Background: Syndecan-1 regulates migration and adhesion in the lung epithelium.
Results: The transmembrane domain modulates focal adhesion disassembly, whereas the extracellular domain regulates cell adhesion.
Conclusion: Lung epithelial migration is governed by the syndecan-1 transmembrane domain and is independent of the extracellular domain control of cell adhesion.
Significance: Syndecan-1 is a receptor that uses multiple domains to facilitate its biological function.
Keywords: Cell Adhesion, Cell Migration, Cell Motility, Integrin, Proteoglycan
Abstract
Syndecan-1 is a cell surface proteoglycan that can organize co-receptors into a multimeric complex to transduce intracellular signals. The syndecan-1 core protein has multiple domains that confer distinct cell- and tissue-specific functions. Indeed, the extracellular, transmembrane, and cytoplasmic domains have all been found to regulate specific cellular processes. Our previous work demonstrated that syndecan-1 controls lung epithelial migration and adhesion. Here, we identified the necessary domains of the syndecan-1 core protein that modulate its function in lung epithelial repair. We found that the syndecan-1 transmembrane domain has a regulatory function in controlling focal adhesion disassembly, which in turn controls cell migration speed. In contrast, the extracellular domain facilitates cell adhesion through affinity modulation of α2β1 integrin. These findings highlight the fact that syndecan-1 is a multidimensional cell surface receptor that has several regulatory domains to control various biological processes. In particular, the lung epithelium requires the syndecan-1 transmembrane domain to govern cell migration and is independent from its ability to control cell adhesion via the extracellular domain.
Introduction
The syndecan family of proteoglycans is composed of four mammalian members with unique distribution and function (1, 2). Syndecan-1–4 are type I transmembrane proteins and, like all proteoglycans, are decorated with glycosaminoglycan side chains (3). The distinct tissue and cellular distribution of the various syndecan family members could account for their non-overlapping functions, but increasing data also suggest that the core protein has unique features that tailor specific roles (4–11).
The syndecan proteins are highly conserved across species. However, individual syndecan proteoglycans have distinct features compared with other family members. The ectodomain is the most unique region across the different syndecan proteins, varying in length and sequence homology (1). Although all of the syndecans have heparan sulfate glycosaminoglycans attached to the extracellular domain, syndecan-1 and syndecan-3 also have chondroitin sulfate side chains. The short cytoplasmic tail contains a variable stretch of sequences (V region) flanked by two highly conserved regions (C1 and C2). Finally, the single-pass transmembrane domain, although relatively conserved among family members, has enough differences to confer unique functions (12).
Although found on most cells in the body, syndecan-1 is most abundant on epithelial and plasma cells (13). Shedding of the syndecan-1 ectodomain is an important mechanism for controlling biological processes such as inflammation (14–17). However, syndecan-1 also functions as a cell surface receptor and governs in vivo processes such as wound repair, gut barrier function, and lipid metabolism (18–23). In contrast to homeostatic functions, malignant cells also co-opt syndecan-1 to regulate cell invasion, angiogenesis, and tumorigenesis (8, 10, 11, 24).
Syndecan-1 has cell- and tissue-specific functions. A prime example is the fact that syndecan-1 augments epithelial cell migration in skin and cornea but restrains migration in lungs (18–20, 25). The divergent actions of syndecan-1 are thought to be largely controlled by the cellular context, where the appropriate co-receptor is presented along with syndecan-1 to create a signaling complex that can then transduce extracellular clues to modulate cellular function (26). Accordingly, the multiple domains of syndecan-1 have different effects in regulating these processes. Although some studies have demonstrated that the extracellular domain facilitates cell spreading (4, 9, 10), others have found that the syndecan-1 cytoplasmic domain is the requisite portion (27–29).
Our previous work showed that syndecan-1 regulates both cell adhesion and migration (18). Here, we used wild-type and mutant syndecan-1 constructs to map out the relevant domains of the core protein that govern the phenotypic response in the lung epithelium. We demonstrate that the syndecan-1 extracellular domain controls cell adhesion through α2β1 integrin affinity modulation. However, the extracellular domain by itself has no effect on cell migration and requires the transmembrane domain to control migration speed and focal adhesion disassembly.
EXPERIMENTAL PROCEDURES
Cloning
The creation of mutant syndecan-1 was described previously (8, 11, 30). cDNAs of various mouse syndecan-1 constructs were subcloned into adeno-associated virus (AAV)2-internal ribosome entry site (IRES)-enhanced GFP (eGFP), AAV-IRES-mCherry, and pBMN-IRES-blasticidin vectors using BamHI and XhoI digestion. AAV vectors with the respective mutant mouse syndecan-1 were produced following the manufacturer's protocol (AAV-DJ helper-free expression system, Cell Biolabs, San Diego, CA). Retroviral vectors were created as described previously using the PhiNx packaging cell line (18). All subcloned plasmid DNA sequences were confirmed by DNA sequencing.
Cell Culture
BEAS-2b cells, a non-malignant immortalized human bronchial epithelial cell line, were cultured in fully supplemented bronchial epithelial growth medium (Lonza, Walkersville, MD). The creation of BEAS-2b cells stably expressing human syndecan-1 shRNA (B2bshRNA.hSdc1) or scrambled control shRNA (B2bshRNA.scr) was validated previously (18). AAV transduction was used for transient overexpression of mutant mouse syndecan-1. Retroviral vectors were used to stably transduce mutant mouse syndecan-1, and cells were maintained in bronchial epithelial growth medium plus blasticidin (10 μg/ml).
Migration Assay
Monolayers of B2bshRNA.scr and B2bshRNA.hSdc1 cells were plated on No. 1.5 chambered coverglass (Corning, Union City, CA) coated with rat tail type I collagen (2 μg/cm2; BD Biosciences). Monolayers were wounded with a sterile P100 pipette tip, and migration was observed under a Nikon TiE inverted widefield fluorescence microscope, which has a humidified chamber to maintain cells at 37 °C and 5% CO2. Differential interference contrast images were obtained every 10–20 min for up to 12 h using a CFI 60×/1.49 NA Apo TIRF oil immersion objective. Migration speed was determined by manually measuring the distance traveled by the cell front over time using Nikon Elements AR software. In transient transduction experiments, cells expressing mutant syndecan-1 were identified by coexpression of eGFP.
Focal Adhesion Disassembly Assays
B2bshRNA.scr and B2bshRNA.hSdc1 cells stably expressing paxillin-eGFP were created previously (31). Cells were transduced with an AAV vector to coexpress mouse syndecan-1 and mCherry. To determine the focal adhesion disassembly rate in migrating cells, monolayers of cells were injured and observed by total internal reflection fluorescence (TIRF) microscopy using a CFI 60×/1.49 NA Apo TIRF oil immersion objective. Additionally, focal adhesion disassembly was induced with a nocodazole washout assay (32, 33). The fluorescence intensity of the focal adhesion was measured over the entire life span (NIH ImageJ). All data were normalized so that the fluorescence intensity was a percentage of the maximum intensity for the individual focal adhesion. The rate of focal adhesion disassembly was determined for mCherry-expressing cells as described previously (31, 32, 34).
