Background: The role of Hsf1 in mammary tumorigenesis and metastasis remains elusive.
Results: Hsf1 deletion inhibits mammary tumorigenesis and metastasis by reducing ERK1/2 activity and epithelial-mesenchymal transition of mammary epithelial cells.
Conclusion: Deletion of Hsf1 in mice carrying Her2/Neu significantly reduces breast cancer and metastasis.
Significance: These findings indicate a powerful inhibitory effect conferred by Hsf1 in ErbB2-induced breast cancer.
Keywords: Breast Cancer, Cancer Biology, Cell Biology, MAPKs (MAPKs), Metastasis, Her2/Neu, Hsf1, Knock-out Mice
Abstract
ErbB2/Neu oncogene is overexpressed in 25% of invasive/metastatic breast cancers. We have found that deletion of heat shock factor Hsf1 in mice overexpressing ErbB2/Neu significantly reduces mammary tumorigenesis and metastasis. Hsf1+/−ErbB2/Neu+ tumors exhibit reduced cellular proliferative and invasive properties associated with reduced activated ERK1/2 and reduced epithelial-mesenchymal transition (EMT). Hsf1+/+Neu+ mammary epithelial cells exposed to TGFβ show high levels of ERK1/2 activity and EMT; this is associated with reduced expression of E-cadherin and increased expression of Slug and vimentin, a mesenchymal marker. In contrast, Hsf1−/−Neu+ or Hsf1+/+Neu+ cells do not exhibit activated ERK1/2 and show reduced EMT in the presence of TGFβ. The ineffective activation of the RAS/RAF/MEK/ERK1/2 signaling pathway in cells with reduced levels of HSF1 is due to the low levels of HSP90 in complex with RAF1 that are required for RAF1 stability and maturation. These results indicate a powerful inhibitory effect conferred by HSF1 downstream target genes in the inhibition of ErbB2-induced breast cancers in the absence of the Hsf1 gene.
Introduction
Amplification or overexpression of oncogenes such as ErbB2/Her2/Neu, a type I transmembrane receptor, occurs in 25% of breast cancer cases and promotes breast cancer invasion and metastasis (1–3). ErbB2/Her2/Neu is an orphan receptor, but it heterodimerizes with Her3 (ErbB3) and Her4 (ErbB4) family members and, following ligand binding, activates MAPK and PI3K/AKT signaling pathways, leading to enhanced cellular proliferation, increased motility, and reduced apoptosis and eventually cellular transformation and invasion (4, 5). During tumorigenesis and metastasis, expression of epithelial cell markers such as E-cadherin, claudin, occludin, and keratins 8, 9, and 18 are down-regulated, and expression of mesenchymal markers such as αSMA,2 fibronectin, vitronectin, FSP1, and vimentin are up-regulated (6, 7). These alterations include appearance of fibroblast-like morphology, leading to tumor cell invasion, metastasis, recurrence, and chemoresistance (7, 8). This epithelial-mesenchymal transition (EMT) is regulated through activation of the transcriptional repressors Slug, Snail, Twist, ZFHX1b, deltaEF1, E12, and E47 or methylation of the E-cadherin promoter (9). Recent studies indicate that activation of ERK/MAPK signaling leads to outgrowth and translocation and proliferation of ErbB2-positive cells using MCF10A acini (10), and in the murine system, mammary epithelial cells undergo EMT when RAS is constitutively activated. Others have suggested that activation of the RAS/MAPK pathway induces EMT, cellular migration, and invasion as well as survival of epithelial cells (11). Data suggest that ErbB2 activation and overexpression alone may not be sufficient to cause tumor cell invasion and metastasis, and there likely are cooperating molecules (e.g. 14-3-3ζ) (12) that drive mammary epithelial cells to acquire invasive properties.
In this study, we have analyzed ErbB2/Neu-induced mammary tumorigenesis in the absence of the Hsf1 gene. HSF1 is expressed in all tissues and is the main regulator of the heat shock response through control of downstream target genes such as heat shock proteins (HSPs) (13–16). HSF1 is activated by a variety of environmental stressors and plays a critical function during development as well as during tumorigenesis (13, 17–21). The Hsf1−/− mice are resistant to skin tumorigenesis induced by 7,1-dimethylbenz(a)anthracene and 12-O-tetradecanoylphorbol-13-acetate, suggesting that the absence of Hsf1 reduces RAS-driven tumor formation; however, the underlying mechanism has not been elucidated (21). The Hsf1−/− mice in the background of TP53 deficiency exhibit a significant reduction in lymphomas (17), and in the background of the p53 mutant, mice exhibit deficiency in the development of sarcomas and carcinomas (21). Hsf1-deficient mice are also resistant to diethylnitrosamine-induced liver tumorigenesis and high fat diet-induced obesity, and the underlying mechanism is associated with altered glucose and lipid metabolism (16). In breast cancer, HSF1 levels have been found to be elevated in 80% of breast carcinomas associated with high histologic grade, large tumor size, and increased mortality (22). HSF1 becomes transcriptionally activated following exposure of the cells to heregulin, leading to increased expression of Hsps (23, 24). Hsf1 has also been found to interact with metastasis-associated protein 1 (MTA1), leading to changes in gene expression (25). Expression levels of Hsf1 target genes such as HSP90, HSP70, and HSP27 are increased in mammary tumors, leading to a decrease in cellular apoptosis (24). Overexpression of ErbB2/Neu has been shown to activate Hsf1 and up-regulate expression of lactate dehydrogenase-A, which leads to an increase in glycolysis. The study shows that glycolysis promoted by ErbB2/Neu occurs, at least in part, through HSF1 (26). HSF1 therefore plays a complex role in tumorigenesis and may impact the process of tumorigenesis through multiple pathways depending on the tumor origin.
To extend our observation regarding the impact of HSF1 on tumorigenesis in vivo, we have crossed Hsf1−/− mice with MMTV-Her2/Neu+ transgenic mice that constitutively express the wild-type form of Her2/Neu in luminal epithelial cells (27). Our results indicate that Hsf1−/− mice exhibit resistance to mammary tumorigenesis, and Hsf1+/−-Her2/Neu+ mice exhibit significant resistance to lung metastasis. Tumor and metastasis inhibitory effects exerted by complete or partial deletion of the Hsf1 gene appear to be by interference with the activation of ERK1/2 in the mammary tumor tissue and primary mammary epithelial cells, which leads to inhibition of tumor cell proliferation, reduced EMT, and cellular migration.
EXPERIMENTAL PROCEDURES
Mice
Generation of Hsf1−/− mice has previously been reported (13). MMTV-Her2/Neu (FVB/N TG (MMTVNeu) 202 MUL/JM) (27) mice were purchased from The Jackson Laboratory. Hsf1−/− mice exhibit embryonic lethality in a pure genetic background. Therefore, the background of MMTV-Neu+, Hsf1+/−-MMTV-Neu+ and Hsf1−/−-MMTV-Neu+ mice was F2 C57BL/6–129/SV-Pasteur/FVB. Cohorts of virgin females of all genotypes were observed for development of mammary gland tumors. All animal procedures were approved by the Institutional Animal Use and Care Committee.
Kaplan-Meier Tumor-free Survival Curves
Wild-type, Hsf1+/−, and Hsf1−/− mice expressing MMTV-Neu were palpated weekly. The date of visible appearance of the tumor was recorded, and mice were euthanized when tumors reached a size of 1–2 cm3. Tumors were fixed in 4% paraformaldehyde for histological analysis and frozen in OCT for immunohistochemical analysis or were snap-frozen for Western blot analyses. Tumor-free survival was estimated using Kaplan-Meier method. Statistical analysis was performed using the log-rank (Mantel-Cox) test. Significant differences were considered at p < 0.05.
