of G-actin (13). Physiological concentrations of F-actin are also high (100–330 μm (14)), posing a stoichiometric problem in which estimated cellular concentrations of each actin disassembly factor (cofilin, 3–30 μm (14); coronin, 1.4–40 μm (13, 15); AIP1, 0.4–0.5 μm (13, 16)) are significantly lower than F-actin concentrations. Although coronin and AIP1 are sufficient to relieve the inhibition of cofilin-mediated actin depolymerization in the presence of physiological concentrations of G-actin, the potential problems associated with physiological concentrations of F-actin have not yet been addressed.
Listeria monocytogenes utilizes cellular machinery to move through cytoplasm by assembling a branched actin network or “comet tail” behind it (17). Host factors are also required for comet tail disassembly, the kinetics of which are similar both in infected cells (18) and in vitro when treated with cell extract (6, 8). Thus, Listeria comet tails offer a physiological actin substrate for the in vitro study of cellular mechanisms of actin filament disassembly. Importantly, comet tail assembly can be experimentally separated from disassembly, and we have taken advantage of this in the past to identify several factors necessary for actin comet tail disassembly under the physiological challenge of high actin monomer concentrations. We have now extended the Listeria system to identify additional factors necessary for actin disassembly when the reaction is challenged by high concentrations of actin polymer.
EXPERIMENTAL PROCEDURES
Proteins and Reagents
All reagents unless otherwise noted were from Sigma-Aldrich. Rhodamine and Alexa Fluor dyes were from Invitrogen. Rabbit skeletal muscle actin, bovine coronin-1A, and AIP1 were purified as described previously (13). Recombinant human cofilin-1 was purified from 1 liter of Escherichia coli. Cells were lysed in 10 mm Tris at pH 8.0, 1 mm EDTA, 0.1 mm PMSF, 2 mm β-mercaptoethanol. The lysate was clarified by centrifugation at 30,000 ×g for 1 h and then passed through a 10-ml Q HP column (GE Healthcare) equilibrated in 10 mm Tris, pH 8.0, 2 mm β-mercaptoethanol. Cofilin flows through this column. The Q flow-through was dialyzed into 10 mm MES, pH 6.0, 2 mm β-mercaptoethanol and applied to a 5-ml S HP column (GE Healthcare) equilibrated in the same buffer. Cofilin was eluted from the column with a 100-ml gradient to 400 mm NaCl in the same buffer. Cofilin eluted from the column at 210 mm NaCl and was typically the only protein remaining at this stage of purification. Cofilin-positive fractions from S were pooled, concentrated using a centrifugal ultrafiltration device and applied to a Superdex 200 16/60 gel filtration column (GE Healthcare) equilibrated in Assay Buffer (100 mm HEPES, 50 mm KCl, 1 mm MgCl2, 1 mm EGTA, 2 mm ATP, pH 7.8). Cofilin eluted at 20 kDa. Filamin was purified from chicken gizzard as described previously (19). Human cyclase-associated protein (CAP) was recombinantly expressed in Rosetta E. coli (EMD) and purified using a nickel-nitrilotriacetic acid-agarose column (Qiagen). CAP expression was induced with 0.1 mm isopropyl β-d-1-thiogalactopyranoside for 7 h at room temperature, and CAP was further purified on a Mono Q column (GE Healthcare) at pH 7.8, eluting at ∼220 mm NaCl.
Standard buffers consisted of 5 mm Tris, 0.2 mm CaCl2, 0.2 mm ATP, pH 8.0 (G Buffer) and Assay Buffer. When used to store proteins, 2 mm β-mercaptoethanol or DTT was added. Adjustments to Assay Buffer are noted, and any version of Assay Buffer was converted to Photo Buffer by the addition of 0.2 mm 6-hydroxy-2,5,7,8-teramethylchroman-2-carboxylic acid (Trolox), 0.4 mg/ml glucose oxidase, and 2.25 mg/ml glucose. Photo Buffers were used within 2 h of initial preparation.
Protein Labeling
Cofilin was labeled with maleimide-activated tetramethylrhodamine by incubation with a 2:1 molar excess of dye for 45 min at room temperature in 20 mm HEPES, pH 7.2, 50 mm KCl, 2 mm tris(2-carboxyethyl)phosphine hydrochloride. Labeling was stopped by addition of 2 mm DTT, and unreacted dye was removed by centrifugation for 1 h at 200,000 × g (k factor, 33.8) at 4 °C followed by dialysis against 20 mm HEPES, pH 7.2 Assay Buffer to remove excess dye. Actin was fluorescently labeled as described previously (20). Briefly, actin was labeled on lysine residues by treating F-actin with N-hydroxysuccinimide-activated fluorophores at approximately stoichiometric ratios for 1 h at room temperature before stopping the reaction with 50 mm Tris buffer and extensively dialyzing against G Buffer with 2 mm β-mercaptoethanol.
DNA Constructs
Human CAP1 cDNA was obtained from Origene and subcloned into the BamHI and SalI sites in the bacterial expression vector pET30a+ (EMD) for expression in Rosetta cells (EMD) with an N-terminal His6 tag.
Purification of CAP from Bovine Thymus
100 g of frozen bovine calf thymus was thawed by placing it in 200 ml of Buffer A (5 mm Tris, pH 7.4, 25 mm NaCl, 1 mm EGTA, 2 mm MgCl2, 14 mm β-mercaptoethanol) at room temperature for 30 min. All subsequent procedures were performed at 4 °C. The tissue was cut into small pieces and transferred to 200 ml of fresh Buffer A pre-equilibrated to 4 °C and homogenized in a Waring-type blender. The homogenate was centrifuged for 30 min at 12,000 × g, and insoluble material was discarded. Polyethyleneimine was added to the supernatant to a final concentration of 0.05% and stirred for 30 min at 4 °C. The slurry was centrifuged at 12,000 × g for 30 min, and the pellet was discarded. The supernatant was centrifuged at 150,000g (k factor, 133) for 90 min. The supernatant was mixed with 100 ml of DE52 (Whatman), stirred for 60 min, and allowed to settle. The liquid was decanted off the beads, and the beads were resuspended in 100 ml of Buffer A. The slurry was poured into a column, and the flow-through was combined with the decanted solution to generate the DE52 flow-through fraction. The disassembly factors flowed through the column under these conditions.
1 m Pipes, pH 6.8 was added to the DE52 flow-through fraction to a final concentration of 40 mm. The extract was then applied to a 70-ml S HP column (GE Healthcare) equilibrated in 10 mm Pipes, pH 6.8, 0.1 mm DTT (Buffer B). The column was washed with 100 ml of 10 mm Pipes, pH 6.8, and this wash was combined with the S flow-through. The pH 6.8 S flow-through fraction was dialyzed overnight against 20 liters of 10 mm MES, pH 6.0, 25 mm NaCl, 0.1 mm DTT (Buffer C) and centrifuged for 1 h at 100,000 × g before being applied to a 70-ml S HP column equilibrated in the same buffer. The column was eluted with a 700-ml gradient to 500 mm NaCl in Buffer C. Activity eluted near 180 mm NaCl. Active fractions were dialyzed into Buffer B and applied to a 20 ml hydroxyapatite column (Bio-Rad) equilibrated in Buffer B. The column was eluted with a 300 ml gradient to 300 mm sodium phosphate. Activity eluted near 130 mm sodium phosphate. Active fractions were combined, concentrated in a centrifugal concentrating device, and applied to a 16/60 Superdex S200 gel filtration column (GE Healthcare) equilibrated in Buffer A with 150 mm NaCl. Active fractions eluted at apparent molecular masses between 500 and 800 kDa. Active fractions from the gel filtration were applied to a 5-ml heparin column (GE Healthcare) equilibrated in Buffer A with 150 mm NaCl and eluted with a 100-ml gradient to 500 mm NaCl in Buffer A. Activity eluted near 300 mm NaCl. At this point, only two bands remained on the gel, and both were excised for identification by mass spectrometry.
