Background: The role of dematin in regulating red cell membrane mechanical function is not well understood.
Results: Dematin facilitates spectrin-actin interaction, and this activity is modulated by PKA phosphorylation of dematin.
Conclusion: Dematin binds to spectrin and dynamically regulates red cell membrane mechanical function.
Significance: A novel role for dematin in regulating red cell membrane mechanical function has been unraveled.
Keywords: Erythrocyte, Membrane Function, Membrane Proteins, Protein Kinase A (PKA), Protein Phosphorylation, Dematin, Membrane Mechanical Stability
Abstract
The membrane skeleton plays a central role in maintaining the elasticity and stability of the erythrocyte membrane, two biophysical features critical for optimal functioning and survival of red cells. Many constituent proteins of the membrane skeleton are phosphorylated by various kinases, and phosphorylation of β-spectrin by casein kinase and of protein 4.1R by PKC has been documented to modulate erythrocyte membrane mechanical stability. In this study, we show that activation of endogenous PKA by cAMP decreases membrane mechanical stability and that this effect is mediated primarily by phosphorylation of dematin. Co-sedimentation assay showed that dematin facilitated interaction between spectrin and F-actin, and phosphorylation of dematin by PKA markedly diminished this activity. Quartz crystal microbalance measurement revealed that purified dematin specifically bound the tail region of the spectrin dimer in a saturable manner with a submicromolar affinity. Pulldown assay using recombinant spectrin fragments showed that dematin, but not phospho-dematin, bound to the tail region of the spectrin dimer. These findings imply that dematin contributes to the maintenance of erythrocyte membrane mechanical stability by facilitating spectrin-actin interaction and that phosphorylation of dematin by PKA can modulate these effects. In this study, we have uncovered a novel functional role for dematin in regulating erythrocyte membrane function.
Introduction
The human erythrocyte undergoes extensive membrane deformations while maintaining its structural integrity during its 120-day life span in the circulation. The erythrocyte membrane skeleton plays a critical role in bestowing the biophysical characteristics of membrane deformability and mechanical stability necessary for the cell to undergo extensive deformations while surviving the rigors of circulatory shear stress (1, 2). The erythrocyte membrane skeleton is a two-dimensional network of α2β2-spectrin tetramers, which are connected to junctional complexes composed of short actin filaments (F-actin) and several actin-binding proteins, including protein 4.1R, adducin, dematin, tropomyosin, and tropomodulin. Protein 4.1R and adducin have been shown to facilitate the interaction between spectrin and F-actin (3–5). Studies on human and mouse red cells deficient in 4.1R, adducin, tropomyosin, tropomodulin, and dematin have shown that these actin-binding proteins play a role in regulating the structural integrity of the membrane (2, 6–10).
The various membrane skeletal proteins (except actin) are phosphorylated by various protein kinases (11). Casein kinase phosphorylates β-spectrin, 4.1R, and dematin (11), and phosphorylation of β-spectrin by casein kinase decreases membrane mechanical stability (12). PKC phosphorylates 4.1R, adducin, and dematin (11), and phosphorylation of 4.1R by PKC decreases membrane stability (13). Similarly, PKA phosphorylates β-spectrin, dematin, adducin, and 4.1R, with dematin being the most preferred substrate (11). However, the effect of PKA phosphorylation on erythrocyte membrane mechanical properties remains to be defined.
Dematin (band 4.9) was originally identified as a major membrane skeletal protein in human erythrocytes (14, 15). Dematin may exist as a trimer consisting of two isoforms of 48 and 52 kDa, with the latter isoform containing an additional 22 amino acids inserted by alternative splicing (14–17). Early studies showed that dematin binds and bundles F-actin (14). Upon PKA phosphorylation, dematin loses actin-bundling activity, whereas the ability to bind F-actin is maintained (17, 18). However, the physiological function of dematin in erythrocytes remains elusive because the mature erythrocytes lack actin bundles.