Plasmids and Retrovirus Infection
shRNAs targeting sequence 1, GTGGACTCCAACCTGGATA, and sequence 2, gatcccGCTCATTCAGTTCCTGATCTTCAAGAGAGATCAGGAACTGAATGAGCttttttggaag, of Hsf1 mRNA were synthesized and subcloned into the retroviral vector pLTHR (pLTHR-shRNA-Hsf1). Ha-RasVal-12 was in the retroviral vector pWZL. For retroviral infection, plasmids were transfected into the packaging cell line, and after 48 h, the supernatant was mixed with 1 μg/ml Polybrene for infection of the appropriate cell line. The virus-infected cells were drug-selected and pooled for analyses. Cells infected with empty vector were used for negative control.
Preparation of Primary Breast Epithelial Cell Cultures
For the primary mouse breast epithelial cell culture, mammary glands were dissected from 2-month-old virgin females and digested in DMEM containing 10% FCS plus 1 mg/ml collagenase D overnight. Cells were resuspended in the medium containing 10% FBS, 10 ng/ml EGF, and 5 μg/ml bovine insulin. Cells were allowed to attach to the tissue culture dish three times to eliminate the contaminating fibroblasts. The epithelial cells in the supernatant were quantitated and seeded in culture dishes for analyses. MCF10A mammary epithelial cells were maintained in DMEM/F-12 media containing 5% horse serum, 10 ng/ml EGF, 5 μg/ml insulin, and 50 μg/ml hydrocortisone. To induce EMT, cells were treated with 5 ng/ml of human TGFβ1 (14-8348-62, eBioscience).
Preparation of Whole-mount Mammary Glands
Numbers four and nine mammary glands were extracted from virgin females and flattened and stretched out on a glass slide. The slides were air-dried for 5 min and fixed in 4% paraformaldehyde. Tissues were transferred to 100% acetone (three times for 1 h) to defat. Tissues were then transferred to 100% and then 90% EtOH (1 h each). Glands were stained in hematoxylin (3 h), rinsed and incubated in 50% EtOH (acidified with 25 ml HCl), and then transferred into 70, 95, and then 100% EtOH (1 h each) and xylene (twice for 1 h). Glands were stored in xylene.
BrdU Labeling
For in situ labeling of mammary epithelial cells, 8-week-old mice were injected with 100 μg/g body weight BrdU three times within a period of 24 h. Mammary glands were fixed, processed, sectioned, and analyzed for number of cells labeled with BrdU. For cultured cells, coverslips containing cells were labeled for 2 h with 10 mm BrdU, and following immunostaining, the number of BrdU-positive cells was quantified.
Transwell Migration Assays
Cell migration was performed as described previously (28). Briefly, the surface under the transwell (8 μm pore size; Costar) was coated with 10 μg/ml collagen I for 16 h at 4 ºC. Mammary epithelial cells cultured from 2-month-old mice were dispersed using trypsin and then resuspended in serum-free medium at a density of 2 × 105 cells/ml. One hundred microliters of cell suspension was added to the upper chamber of the transwell. Cells were allowed to migrate for 48 h in presence or absence of 5 ng/ml TGFβ1. After this time, cells remaining in the upper chamber were removed, and the cells on the undersurface of the transwells were fixed and stained with crystal violet. The number of cells that had migrated to the undersurface was quantified using a phase-contrast microscope.
Immunoblotting
Immunoblotting experiments were performed according to standard protocols (29). Cells were lysed in lysis buffer, and 30 μg of protein from each sample was resolved by SDS-PAGE and processed for immunoblotting analyses.
Antibodies
The following antibodies were used: β-actin, SMA, BrdU (Sigma); vimentin, β-catenin, p-Smad 3, p-Raf, Raf, p-MEK, MEK, p-AKT/T308, p-ERK1/2, ERK1/2, HSF1, Slug, Smad 3 (Cell Signaling); αSMA and keratin 18, ErbB2 (Abcam); Myc tag, p23 (StressGen); E-cadherin, CD31 (BD Biosciences); AKT, p-AKT S473, Ha-Ras, CDC37, Hop (Santa Cruz Biotechnology); N-cadherin (Invitrogen); HSP90a (StressMarq); Ki67 (NeoMarkers).
Histology and Immunohistochemical and Immunofluorescence Staining
For histology, tissues were fixed in 4% paraformaldehyde and embedded in paraffin, and tissue sections were stained with H&E and analyzed using the Elston and Ellis technique (30) for the histological grading system. Tumors were analyzed according to tube formation, nuclear pleomorphism, and number of mitotic cells (30). Analyses were performed to estimate whether tumors were well, moderately, or poorly differentiated. For immunocytochemistry, cells were cultured on glass coverslips, fixed in 4% paraformaldehyde, permeabilized (0.1% Triton X-100 in PBS), and blocked (5% milk in PBS) for 30 min at 25 ºC. Fixed cells were incubated with primary antibodies overnight at 4 ºC, rinsed, and incubated for 1 h with fluorescent-conjugated secondary antibody (Molecular Probes). Cells were further rinsed and mounted with Vectashield containing DAPI (Vector Laboratories) and analyzed using a confocal or Zeiss fluorescence microscope. For immunohistochemistry, 7-μm tissue sections were deparaffinized in xylene and rehydrated in a series of alcohol/water mixtures. Antigen retrieval was performed by placing tissue sections in 10 mm sodium citrate, pH 6.0, and steamed for 30 min. After rinsing in PBS, tissue sections were blocked (3% BSA in PBS for 2 h at 4 ºC) and then incubated in primary antibody (in 3% BSA in PBS plus 1% Tween 20) overnight at 4 ºC. Antibody/antigen was detected using Cy3 fluorescent-conjugated anti-mouse (or rabbit) IgG secondary antibody. Nuclei were stained with DAPI, and sections were processed, mounted, and analyzed as above (31, 32).
Statistical Analyses
All experiments were performed with at least 3–40 mice. Data are presented as means ± S.D. Statistical significance between experimental groups was assessed using Student's t test and p < 0.05 was considered significant.
RESULTS
Hsf1 Promotes Development of Her2/Neu-induced Mammary Tumors and Lung Metastasis
To determine the function of Hsf1 in mammary tumorigenesis, we selected the MMTV-Her2/Neu+ transgenic mouse model crossed with Hsf1−/− mice (27). In this model, Her2/Neu expression in mammary epithelial cells induces mammary tumors and lung metastasis in high percentages of mice. For Her2/Neu+ mice (thereafter named Neu+), a cohort of virgin female mice expressing Neu in the presence or absence of the Hsf1 gene was palpated weekly to detect the presence of breast tumors. In contrast to Hsf1−/− Neu+ mice where development of mammary tumors was rare (7%), high percentages of Hsf1+/+Neu+ mice developed tumors (74%) (Fig. 1A). At 499 days post-observation, 22% of the Hsf1+/+Neu+ mice developed tumors, whereas none of the Hsf1+/−Neu+ mice did. At 599 days, 14% of the Hsf1+/+Neu+ mice and 16% of Hsf1+/−Neu+ mice developed tumors. These data suggest a period of delay in the onset of tumorigenesis in the absence of one copy of the Hsf1 gene. There were, however, no significant overall differences between these two groups in terms of tumor-free survival. During the period of observation, only one Hsf1−/−Neu+ mouse developed a small mammary tumor and that occurred at 707 days.
FIGURE 1.