Fluorescence Recovery after Photobleaching
A Nikon A1R scanning laser confocal microscope using a CFI 60×/1.40 NA Plan Apo VC oil immersion objective fitted with a Tokai Hit stage-top incubator to maintain cells in a humidified chamber at 37 °C and 5% CO2 was used for fluorescence recovery after photobleaching experiments. Cells were transduced with an AAV vector to coexpress mouse syndecan-1 and mCherry. Migrating B2bshRNA.scr and B2bshRNA.hSdc1 cells stably expressing paxillin-eGFP had leading edge focal adhesions selectively photobleached in mCherry-expressing cells. With the 405 and 488 nm lasers set to 50% power, a 1-s pulse was applied over the entire focal adhesion, which would consistently photobleach the focal adhesion to 80% of the prebleaching intensity. The fluorescence recovery was measured with Nikon Elements AR software, and the half-time of recovery was determined as described previously (31).
Integrin Activation Experiments
Assays measuring cell adhesion to type I collagen were performed following published protocols (18). So that data from independent experiments could be compared, all adhesion data were normalized to the percentage of adherent B2bshRNA.scr cells. Additionally, cells stably expressing mutant mouse syndecan-1 were stained with activation state-specific antibodies (clones 12G10 and TS2/16) to determine the affinity state of the β1 integrin subunit. Flow cytometry was performed with a Guava bench-top flow cytometer (Millipore, Billerica, MA).
RESULTS
Syndecan-1 Transmembrane Domain Restrains Cell Migration
Syndecan-1 restrains migration of lung epithelial cells (18). Here, we used shRNA to suppress syndecan-1 expression in lung epithelial cells and found that cells with suppressed syndecan-1 expression (B2bshRNA.hSdc1) had a faster migration speed than cells expressing a control shRNA (B2bshRNA.scr) (7.56 ± 0.47 μm/h versus 4.99 ± 0.44 μm/hr; p < 0.0005) (Fig. 1 and supplemental Video 1).
FIGURE 1.
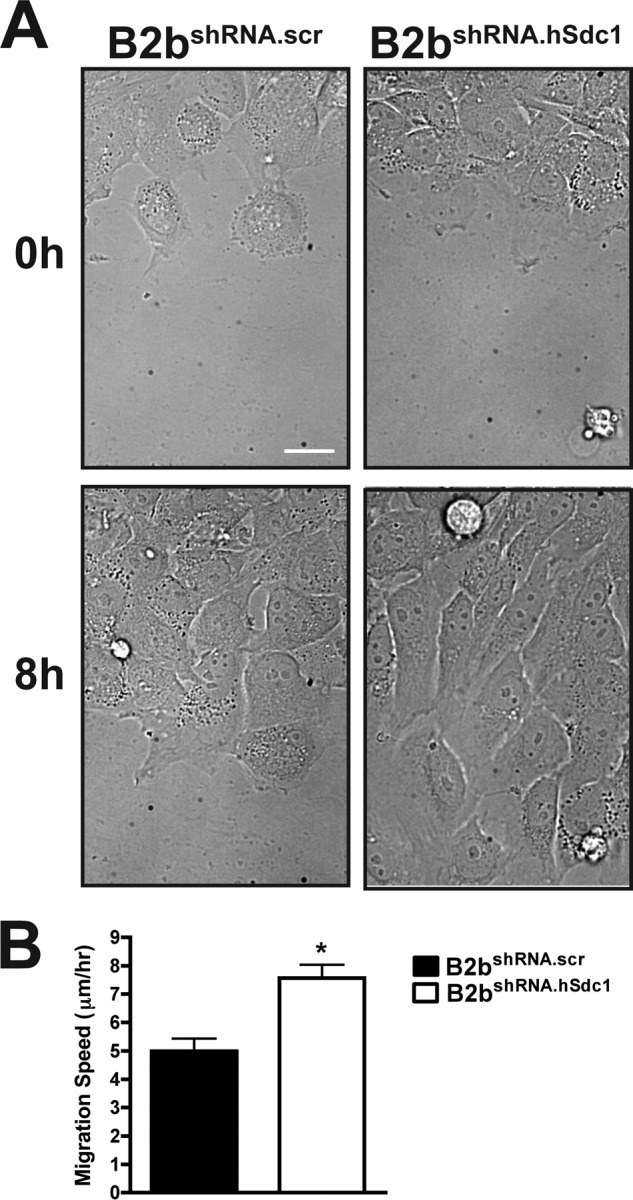
Syndecan-1 restrains cell migration. A, monolayers of B2bshRNA.scr and B2bshRNA.hSdc1 cells were injured, and the wound closure was observed over time. See supplemental Video 1 for the entire image series. Scale bar = 20 μm. B, migration speed was measured for cells at the injury front. *, p < 0.0005 by Student's t test (n = four independent experiments). Migration speed for a minimum of 10 cells was measured for each condition in each experiment.