Recently, it has been demonstrated that deletion of the headpiece domain of dematin results in mild spherocytosis accompanied by hemolytic anemia and reduced erythrocyte membrane stability (10). Furthermore, the combined deletion of the dematin headpiece and β-adducin leads to a more severe phenotype (20). Dematin has recently been shown to serve as an anchoring site for the membrane skeleton to the lipid bilayer via binding to glucose transporter-1 (21). These findings imply an important role for dematin in erythrocyte membrane function. In this study, we explored the effect of PKA phosphorylation on erythrocyte membrane function, and in the process, we identified an important role for dematin in regulating mechanical stability by facilitating the interaction of spectrin with actin.
EXPERIMENTAL PROCEDURES
Materials
[γ-32P]ATP was obtained from PerkinElmer Life Sciences. Non-muscle actin from human platelets was from Cytoskeleton. The catalytic subunit of PKA from bovine heart, cAMP, ATP, and calyculin A were from Sigma. Q Sepharose was from GE Healthcare. Anti-dematin mAb was purchased from BD Transduction Laboratories.
Phosphorylation of Membrane Proteins by PKA
Blood was drawn from healthy human volunteers after obtaining informed consent and used within 4 days of collection. Blood was filtered using porous polyurethane filters (Immuguard III, Terumo, Tokyo, Japan) to deplete white cells. PBS-washed erythrocytes were incubated in 4 volumes of hypotonic buffer (5 mm sodium Pi (pH 7.5), 1 mm [γ-32P]ATP (100 Ci/mmol), and 2.4 mm MgCl2) for 10 min on ice with gentle stirring in the presence or absence of 100 μm cAMP and 0.2 μm calyculin A. Following the addition of 0.1 volume of 1.5 m NaCl, the red cell ghosts were incubated for 45 min at 37 °C to facilitate phosphorylation by endogenous PKA. The ghosts were then washed once with 20 volumes of 5 mm Tris-Cl (pH 7.4) and 5 mm KCl in the presence or absence of 0.05 μm calyculin A, followed by resealing using 150 mm NaCl at 37 °C for 45 min.
Identification and Quantitation of Protein Phosphorylation
Three μl of the ghosts prepared as described above were subjected to SDS-PAGE, followed by autoradiography. The intensities of various phosphorylated protein bands were analyzed by densitometric scanning. The derived values were normalized based on the copy number of each protein, i.e. β-spectrin, 240,000; adducin, 60,000; 4.1R, 200,000; and dematin, 40,000 trimers (22).
Measurement of Membrane Stability
PKA phosphorylation was carried out as described above except that nonradioactive ATP was used instead of [γ-32P]ATP. Membrane mechanical stability was quantified using an ektacytometer as described previously (23) with minor modifications. The mean T90 values, the time required for the deformability index (DI)2 to reach 90% of DImax, were obtained from five independent experiments and compared with that of the untreated control.
Purification of Dematin
Dematin was purified from human erythrocyte ghosts as described previously (14) with some modifications. In brief, band 6-depleted white ghosts were treated with 9 volumes of 0.5% (v/v) Triton X-100 in buffer A (5 mm sodium Pi (pH 7.5) and 1 mm EDTA) in the presence of 0.8 mm PMSF for 20 min on ice and centrifuged for 25 min at 40,000 × g. The pellet was washed once with buffer A with Triton X-100 and then once with buffer A without Triton X-100. The resulting Triton X-100 shells were incubated in 10 volumes of 3 mm Tris-Cl (pH 8.5), 0.5 mm EDTA, 2 mm DTT, and 0.8 mm PMSF for 45 min at 37 °C, followed by centrifugation at 40,000 × g for 3 h. The supernatant was loaded onto a Q Sepharose column (2.5 (inner diameter) × 29 cm) and eluted with a linear gradient of 20–300 mm KCl in buffer B (20 mm Tris-Cl (pH 8.3), 20 mm KCl, 0.5 mm DTT, 1 mm EGTA, 0.02% NaN3, and 0.8 mm PMSF). The fractions containing dematin were mixed and diluted with buffer B to reduce the concentration of KCl to <100 mm. The diluted sample was reloaded onto the same Q Sepharose column and eluted with a linear gradient of 150–300 mm KCl in buffer B. The fractions containing dematin were mixed and diluted as described above and loaded onto a different Q Sepharose column (2.5 (inner diameter) × 3 cm). Pure dematin was obtained by elution with 200 mm KCl in buffer B.