Hsf1-deficient cells exhibit reduced numbers of mammary gland tumors. A, Kaplan-Meier tumor-free survival (%) in days is presented for Hsf1+/+Neu+ (n = 27), Hsf1+/−-Her2/Neu+ (n = 30), and Hsf1−/−Neu+ (n = 13) mice. p values for Hsf1−/−Neu+ versus Hsf1+/+Neu+ and Hsf1+/−Neu+ was <0.0006 using log-rank test. B, upper panel, representative H&E-stained tumor tissue sections extracted from the indicated genotype. Lower panel, representative H&E-stained section of lung tumor metastases extracted from the indicated genotypes. Bars, 100 μm. C, Ki-67 immunohistochemical staining of tumor tissue sections of the indicated genotypes. Bar, 50 μm. Red color indicates Ki-67 (arrowheads), and blue color indicates DAPI (nuclei) immunostaining. Quantification of Ki-67-positive cells between the indicated genotypes is presented in the right panel. Bars are means ± S.D. (n = 3 mice). Statistical significance is as follows: *, p < 0.05. D, TUNEL assays of the tumor tissue sections of the indicated genotypes. Arrowheads show some of the TUNEL-positive cells. Bar, 50 μm. E, CD31 immunohistochemical staining of tumor tissue sections of the indicated genotypes. Arrowheads show some of the CD31 positive cells. Bar, 50 μm. Quantification of the CD-31-positive cells in tumor tissue sections of the indicated genotypes is shown in the right panel. Bars are means ± S.D. (n = 3 mice, with the exception of Hsf1−/−Neu+ mice that only generated one tumor). Statistical significance is as follows: **, p < 0.01; ***, p < 0.001.
Representative H&E staining of the mammary tumors of Hsf1+/+Neu+ and Hsf1+/−Neu+ mice are presented in Fig. 1B. Histological examination of the tumors was performed using criteria defined by Elston and Ellis (30). Using histological criteria, the tumors that developed in Hsf1+/+Neu+ and Hsf1+/−Neu+ mice were approximately comparable. However, significant differences between Hsf1+/+Neu+ and Hsf1+/−Neu+ tumors are noted below.
As mentioned before, tumors arising in Neu+ mice metastasize to the lungs with about 70% frequency (27). To determine whether there were differences in metastasis ability between tumors that developed in Hsf1+/+Neu+ or Hsf1+/−Neu+ mice, lungs were collected from tumor-bearing mice and examined for the presence of tumor nodules. Histological analyses indicate that 26% of Hsf1+/+Neu+ mice exhibited lung metastasis, in contrast to 0% of the Hsf1+/−Neu+ mice (Fig. 1B).
Interestingly, there were significant differences in multiple parameters in the tumors that developed in Hsf1+/−Neu+ mice compared with Hsf1+/+Neu+ mice. For example, tumors in the Hsf1+/−Neu+ mice exhibited a 40% reduction in Ki-67 immunostaining compared with tumors in the Hsf1+/+Neu+ mice, indicating a lower proliferating capacity of tumor cells in this genetic background (Fig. 1C). Furthermore, Hsf1+/−Neu+ tumors exhibited a significant increase in TUNEL-positive cells indicating cells were undergoing apoptosis (Fig. 1D). The tumors arising in Hsf1−/−Neu+ and Hsf1+/−Neu+ mice also exhibited a significant reduction in vascularization compared with tumors arising in Hsf1+/+Neu+ mice. This was confirmed by CD31 immunostaining (Fig. 1E).
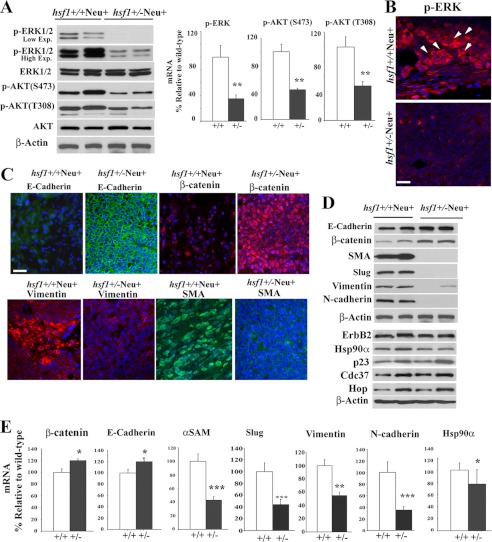
ErbB2 expression in cells activates the RAS/RAF/MEK/ERK1/2 and PI3K/AKT signaling pathways, leading to cell growth and inhibition of apoptosis (33). To detect expression of p-ERK1/2 and p-AKT in Hsf1+/+Neu+ and Hsf1+/−Neu+ tumors, immunoblotting was performed (Fig. 2A). The results show lower levels of p-ERK1/2, p-AKT Ser-473, and Thr-308 in Hsf1+/−Neu+ tumors compared with tumors in Hsf1+/+Neu+ mice. ERK1/2 activity has been implicated in clonal outgrowth and translocation and proliferation of ErbB2-positive cells, suggesting its relevance in the initial steps in ErbB2-mediated tumorigenesis (10). To determine differences in p-ERK1/2 expression between Hsf1+/+Neu+ and Hsf1+/−Neu+ tumors, immunohistochemical (IHC) staining was performed using antibody to detect p-ERK1/2. The data presented in Fig. 2B indicate a significant increase in the number of tumor cells expressing p-ERK1/2 in Hsf1+/+Neu+ compared with Hsf1+/−Neu+ tumors. Similar studies using antibody to p-AKT Ser-473 indicated that the expression of p-AKT in tumors was approximately comparable between the genotypes (data not shown).
FIGURE 2.
Mammary tumors from Hsf1+/−Neu+ mice exhibit lower ERK1/2 activation and reduced EMT. A, tumor cell extracts from the indicated genotypes were used in representative immunoblotting to detect expression of total and phosphorylated ERK1/2 (low and high exposures (Exp.) are indicated) and AKT. β-Actin is loading control. Quantification of Western blots are presented in the right panels. Bars are mean ± S.D. (n = 3 mice). Statistical significance is as follows: **, p < 0.01. B, tumors of indicated genotypes were fixed and immunostained using antibody to p-ERK1/2 (red). Nuclei were stained with DAPI (blue). Arrows indicate some of the p-ERK1/2-positive cells. Bar, 20 μm. C, tumors of the indicated genotypes were fixed and immunostained using antibody to E-cadherin, β-catenin, vimentin, and αSMA. Nuclei were stained with DAPI (blue). Bar, 20 μm. D and E, tumor cell extracts from the indicated genotypes were used in representative immunoblotting to detect expression of epithelial and mesenchymal markers as well as HSP90 and its cochaperones. β-Actin is loading control. Quantification of Western blots with significant changes are presented in E. Bars are mean ± S.D. (n = 3 mice). Statistical significance is as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Activation of ERK1/2 through ErbB2 and RAS has been shown to induce EMT, which plays an important role during development and in tumorigenesis, resulting in tumor cell spreading and increased cellular migration and invasiveness to the surrounding tissue (11, 34). As we observed significantly less p-ERK1/2 and a lower number of tumors that metastasized to the lungs in Hsf1+/−Neu+ compared with Hsf1+/+Neu+ tumors, we examined the expression of epithelial and mesenchymal markers in tumors that evolved in Hsf1+/+Neu+ and Hsf1+/−Neu+ mice. IHC staining of epithelial and mesenchymal markers showed lower expression of E-cadherin in Hsf1+/+Neu+ tumors, whereas expression of E-cadherin remained elevated in Hsf1+/−Neu+ tumors (Fig. 2C). Loss of β-catenin, which destabilizes the epithelial cell-cell junction, was also examined, and Hsf1+/+Neu+ tumors exhibited significant loss of β-catenin expression compared with Hsf1+/−Neu+ tumors (Fig. 2C). Loss of epithelial cell markers correlated with increased expression of mesenchymal markers vimentin and αSMA in Hsf1+/+Neu+ tumors as expected, whereas the expression of these mesenchymal markers was significantly lower in Hsf1+/−Neu+ tumors. IHC data were confirmed using immunoblotting (Fig. 2, D and E). The data indicated a reduction in the expression of the Slug transcription factor in Hsf1+/−Neu+ tumors as a potential regulator of E-cadherin expression, leading to a reduction in EMT.