F-actin Co-sedimentation
In each experiment, either actin or CAP was held constant, and the other was varied; both were run together using SDS-PAGE (10%). Actin was polymerized in 10 mm HEPES, pH 7.4 Assay Buffer to which an appropriate amount of CAP and/or Assay Buffer was added. Either actin was held constant at 10 μm and CAP was varied, or CAP was held constant at 3 μm, and actin was varied. In either case, actin was allowed to polymerize for 1 h before the addition of CAP after which 20 min was allowed for binding and to re-establish steady state. Of the final 40-μl volume, 20 μl was taken immediately (uncentrifuged), whereas the remaining 20 μl was centrifuged in a Beckman TLA 100 rotor at 350,000 × g (k factor, 8.1) for 20 min at 4 °C, providing a supernatant fraction and after addition of an equal volume of Assay Buffer and resuspension a pellet fraction. Equal volumes of each sample were then separated using SDS-PAGE. Densitometry of Coomassie-stained bands was accomplished using NIH ImageJ software and used to derive a dissociation constant by the method of Wachsstock et al. (21).
L. monocytogenes Comet Tail Microscopy
Listeria actin comet tails were assembled in the presence of HEK 293 cell extract in homemade perfusion chambers as described previously (13). After rinsing, the chamber was filled with actin disassembly factors under the indicated conditions, and a fluorescence time lapse sequence was acquired to obtain the fluorescence intensity decay and to derive an apparent actin koff as described previously (13). Excess F-actin, if supplied in a given experiment, was unlabeled and prepolymerized in Assay Buffer at least 45 min before use. Actin disassembly factors were added to a mixture containing F-actin at the indicated final concentrations, and this mixture was applied to chambers 2 min thereafter. Visualization was achieved with a Zeiss 20× air objective (numerical aperture, 0.8) on a Zeiss Imager M1 stand and recorded with a Hamamatsu ORCA-ER charge-coupled device camera driven with Zeiss Axiovision 4.7 software.
Cofilin Loading
Utilizing L. monocytogenes actin comet tails as a fluorescent actin substrate, we preincubated the comet tails with either CAP or coronin for 5 min before rinsing twice with 1.5 chamber volumes of Assay Buffer and perfusing in fluorescently labeled cofilin. After rinsing and incubation with zero length cross-linker 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (100 nm; 30 s), chambers were rinsed twice before being perfused with Photo Buffer and visualized as described above except with a Zeiss 40× air objective (numerical aperture, 0.75), taking five images of each chamber (center and one from each quadrant). Images were recorded in both the cofilin and actin channels and corrected separately for background, and finally cofilin intensity was normalized to actin intensity to control for variance between individual chambers, fields, and comet tails.
Single Actin Filament Microscopy
Actin filaments were assembled in perfusion chambers as described previously (21). Briefly, actin-bundling protein filamin was adsorbed onto the glass surface before blocking the glass with 10 mm HEPES, pH 7.2 Assay Buffer containing 10 mg/ml bovine casein, 0.2% Tween 20, and 0.05% Pluronic F-127. A 4 μm solution of Alexa Fluor 647-G-actin (20% labeled) in either 50 mm Imidazole Assay Buffer at the indicated pH or 50 mm MES Assay Buffer at pH 6 was allowed to polymerize in the chamber before rinsing with Photo Buffer. Actin disassembly factors were then perfused into the chamber in Photo Buffer, and actin filament disassembly was observed using a Zeiss 63× oil objective (numerical aperture, 1.4) and the microscopy equipment and software described above.
To image actin polymerization off of new filament ends generated through severing reactions, disassembly was allowed to occur as described for 90 s before rinsing the chambers with buffer and reperfusing with G-actin labeled with a different fluorophore. Photo Buffer was then perfused into the chamber for imaging. Filaments that had been severed during the disassembly step were compared with the dual color image to identify whether new F-actin polymerization happened after a severing event; not all filaments were severed, and not all filaments nucleated growth in the times allotted. To test whether CAP-mediated or CAP/cofilin-mediated severing reactions depend upon phosphate release from F-actin, 20 mm phosphate was added to Assay Buffer from stock phosphate buffers and then adjusted to the final pH value indicated (6 or 7.4).
Microscopy Data Analysis
Original movies were acquired and converted to “.tif” stacks using Zeiss Axiovision 4.7 software. In the case of L. monocytogenes actin comet tails, data analysis was accomplished through measuring background-corrected intensity decay over time and either displaying this normalized decay directly or using a single exponential model to plot an apparent actin koff from normalized data as described previously (13). To determine the severing rate from imaging of single filaments, we measured the total length of the actin polymer in a field using a custom routine in Matlab (R2011b; The Mathworks, Inc.). Severing rates are thus reported as the number of events per second over the time course of the experiment normalized to the starting total length of actin in micrometers.
Pyrene Fluorescence Experiments
To determine whether severed actin filaments produced barbed ends capable of seeding actin polymerization, a solution of 10 μm unlabeled F-actin was mixed with an equal volume of Assay Buffer containing 6 μm CAP and 4 μm cofilin at pH 7.4 or 6 μm CAP at pH 6. After incubating at room temperature for 5 min, this solution was diluted 1:20 into a solution of 2 μm G-actin labeled with pyrene (25% label) and Assay Buffer at pH 7.4 with or without 300 nm cytochalasin D. Pyrene fluorescence was monitored in a Spectromax M2 plate reader.