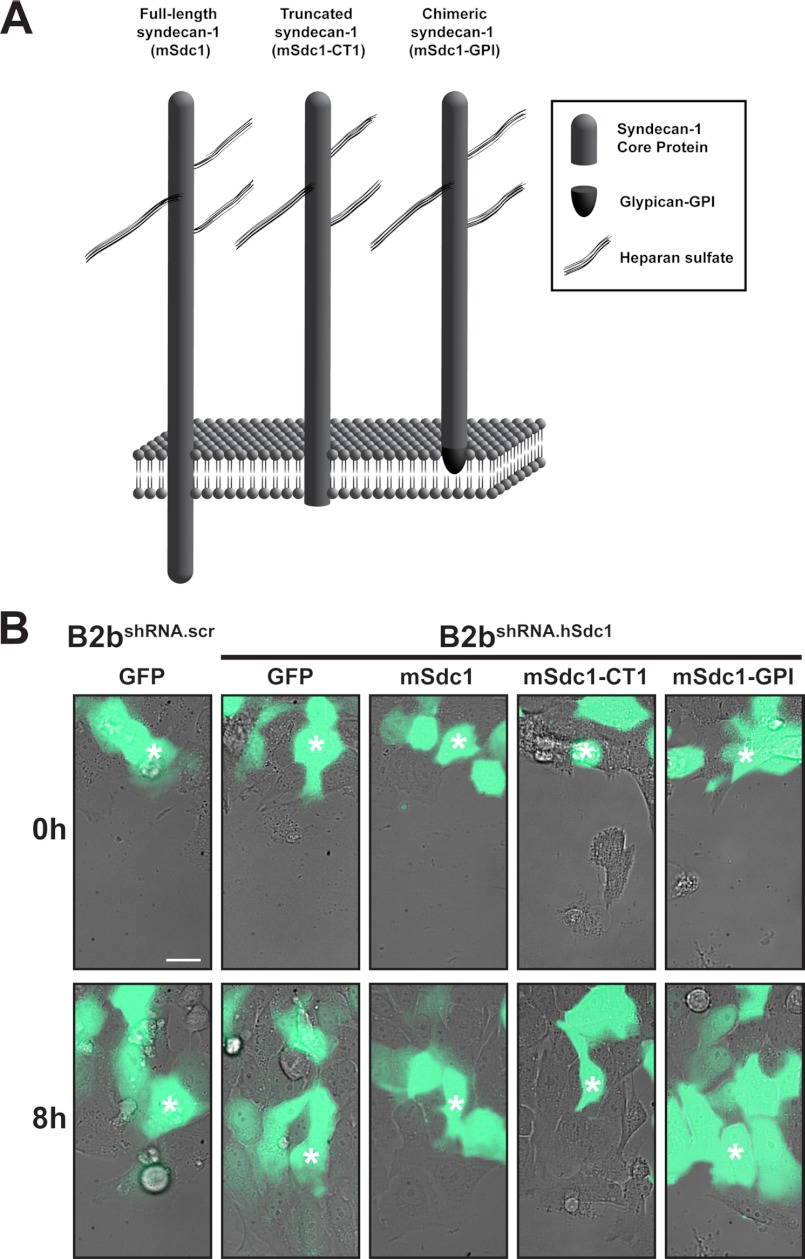
To map the domains of the syndecan-1 core protein that control cell migration, we expressed wild-type and mutant syndecan-1 cDNAs into B2bshRNA.hSdc1 cells and compared their migration speed with that of control B2bshRNA.scr and B2bshRNA.hSdc1 cells (eGFP expression only). In contrast to full-length syndecan-1 (mSdc1, 311-amino acid core protein), mSdc1-CT1 lacked the cytoplasmic domain (amino acids 1–279), and mSdc1-GPI lacked both the transmembrane and cytoplasmic domains (amino acids 1–252) (Fig. 2A) (10). The entire syndecan-1 extracellular domain was present in all of the constructs. The expression levels of full-length and mutant mSdc1 proteins were similar under all conditions (supplemental Fig. S1).
FIGURE 2.
Transmembrane domain of syndecan-1 regulates migration speed. A, schematic of wild-type and mutant syndecan-1 used in these experiments. B, B2bshRNA.scr and B2bshRNA.hSdc1 cells transduced as indicated were injured, and migration of eGFP cells was observed. White asterisks identify the same cell at 0 and 8 h after injury. See supplemental Video 2 for the entire image series. Scale bar = 20 μm.
We found that B2bshRNA.hSdc1 cells migrated faster than B2bshRNA.scr cells (Fig. 2B, Table 1, and supplemental Video 2). Both mSdc1 and mSdc1-CT1 slowed migration of B2bshRNA.hSdc1 cells to a similar rate compared with B2bshRNA.scr cells. Only mSdc1-GPI, which contains the entire syndecan-1 ectodomain but is anchored to the cell membrane by a glycosylphosphatidylinositol domain (11, 35–37), lacked the ability to slow cell migration (Table 1), indicating that the transmembrane region is the crucial domain regulating lung epithelial migration.
TABLE 1.
Syndecan-1 domains and migration speed
B2bshRNA.hSdc1 and B2bshRNA.hSdc1 + mSdc1-GPI cells were significantly faster than B2bshRNA.scr cells by one-way ANOVA and Bonferroni post hoc analysis (p < 0.01; n = three independent experiments). Migration speed for a minimum of 10 cells was measured for each condition in each experiment. All data are presented as means ± S.E.
| Cell line | Migration speed |
|---|---|
| μm/h | |
| B2bshRNA.scr | 4.57 ± 0.40 |
| B2bshRNA.hSdc1 | 6.45 ± 0.48 |
| B2bshRNA.hSdc1 + mSdc1 | 3.67 ± 0.36 |
| B2bshRNA.hSdc1 + mSdc1-CT1 | 4.07 ± 0.34 |
| B2bshRNA.hSdc1 + mSdc1-GPI | 6.47 ± 0.44 |
To ensure that the glycosylphosphatidylinositol anchor did not have any inhibitor actions on syndecan-1, we used a mutant syndecan-1 that had polyleucine substitutions in the transmembrane domain (mSdc1-pL) (supplemental Fig. S2) (38). In concordance with the data from the mSdc1-CT1 and mSdc1-GPI mutants, the mSdc1-pL mutant did not slow migration of B2bshRNA.hSdc1 cells.
Syndecan-1 Transmembrane Domain Controls Focal Adhesion Disassembly
Cell migration speed is largely determined by the focal adhesion turnover rate (32, 37, 39). Our previous work demonstrated syndecan-1 restrains cell migration by slowing focal adhesion disassembly (31). Therefore, we evaluated the disassembly of focal adhesions in migrating cells (Fig. 3). Kymographs of individual focal adhesions best represented the turnover through time (Fig. 3A). Moreover, the intensity of the focal adhesion was measured, and the downward slope of intensity correlated with the disassembly rate (Fig. 3B) (31, 32, 37). Migrating B2bshRNA.hSdc1 cells were compared with B2bshRNA.scr cells and had a significantly shorter focal adhesion life span and faster disassembly (Table 2), consistent with our prior observations (31). Whereas mSdc1-GPI had no effect, mSdc1 and mSdc1-CT1 both reversed the focal adhesion life span and disassembly differences in B2bshRNA.hSdc1 cells to become more similar to those in B2bshRNA.scr cells, which trends with the pattern seen with the migration speed.
FIGURE 3.