Previous data indicated that downstream targets of Hsf1 (e.g. Hsp90a and its cochaperones) regulate expression of ErbB2 and its downstream signaling components, RAF1 and AKT (35). To determine whether expression of the Hsf1 target genes is affected in tumors derived from Hsf1+/−Neu+ compared with Hsf1+/+Neu+ mice, we performed microarray analyses and examined the expression of HSPs by immunoblotting. Microarray data as well as quantification of the immunoblots indicate a reduction in the expression levels of Hsp90a (Hsp90aa1) by 1.4 (20–40% reduction by immunoblotting), Hsp70 (Hspa8, Hspa1l, Hspa13, and Hspa2) by 1.2, and small Hsps (HspB1, HspB2, HspB6, HspB7, and HspB8) by 1.2–2.6-fold in Hsf1+/−Neu+ tumors compared with Hsf1+/+Neu+ tumors. There was no significant reduction in the levels of CDC37, HOP, HSP40, or p23, which are known to be Hsp90a cochaperones (Fig. 2D and data not presented).
Taken together, these data indicate that absence of the Hsf1 gene inhibits mammary tumorigenesis. Furthermore, a reduction in HSF1 expression level in Hsf1+/−Neu+ mice reduces lung metastasis and is associated with reduced cellular proliferation and a reduction in p-ERK1/2 and EMT.
Hsf1 Promotes Mammary Gland Morphogenesis
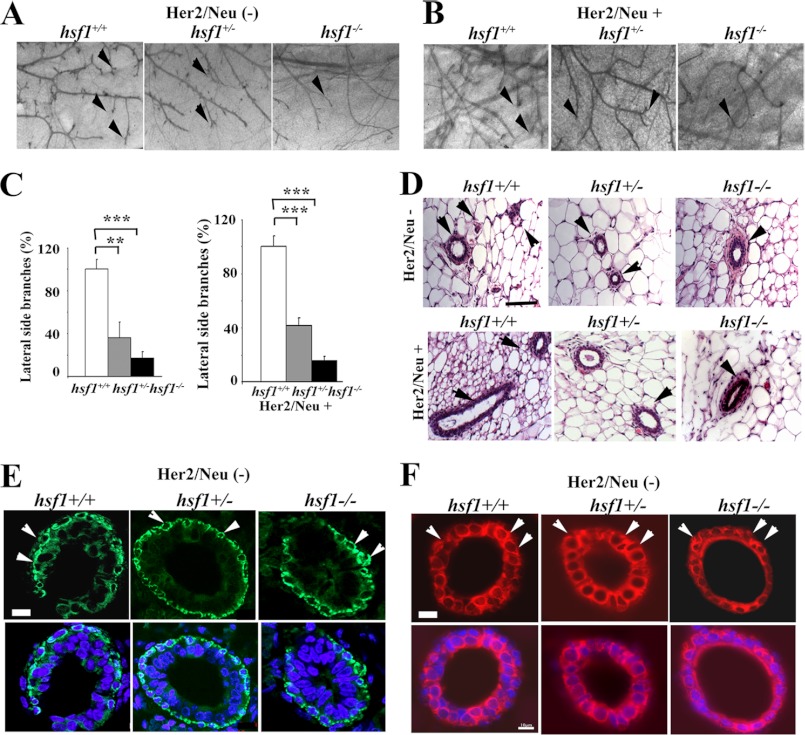
To determine the underlying mechanism for the significant reduction in tumor development in Hsf1−/−Neu+ mice, we analyzed mammary glands of adult Hsf1+/+, Hsf1+/−, and Hsf1−/− mice in the presence or absence of the Neu transgene. Maturation of the mammary gland occurs mainly postnatally (36). Cellular differentiation and migration are limited to the cap layer at the terminal end buds that migrate to the fat pad and stroma. Terminal end bud growth is regulated by a variety of growth factors, leading to ordered ductal development (36). Therefore, mammary gland numbers 4 and 9 of virgin female mice were examined for ductal morphogenesis. At 8 weeks of age, the ductal tree of Hsf1+/+ mice filled the entire fat pad with substantial alveolar development (Fig. 3A) (36). The ductal tree of Hsf1+/− but specifically Hsf1−/− mice appeared to possess fewer lateral side branches. Interestingly, expression of Neu+ did not increase the number of ductal branches observed in Hsf1−/− mice, but it did increase slightly the number of ductal branches in Hsf1+/+Neu+ and Hsf1+/−Neu+ mice (Fig. 3B). Quantification of lateral side branches of the indicated genotypes is presented in Fig. 3C.
FIGURE 3.
Whole-mount preparation and histological analyses of mammary glands from mice with or without Hsf1 gene or Neu oncogene. A–C, whole-mount preparation of number 4 mammary glands from 8- to 10-week-old virgin Hsf1+/+, Hsf1+/−, and Hsf1−/− female mice, without Neu (A), or with Neu (B) expression. Arrowheads indicate branches and terminal end buds. Quantification of the lateral side branches in the three genotypes is indicated in C. Bars are mean ± S.D. Magnification is ×50. n = 3 mice. Statistical significance is as follows: **, p < 0.01 and ***, p < 0.001. D, H&E-stained cross-sections of mammary glands from the indicated genotypes. Bar, 50 μm. Arrowheads indicate tubules and branches. E and F, immunohistochemical staining of cross-sections of mammary glands from the indicated genotypes using αSMA (E) or keratin 18 (F) as the primary antibody. Arrowheads indicate some of the positively stained myoepithelial (E) or luminal epithelial cells (F) cells. Bar, 10 μm.
Mammary glands from all genotypes were analyzed for histological changes (Fig. 3D). A decrease in the number of ductal side branches is evident following histological analyses of the cross-sections of the mammary glands in mice deficient in Hsf1, but this is less evident in Hsf1+/− mice. To investigate if the expressions of αSMA, a myoepithelial marker, and keratin 18, a luminal epithelial marker, were comparable between the genotypes, IHC staining of cross-sections of the mammary glands was performed (Fig. 3, E and F). The results show that both myoepithelial cells and luminal epithelial cells were present, and the relative expression levels of αSMA and keratin 18 were comparable between the genotypes.
Hsf1 Protects Mammary Epithelial Cells from Apoptosis and Increases Their Proliferative Capacity
Hsf1 and its downstream target genes protect cells against apoptotic cell death and reverse the senescence phenotype (15, 37–39). To determine whether the reduction in ductal branching observed in Hsf1−/− mice correlated with increased epithelial cell apoptosis, we performed TUNEL assays on mammary gland tissue sections prepared from Hsf1+/+, Hsf1+/−, and Hsf1−/− mice in the presence or absence of the Neu+ transgene. Data indicate that the number of apoptotic cells is significantly increased in the absence of the Hsf1 gene (Fig. 4, A and B). The number of apoptotic cells decreased to a small extent in mice expressing the Neu transgene.
FIGURE 4.
Loss of Hsf1 affects cellular apoptosis and proliferation of mammary epithelial cells. A and B, cross-sections of mammary gland tissue sections of the indicated genotypes were stained for apoptotic cells using TUNEL. Bar, 50 μm. Red color indicates TUNEL-positive cells (arrowheads), and blue color indicates nuclear (DAPI) staining. Quantification of TUNEL-positive mammary epithelial cells for the indicated genotypes are presented in B. Bars are mean ± S.D. n = 3 mice. Statistical significance is as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001. C and D, mice of indicated genotypes were labeled with BrdU in situ. Mammary epithelial cells were immunostained with BrdU antibody. Arrowheads show some of the BrdU-positive cells. Nuclei were stained with DAPI. Bar, 50 μm. Quantification of BrdU-positive cells between the indicated genotypes is presented in D. Bars are mean ± S.D. n = 3 mice. Statistical significance is as follows: *, p < 0.05 and **, p < 0.01. E and F, mammary epithelial cells were prepared from number 4 mammary glands of 2-month-old virgin female mice for the indicated genotypes. Cultured cells were labeled with BrdU and immunostained using antibody to BrdU (arrowheads; green). Propidium iodide (red) indicate nuclei staining. Bar, 50 μm. Quantification of BrdU-positive cells between the indicated genotypes is presented in F. Bars are mean ± S.D. n = 3 mice. Statistical significance is as follows: *, p < 0.05 and **, p < 0.01.