RESULTS
Cofilin, Coronin, and AIP1 Are Insufficient to Disassemble Actin in the Presence of Excess F-actin
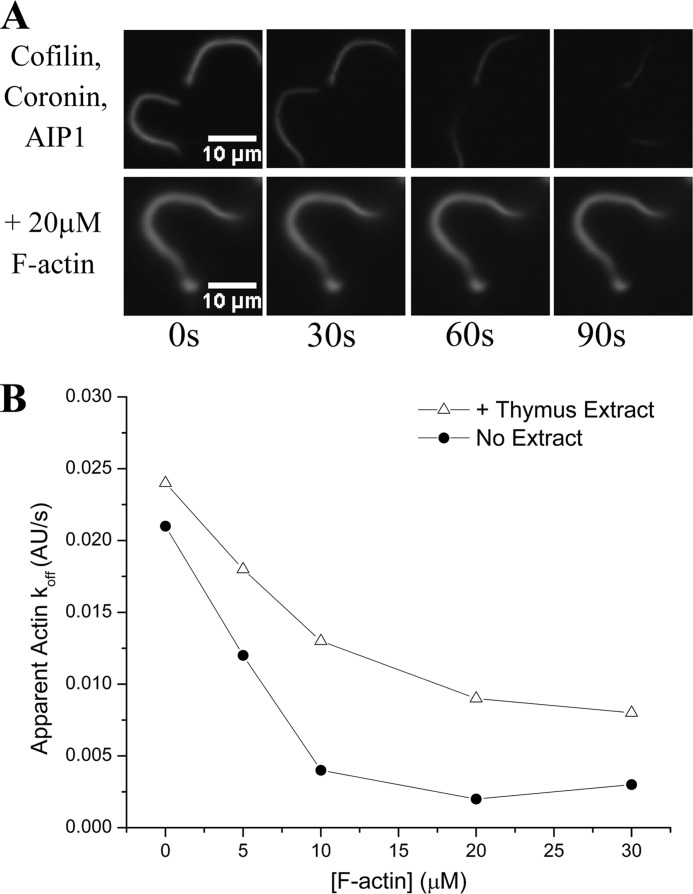
We have previously demonstrated that cofilin, coronin, and AIP1 are each necessary and together are sufficient to disassemble the actin comet tails assembled by L. monocytogenes in our perfusion chamber setup despite the presence of a physiological excess of G-actin (13). However, it is also true that high concentrations of F-actin exist in vivo (14). To address the question of whether cofilin, coronin, and AIP1 would retain efficacy in the face of a physiological excess of F-actin, we assessed whether this ternary mixture could still disassemble fluorescent actin comet tails in the presence of unlabeled prepolymerized F-actin. After assembling fluorescent comet tails in a perfusion chamber, we perfused a limiting amount of cofilin (2 μm) with saturating amounts (13) of coronin (2 μm) and AIP1 (0.2 μm) into the chamber along with varying concentrations of prepolymerized F-actin. Although sufficient to disassemble actin comet tails in the presence of G-actin, the activity of the cofilin, coronin, and AIP1 mixture was inhibited by the presence of F-actin (Fig. 1A) in a dose-dependent manner (Fig. 1B). A high speed supernatant from bovine thymus extract rescued this inhibition, implying that an additional actin disassembly factor was present in the extract (Fig. 1B). This activity can be chromatographically separated from each of the three known factors in the extract, first from coronin and AIP1 by flowing through DE52 beads and then from cofilin by flowing through an S column at physiological pH, thus indicating that this activity is a fourth actin disassembly factor that restores actin disassembly activity under conditions of physiological F-actin (Table 1).
FIGURE 1.
Physiological concentrations of F-actin inhibit disassembly of an F-actin substrate; an unknown factor protects against this inhibition. A, cofilin, coronin, and AIP1 readily disassemble a fluorescently labeled actin substrate (L. monocytogenes comet tails) on the required cellular time scale of tens of seconds (top series) but are inhibited when challenged with a physiological excess of F-actin (bottom series). B, Actin disassembly activity of this same mixture is markedly sensitive to increasing concentrations of F-actin, nearing complete inhibition by 10 μm F-actin (circles). The addition of a high speed supernatant derived from thymus extract, however, protected against this inhibition (triangles). Experiments were done in the presence of cofilin (2 μm), coronin (2 μm), and AIP1 (200 nm) with prepolymerized F-actin at the concentrations indicated. AU, arbitrary units.
TABLE 1.
Purification of cyclase-associated protein
| Column | Total protein | Protein concentration | Total activity | Specific activity |
|---|---|---|---|---|
| mg | mg/ml | units | units/mg | |
| DE52 | 1,320 | 11 | 20,000 | 18 |
| pH 6.8 S | 915 | 6.1 | 9,000 | 9.7 |
| pH 6.0 S | 111 | 3.1 | 6,000 | 54 |
| HAP/S200 | 3.8 | 1.9 | 2,000 | 526 |
| Heparin | 1.2 | 1.2 | 667 | 556 |
Purification of CAP as a Fourth Actin Disassembly Factor
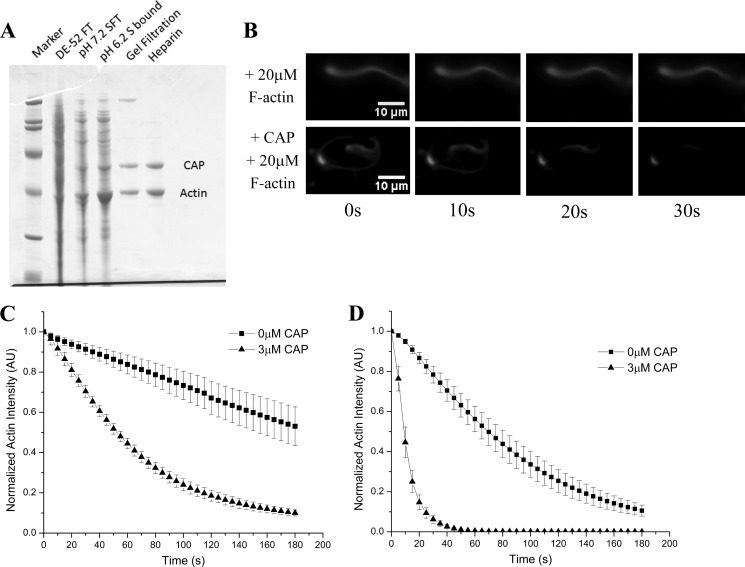
To identify the factor responsible for ameliorating the observed F-actin mediated inhibition of actin substrate disassembly, we continued to test the activity of our ternary actin disassembly mixture (cofilin, AIP1, and coronin) in the presence of both excess F-actin and chromatographic fractions from thymus extract. The activity behaved as a single factor through multiple chromatographic steps (Table 1) resulting in the purification of two bands of 57 and 43 kDa (Fig. 2A). The 43-kDa band was identified by mass spectrometry as actin, and the 57-kDa band was identified as CAP. We expressed the human CAP isoform with the widest tissue distribution (CAP1; hereafter “CAP”) (22). Recombinant human CAP scored as the factor responsible for imparting resistance to excess F-actin, allowing cofilin, coronin, and AIP1 to efficiently depolymerize Listeria actin comet tails despite the presence of inhibiting levels of F-actin (Fig. 2B).
FIGURE 2.
CAP relieves F-actin-mediated inhibition of actin disassembly. A, SDS-PAGE summarizing purification of activity from bovine thymus extract. The activity was separated from all three known factors, first from coronin and AIP1 by flowing through DE52 beads and then from cofilin by flowing through an S column at physiological pH, thus indicating that this activity is a fourth actin disassembly factor. Mass spectrometry identified the lower band as actin, whereas the higher band was identified as CAP. B, excess F-actin inhibits the cofilin, coronin, and AIP1 mixture (top series), but recombinant CAP confers resistance to this inhibition (bottom series). C, quantification of CAP-mediated resistance to inhibition induced by excess (30 μm) F-actin. In the absence of CAP (squares), actin disassembly is greatly attenuated, but when CAP is added to the disassembly mixture (triangles), inhibition is lifted and efficient actin depolymerization is restored. D, in the absence of excess F-actin, CAP (triangles) markedly increases actin disassembly activity over the tripartite disassembly mixture alone (cofilin, coronin, and AIP1; squares). All experiments were done in the presence of cofilin (2 μm), coronin (2 μm), AIP1 (200 nm), and where noted CAP (3 μm). Displayed for each condition in C and D is the mean normalized actin fluorescence intensity resolved over time of at least three independent experiments ±S.D. Error bars represent S.D. AU, arbitrary units.
Under the challenges of excess F-actin concentrations, recombinant CAP proved necessary to depolymerize fluorescent actin comet tails, and all four factors (cofilin, coronin, AIP1, and CAP) were together sufficient for actin disassembly (Fig. 2C). CAP has already been implicated in actin turnover dynamics and is known to accelerate the release of cofilin from its high affinity interaction with ADP·G-actin for another round of disassembly (23–26). Although CAP-mediated cofilin recycling might help explain how CAP protects actin depolymerization from high concentrations of F-actin, we noticed that CAP increased the rate of actin disassembly even in the absence of challenging F-actin (Fig. 2D). This suggests that CAP in addition to its established function in cofilin recycling also acts directly on F-actin to enhance depolymerization.