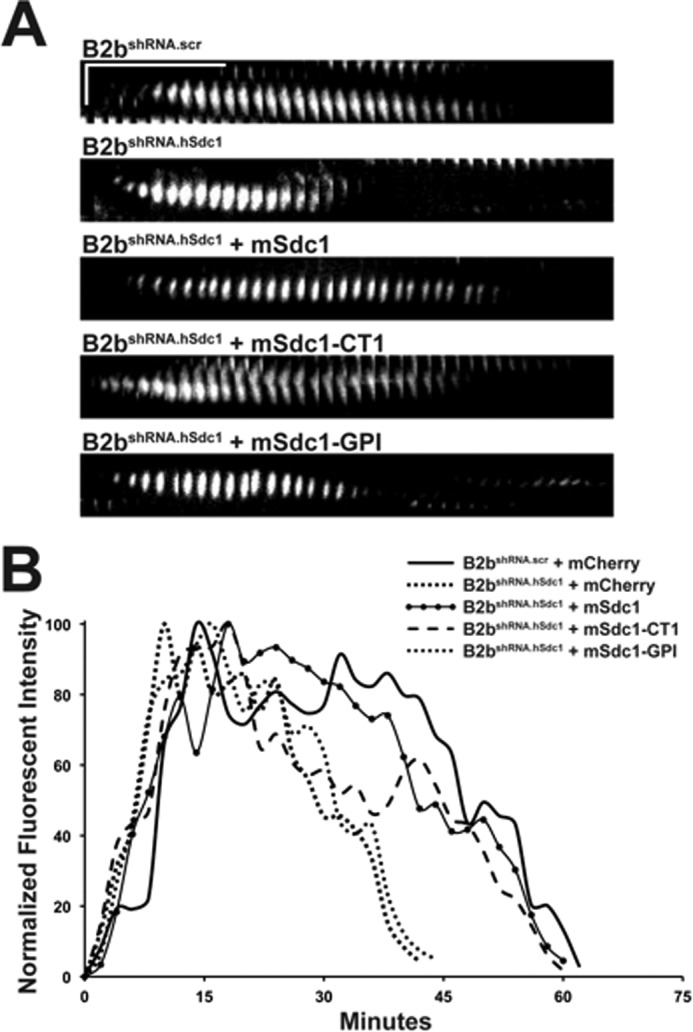
Syndecan-1 transmembrane domain slows focal adhesion disassembly in migrating lung epithelial cells. Cells stably expressing paxillin-eGFP were injured, and the turnover of focal adhesions in migrating cells was evaluated. A, kymographs of a focal adhesion at the leading edge of a migrating cell. Horizontal line = 10 min; vertical line = 5 μm. B, the normalized intensity of the focal adhesion in A was measured and plotted over time.
TABLE 2.
Syndecan-1 domains and focal adhesion dynamics
All data are presented as means ± S.E.
| Cell line | Life spana | Disassembly rateb |
|
|---|---|---|---|
| Migrating cella | Nocodazole washoutc | ||
| min | normalized intensity/min | ||
| B2bshRNA.scr | 49.05 ± 1.81 | −3.23 ± 0.18 | −7.93 ± 0.74 |
| B2bshRNA.hSdc1 | 35.08 ± 1.49 | −6.10 ± 0.51 | −13.02 ± 0.85 |
| B2bshRNA.hSdc1 + mSdc1 | 48.96 ± 1.76 | −3.60 ± 0.28 | −8.64 ± 0.61 |
| B2bshRNA.hSdc1 + mSdc1-CT1 | 50.43 ± 1.76 | −3.77 ± 0.21 | −10.38 ± 0.65 |
| B2bshRNA.hSdc1 + mSdc1-GPI | 35.70 ± 1.35 | −5.13 ± 0.31 | −16.13 ± 1.28 |
a B2bshRNA.hSdc1 and B2bshRNA.hSdc1 + mSdc1-GPI cells were significantly different from B2bshRNA.scr cells by one-way ANOVA and Bonferroni post hoc analysis (p < 0.001; n = four independent experiments).
b The slope of the descending limb of the normalized intensity curve (Figs. 3B and 4F) reflects the disassembly rate. A more negative value equals faster focal adhesion disassembly. A minimum of 10 focal adhesions were measured for each condition in each experiment.
c B2bshRNA.hSdc1 and B2bshRNA.hSdc1 + mSdc1-GPI cells were significantly different from B2bshRNA.scr cells by one-way ANOVA and Bonferroni post hoc analysis (p < 0.01; n = three independent experiments).
Microtubules target focal adhesions and induce disassembly (32, 33). Forced disassembly of focal adhesions occurred within minutes of microtubule polymerization after nocodazole washout (Fig. 4, A–E). The intensity of the focal adhesion was measured (Fig. 4F), and the disassembly rate was calculated (Table 2). B2bshRNA.hSdc1 cells had faster disassembly of focal adhesions compared with B2bshRNA.scr cells. These findings are congruous with the focal adhesion disassembly rate of migrating cells (31). Moreover, the transmembrane domain once again was the relevant domain that could reverse the accelerated focal adhesion disassembly rate in B2bshRNA.hSdc1 cells (Table 2).
FIGURE 4.
Syndecan-1 transmembrane domain attenuates focal adhesion disassembly after nocodazole washout. Cells stably expressing paxillin-eGFP were treated with nocodazole (10 μm, 4 h), and the disassembly of focal adhesions was evaluated after washout. A–E, focal adhesion disassembly was observed in B2bshRNA.scr cells, B2bshRNA.hSdc1 cells, and B2bshRNA.hSdc1 cells expressing wild-type and mutant mouse syndecan-1. F, intensity curves of the focal adhesions in A–E.
Syndecan-1 Transmembrane Domain Facilitates Exchange of Adhesion Complex Proteins
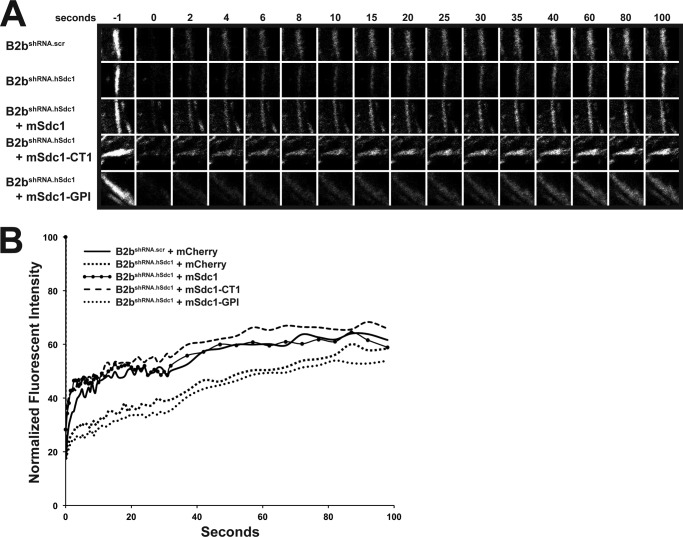
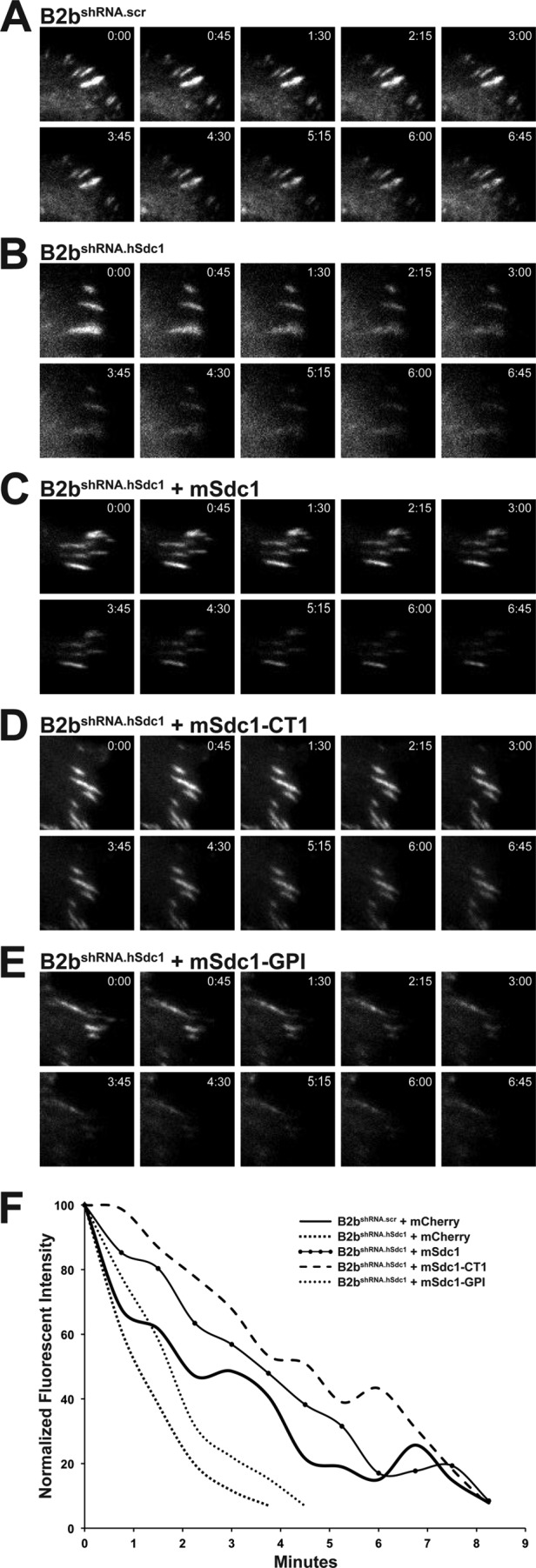