The increase in apoptosis in mammary epithelial cells in Hsf1−/− mice also coincided with lower numbers of proliferating cells that were detected following in situ BrdU labeling of ductal epithelial cells (Fig. 4, C and D). The reduction in cellular proliferation using BrdU labeling also was present in cultured primary mammary epithelial cells prepared from Hsf1+/+, Hsf1+/− or Hsf1−/− mice with or without expression of the Neu transgene (Fig. 4, E and F). Taken together, these data indicate that ductal epithelial cells undergo increased apoptotic cell death and reduced cellular proliferation in the absence of one or both copies of the Hsf1 gene.
HSF1 Promotes EMT and Increases Epithelial Cell Migration in Response to TGFβ
TGFβ cooperates with signaling pathways such as RAS and WNT to induce EMT; this is a critical process during tumorigenesis (34). To further examine whether the EMT program is affected in Hsf1−/− mammary epithelial cells and whether this affects cellular migration, Neu+ primary cultured epithelial cells from Hsf1+/+Neu+, Hsf1+/−Neu+, and Hsf1−/−Neu+ cells were exposed to TGFβ and immunostained for the presence of E-cadherin, β-catenin, and the mesenchymal marker αSMA. The expression levels of E-cadherin and β-catenin are reduced in response to TGFβ in Hsf1+/+Neu+ cells as expected (Fig. 5, A and B). This was associated with an increase in the expression of αSMA 24–48 h following exposure of cells to TGFβ. However, reduced levels of HSF1 led to a reduction in E-cadherin and β-catenin degradation as they remain elevated in Hsf1+/− and Hsf1−/− cells compared with Hsf1+/+ cells (Fig. 5A). The level of mesenchymal marker αSMA is increased during the 48-h incubation in the presence of TGFβ in Hsf1+/+, but this was much less pronounced in Hsf1+/−Neu+ and Hsf1−/−Neu+ cells. This also was associated with an increase in fibroblast cell morphology in Hsf1+/+Neu+ cells. Immunoblot analyses confirmed activation of TGFβ signaling through activation of p-Smad 3, increase in Slug, reduction in E-cadherin (2–5-fold at 24–48 h) and β-catenin (5-fold at 24–48 h) levels, and an increase in the mesenchymal markers N-cadherin (1.5-fold at 24–48 h) and vimentin (3- and 2-fold at 24–48 h) in Hsf1+/+Neu+ cells but not in Hsf1+/−Neu+ cells. We found that exposure of cells to TGFβ increased the activities of ERK1/2 and AKT (Ser(P)-473), which are downstream of TGFβ signaling and are known inducers of EMT. The levels of the phosphorylated forms of ERK1/2 and AKT were also reduced by 3–5-fold in response to TGFβ in Hsf1+/−Neu+ cells compared with Hsf1+/+Neu+ cells, which increased by 3–5-fold relative to untreated control cells (Fig. 5D). The expression of total ERK1/2 and AKT was not significantly different between the genotypes.
FIGURE 5.
Reduction in HSF1 levels inhibits EMT and cellular migration. A–C, mammary epithelial cells were cultured from number 4 mammary glands of 2-month-old virgin female mice. Cells were exposed to TGFβ for 0, 24, or 48 h. Cells were then fixed and stained with antibody to E-cadherin (green), β-catenin (red), or αSMA (green). Propidium iodide (red) (A) and DAPI (blue) (B and C) show nuclear staining. Bar, 20 μm. D, immunoblot analyses of mammary epithelial cells prepared from Hsf1+/+ Neu+ or Hsf1+/−Neu+ mice (as in A) showing the levels of the indicated proteins following exposure to TGFβ. β-Actin is loading control. E, transwell assay. Mammary epithelial cells were cultured from number 4 mammary glands of 2-month-old virgin female mice. Cells were placed in the transwell as described under “Experimental Procedures” and allowed to migrate to the undersurface. Cells were then fixed, stained, and photographed. Quantification of the cells migrated in the undersurface is presented in the lower panel. Bars are mean ± S.D. Experiments were repeated three times. Statistical significance is as follows: **, p < 0.01; ***, p < 0.001.
EMT is critical during the progression of carcinomas to an invasive state (34). We therefore used a transwell migration assay to examine whether the reduced EMT observed in Hsf1+/− and Hsf1−/− cells reduces the migratory ability of Neu+ primary epithelial cells harboring reduced HSF1 compared with Hsf1+/+Neu+ cells. The data presented in Fig. 5E indicate that a significant reduction in the migratory ability of Neu+ primary mammary epithelial cells that are deficient in or have lower levels of HSF1. The reduced cellular migration of cells with lower levels of HSF1 and expressing Neu was evident in the presence or absence of TGFβ. Treatment of cells with TGFβ increased the ability of Hsf1+/+Neu+ cells to migrate, but it was less effective when Hsf1 level was reduced (Fig. 5E, lower panel).
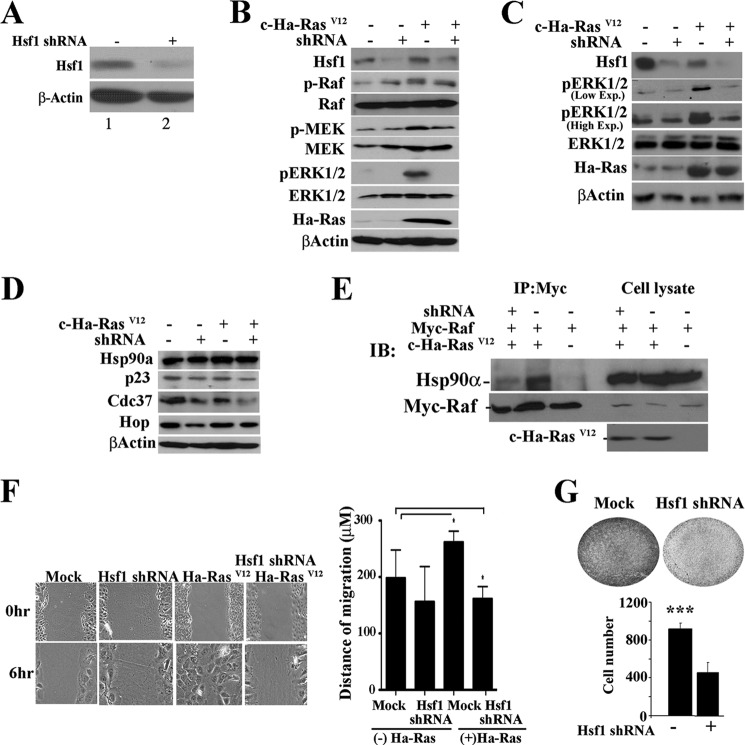
The results obtained using mouse mammary tumors expressing lower HSF1 levels or using primary epithelial cells prepared from Hsf1+/− or Hsf1−/− mammary glands suggest that they respond poorly to activate ERK1/2 even in the presence of TGFβ stimulation compared with wild-type cells. To recapitulate some of the effects observed in mouse mammary epithelial cells in the absence of the Hsf1 gene, we knocked down HSF1 in MCF10A cells using shRNA (sequence 1, see “Experimental Procedures”) (Fig. 6A). We then determined whether MCF10A cells can activate ERK1/2 using constitutively active Ha-RasVal-12 as an efficient inducer of ERK1/2 signaling pathway downstream of ErbB2, and TGFβ receptors when Hsf1 levels are reduced (33). Immunoblotting experiments show that MCF10A cells expressing Hsf1 shRNA and c-Ha-RasVal-12 exhibit significantly lower levels of p-ERK1/2 (10-fold reduction compared with MCF10A cells expressing scrambled shRNA). We also observed a 2-fold reduction in the levels of p-RAF1 and p-MEK in cells expressing Hsf1 shRNA that also expressed Ha-RasVal-12, compared with cells that expressed Ha-RasVal-12 alone. These data indicate that lowering the Hsf1 levels leads to alterations in MCF10A cells, reducing their ability to fully activate ERK1/2. No reductions in the total levels of RAF1, MEK, ERK1/2, AKT, or p-AKT were observed by immunoblotting (Fig. 6B and data not shown). We also tested another Hsf1 shRNA (sequence 2, see “Experimental Procedures”) to ensure that P-ERK1/2 was reduced in Ha-RAS-expressing cells when Hsf1 level was reduced (Fig. 6C).