CAP Partially Substitutes for Coronin Actin Disassembly Activity through a Distinct Mechanism
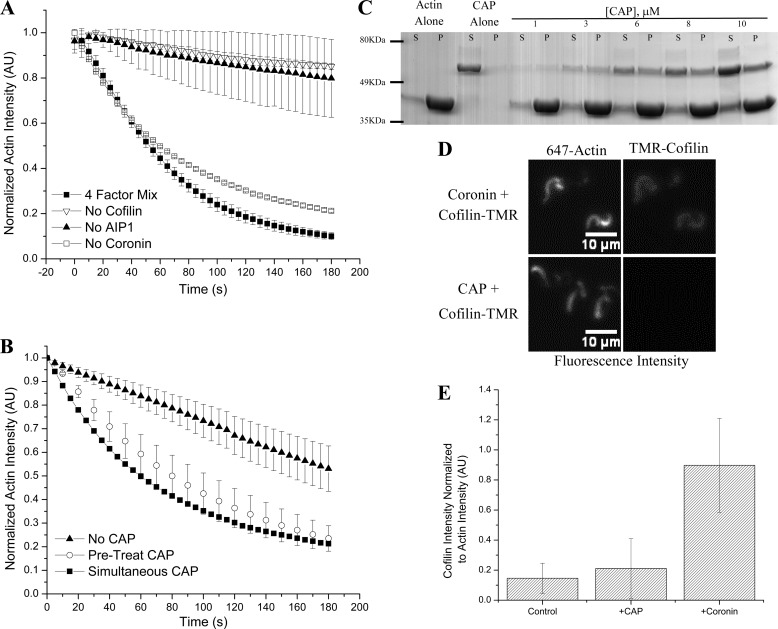
We tested whether CAP could substitute for any of the other actin depolymerization factors (cofilin, coronin, or AIP1) to disassemble Listeria actin comet tails in the presence of excess F-actin. We found that CAP could substitute for coronin; however, cofilin and AIP1 were still necessary for comet tail disassembly (Fig. 3A). We previously demonstrated that coronin binds F-actin; after preincubation with actin comet tails, excess coronin can be rinsed away; and then upon addition of cofilin and AIP1, actin disassembly is accelerated in a manner similar to when all three proteins are added simultaneously (13). To determine whether CAP can score similarly in a pretreatment assay, comet tails were assembled in perfusion chambers, incubated with CAP or Assay Buffer, and subsequently treated with cofilin, AIP1, and excess F-actin. Comet tail disassembly was then monitored as described. We found that CAP scores in such a pretreatment assay to disassemble actin comet tails in the presence of cofilin and AIP1 despite the presence of excess F-actin (Fig. 3B). This result is consistent with our hypothesis that CAP is acting at the level of the actin filament and thus directly participating in actin disassembly.
FIGURE 3.
CAP shows partial redundancy with coronin but remains mechanistically distinct. A, testing the four-factor actin disassembly mixture of cofilin, coronin, AIP1, and CAP for redundancy between CAP and each of the other factors in the presence of excess F-actin, we found that in the absence of coronin (open squares) activity was virtually unchanged (versus four-factor mixture; closed squares). Cofilin (inverted triangles) and AIP1 (closed triangles) remain necessary as their absence greatly attenuates actin depolymerization. B, CAP scores in a pretreatment assay (open circles) with activity similar to when CAP is added simultaneously with cofilin and AIP1 (squares). Triangles represent cofilin and AIP1 control. C, CAP, like coronin, directly binds F-actin as demonstrated in this Coomassie-stained SDS-PAGE gel showing supernatant (S) and pellet (P) fractions after sedimentation of 10 μm F-actin with an indicated amount of CAP. His6-CAP is the upper band (∼56 kDa), and actin is the lower band (43 kDa). D, unlike coronin (top series), CAP (bottom series) does not increase fluorescently labeled cofilin loading onto differentially labeled actin comet tails. E, quantification of cofilin loading. Although coronin significantly increases cofilin loading, CAP does not. Cofilin intensity is normalized to actin intensity in each image; bars represent the mean of 10 data points per condition ±S.D. All experiments except sedimentation utilized L. monocytogenese actin comet tails assembled as described. Kinetic data (A and B) are challenged with excess F-actin at 30 μm. Unless noted otherwise, 2 μm cofilin, 2 μm coronin, 2 μm CAP, and 200 nm AIP1 were used. Error bars represent S.D. TMR, tetramethylrhodamine; AU, arbitrary units.
In Saccharomyces cerevisiae, CAP has been shown to bind F-actin indirectly through an intermediary protein known as Abp1 (27). To determine whether CAP could bind directly to F-actin, we tested whether CAP would co-sediment with F-actin in a defined system. CAP bound directly to F-actin in a substoichiometric fashion as indicated by SDS-PAGE stained with Coomassie (Fig. 3C). In further co-sedimentation experiments, we estimated the dissociation constant for CAP with respect to F-actin as ∼2 μm by the method of Wachsstock et al. (21). Thus, we found that, like coronin, CAP binds directly to F-actin. As coronin enhances cofilin-mediated comet tail disassembly by facilitating cofilin binding to the comet tail (13), we tested whether CAP shares this mechanistic function with coronin by assessing the ability of CAP to load fluorescently labeled cofilin onto actin comet tails. We found that, unlike coronin, CAP does not act to significantly facilitate cofilin loading (Fig. 3, D and E). Thus, although CAP and coronin are each capable of binding to comet tails to increase the rate of actin depolymerization, they each act through distinct mechanisms.
CAP Augments Cofilin-mediated Severing
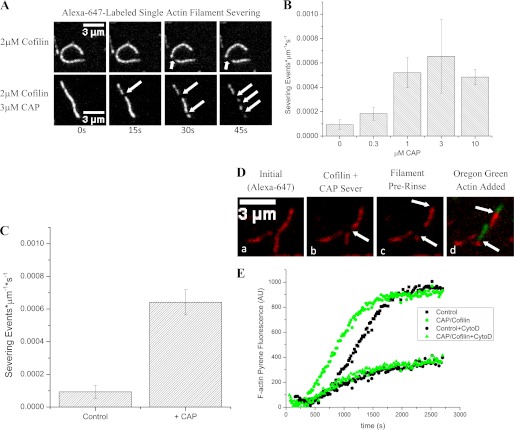
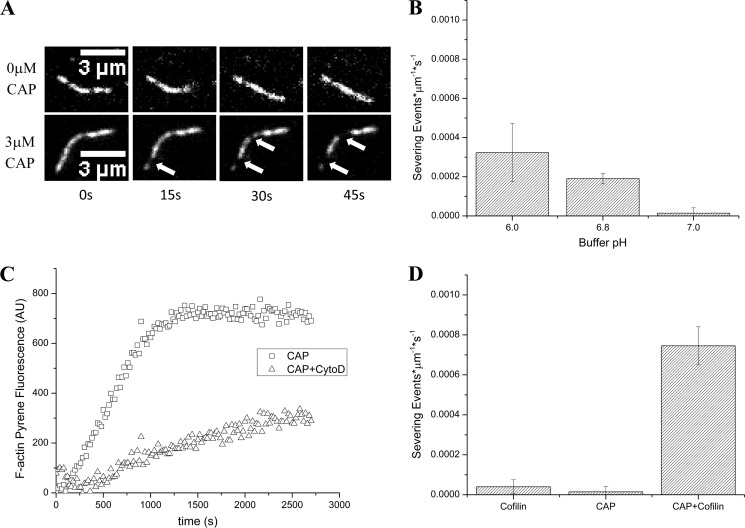
To gain more mechanistic insight into how CAP enhances cofilin-mediated actin disassembly, we used wide field fluorescence microscopy to image disassembly of single filaments as a function of CAP. Actin filaments were assembled in perfusion chambers coated with the actin-bundling protein filamin after which the assembly solution was replaced with a disassembly solution containing 2 μm cofilin and increasing concentrations of CAP. We found that CAP increased the frequency of cofilin-mediated actin severing events ∼7-fold relative to cofilin alone (Fig. 4, A and B). Under these conditions at pH 7.4, CAP did not have any significant activity on its own (see below and Fig. 5). This allowed us to test whether CAP would bind single actin filaments and whether its presence would significantly affect cofilin activity. In agreement with our Listeria comet tail assays, CAP scored in such a pretreatment experiment in the context of single actin filaments in which we pretreated actin filaments with CAP and after rinsing supplied cofilin, resulting in severing activity quantitatively similar to that seen when adding both CAP and cofilin simultaneously (Fig. 4C). Therefore, CAP binds directly to F-actin to accelerate cofilin-mediated actin severing.