Cycling of adhesion complex proteins (e.g. paxillin and focal adhesion kinase) between focal adhesions and the cytosol controls migration speed by altering the stability of the focal adhesions (31–34, 40). Fluorescence recovery after photobleaching was used to evaluate the exchange rate of adhesion complex proteins (Fig. 5, A and B). Consistent with our previous observations (31), the dynamic cycling of paxillin was faster in B2bshRNA.scr cells than in B2bshRNA.hSdc1 cells (Table 3). The kinetics of adhesion complex protein exchange was reversed and similar to those in B2bshRNA.scr cells only when mSdc1 and mSdc1-CT1 were introduced into B2bshRNA.hSdc1 cells.
FIGURE 5.
Syndecan-1 transmembrane domain facilitates recovery of fluorescence after photobleaching in migrating lung epithelial cells. Cells stably expressing paxillin-eGFP were injured, and the focal adhesion at the leading edge of the migrating cells was selectively photobleached. A, focal adhesion prior to (−1 s) and after photobleaching. B, normalized intensity of the representative focal adhesion in A plotted over time.
TABLE 3.
Syndecan-1 domains and fluorescence recovery half-time
All data are presented as means ± S.E.
| Cell line | t½a |
|---|---|
| s | |
| B2bshRNA.scr | 15.50 ± 2.43 |
| B2bshRNA.hSdc1 | 34.17 ± 1.53 |
| B2bshRNA.hSdc1 + mSdc1 | 28.41 ± 2.32 |
| B2bshRNA.hSdc1 + mSdc1-CT1 | 24.17 ± 2.72 |
| B2bshRNA.hSdc1 + mSdc1-GPI | 39.35 ± 2.21 |
a B2bshRNA.hSdc1 and B2bshRNA.hSdc1 + mSdc1-GPI cells were significantly longer than B2bshRNA.scr cells by one-way ANOVA and Bonferroni post hoc analysis (p < 0.001; n = three independent experiments). A minimum of five focal adhesions were measured for each condition in each experiment.
Syndecan-1 Ectodomain Is Required for Integrin Activation
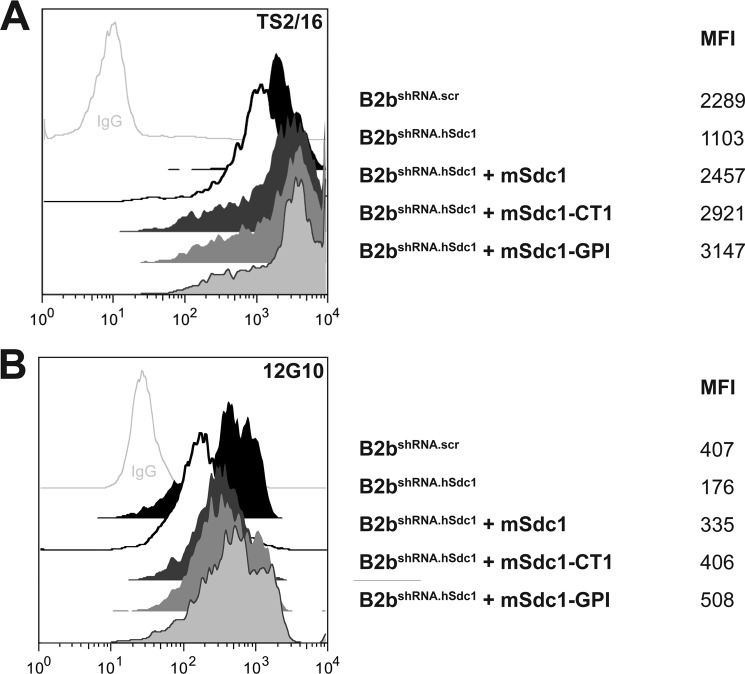
Changes to the integrin affinity state can modulate cell migration (32, 41). Because syndecan-1 regulates both cell migration and α2β1 integrin activation (18), we evaluated the relevant domains of syndecan-1 that control α2β1 integrin activation. Using activation state-specific antibodies, we quantified the level of the high affinity β1 integrin subunit (Fig. 6). The high affinity β1 subunit was less abundant in B2bshRNA.hSdc1 cells compared with B2bshRNA.scr cells, replicating our prior findings (18). However, in contrast to cell migration, where the transmembrane domain regulated migration speed and focal adhesion dynamics, integrin activation was controlled by the syndecan-1 ectodomain. Indeed, mSdc1, mSdc1-CT1, and mSdc1-GPI were all capable of increasing the level of the activated β1 integrin subunit in B2bshRNA.hSdc1 cells compared with B2bshRNA.scr cells (Fig. 6).
FIGURE 6.
Syndecan-1 ectodomain regulates activation of β1 integrin subunit. Shown are representative histograms of the high affinity β1 integrin subunit using antibody clones TS2/16 (A) and 12G10 (B). The cell line and geometric mean fluorescence intensity (MFI) are indicated to the right of the respective histogram.