FIGURE 6.
Knockdown of HSF1 in MCF10A cells affects p-ERK activity. A, immunoblot analyses showing the expression of HSF1 in MCF10A cells expressing scrambled shRNA vector (lane 1) or Hsf1-specific shRNA (lane 2). β-Actin is loading control. B–D, immunoblot analyses showing the level of indicated proteins in MCF10A cells expressing scrambled shRNA vector (−) or Hsf1-specific shRNA (+) in the presence (+) or absence (−) of c-Ha-RasVal-12. MCF10A cells were infected with retroviral vectors containing scrambled or Hsf1 shRNA, together with mock vector or vector encoding c-Ha-RasVal-12. β-Actin is loading control. The p-ERK and ERK antibodies recognize the ERK2 isoform in human MCF10A cells more efficiently. B and C represent two different Hsf1-specific shRNA. E, 293T cells were transduced with the indicated expression plasmids (+). 48 h following infection, Myc-Raf1 was immunoprecipitated (IP) using antibody to Myc tag, and immunoprecipitated materials were detected using antibody to HSP90α (upper panel) or Myc-Raf1 (lower panel). Cell lysates from the indicated groups show expression of HSP90α, Myc-Raf1, and c-Ha-RasVal-12. IB, immunoblot. F, MCF10A cells were infected with retroviral vectors expressing scrambled shRNA (Mock), Hsf1 shRNA, c-Ha-RasVal-12, or c-Ha-RasVal-12 plus Hsf1 shRNA. Cells were cultured until reaching confluence. Cells were scratched with a small sterile tip followed by incubation for 6 h at 37 ºC. Cells were photographed at 0 and 6 h. Magnification was ×100. The migration distance was determined using Axiovision LE software and is presented in F. Bars are mean ± S.D. Statistical significance is as follows: *, p < 0.05. G, 103 MCF10A cells were infected with mock vector or vector containing Hsf1 shRNA. Cells were cultured in transwell, and the number of cells migrating to the undersurface were quantified after 6 h (lower panel). Bars are mean ± S.D. Statistical significance is as follows: ***, p < 0.001.
Previous studies showed that the maturation of RAF1 is regulated by HSP90 and its co-chaperones (35, 40). HSF1 regulates the expression of HSP90a and HSP70, as well as their cochaperones, which contain heat shock elements in their promoters (13, 14). MCF10A cells expressing Hsf1 shRNA showed 20–40% reduction in the levels of Hsp90a cochaperones CDC37, p23, and HOP (Fig. 6D). To determine whether HSP90a-RAF1 complexes were affected in cells expressing Hsf1 shRNA, we ectopically expressed myc-Raf1 together with Hsf1 shRNA with or without Ha-RasVal-12. Myc-Raf1 was immunoprecipitated, and immunoblotting was performed to detect HSP90a in the complex. Fig. 6E shows that the amount of RAF1 binding to HSP90a was significantly reduced in cells expressing Hsf1 shRNA compared with cells that expressed scrambled shRNA. These data indicate a mechanism by which ERK1/2 activity may be reduced in Hsf1 shRNA-containing cells. This suggests that a small reduction in the levels of HSPs and their cochaperones affects complexes of HSP90a with their client proteins more significantly than their total expression in cells.
As noted before, the process of tumorigenesis involves increased cellular migration (34). To examine whether knockdown of Hsf1 leads to changes in MCF10A cell migration, we performed wound healing and transwell assays. Although cellular migration was comparable between mock-treated cells and cells expressing Hsf1 shRNA, cells expressing c-Ha-RasVal-12 were able to migrate significantly more distances when they expressed wild-type levels of HSF1 compared with cells expressing Hsf1 shRNA (Fig. 6, F and G). We also evaluated the ability of Hsf1 shRNA-containing cells to migrate using a transwell assay. Fig. 6G shows that MCF10A cells containing Hsf1 shRNA showed a significant reduction in migration ability compared with mock-transduced cells using the transwell assay. Taken together, the above results indicate that Hsf1 deficiency leads to reduced p-ERK1/2 activity, inefficient EMT, and reduced cellular migration in response to TGFβ in mammary epithelial cells.
DISCUSSION
We sought to determine the role of Hsf1 in breast cancer in a cohort of mice deficient in the Hsf1 gene that overexpressed the Neu oncogene in luminal epithelial cells of the mammary gland. The results show that Hsf1−/−Neu+ mice are resistant in developing breast cancer, whereas Hsf1+/−Neu+ mice exhibit some delay in the onset of breast cancer development. However, the overall tumor-free survival was not significantly delayed when compared with the Hsf1+/+Neu+ mice. Interestingly, however, Hsf1+/−Neu+ mice exhibit a significant reduction in lung metastasis compared with Hsf1+/+Neu+ mice. To elucidate the mechanism underlying the inhibition of tumorigenesis and metastasis observed in Hsf1−/− or Hsf1+/−Neu+ mice, respectively, we analyzed multiple parameters such as proliferative capacity, apoptotic rate, and EMT in the tumors and in mammary epithelial cells expressing reduced levels of the Hsf1 gene. We found alterations of multiple parameters that could reduce tumor growth and metastasis in the absence of the Hsf1 gene. We observed a significant reduction in proliferative capacity of cells deficient in HSF1 following both in situ as well as in vitro BrdU labeling of cells, and a significant increase in the in situ detection of apoptotic cell death of mammary epithelial cells in the absence of the Hsf1 gene. Other alterations that appear to significantly inhibit tumor growth and metastasis is the inability of Hsf1+/− and Hsf1−/− cells to effectively show ERK1/2 activity. ERK1/2 are downstream of ErbB2 receptor signaling, and ErbB2-positive mammary epithelial cells have been shown to require activity of MEK/ERK1/2, and not AKT, to drive the translocation of cells to the ductal lumen and their subsequent proliferation (10). Other studies have shown that expression of the active form of RAF in MCF10A acini leads to an increase in cell movement (41). ErbB2-driven AKT activation has been shown to activate NF-κB, suppressing apoptosis, and to activate mammalian target of rapamycin, leading to an increase in cellular proliferation (33). Both activation of ERK1/2 and AKT led to an increase in the expression of a number of genes, including VegfA and CCNDI (cyclin D1), that led to an increase in angiogenesis and cellular proliferation (33). We therefore anticipate that lower levels of p-ERK1/2 and p-AKT lead to increased apoptotic cell death (Fig. 1D) and a decrease in cellular proliferation (Fig. 1C) and in angiogenesis (Fig. 1E), leading to reduced tumor cell proliferation and metastasis when the level of HSF1 is reduced in Hsf1+/−Neu+ mice. There is increasing evidence that ERK1/2 (and more recently ERK2 (42)) induce EMT in mammary epithelial cells (11, 34). Therefore, reduction in ERK1/2 activity could lead to the reduction in EMT that we observe in tumors formed in Hsf1+/−Neu+ mice compared with Hsf1+/+Neu+ mice. A lower level of EMT also was observed in an in vitro response to TGFβ stimulation, which is a strong inducer of EMT. The results showed reduced activated p-ERK1/2 in Hsf1+/− Neu+ cells compared with Hsf1+/+Neu+ cells, and a reduction in EMT was associated with reduced levels of Slug transcriptional repressor in Hsf1+/−Neu+ cells, which leads to increased expression of E-cadherin and reduced expression of N-cadherin, a mesenchymal marker, as expected (11, 34). Downstream effects of reduction in EMT led to reduced cellular migration in Hsf1+/−Neu+ cells compared with Hsf1+/+Neu+ as expected.