FIGURE 4.
CAP accelerates cofilin severing activity without capping filament ends. A, cofilin severs actin filaments as expected (top series), and with the addition of CAP, both proteins sever actin filaments at an elevated rate (bottom series). Arrows indicate severing events. B, quantification of actin severing activity with increasing concentrations of CAP in the presence of 2 μm cofilin at pH 7.4. C, CAP scores in a pretreatment assay with single actin filaments, resulting in activity quantitatively similar to that seen when adding both CAP and cofilin simultaneously. D, severed ends can polymerize new actin. To Alexa Fluor 647-labeled actin filaments (panel a; pseudo-colored red), we added CAP and cofilin and tracked severing for 90 s. The image in panel b demonstrates a severing event at 45 s (arrow), producing a severed barbed end (panel c, lower arrow) and an unsevered barbed end (panel c, upper arrow). After rinsing and supplying Oregon Green-labeled G-actin, both the severed and unsevered barbed ends display the ability to nucleate new growth (panel d). E, unlabeled CAP/cofilin-severed filaments or unlabeled control filaments seeded pyrene-actin growth with CAP/cofilin-induced severing producing a decreased lag (green circles) relative to untreated control (squares), consistent with a greater number of nucleating ends. The addition of barbed end-capping drug cytochalasin D (CytoD) gave similar decreases in actin assembly rates in both control F-actin-nucleated and CAP/cofilin-treated F-actin-nucleated experiments (black circles and green triangles, respectively), consistent with a process dependent upon free barbed ends. Traces from one representative experiment are shown; bar graphs indicate mean values of at least three experiments ±S.D. Pyrene-actin assays were performed using 2 μm cofilin and 2 μm CAP during severing and 2 μm pyrene-actin and 300 nm cytochalasin D during polymerization. Error bars represent S.D. AU, arbitrary units.
FIGURE 5.
CAP alone is sufficient to sever actin at acidic pH and rescues cofilin severing at neutral pH. A, time-lapsed images showing single actin filaments diluted into Assay Buffer at pH 6 (top series) or into 3 μm CAP at pH 6 (bottom series). Arrows indicate severing events. B, CAP scores as an actin severing factor at non-physiological, acidic pH but loses activity as the pH is raised to neutral. C, pyrene-actin polymerization seeded with CAP-severed F-actin treated at pH 6 (squares). Polymerization is inhibited by the addition of the barbed end-capping drug cytochalasin D (CytoD) (triangles), indicating that CAP-severed F-actin provides free barbed ends. Traces from one representative experiment are shown. D, quantification of filament severing rates at pH 7 in the presence of cofilin alone, CAP alone, or cofilin and CAP in combination. Neither cofilin nor CAP alone are sufficient to sever F-actin, but in combination (2 μm cofilin and 3 μm CAP), the two proteins sever F-actin at an accelerated rate. Single filament assays display the mean of at least three experiments ±S.D. Pyrene-actin assays were performed using 2 μm CAP during severing and 2 μm pyrene-actin and 300 nm cytochalasin D during polymerization. Error bars represent S.D. AU, arbitrary units.
AIP1 is another auxiliary factor capable of enhancing cofilin activity, and although it is thought to occlude the barbed end of cofilin-decorated filaments to prevent reannealing of severed daughter filaments (28), it may also have additional roles in actin disassembly (16, 29, 30). We conclude that CAP, however, is not augmenting cofilin-mediated severing through filament end capping and blocking of reannealing because filament ends created by severing events in the presence of CAP could extend new actin polymer (Fig. 4D). In addition, bulk pyrene-actin experiments demonstrated that the products of disassembly reactions generated by the combination of cofilin and CAP reduce the lag phase normally associated with actin polymerization, again demonstrating that the ends of the filaments are free and can seed actin assembly (Fig. 4E). From these results, we conclude that CAP acts directly on F-actin to accelerate cofilin-mediated actin filament severing.
CAP Severs F-actin at Acidic pH
Twinfilin, like CAP, was originally identified as a G-actin sequestration factor (31) but is now known to also sever F-actin at acidic pH (32). Therefore, we imaged single filaments in the presence of CAP alone at varying pH and found that at acidic pH values CAP is sufficient to sever actin filaments (Fig. 5A). CAP severing activity decreased as pH was increased until it was all but inactive at neutral pH (Fig. 5B) and had no detectable severing activity by itself at basic pH as demonstrated in pretreatment assays conducted at pH 7.4 (Fig. 4C). Furthermore, actin severing reactions driven by CAP alone at pH 6 produced filament ends that could seed actin assembly reactions, demonstrating that CAP does not occlude filament ends (Fig. 5C).
CAP Rescues Cofilin Function at Neutral pH
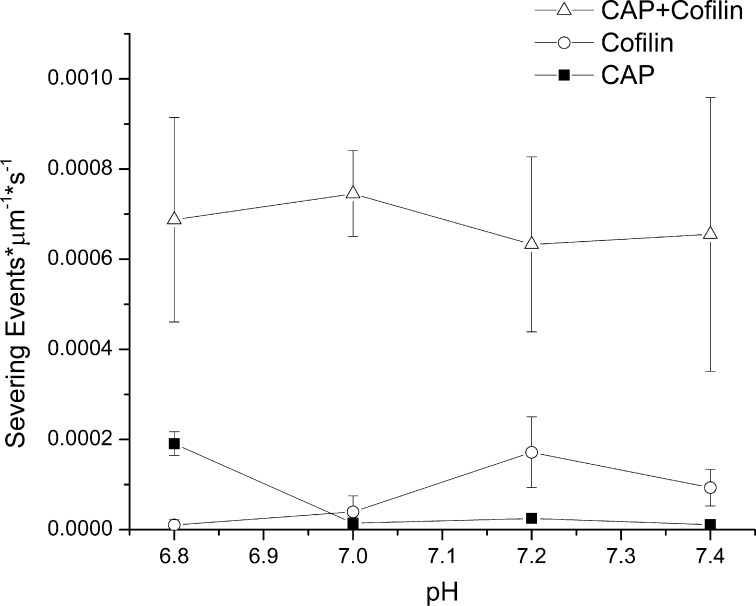
Cofilin function is highly sensitive to pH, and at neutral or acidic pH, cofilin loses all severing activity, although it can still bind actin (33, 34). Cytosolic pH in many cell types is near neutral or even slightly acidic (35). It is possible, however, that both CAP and cofilin could act together to disassemble filaments at neutral pH. We first verified that cofilin severing activity is lost in our assay at neutral pH. Cofilin severed actin filaments at pH 7.2 and greater, but the severing activity of cofilin alone approached background severing rates at pH 7.0 (Fig. 5D), which is well within the range of physiologically relevant intracellular pH values (36). The addition of 3 μm CAP, however, rescued cofilin-mediated actin severing activity. Extending this result to other relevant pH values, we found that CAP rescues and accelerates cofilin severing activity across the whole physiological range of cytosolic pH from 6.8 to 7.4 (Fig. 6). CAP, therefore, accelerates actin severing reactions in the presence of cofilin, yielding a constant, accelerated severing rate that is independent of pH.