Functionally, we determined whether the syndecan-1 ectodomain also controls cell adhesion to type I collagen. As expected, B2bshRNA.hSdc1 cells were less adherent than B2bshRNA.scr cells (Table 4) (9, 18). However, adhesion of B2bshRNA.hSdc1 cells was augmented with the expression of mSdc1, mSdc1-CT1, and mSdc1-GPI, all of which contain the entire syndecan-1 ectodomain.
TABLE 4.
Syndecan-1 domains and cell adhesion
All data are presented as means ± S.E.
| Cell line | Normalized adhesiona |
|---|---|
| B2bshRNA.scr | 99.81 ± 2.30 |
| B2bshRNA.hSdc1 | 63.42 ± 6.02 |
| B2bshRNA.hSdc1 + mSdc1 | 90.42 ± 11.04 |
| B2bshRNA.hSdc1 + mSdc1-CT1 | 88.93 ± 7.00 |
| B2bshRNA.hSdc1 + mSdc1-GPI | 87.82 ± 8.02 |
a B2bshRNA.hSdc1 cells were significantly less adherent than other cells by one-way ANOVA (p < 0.005; n = four independent experiments). The percentage of adherent cells under all conditions was normalized to the percentage of adherent B2bshRNA.scr cells.
DISCUSSION
After epithelial injury, cell-cell and cell-matrix interactions form a contextual framework vital to guiding and optimizing collective cell migration (42, 43). Although the cells in an epithelial surface migrate in concert while maintaining their cellular interconnections, each individual cell retains characteristics of single-cell migration (44). Syndecan-1 restrains cell migration and facilitates the activation of α2β1 integrin (18). Here, we have demonstrated that syndecan-1 regulates cell migration independent of the ability to control the α2β1 integrin activation state. The transmembrane domain is the critical portion of the syndecan-1 core protein that controls cell migration by controlling focal adhesion disassembly. In contrast, cell adhesion is controlled by the syndecan-1 ectodomain through integrin affinity modulation.
Various functions have been identified for the syndecan-1 extracellular domain. Angiogenesis and cancer cell invasion are regulated by the syndecan-1 ectodomain (8, 10, 11, 45). Additionally, the syndecan-1 extracellular domain is required for cell adhesion and spreading (4, 8–10). Affinity modulation of the αvβ3 and αvβ5 integrins also requires the extracellular domain (5, 7, 8). Moreover, the heparan sulfate side chains are required to engage the matrix, but distinct portions of the syndecan-1 core protein are required for its cell-specific functions (5, 27, 28). Although heparan sulfate can bind collagen, this interaction is most likely not the main determinant of cell adhesion (9, 10, 46). These findings are consistent with ours, which indicate that the syndecan-1 extracellular domain mediates the adhesive properties of the cells. Furthermore, at least in the lung epithelium, syndecan-1 facilitates cell adhesion through affinity modulation of α2β1 integrin (18).
Syndecan-1 is a multidimensional protein that coordinates several events for a specific cellular function. For example, αvβ3 integrin and insulin-like growth factor receptor-1 coalesce through syndecan-1 to form a fully functional ternary complex (47). Furthermore, murine B82L fibroblasts send out filopodial projections upon syndecan-1 ligation but also require αvβ5 integrin engagement for a spread phenotype (7). Both the extracellular and transmembrane domains are necessary to induce cell spreading and polarization in Raji lymphoid cells (38). Additionally, the syndecan-1 extracellular domain can augment cell adhesion but in itself is not sufficient to promote cell spreading and invasion (9, 10).
Our data show that syndecan-1 truncated at the first cytoplasmic amino acid but retaining the endogenous transmembrane domain (i.e. mSdc1-CT1) is functionally conserved. In contrast, substitutions with a glycosylphosphatidylinositol anchor or with polyleucines in the transmembrane domain both lose the ability to control cell migration. Because the transmembrane domain is believed to facilitate dimerization of the syndecan receptor (1), syndecan-1 may require the transmembrane domain to dimerize in mediating its effects on cell migration. Of particular note, glycine-to-leucine mutations within the transmembrane domain of syndecan-2 and syndecan-4 abrogate both dimerization and effects on migration (48).
Unlike syndecan-2, -3, and -4, which form strong homo- and heterodimers, the syndecan-1 transmembrane domain has relatively weak interactions with itself and other members of the syndecan family (12). Thus, alternatively, the transmembrane domain may not necessarily mediate syndecan-1 function through dimerization but through organizing into distinct cell surface domains. Indeed, Raji cells require lipid rafts for syndecan-1-mediated spreading (38). Syndecan-1 may coalesce in specific membrane pools, bringing it in proximity to other co-receptors to form a multimeric complex required to carry out its cellular function. In fact, the GxxxG sequence within the transmembrane domain of syndecan-1 is found in many transmembrane proteins (49). Thus, syndecan-1 is primed for interactions with other transmembrane proteins carrying a similar GxxxG motif. Moreover, flanking sequences help determine the strength of homo- and heterodimeric bonds, adding specificity of these interactions to the various syndecans (12, 50, 51). Certainly, the transmembrane and extracellular domains may function in combination to bring together co-receptors on the cell surface. Upon injury, syndecan-1 is shed from epithelial surfaces (17, 18, 52), and the loss of the syndecan-1 ectodomain could break down these complexes and alter the intracellular signals to induce a migratory phenotype.
Cell must balance their matrix adhesiveness to maximize traction forces without compromising cell body translocation (35–37). The ability of a cell to migrate can be regulated by changes to the integrin affinity state (32, 41). However, our findings demonstrate that alteration to cell adhesion by itself does not affect cell migration. Although these findings indicate the necessity of the transmembrane domain in cell migration, we cannot exclude the additional requirement of the extracellular domain. Our data only show that the extracellular domain in isolation does not regulate cell migration. Interestingly, the extracellular domain enhances cell adhesion, whereas the transmembrane and cytoplasmic domains slows migration when syndecan-1 is exogenously expressed in fibrosarcoma cells, which lack endogenous syndecan-1 expression (53). These results further substantiate the notion that cells use multiple syndecan-1 domains to fully transduce signals from the extracellular matrix into the cell.
Cell migration speed can be controlled by altering the focal adhesion turnover rate (39). Cells can also alter the optimum substrate concentration by regulating focal adhesion dynamics (32, 37). Syndecan-1 restrains cell migration by attenuating focal adhesion disassembly, which prolongs focal adhesion life span and slows migration speed (31). Here, we have shown that the syndecan-1 transmembrane domain regulates cell migration. Moreover, our fluorescence recovery after photobleaching experiments demonstrate that the transmembrane domain controls the cycling of adhesion complex proteins, which regulates focal adhesion turnover. Our findings are consistent with the fact that faster exchange of focal adhesion proteins slows migration by attenuating focal adhesion turnover (31, 34, 40, 54, 55).