In general, Hsf1-deficient cells are specifically deficient in inducing a heat shock response when exposed to elevated temperature or other forms of stressors (13). However, in different cell types, and dependent on whether cells are primary or express different oncogenes, Hsf1-deficient cells exhibit reduced levels of different HSPs (13, 16, 43, 44). Hsf1 is known to drive the expression of HSP110, HSP90a, HSP70 (inducible), and HSP40, as well as other heat shock element-containing genes such as p23, CDC37, and HOP, which are cochaperones for HSP90. The HSP90 complexes have been shown to be involved in ErbB2, RAF, and AKT maturation, and they are degraded when cells are exposed to HSP90 inhibitors (35, 40, 44). In addition, tumorigenesis process is known to increase the levels of molecular chaperones, and it places a higher demand on the function of molecular chaperones (45, 46). As noted before, microarray and immunoblotting analyses of Neu+ tumors, Neu+ primary mammary epithelial cells expressing lower levels of HSF1, or human mammary epithelial MCF10A cells expressing Hsf1 shRNA did not show significantly lower levels of all the components of the HSP90 machinery. However, we noted lower levels of HSP70 and HSP90a in Hsf1+/−Neu+ tumors by 20–40% and no significant reduction in HOP, CDC37, or p23. In MCF10A cells expressing Hsf1 shRNA, we observed 20–40% reduction in the level of HSP90 cochaperones CDC37, HOP, and p23. Interestingly, however, immunoprecipitation experiments show a significant reduction in RAF1 association with HSP90a in cells expressing Hsf1 shRNA if there is some perturbation in the expression of HSPs or their cochaperones. We therefore anticipate that lower HSF1 levels leads to a base-line reduction in multiple chaperones, causing improper formation of HSP90 with its client proteins, such as RAF1, leading to inefficient activation of RAS/RAF/MEK/ERK1/2 signaling. Although AKT is also a client protein of Hsp90, a comparable reduction in pAKT levels was not observed in MCF10A. We therefore envision where reduction in HSF1 levels could impact ErbB2 and TGFβ signaling pathways, affecting tumor growth. Inhibition of HSP90 leads to degradation of its many client proteins such as RAF1, ErbB2 (and other receptor tyrosine kinases), and AKT as has been reported previously (35). Hsf1 deficiency reduces the levels of HSP90a and other chaperones such as HSP70 and their client proteins. Although the client proteins may not be degraded entirely, there is a reduction in their levels (such as RAF1 presented here) in chaperone complexes. This leads to reduced signaling through receptor tyrosine kinases and reduced tumor growth or metastasis. Recent studies show that inhibition of HSP90 using geldanamycin reduced levels of TGFβ receptor and signaling leading to reduction in TGFβ-induced p-Smad3 (47) and EMT (48). Although in both studies induction of Hsf1-induced HSP70 was associated with this effect and this would not be relevant in our studies where Hsf1 has been deleted, nevertheless, a reduction in the levels of HSP90 and/or its cochaperones as presented in our studies could potentially reduce TGFβ-induced signaling through the TGFβ receptor leading to a reduction in EMT. Significant reduction in mammary tumorigenesis and metastasis in the absence of the Hsf1 gene in Hsf1−/−Neu+ or Hsf1+/−Neu+ mice could be due to the reduced level of expression and signaling through the ErbB2 receptor. Previous reports indicate that overexpression of Her2 in MCF10A cells carrying Hsf1 shRNA leads to reduced ability of cells to grow on soft agar or in nude mice (49). The mechanism proposed was higher levels of p21 and down-regulation of HSP70 and HSP27. In addition, using the constitutively active form of Her2, it has recently been shown that Hsf1−/− mice exhibit resistance to tumorigenesis (50). The underlying mechanism is thought to be suppression of angiogenesis through Hsf1 regulation of the HuR-HIF1 pathway. The above data are consistent with our results suggesting that Hsf1 deficiency leads to reduced levels of HSPs, leading to inhibition of critical signaling molecules that are known clients of Hsf1 downstream targets leading to suppression of tumorigenesis.
This work was supported, in whole or in part, by National Institutes of Health Grants CA062130 and CA132640 (to N. F. M.) and CA121951 (to D. M.). Georgia Health Sciences University was formerly known as the Medical College of Georgia.
- αSMA
- α-smooth muscle actin
- EMT
- epithelial-mesenchymal transition
- Hsp
- heat shock protein
- IHC
- immunohistochemical.
REFERENCES
- 1. Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244, 707–712 [DOI] [PubMed] [Google Scholar]
- 2. Lee E. Y., Muller W. J. (2010) Oncogenes and tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2, a003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weigelt B., Peterse J. L., van 't Veer L. J. (2005) Breast cancer metastasis. Markers and models. Nat. Rev. Cancer 5, 591–602 [DOI] [PubMed] [Google Scholar]
- 4. Ciocca D. R., Gago F. E., Fanelli M. A., Calderwood S. K. (2006) Co-expression of steroid receptors (estrogen receptor α and/or progesterone receptors) and Her-2/neu. Clinical implications. J. Steroid Biochem. Mol. Biol. 102, 32–40 [DOI] [PubMed] [Google Scholar]
- 5. Foley J., Nickerson N. K., Nam S., Allen K. T., Gilmore J. L., Nephew K. P., Riese D. J., 2nd (2010) EGFR signaling in breast cancer. Bad to the bone. Semin. Cell Dev. Biol. 21, 951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polyak K., Weinberg R. A. (2009) Transitions between epithelial and mesenchymal states. Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265–273 [DOI] [PubMed] [Google Scholar]
- 7. Iwatsuki M., Mimori K., Yokobori T., Ishi H., Beppu T., Nakamori S., Baba H., Mori M. (2010) Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 101, 293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu D., Hung M. C. (2000) Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 19, 6115–6121 [DOI] [PubMed] [Google Scholar]
- 9. Peinado H., Portillo F., Cano A. (2004) Transcriptional regulation of cadherins during development and carcinogenesis. Int. J. Dev. Biol. 48, 365–375 [DOI] [PubMed] [Google Scholar]
- 10. Leung C. T., Brugge J. S. (2012) Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature 482, 410–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thiery J. P. (2003) Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15, 740–746 [DOI] [PubMed] [Google Scholar]
- 12. Lu J., Guo H., Treekitkarnmongkol W., Li P., Zhang J., Shi B., Ling C., Zhou X., Chen T., Chiao P. J., Feng X., Seewaldt V. L., Muller W. J., Sahin A., Hung M. C., Yu D. (2009) 14-3-3ζ cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial-mesenchymal transition. Cancer Cell 16, 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y., Huang L., Zhang J., Moskophidis D., Mivechi N. F. (2002) Targeted disruption of hsf1 leads to lack of thermotolerance and defines tissue-specific regulation for stress-inducible Hsp molecular chaperones. J. Cell. Biochem. 86, 376–393 [DOI] [PubMed] [Google Scholar]
- 14. Morimoto R. I. (1998) Regulation of the heat shock transcriptional response. Cross-talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12, 3788–3796 [DOI] [PubMed] [Google Scholar]
- 15. Akerfelt M., Morimoto R. I., Sistonen L. (2010) Heat shock factors. Integrators of cell stress, development, and life span. Nat. Rev. Mol. Cell Biol. 11, 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin X., Moskophidis D., Mivechi N. F. (2011) Heat shock transcription factor 1 is a key determinant of HCC development by regulating hepatic steatosis and metabolic syndrome. Cell Metab. 14, 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Min J. N., Huang L., Zimonjic D. B., Moskophidis D., Mivechi N. F. (2007) Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene 26, 5086–5097 [DOI] [PubMed] [Google Scholar]