Phosphorylation of Dematin in Solution
Purified dematin was dialyzed against sedimentation buffer (10 mm sodium Pi (pH 7.4), 100 mm NaCl, 1 mm EDTA, 0.2 mm DTT, and 0.8 mm PMSF) and mixed with 1 mm ATP, 2.4 mm MgCl2, and 100 units/ml bovine PKA catalytic subunit. The mixture was incubated for 1.5 h at 37 °C, followed by inactivation of PKA at 68 °C for 5 min.
Preparation of Phospho-dematin-specific Antibody
Because Ser381 (corresponding to Ser403 in the 52-kDa isoform) was shown to be a PKA phosphorylation site (17), an antibody against phospho-dematin was generated by immunization of rabbits with the synthetic peptide CNELKKKA(pS)LF, corresponding to Asn396–Phe405 of human dematin (52-kDa isoform), conjugated to keyhole limpet hemocyanin via an N-terminally added Cys residue. The serum from immunized rabbits was collected and characterized for the specificity of the antibody generated.
Co-sedimentation of Spectrin with Actin in the Presence or Absence of Dematin
Spectrin was purified from human erythrocytes as described previously (24). Non-muscle actin (5 mg/ml) from human platelets was polymerized in 50 mm KCl, 2 mm MgCl2, 1 mm ATP, and 1 mm EGTA for 30 min at room temperature. Spectrin (1.13 μm) and F-actin (9.3 μm) were incubated on ice for 1 h in sedimentation buffer with either dematin or phospho-dematin (0–0.6 μm). The mixture was centrifuged through a 10% sucrose cushion at 195,000 × g for 30 min at 2 °C. The pellet was rinsed once with the same sucrose solution and subjected to SDS-PAGE. The amount of spectrin co-sedimenting with F-actin was quantitated by densitometric analysis of the gel stained with Coomassie Brilliant Blue.
In another series of experiments, spectrin, F-actin, and unphosphorylated dematin were mixed and incubated on ice for 1 h. At the end of the incubation period, the reaction mixture was divided into two aliquots: 1 mm ATP and 2.4 mm MgCl2 were added to one aliquot, whereas 1 mm ATP and 2.4 mm MgCl2 along with 133 units/ml bovine PKA catalytic subunit were added to the second aliquot. Following incubation at 37 °C for 1.5 h, the samples were centrifuged through the same sucrose cushion and subjected to SDS-PAGE.
Preparation of Recombinant Fragments of Spectrin
Constructs pGEX-βN-2 (N-terminal actin-binding domain and repeats 1 and 2 of human βI-spectrin, amino acid residues 1–527) and pET-α20-C (repeats 20 and 21 and C-terminal EF-domain of human αI-spectrin, amino acid residues 2044–2419) were generous gifts from Dr. Xiuli An (New York Blood Center). α20-C was subcloned into pGEX-6P-3 at the EcoRI and XhoI sites (pGEX-α20-C).
The plasmids were introduced into BL21 Star(DE3)pLysS cells. After induction with 1 mm isopropyl β-d-thiogalactopyranoside, the bacterial pellet was lysed in TBS (50 mm Tris-Cl (pH 8.0), 200 mm NaCl, 1 mm EDTA, and 1 mm 2-mercaptoethanol) containing 0.05% Tween 20 in the presence of Complete protease inhibitors (Roche Applied Science) by sonication. GST fusion proteins were collected using glutathione-Sepharose 4B.
Preparation of Mini-spectrin Dimer
In brief, following elution from glutathione-Sepharose beads, GST-βN-2 was digested with thrombin. The mixture of digested GST and βN-2 was mixed and incubated with GST-α20-C on ice for 1 h in sedimentation buffer and separated on prepacked Sephacryl S-200 at 0.2 ml/min. Fractions (2 ml) were collected. Fractions with equimolar amounts of GST-α20-C and βN-2 (mini-spectrin) determined on SDS-PAGE were pooled and used for binding assay.