In sum, syndecan-1 most likely interacts with one or more co-receptors to form a signaling complex that transduces signals to augment cell adhesion and restrain cell migration. Although the extracellular domain is necessary for integrin affinity modulation, the transmembrane domain is crucial for controlling focal adhesion disassembly and migration speed in wounded lung epithelial cells. These data support the notion that syndecan-1 coordinates co-receptor complex formation through various domains of its core protein to mediate cellular function. The specific functional outcome is largely determined by the cellular context and regulated coexpression of signaling receptors.
Supplementary Material
Acknowledgments
We thank Dr. Ron Seifert for technical assistance. The imaging studies were supported in part by the Mike and Lynn Garvey Cell Imaging Lab at the University of Washington Institute for Stem Cell and Regenerative Medicine.
This work was supported, in whole or in part, by National Institutes of Health Grants HL084396 and HL103868 (to P. C.), HL086883 (to W. A. A.), and HL029594 (to W. C. P.). This work was also supported by an American Heart Association beginning grant-in-aid and the American Lung Association (to P. C.) and by the Cystic Fibrosis Foundation Research Development Program (to W. C. P.).

This article contains supplemental Figs. S1 and S2 and Videos 1 and 2.
- AAV
- adeno-associated virus
- IRES
- internal ribosomal entry site
- eGFP
- enhanced GFP.
REFERENCES
- 1. Couchman J. R. (2003) Syndecans: proteoglycan regulators of cell-surface microdomains? Nat. Rev. Mol. Cell Biol. 4, 926–937 [DOI] [PubMed] [Google Scholar]
- 2. Teng Y. H., Aquino R. S., Park P. W. (2012) Molecular functions of syndecan-1 in disease. Matrix Biol. 31, 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Couchman J. R. (2010) Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 26, 89–114 [DOI] [PubMed] [Google Scholar]
- 4. Beauvais D. M., Rapraeger A. C. (2003) Syndecan-1-mediated cell spreading requires signaling by αvβ3 integrins in human breast carcinoma cells. Exp. Cell Res. 286, 219–232 [DOI] [PubMed] [Google Scholar]
- 5. Beauvais D. M., Burbach B. J., Rapraeger A. C. (2004) The syndecan-1 ectodomain regulates αvβ3 integrin activity in human mammary carcinoma cells. J. Cell Biol. 167, 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burbach B. J., Ji Y., Rapraeger A. C. (2004) Syndecan-1 ectodomain regulates matrix-dependent signaling in human breast carcinoma cells. Exp. Cell Res. 300, 234–247 [DOI] [PubMed] [Google Scholar]
- 7. McQuade K. J., Beauvais D. M., Burbach B. J., Rapraeger A. C. (2006) Syndecan-1 regulates αvβ5 integrin activity in B82L fibroblasts. J. Cell Sci. 119, 2445–2456 [DOI] [PubMed] [Google Scholar]
- 8. Beauvais D. M., Ell B. J., McWhorter A. R., Rapraeger A. C. (2009) Syndecan-1 regulates αvβ3 and αvβ5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J. Exp. Med. 206, 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lebakken C. S., Rapraeger A. C. (1996) Syndecan-1 mediates cell spreading in transfected human lymphoblastoid (Raji) cells. J. Cell Biol. 132, 1209–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu W., Litwack E. D., Stanley M. J., Langford J. K., Lander A. D., Sanderson R. D. (1998) Heparan sulfate proteoglycans as adhesive and anti-invasive molecules. Syndecans and glypican have distinct functions. J. Biol. Chem. 273, 22825–22832 [DOI] [PubMed] [Google Scholar]
- 11. Langford J. K., Yang Y., Kieber-Emmons T., Sanderson R. D. (2005) Identification of an invasion regulatory domain within the core protein of syndecan-1. J. Biol. Chem. 280, 3467–3473 [DOI] [PubMed] [Google Scholar]
- 12. Dews I. C., Mackenzie K. R. (2007) Transmembrane domains of the syndecan family of growth factor co-receptors display a hierarchy of homotypic and heterotypic interactions. Proc. Natl. Acad. Sci. U.S.A. 104, 20782–20787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim C. W., Goldberger O. A., Gallo R. L., Bernfield M. (1994) Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell 5, 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Q., Park P. W., Wilson C. L., Parks W. C. (2002) Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111, 635–646 [DOI] [PubMed] [Google Scholar]
- 15. Hayashida K., Parks W. C., Park P. W. (2009) Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood 114, 3033–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu J., Park P. W., Kheradmand F., Corry D. B. (2005) Endogenous attenuation of allergic lung inflammation by syndecan-1. J. Immunol. 174, 5758–5765 [DOI] [PubMed] [Google Scholar]
- 17. Bass M. D., Morgan M. R., Humphries M. J. (2009) Syndecans shed their reputation as inert molecules. Sci. Signal. 2, pe18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen P., Abacherli L. E., Nadler S. T., Wang Y., Li Q., Parks W. C. (2009) MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting α2β1 integrin activation. PLoS ONE 4, e6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stepp M. A., Gibson H. E., Gala P. H., Iglesia D. D., Pajoohesh-Ganji A., Pal-Ghosh S., Brown M., Aquino C., Schwartz A. M., Goldberger O., Hinkes M. T., Bernfield M. (2002) Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J. Cell Sci. 115, 4517–4531 [DOI] [PubMed] [Google Scholar]
- 20. Pal-Ghosh S., Tadvalkar G., Jurjus R. A., Zieske J. D., Stepp M. A. (2008) BALB/c and C57BL/6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Exp. Eye Res. 87, 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ojeh N., Hiilesvuo K., Wärri A., Salmivirta M., Henttinen T., Määttä A. (2008) Ectopic expression of syndecan-1 in basal epidermis affects keratinocyte proliferation and wound re-epithelialization. J. Invest. Dermatol. 128, 26–34 [DOI] [PubMed] [Google Scholar]
- 22. Bode L., Salvestrini C., Park P. W., Li J. P., Esko J. D., Yamaguchi Y., Murch S., Freeze H. H. (2008) Heparan sulfate and syndecan-1 are essential in maintaining murine and human intestinal epithelial barrier function. J. Clin. Invest. 118, 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stanford K. I., Bishop J. R., Foley E. M., Gonzales J. C., Niesman I. R., Witztum J. L., Esko J. D. (2009) Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J. Clin. Invest. 119, 3236–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liebersbach B. F., Sanderson R. D. (1994) Expression of syndecan-1 inhibits cell invasion into type I collagen. J. Biol. Chem. 269, 20013–20019 [PubMed] [Google Scholar]