- 18. Xiao X., Zuo X., Davis A. A., McMillan D. R., Curry B. B., Richardson J. A., Benjamin I. J. (1999) HSF1 is required for extra-embryonic development, postnatal growth, and protection during inflammatory responses in mice. EMBO J. 18, 5943–5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christians E., Davis A. A., Thomas S. D., Benjamin I. J. (2000) Maternal effect of Hsf1 on reproductive success. Nature 407, 693–694 [DOI] [PubMed] [Google Scholar]
- 20. Wang G., Ying Z., Jin X., Tu N., Zhang Y., Phillips M., Moskophidis D., Mivechi N. F. (2004) Essential requirement for both hsf1 and hsf2 transcriptional activity in spermatogenesis and male fertility. Genesis 38, 66–80 [DOI] [PubMed] [Google Scholar]
- 21. Dai C., Whitesell L., Rogers A. B., Lindquist S. (2007) Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 130, 1005–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santagata S., Hu R., Lin N. U., Mendillo M. L., Collins L. C., Hankinson S. E., Schnitt S. J., Whitesell L., Tamimi R. M., Lindquist S., Ince T. A. (2011) High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc. Natl. Acad. Sci. U.S.A. 108, 18378–18383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khaleque M. A., Bharti A., Sawyer D., Gong J., Benjamin I. J., Stevenson M. A., Calderwood S. K. (2005) Induction of heat shock proteins by heregulin β1 leads to protection from apoptosis and anchorage-independent growth. Oncogene 24, 6564–6573 [DOI] [PubMed] [Google Scholar]
- 24. Calderwood S. K. (2010) Heat shock proteins in breast cancer progression. A suitable case for treatment? Int. J. Hyperthermia 26, 681–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khaleque M. A., Bharti A., Gong J., Gray P. J., Sachdev V., Ciocca D. R., Stati A., Fanelli M., Calderwood S. K. (2008) Heat shock factor 1 represses estrogen-dependent transcription through association with MTA1. Oncogene 27, 1886–1893 [DOI] [PubMed] [Google Scholar]
- 26. Zhao Y. H., Zhou M., Liu H., Ding Y., Khong H. T., Yu D., Fodstad O., Tan M. (2009) Up-regulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene 28, 3689–3701 [DOI] [PubMed] [Google Scholar]
- 27. Guy C. T., Webster M. A., Schaller M., Parsons T. J., Cardiff R. D., Muller W. J. (1992) Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. U.S.A. 89, 10578–10582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bian D., Mahanivong C., Yu J., Frisch S. M., Pan Z. K., Ye R. D., Huang S. (2006) The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene 25, 2234–2244 [DOI] [PubMed] [Google Scholar]
- 29. Hu Y., Mivechi N. F. (2006) Association and regulation of heat shock transcription factor 4b with both extracellular signal-regulated kinase mitogen-activated protein kinase and dual-specificity tyrosine phosphatase DUSP26. Mol. Cell. Biol. 26, 3282–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elston C. W., Ellis I. O. (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer. Experience from a large study with long term follow-up. Histopathology 19, 403–410 [DOI] [PubMed] [Google Scholar]
- 31. Eroglu B., Moskophidis D., Mivechi N. F. (2010) Loss of Hsp110 leads to age-dependent τ hyperphosphorylation and early accumulation of insoluble amyloid β. Mol. Cell. Biol. 30, 4626–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Homma S., Jin X., Wang G., Tu N., Min J., Yanasak N., Mivechi N. F. (2007) Demyelination, astrogliosis, and accumulation of ubiquitinated proteins, hallmarks of CNS disease in hsf1-deficient mice. J. Neurosci. 27, 7974–7986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baselga J., Swain S. M. (2009) Novel anticancer targets. Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 9, 463–475 [DOI] [PubMed] [Google Scholar]
- 34. Thiery J. P., Acloque H., Huang R. Y., Nieto M. A. (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 [DOI] [PubMed] [Google Scholar]
- 35. Citri A., Kochupurakkal B. S., Yarden Y. (2004) The achilles heel of ErbB-2/HER2. Regulation by the Hsp90 chaperone machine and potential for pharmacological intervention. Cell Cycle 3, 51–60 [PubMed] [Google Scholar]
- 36. Watson C. J., Khaled W. T. (2008) Mammary development in the embryo and adult. A journey of morphogenesis and commitment. Development 135, 995–1003 [DOI] [PubMed] [Google Scholar]
- 37. Meng L., Hunt C., Yaglom J. A., Gabai V. L., Sherman M. Y. (2011) Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene 30, 2836–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salinthone S., Tyagi M., Gerthoffer W. T. (2008) Small heat shock proteins in smooth muscle. Pharmacol. Ther. 119, 44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jego G., Hazoumé A., Seigneuric R., Garrido C. (2010) Targeting heat shock proteins in cancer. Cancer Lett., in press [DOI] [PubMed] [Google Scholar]
- 40. Okawa Y., Hideshima T., Steed P., Vallet S., Hall S., Huang K., Rice J., Barabasz A., Foley B., Ikeda H., Raje N., Kiziltepe T., Yasui H., Enatsu S., Anderson K. C. (2009) SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood 113, 846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pearson G. W., Hunter T. (2007) Real-time imaging reveals that noninvasive mammary epithelial acini can contain motile cells. J. Cell Biol. 179, 1555–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shin S., Dimitri C. A., Yoon S. O., Dowdle W., Blenis J. (2010) ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol. Cell 38, 114–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jin X., Moskophidis D., Hu Y., Phillips A., Mivechi N. F. (2009) Heat shock factor 1 deficiency via its downstream target gene αB-crystallin (Hspb5) impairs p53 degradation. J. Cell Biochem. 107, 504–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schulz R., Marchenko N. D., Holembowski L., Fingerle-Rowson G., Pesic M., Zender L., Dobbelstein M., Moll U. M. (2012) Inhibiting the HSP90 chaperone destabilizes macrophage migration inhibitory factor and thereby inhibits breast tumor progression. J. Exp. Med. 209, 275–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jolly C., Morimoto R. I. (2000) Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J. Natl. Cancer Inst. 92, 1564–1572 [DOI] [PubMed] [Google Scholar]
- 46. Mosser D. D., Morimoto R. I. (2004) Molecular chaperones and the stress of oncogenesis. Oncogene 23, 2907–2918 [DOI] [PubMed] [Google Scholar]
- 47. Yun C. H., Yoon S. Y., Nguyen T. T., Cho H. Y., Kim T. H., Kim S. T., Kim B. C., Hong Y. S., Kim S. J., Lee H. J. (2010) Geldanamycin inhibits TGF-β signaling through induction of Hsp70. Arch. Biochem. Biophys. 495, 8–13 [DOI] [PubMed] [Google Scholar]
- 48. Li Y., Kang X., Wang Q. (2011) HSP70 decreases receptor-dependent phosphorylation of Smad2 and blocks TGF-β-induced epithelial-mesenchymal transition. J. Genet. Genomics 38, 111–116 [DOI] [PubMed] [Google Scholar]
- 49. Meng L., Gabai V. L., Sherman M. Y. (2010) Heat-shock transcription factor HSF1 has a critical role in human epidermal growth factor receptor-2-induced cellular transformation and tumorigenesis. Oncogene 29, 5204–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gabai V. L., Meng L., Kim G., Mills T. A., Benjamin I. J., Sherman M. Y. (2012) Heat shock transcription factor Hsf1 is involved in tumor progression via regulation of hypoxia-inducible factor 1 and RNA-binding protein HuR. Mol. Cell. Biol. 32, 929–940 [DOI] [PMC free article] [PubMed] [Google Scholar]