FIGURE 6.
The combination of CAP and cofilin yields pH-independent actin severing. Across the physiological range of pH values, CAP alone (squares) and cofilin alone (circles) each have individually limited actin severing activities, and each is pH-dependent. When added together (3 μm CAP and 2 μm cofilin), however, the two have a severing activity ∼7-fold higher than either can achieve independently over this pH range (triangles). Each data point represents the mean of at least three experiments ±S.D. Error bars represent S.D.
CAP-mediated and CAP/Cofilin-mediated F-actin Severing Respects Nucleotide State
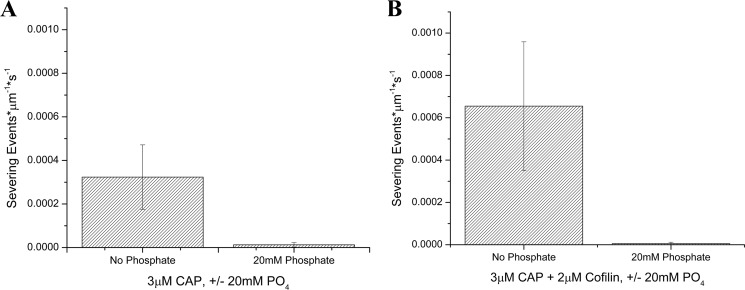
Binding of pure cofilin to F-actin and cofilin-mediated actin filament severing are nucleotide-dependent (24, 26, 37), and inorganic phosphate must be released from F-actin following ATP hydrolysis before cofilin can bind. Therefore, we tested whether CAP-dependent actin severing reactions were also dependent upon the release of inorganic phosphate from F-actin by challenging severing reactions with the inclusion of inorganic phosphate in the reaction buffer at every step of the experiment. We found that both CAP-mediated severing at acidic pH (Fig. 7A) and CAP/cofilin-mediated severing at basic pH (Fig. 7B) remain dependent upon phosphate release.
FIGURE 7.
CAP-mediated and CAP/cofilin-mediated F-actin severing is inhibited by inorganic phosphate. A, CAP severing activity at pH 6 in the absence (left bar) and presence (right bar) of 20 mm inorganic phosphate. B, cofilin- and CAP-mediated (3 μm CAP and 2 μm cofilin) severing at pH 7.4 in the absence (left bar) and presence (right bar) of 20 mm inorganic phosphate. Both CAP-mediated severing at acidic pH and CAP/cofilin-mediated severing at basic pH are severely inhibited by excess phosphate. Error bars represent S.D.
DISCUSSION
Fast actin disassembly is necessary for cells to rapidly reorganize their actin cytoskeleton in response to both internal and external cues as well as to maintain a high concentration of actin monomer to drive fast actin assembly reactions. The importance of cofilin as the central and essential component of the actin disassembly machinery of the cell has long been recognized (6, 10, 38–40). However, although cofilin is necessary for actin filament disassembly, it is increasingly clear that cofilin alone cannot account for actin disassembly in cells where high concentrations of both polymerizable G-actin and F-actin in addition to neutral pH either favor actin assembly or limit cofilin activity. Understanding how these in vivo challenges are overcome to allow actin disassembly is essential for understanding how actin dynamics are managed and utilized effectively in cells. Our overall approach to this issue has been to design in vitro depolymerization assays that mimic the challenges to depolymerization found in cells and fractionate tissue extracts to identify the factors that restore fast cofilin-mediated disassembly. Here, we demonstrated that although the combination of cofilin, coronin, and AIP1 can rapidly depolymerize actin in the presence of G-actin the activity of this three-protein mixture is easily inhibited by high concentrations of F-actin. We identified CAP as a fourth factor that enhances cofilin-mediated actin depolymerization even in the presence of high concentrations of F-actin. The ability of CAP to recycle cofilin from its high affinity interaction with ADP·G-actin for another round of disassembly probably contributes to restoring fast actin disassembly under these conditions. However, in addition to its role as a cofilin recycling factor, we discovered a novel role for CAP in acting directly on F-actin to enhance actin filament severing. Interestingly, we also found that CAP in combination with cofilin was also sufficient to overcome the barrier to depolymerization posed by neutral pH.
Although a number of auxiliary factors act directly on the actin filament to augment cofilin-mediated actin depolymerization, their associated mechanisms are distinct. Coronin for example enhances cofilin-mediated actin disassembly by facilitating cofilin binding to F-actin (13) and possibly by acting to selectively disassemble filaments that have released inorganic phosphate (41). AIP1 on the other hand accelerates cofilin-mediated actin filament severing by capping barbed ends of cofilin-severed filaments to block the back-reaction of filament reannealing (28, 42, 43). In addition to its role in barbed end capping, evidence also suggests that AIP1 acts more directly on the filament, further weakening F-actin to enhance cofilin-mediated severing (29, 30, 44). We found that CAP apparently acts in a unique fashion, neither increasing cofilin loading like coronin has been shown to do nor acting to bar filament reannealing after severing as AIP1 is proposed to do. CAP may be acting to increase cofilin function by binding to and further destabilizing actin filaments, producing a substrate more susceptible to cofilin-mediated severing. Alternatively, CAP may be producing a more effective cofilin as a consequence of a CAP-cofilin interaction. The N-terminal domain of CAP has been shown to directly interact with cofilin in all systems studied (27, 45), possibly affecting cofilin activity in such a way. However, the C-terminal actin-binding domain of CAP is sufficient to rescue morphological changes observed in CAP-deficient systems (46), which combined with our data showing that CAP is capable of directly severing actin filaments at acidic pH leads us to favor the first model: CAP is acting on the filament to make it more susceptible to cofilin action.