Pulldown of Dematin by Mini-spectrin Dimer
Recombinant mini-spectrin dimer immobilized on glutathione beads was mixed with increasing concentrations of dematin and incubated for 2 h at room temperature. The beads were then washed with 0.05% (v/v) Tween 20 in sedimentation buffer and boiled in SDS sample buffer. The samples were resolved by SDS-PAGE, followed by immunoblot analysis using anti-dematin mAb and IRDye 800CW-conjugated anti-mouse antibody (LI-COR Biosciences). Near-infrared fluorescence was detected and quantified using Odyssey (LI-COR Biosciences). Serial dilution of purified dematin quantified in parallel gave a linear standard curve over the entire range of concentrations used in the study. Similarly, pulldown assay was performed for phospho-dematin. Phospho-dematin was detected using ECL (Pierce).
Quartz Crystal Microbalance Measurement
Purified dematin (ligand) was immobilized on a gold sensor chip, and binding of the mini-spectrin dimer prepared as described above (analyte) was monitored in PBS using Single Q 0500 (As One Corp., Tokyo, Japan), a quartz crystal microbalance device. Curve fitting and calculation of the dissociation constant (KD) and the Hill coefficient were conducted using SigmaPlot Version 11.2 (Systat Software).
Binding of Dematin to F-actin
Dematin or phospho-dematin (0–0.4 μm) and F-actin (11.6 μm) in sedimentation buffer were incubated on ice for 1 h and subjected to centrifugation through a 10% sucrose cushion in the same buffer. The protein pellet was resolved on SDS gel, followed by immunoblot analysis using anti-dematin monoclonal antibody. The amount of dematin or phospho-dematin co-sedimenting with F-actin was quantitated by densitometric analysis.
RESULTS
PKA Predominantly Phosphorylates Dematin and Leads to Decreased Membrane Mechanical Stability
Activation of endogenous PKA by cAMP in the presence of calyculin A, a phosphatase inhibitor, showed a marked increase in phosphorylation of dematin and also of protein 4.1R but to a much lesser extent (Fig. 1, A and B). This observation is in agreement with findings from earlier studies (11). It is interesting to note that in the presence of the phosphatase inhibitor calyculin A alone, increased phosphorylation of β-spectrin and dematin, but not of adducin and 4.1R, over the basal levels was observed (Fig. 1B). However, in contrast to dematin, cAMP caused no further increases in phosphorylation of either β-spectrin or adducin. These findings clearly show that of all erythrocyte membrane skeletal proteins, dematin in situ is the preferred substrate for PKA.
FIGURE 1.
PKA phosphorylation of membrane skeletal proteins and its effect on the membrane mechanical stability. A, autoradiogram of 32P-labeled erythrocyte membrane proteins phosphorylated in the presence or absence of 0.2 μm calyculin A (CA) and/or 100 μm cAMP. Major membrane skeletal proteins are indicated. CBB, Coomassie Brilliant Blue. B, band intensities in the autoradiogram were determined by densitometric scanning and normalized by the copy number of each protein (see “Experimental Procedures” for details). White bars, control (without calyculin A); hatched bars, calyculin A alone; black bars, calyculin A + cAMP. Data are means ± S.D. (n = 3). *, p < 0.05; **, p < 0.01. C, erythrocyte membrane mechanical stability was examined using an ektacytometer. A decrease in DI reflects membrane fragmentation by applied shear stress. Representative results from five independent experiments are shown. D, erythrocyte membrane mechanical stability is expressed as T90 values, which is the time required for the DI to reach 90% of DImax. Data are means ± S.D. (n = 5). *, p < 0.05.