- 25. Stepp M. A., Liu Y., Pal-Ghosh S., Jurjus R. A., Tadvalkar G., Sekaran A., Losicco K., Jiang L., Larsen M., Li L., Yuspa S. H. (2007) Reduced migration, altered matrix, and enhanced TGFβ1 signaling are signatures of mouse keratinocytes lacking Sdc1. J. Cell Sci. 120, 2851–2863 [DOI] [PubMed] [Google Scholar]
- 26. Morgan M. R., Humphries M. J., Bass M. D. (2007) Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 8, 957–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adams J. C., Kureishy N., Taylor A. L. (2001) A role for syndecan-1 in coupling fascin spike formation by thrombospondin-1. J. Cell Biol. 152, 1169–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chakravarti R., Sapountzi V., Adams J. C. (2005) Functional role of syndecan-1 cytoplasmic V region in lamellipodial spreading, actin bundling, and cell migration. Mol. Biol. Cell 16, 3678–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carey D. J., Stahl R. C., Tucker B., Bendt K. A., Cizmeci-Smith G. (1994) Aggregation-induced association of syndecan-1 with microfilaments mediated by the cytoplasmic domain. Exp. Cell Res. 214, 12–21 [DOI] [PubMed] [Google Scholar]
- 30. Yang Y., Børset M., Langford J. K., Sanderson R. D. (2003) Heparan sulfate regulates targeting of syndecan-1 to a functional domain on the cell surface. J. Biol. Chem. 278, 12888–12893 [DOI] [PubMed] [Google Scholar]
- 31. Altemeier W. A., Schlesinger S. Y., Buell C. A., Parks W. C., Chen P. (2012) Syndecan-1 controls cell migration by activating Rap1 to regulate focal adhesion disassembly. J. Cell Sci., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Millon-Frémillon A., Bouvard D., Grichine A., Manet-Dupé S., Block M. R., Albiges-Rizo C. (2008) Cell adaptive response to extracellular matrix density is controlled by ICAP-1-dependent β1 integrin affinity. J. Cell Biol. 180, 427–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaverina I., Krylyshkina O., Small J. V. (1999) Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 146, 1033–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F. (2004) FAK-Src signaling through paxillin, ERK, and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6, 154–161 [DOI] [PubMed] [Google Scholar]
- 35. DiMilla P. A., Stone J. A., Quinn J. A., Albelda S. M., Lauffenburger D. A. (1993) Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J. Cell Biol. 122, 729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palecek S. P., Loftus J. C., Ginsberg M. H., Lauffenburger D. A., Horwitz A. F. (1997) Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385, 537–540 [DOI] [PubMed] [Google Scholar]
- 37. Gupton S. L., Waterman-Storer C. M. (2006) Spatiotemporal feedback between actomyosin and focal adhesion systems optimizes rapid cell migration. Cell 125, 1361–1374 [DOI] [PubMed] [Google Scholar]
- 38. McQuade K. J., Rapraeger A. C. (2003) Syndecan-1 transmembrane and extracellular domains have unique and distinct roles in cell spreading. J. Biol. Chem. 278, 46607–46615 [DOI] [PubMed] [Google Scholar]
- 39. Webb D. J., Parsons J. T., Horwitz A. F. (2002) Adhesion assembly, disassembly, and turnover in migrating cells–over and over and over again. Nat. Cell Biol. 4, E97–E100 [DOI] [PubMed] [Google Scholar]
- 40. von Wichert G., Haimovich B., Feng G. S., Sheetz M. P. (2003) Force-dependent integrin-cytoskeleton linkage formation requires down-regulation of focal complex dynamics by Shp2. EMBO J. 22, 5023–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huttenlocher A., Ginsberg M. H., Horwitz A. F. (1996) Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand binding affinity. J. Cell Biol. 134, 1551–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Friedl P., Gilmour D. (2009) Collective cell migration in morphogenesis, regeneration, and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457 [DOI] [PubMed] [Google Scholar]
- 43. Friedl P., Wolf K. (2010) Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 188, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farooqui R., Fenteany G. (2005) Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell sheet movement. J. Cell Sci. 118, 51–63 [DOI] [PubMed] [Google Scholar]
- 45. Burbach B. J., Friedl A., Mundhenke C., Rapraeger A. C. (2003) Syndecan-1 accumulates in lysosomes of poorly differentiated breast carcinoma cells. Matrix Biol. 22, 163–177 [DOI] [PubMed] [Google Scholar]
- 46. Koda J. E., Rapraeger A., Bernfield M. (1985) Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J. Biol. Chem. 260, 8157–8162 [PubMed] [Google Scholar]
- 47. Beauvais D. M., Rapraeger A. C. (2010) Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation. J. Cell Sci. 123, 3796–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choi S., Lee E., Kwon S., Park H., Yi J. Y., Kim S., Han I. O., Yun Y., Oh E. S. (2005) Transmembrane domain-induced oligomerization is crucial for the functions of syndecan-2 and syndecan-4. J. Biol. Chem. 280, 42573–42579 [DOI] [PubMed] [Google Scholar]
- 49. Senes A., Gerstein M., Engelman D. M. (2000) Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with β-branched residues at neighboring positions. J. Mol. Biol. 296, 921–936 [DOI] [PubMed] [Google Scholar]
- 50. Asundi V. K., Carey D. J. (1995) Self-association of N-syndecan (syndecan-3) core protein is mediated by a novel structural motif in the transmembrane domain and ectodomain flanking region. J. Biol. Chem. 270, 26404–26410 [DOI] [PubMed] [Google Scholar]
- 51. Senes A., Engel D. E., DeGrado W. F. (2004) Folding of helical membrane proteins: the role of polar, GxxxG-like, and proline motifs. Curr. Opin. Struct. Biol. 14, 465–479 [DOI] [PubMed] [Google Scholar]
- 52. Endo K., Takino T., Miyamori H., Kinsen H., Yoshizaki T., Furukawa M., Sato H. (2003) Cleavage of syndecan-1 by membrane-type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 278, 40764–40770 [DOI] [PubMed] [Google Scholar]
- 53. Zong F., Fthenou E., Mundt F., Szatmári T., Kovalszky I., Szilák L., Brodin D., Tzanakakis G., Hjerpe A., Dobra K. (2011) Specific syndecan-1 domains regulate mesenchymal tumor cell adhesion, motility, and migration. PLoS ONE 6, e14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hamadi A., Bouali M., Dontenwill M., Stoeckel H., Takeda K., Rondé P. (2005) Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J. Cell Sci. 118, 4415–4425 [DOI] [PubMed] [Google Scholar]
- 55. Deramaudt T. B., Dujardin D., Hamadi A., Noulet F., Kolli K., De Mey J., Takeda K., Rondé P. (2011) FAK phosphorylation at Tyr-925 regulates cross-talk between focal adhesion turnover and cell protrusion. Mol. Biol. Cell 22, 964–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.