Cofilin-mediated actin depolymerization is pH-dependent, and cofilin-mediated actin severing itself titrates with a pKa of 7.4 (47). Consistent with these results, we found that cofilin-mediated actin severing is undetectable at pH 7.0. Cytosolic pH varies by tissue and cell type but is typically kept within a narrow range, usually between 6.8 and 7.4 with most cells tending toward neutral or slightly acidic pH rather than pH 7.4, which is typical of extracellular space (35, 48, 49). Therefore, cofilin-mediated actin disassembly inside cells requires either alkalinization of cytosol or the use of auxiliary depolymerization factors to overcome pH-dependent inhibition of cofilin function. Certain signaling molecules such as PDGF transiently elevate cytosolic pH, and rapid actin reorganization might be coupled to controlled changes in pH (50). In addition, the chronically elevated cytosolic pH of cancer cells helps drive faster actin turnover dynamics to increase their metastatic potential (10). Finally, it is increasingly evident that systems responsible for controlling cytosolic pH are coupled to the actin depolymerization machinery (51, 52). Thus, the regulation of cytosolic pH may be an important determinant of actin turnover dynamics. However, fast actin disassembly is still required in normal cells, both motile and non-motile, where cytosolic pH is often near neutral. In these cases, all cofilin-mediated actin disassembly reactions most likely require auxiliary factors such as CAP, a point supported by in vivo evidence that CAP insufficiencies phenocopy cofilin insufficiencies (53). Our data demonstrating that the combination of CAP and cofilin sever actin filaments at neutral pH provide at least one mechanism for driving fast actin disassembly without having to alter cytosolic pH, which would have consequences for many cellular reactions beyond those affecting actin.
Our identification of CAP as an actin filament-severing protein should help inform interpretation of earlier results with respect to how CAP contributes to actin-dependent processes in both physiological and pathophysiological settings. For example, the observation that CAP locally controls the amount of actin at apical cell-cell adhesive junctions (54) whereas cofilin controls total F-actin levels throughout Drosophila epithelial cells (55) is easier to reconcile with CAP-enhanced actin filament severing than with its ability to recycle cofilin. In addition, there is evidence that CAP overexpression strongly correlates with invasiveness in at least one highly metastatic cancer type. In aggressive pancreatic cancers, CAP overexpression was recognized in 100% of clinical cases studied and correlated with invasive behavior and poor prognosis (56). Our identification of CAP as an actin severing factor provides a mechanism for understanding how CAP can locally control the stability of specific actin arrays in distinct regions of the cell and how elevated CAP activity might also contribute to the accelerated actin turnover dynamics that appear to characterize metastatic cells through the cofilin pathway.
Acknowledgments
We thank members of the Brieher Laboratory for critical discussions and support and extend our sincere gratitude to the Jim and Kathy Kemp family of Dubuque, IA for gracious hospitality and for chicken gizzards from which filamin was purified.
This work was supported, in whole or in part, by National Institutes of Health Grant DK 091169 from the NIDDK (to K. P. M. N.) and a Ruth L. Kirschstein National Research Service Award predoctoral fellowship (to K. P. M. N.). This work was also supported by a grant from the March of Dimes.
- G
- globular
- F
- filamentous
- AIP1
- actin-interacting protein 1
- CAP
- cyclase-associated protein.
REFERENCES
- 1. Kessels M. M., Schwintzer L., Schlobinski D., Qualmann B. (2011) Controlling actin cytoskeletal organization and dynamics during neuronal morphogenesis. Eur. J. Cell Biol. 90, 926–933 [DOI] [PubMed] [Google Scholar]
- 2. Suzuki M., Morita H., Ueno N. (2012) Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure. Dev. Growth Differ. 54, 266–276 [DOI] [PubMed] [Google Scholar]
- 3. Ananthakrishnan R., Ehrlicher A. (2007) The forces behind cell movement. Int. J. Biol. Sci. 3, 303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ponti A., Vallotton P., Salmon W. C., Waterman-Storer C. M., Danuser G. (2003) Computational analysis of F-actin turnover in cortical actin meshworks using fluorescent speckle microscopy. Biophys. J. 84, 3336–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Theriot J. A., Mitchison T. J. (1991) Actin microfilament dynamics in locomoting cells. Nature 352, 126–131 [DOI] [PubMed] [Google Scholar]
- 6. Rosenblatt J., Agnew B. J., Abe H., Bamburg J. R., Mitchison T. J. (1997) Xenopus actin depolymerizing factor/cofilin (XAC) is responsible for the turnover of actin filaments in Listeria monocytogenes tails. J. Cell Biol. 136, 1323–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watanabe N., Mitchison T. J. (2002) Single-molecule speckle analysis of actin filament turnover in lamellipodia. Science 295, 1083–1086 [DOI] [PubMed] [Google Scholar]
- 8. Kueh H. Y., Brieher W. M., Mitchison T. J. (2010) Quantitative analysis of actin turnover in Listeria comet tails: evidence for catastrophic filament turnover. Biophys. J. 99, 2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pollard T. D. (1986) Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 103, 2747–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oser M., Condeelis J. (2009) The cofilin activity cycle in lamellipodia and invadopodia. J. Cell. Biochem. 108, 1252–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Theriot J. A. (1997) Accelerating on a treadmill: ADF/cofilin promotes rapid actin filament turnover in the dynamic cytoskeleton. J. Cell Biol. 136, 1165–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carlier M. F., Laurent V., Santolini J., Melki R., Didry D., Xia G. X., Hong Y., Chua N. H., Pantaloni D. (1997) Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J. Cell Biol. 136, 1307–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brieher W. M., Kueh H. Y., Ballif B. A., Mitchison T. J. (2006) Rapid actin monomer-insensitive depolymerization of Listeria actin comet tails by cofilin, coronin, and Aip1. J. Cell Biol. 175, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pollard T. D., Blanchoin L., Mullins R. D. (2000) Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29, 545–576 [DOI] [PubMed] [Google Scholar]
- 15. Yan M., Di Ciano-Oliveira C., Grinstein S., Trimble W. S. (2007) Coronin function is required for chemotaxis and phagocytosis in human neutrophils. J. Immunol. 178, 5769–5778 [DOI] [PubMed] [Google Scholar]
- 16. Okreglak V., Drubin D. G. (2010) Loss of Aip1 reveals a role in maintaining the actin monomer pool and an in vivo oligomer assembly pathway. J. Cell Biol. 188, 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tilney L. G., Portnoy D. A. (1989) Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109, 1597–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Theriot J. A., Mitchison T. J., Tilney L. G., Portnoy D. A. (1992) The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature 357, 257–260 [DOI] [PubMed] [Google Scholar]
- 19. Brieher W. M., Coughlin M., Mitchison T. J. (2004) Fascin-mediated propulsion of Listeria monocytogenes independent of frequent nucleation by the Arp2/3 complex. J. Cell Biol. 165, 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kueh H. Y., Brieher W. M., Mitchison T. J. (2008) Dynamic stabilization of actin filaments. Proc. Natl. Acad. Sci. U.S.A. 105, 16531–16536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wachsstock D. H., Schwartz W. H., Pollard T. D. (1993) Affinity of α-actinin for actin determines the structure and mechanical properties of actin filament gels. Biophys. J. 65, 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swiston J., Hubberstey A., Yu G., Young D. (1995) Differential expression of CAP and CAP2 in adult rat tissues. Gene 165, 273–277 [DOI] [PubMed] [Google Scholar]