Measurement of membrane mechanical stability of ghosts prepared from PKA-activated erythrocytes showed a more rapid rate of decrease in DI at a constant value of applied shear stress compared with that of control ghosts, implying decreased membrane mechanical stability (Fig. 1C). Calyculin A treatment alone also resulted in measurable but a lesser extent of decreased membrane mechanical stability compared with control ghosts. The membrane mechanical stability (T90; see “Experimental Procedures”) following activation of PKA was decreased by 31.1 ± 8.2% compared with the control, whereas following treatment with calyculin A alone, the decrease was only 10 ± 8.6% (Fig. 1D). These findings imply that PKA phosphorylation of dematin decreases membrane mechanical stability. As membrane mechanical stability is regulated primarily by lateral interactions of the protein components of the membrane skeleton, i.e. spectrin-actin interaction and spectrin-spectrin interaction, perturbation of either or both of these two specific protein-protein interactions by PKA phosphorylation could account for decreased membrane mechanical stability.
Dematin Facilitates Spectrin-Actin Interaction
As dematin has previously been shown to be a constituent of the spectrin-actin junctional complex, we explored the consequences of dematin phosphorylation on its ability to modulate the interaction between spectrin and actin. Dematin was purified from erythrocytes to >95% purity as determined by densitometric scanning of the Coomassie Brilliant Blue-stained gel (Fig. 2A). An anti-dematin monoclonal antibody recognized both unphosphorylated and in vitro phosphorylated dematin with equal efficiency (Fig. 2B, upper panel). In contrast, a rabbit anti-phospho-dematin polyclonal antibody raised against the synthetic peptide CNELKKKA(pS)403LF specifically recognized only the phosphorylated dematin (Fig. 2B, middle panel). This polyclonal antibody also recognized dematin that was phosphorylated in situ on the membrane (Fig. 2B, lower panel), documenting that Ser403 is phosphorylated by PKA in situ as well as in vitro. Note that phospho-dematin showed lower mobility on SDS gels probably due to the addition of a phosphate group. Hence, phosphorylation of dematin could be monitored by the mobility shift using anti-dematin monoclonal antibody.
FIGURE 2.

Effects of dematin and its phosphorylation on spectrin-actin interaction. A, dematin purified as described under “Experimental Procedures” was >95% pure as determined by densitometric scanning of the Coomassie Brilliant Blue (CBB)-stained gel. B, purified dematin and purified dematin phosphorylated in vitro were probed with anti-dematin monoclonal antibody (upper panel) and anti-phospho-dematin polyclonal antibody raised against the synthetic peptide CNELKKKA(pS)403LF (middle panel). The ghosts prepared from PKA-activated erythrocytes were subjected to SDS-PAGE and probed with anti-phospho-dematin polyclonal antibody (lower panel). C, upper panel, purified spectrin (1.13 μm) was incubated with F-actin (9.3 μm) on ice for 1 in the presence or absence of dematin or phospho-dematin (pDematin; ∼0.6 μm). Proteins co-sedimenting with F-actin were resolved on SDS gel. Spectrin, actin, and dematin are indicated. Lower panel, an immunoblot (IB) using anti-dematin mAb to unambiguously indicate co-sedimenting dematin is shown. D, the band intensity of spectrin co-sedimenting with F-actin was determined by densitometric scanning of a Coomassie Brilliant Blue-stained gel and is shown as the relative amount to the control (without dematin; None). Data are means ± S.D. (n = 3). **, p < 0.01. E, upper panel, spectrin was incubated with F-actin on ice for 1 h in the presence of increasing concentrations of dematin or phospho-dematin (0–0.6 μm). Proteins co-sedimenting with F-actin were resolved by SDS-PAGE. Lower panel, an immunoblot using anti-dematin mAb to unambiguously indicate dematin is shown. F, the band intensity of spectrin shown in C was determined by densitometric scanning and is shown as the relative amount to that obtained without dematin.
Co-sedimentation assay performed using purified spectrin and actin showed that spectrin interacts with F-actin weakly (Fig. 2C, upper panel, lane 3) as reported previously (13). In the presence of dematin, the amount of spectrin co-sedimenting with F-actin was significantly increased, implying that dematin facilitates spectrin-actin interaction (Fig. 2C, upper panel, lane 4, and Fig. 2D). In marked contrast, the ability of phosphorylated dematin to enhance interaction between spectrin and actin was markedly compromised (Fig. 2C, upper panel, lane 5, and Fig. 2D). The mobility shift of phospho-dematin gave assurance that the dematin used in the co-sedimentation assay was indeed phosphorylated (Fig. 2C, lower panel, lanes 4 and 5). Use of increasing concentrations of dematin in the co-sedimentation assay resulted in increasing amounts of spectrin co-sedimenting with actin (Fig. 2E, lanes 2–5, and Fig. 2F). In marked contrast, increasing concentrations of phospho-dematin failed to enhance spectrin-actin interaction (Fig. 2E, lanes 6–9, and Fig. 2F). Note also that although increasing amounts of dematin were found in the pellet, much reduced amounts of phospho-dematin co-sedimented with spectrin and actin (Fig. 2E, lower panel).
The effect of dematin phosphorylation on the interaction between spectrin and F-actin was tested in a preformed spectrin-actin-dematin complex. The ability of dematin to facilitate and maintain spectrin-actin interaction in the preformed complex was sustained during incubation at 37 °C for an additional 1.5 h (Fig. 3A, compare lanes 1 and 2 and lanes 3 and 4). In marked contrast, when the preformed complex was incubated in the presence of PKA, dematin was phosphorylated, and both spectrin and dematin dissociated from F-actin (Fig. 3A, lane 5). These results clearly demonstrate that dematin facilitates spectrin-actin interaction, and PKA phosphorylation of dematin markedly diminishes its ability to facilitate this interaction.
FIGURE 3.
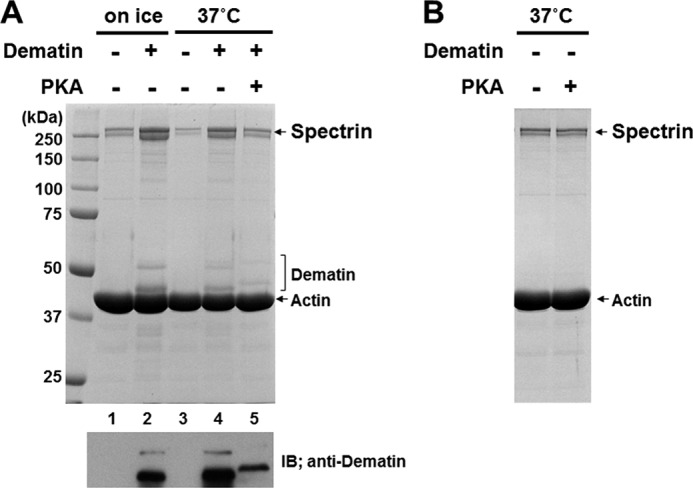
Phosphorylation of dematin in the preformed complex. A, dematin was incubated with spectrin and F-actin to form a ternary complex. Then, 1 mm ATP and 2.4 mm MgCl2 were added to the reaction mixture and further incubated in the presence or absence of 133 units/ml bovine PKA catalytic subunit for 1.5 h at 37 °C. Proteins co-sedimenting with F-actin were analyzed by SDS-PAGE. An immunoblot (IB) using anti-dematin mAb to unambiguously indicate co-sedimenting dematin is shown. B, PKA treatment of the preformed spectrin-actin complex as described above did not affect spectrin binding to F-actin.
Because PKA also phosphorylates spectrin (see Fig. 1A), the effect of PKA treatment on spectrin binding to F-actin was examined. No significant change in the amount of spectrin co-sedimenting with F-actin was observed following incubation of a preformed spectrin-actin complex with PKA at 37 °C for 1.5 h (Fig. 3B). The activity of PKA used in these experiments was confirmed by showing that the same PKA preparation phosphorylated purified dematin (data not shown). This finding enables us to conclude that dissociation of spectrin from the spectrin-F-actin-dematin complex is due to phosphorylation of dematin, but not of spectrin itself.
Dematin Binding to Spectrin
To document direct interaction between dematin and spectrin, we performed pulldown assays using purified dematin and mini-spectrin dimers composed of α20-C and βN-2. Binding of dematin to mini-spectrin was documented using an anti-dematin mAb (Fig. 4A). With increasing concentrations of dematin, the amount of dematin pulled down by mini-spectrin increased (Fig. 4B), suggesting that dematin directly binds to mini-spectrin. In contrast, phospho-dematin failed to bind to mini-spectrin dimers (Fig. 4A). GST alone did not bind either dematin or phospho-dematin.