- 23. Ressad F., Didry D., Xia G. X., Hong Y., Chua N. H., Pantaloni D., Carlier M. F. (1998) Kinetic analysis of the interaction of actin-depolymerizing factor (ADF)/cofilin with G- and F-actins. Comparison of plant and human ADFs and effect of phosphorylation. J. Biol. Chem. 273, 20894–20902 [DOI] [PubMed] [Google Scholar]
- 24. Maciver S. K., Weeds A. G. (1994) Actophorin preferentially binds monomeric ADP-actin over ATP-bound actin: consequences for cell locomotion. FEBS Lett. 347, 251–256 [DOI] [PubMed] [Google Scholar]
- 25. Blanchoin L., Pollard T. D. (1998) Interaction of actin monomers with Acanthamoeba actophorin (ADF/cofilin) and profilin. J. Biol. Chem. 273, 25106–25111 [DOI] [PubMed] [Google Scholar]
- 26. Hayden S. M., Miller P. S., Brauweiler A., Bamburg J. R. (1993) Analysis of the interactions of actin depolymerizing factor with G- and F-actin. Biochemistry 32, 9994–10004 [DOI] [PubMed] [Google Scholar]
- 27. Balcer H. I., Goodman A. L., Rodal A. A., Smith E., Kugler J., Heuser J. E., Goode B. L. (2003) Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 13, 2159–2169 [DOI] [PubMed] [Google Scholar]
- 28. Ono S. (2003) Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1: new blades for twisted filaments. Biochemistry 42, 13363–13370 [DOI] [PubMed] [Google Scholar]
- 29. Ono S., Mohri K., Ono K. (2004) Microscopic evidence that actin-interacting protein 1 actively disassembles actin-depolymerizing factor/cofilin-bound actin filaments. J. Biol. Chem. 279, 14207–14212 [DOI] [PubMed] [Google Scholar]
- 30. Okada K., Obinata T., Abe H. (1999) XAIP1: a Xenopus homologue of yeast actin interacting protein 1 (AIP1), which induces disassembly of actin filaments cooperatively with ADF/cofilin family proteins. J. Cell Sci. 112, 1553–1565 [DOI] [PubMed] [Google Scholar]
- 31. Goode B. L., Drubin D. G., Lappalainen P. (1998) Regulation of the cortical actin cytoskeleton in budding yeast by twinfilin, a ubiquitous actin monomer-sequestering protein. J. Cell Biol. 142, 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moseley J. B., Okada K., Balcer H. I., Kovar D. R., Pollard T. D., Goode B. L. (2006) Twinfilin is an actin-filament-severing protein and promotes rapid turnover of actin structures in vivo. J. Cell Sci. 119, 1547–1557 [DOI] [PubMed] [Google Scholar]
- 33. Yonezawa N., Nishida E., Sakai H. (1985) pH control of actin polymerization by cofilin. J. Biol. Chem. 260, 14410–14412 [PubMed] [Google Scholar]
- 34. Blondin L., Sapountzi V., Maciver S. K., Lagarrigue E., Benyamin Y., Roustan C. (2002) A structural basis for the pH-dependence of cofilin. F-actin interactions. Eur. J. Biochem. 269, 4194–4201 [DOI] [PubMed] [Google Scholar]
- 35. Rottenberg D. A., Ginos J. Z., Kearfott K. J., Junck L., Dhawan V., Jarden J. O. (1985) In vivo measurement of brain tumor pH using [11C]DMO and positron emission tomography. Ann. Neurol. 17, 70–79 [DOI] [PubMed] [Google Scholar]
- 36. Webb B. A., Chimenti M., Jacobson M. P., Barber D. L. (2011) Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer 11, 671–677 [DOI] [PubMed] [Google Scholar]
- 37. Muhlrad A., Pavlov D., Peyser Y. M., Reisler E. (2006) Inorganic phosphate regulates the binding of cofilin to actin filaments. FEBS J. 273, 1488–1496 [DOI] [PubMed] [Google Scholar]
- 38. Lappalainen P., Drubin D. G. (1997) Cofilin promotes rapid actin filament turnover in vivo. Nature 388, 78–82 [DOI] [PubMed] [Google Scholar]
- 39. Bamburg J. R. (1999) Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 15, 185–230 [DOI] [PubMed] [Google Scholar]
- 40. Lin M. C., Galletta B. J., Sept D., Cooper J. A. (2010) Overlapping and distinct functions for cofilin, coronin and Aip1 in actin dynamics in vivo. J. Cell Sci. 123, 1329–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gandhi M., Achard V., Blanchoin L., Goode B. L. (2009) Coronin switches roles in actin disassembly depending on the nucleotide state of actin. Mol. Cell 34, 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Okada K., Ravi H., Smith E. M., Goode B. L. (2006) Aip1 and cofilin promote rapid turnover of yeast actin patches and cables: a coordinated mechanism for severing and capping filaments. Mol. Biol. Cell 17, 2855–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Okada K., Blanchoin L., Abe H., Chen H., Pollard T. D., Bamburg J. R. (2002) Xenopus actin-interacting protein 1 (XAip1) enhances cofilin fragmentation of filaments by capping filament ends. J. Biol. Chem. 277, 43011–43016 [DOI] [PubMed] [Google Scholar]
- 44. Clark M. G., Teply J., Haarer B. K., Viggiano S. C., Sept D., Amberg D. C. (2006) A genetic dissection of Aip1p's interactions leads to a model for Aip1p-cofilin cooperative activities. Mol. Biol. Cell 17, 1971–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moriyama K., Yahara I. (2002) Human CAP1 is a key factor in the recycling of cofilin and actin for rapid actin turnover. J. Cell Sci. 115, 1591–1601 [DOI] [PubMed] [Google Scholar]
- 46. Mattila P. K., Quintero-Monzon O., Kugler J., Moseley J. B., Almo S. C., Lappalainen P., Goode B. L. (2004) A high affinity interaction with ADP-actin monomers underlies the mechanism and in vivo function of Srv2/cyclase-associated protein. Mol. Biol. Cell 15, 5158–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yeoh S., Pope B., Mannherz H. G., Weeds A. (2002) Determining the differences in actin binding by human ADF and cofilin. J. Mol. Biol. 315, 911–925 [DOI] [PubMed] [Google Scholar]
- 48. Vaupel P., Kallinowski F., Okunieff P. (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 49, 6449–6465 [PubMed] [Google Scholar]
- 49. Roos A., Boron W. F. (1981) Intracellular pH. Physiol. Rev. 61, 296–434 [DOI] [PubMed] [Google Scholar]
- 50. Magalhaes M. A., Larson D. R., Mader C. C., Bravo-Cordero J. J., Gil-Henn H., Oser M., Chen X., Koleske A. J., Condeelis J. (2011) Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. J. Cell Biol. 195, 903–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choi C. H., Patel H., Barber D. L. (2010) Expression of actin-interacting protein 1 suppresses impaired chemotaxis of Dictyostelium cells lacking the Na+-H+ exchanger NHE1. Mol. Biol. Cell 21, 3162–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sultana H., Rivero F., Blau-Wasser R., Schwager S., Balbo A., Bozzaro S., Schleicher M., Noegel A. A. (2005) Cyclase-associated protein is essential for the functioning of the endo-lysosomal system and provides a link to the actin cytoskeleton. Traffic 6, 930–946 [DOI] [PubMed] [Google Scholar]
- 53. Bertling E., Hotulainen P., Mattila P. K., Matilainen T., Salminen M., Lappalainen P. (2004) Cyclase-associated protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian nonmuscle cells. Mol. Biol. Cell 15, 2324–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baum B., Perrimon N. (2001) Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat. Cell Biol. 3, 883–890 [DOI] [PubMed] [Google Scholar]
- 55. Gunsalus K. C., Bonaccorsi S., Williams E., Verni F., Gatti M., Goldberg M. L. (1995) Mutations in twinstar, a Drosophila gene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. J. Cell Biol. 131, 1243–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yamazaki K., Takamura M., Masugi Y., Mori T., Du W., Hibi T., Hiraoka N., Ohta T., Ohki M., Hirohashi S., Sakamoto M. (2009) Adenylate cyclase-associated protein 1 overexpressed in pancreatic cancers is involved in cancer cell motility. Lab. Invest. 89, 425–432 [DOI] [PubMed] [Google Scholar]