FIGURE 4.

Specific interaction between dematin and spectrin. A, dematin or phospho-dematin was incubated with mini-spectrin immobilized on glutathione-Sepharose beads. Bound dematin and phospho-dematin (pDematin) were detected by immunoblotting using anti-dematin monoclonal antibody, which recognizes both phosphorylated and unphosphorylated dematin. The upper panel shows that equal amounts of dematin and phospho-dematin were used as input. CBB, Coomassie Brilliant Blue. B, increasing concentrations of purified dematin was pulled down by the mini-spectrin dimer (GST-α20-C + βN-2) immobilized on glutathione-Sepharose beads. C, increasing concentrations of the mini-spectrin dimer (GST-α20-C + βN-2) was added to a sensor chip on which purified dematin had been immobilized. The amount of mini-spectrin bound was quantified as change in frequency (ΔF). Representative results from three independent experiments are shown. All three experiments showed saturable and sigmoidal binding curves. A dissociation constant of 14.8 ± 1.6 nm and a Hill coefficient of 1.75 ± 0.19 were derived from analysis of the binding curves.
To obtain quantitative assessment of the affinity of the interaction between dematin and spectrin, we used the quartz crystal microbalance measurement. As shown in Fig. 4C, the binding of the mini-spectrin dimer to immobilized dematin was saturable, demonstrating the specificity of the binding. A dissociation constant of 14.8 ± 1.6 nm was derived from the analysis of the binding profile. As the binding curve was sigmoidal, it appears that the spectrin dimer binds dematin in a positive cooperative manner (Hill coefficient = 1.75 ± 0.19). It is likely that this could be the result of the binding of the mini-spectrin dimer to the trimeric dematin immobilized on the sensor chip. These results enable us to document that dematin specifically binds to the tail region of the spectrin dimer, which facilitates the binding of the spectrin dimer to F-actin at the junctional complex.
Interaction of Dematin with F-actin
It is well established that dematin binds to F-actin, and the binding is affected by PKA phosphorylation (18, 25). We confirmed these earlier findings using the co-sedimentation assay by documenting a 70% reduction in the ability of dematin to bind F-actin upon phosphorylation by PKA (data not shown). These results are in very good agreement with earlier findings that showed that PKA phosphorylation reduces the binding affinity of dematin for F-actin by 3–5-fold (18, 25).
PKA Phosphorylation Does Not Alter Spectrin-Spectrin Interaction
To determine the potential contribution of altered spectrin dimer-dimer interaction to the PKA-mediated decrease in membrane mechanical stability, we compared the dimer/tetramer ratio of spectrin extracted from PKA-activated erythrocytes to that from control cells. No differences were noted in the spectrin dimer/tetramer ratio between PKA-activated and control erythrocytes (data not shown), implying that altered spectrin-spectrin interaction does not contribute to PKA-mediated reduced membrane mechanical stability.
DISCUSSION
The spectrin-actin junctional complex is a key structural feature of the erythrocyte membrane skeleton, where the tail of the αβ-spectrin dimer binds to a short actin filament and where several skeletal proteins, including 4.1R, adducin, tropomyosin, tropomodulin, and dematin, are localized (1, 2). A detailed understanding of the contribution of interactions among spectrin, actin, and 4.1R in the junctional complex to membrane mechanical function has been developed (2, 3, 6). Furthermore, phosphorylation of 4.1R by PKC and of β-spectrin by casein kinase has been shown to reduce membrane mechanical stability (12, 13). However, the regulatory role of PKA phosphorylation and the contribution of dematin to red cell membrane mechanical function remain unclear.
In this study, we documented that like 4.1R, dematin enhances the interaction of spectrin with actin and that phosphorylation of dematin by PKA reduces this enhancing ability. Importantly, we showed that phosphorylation of membrane skeletal proteins by PKA reduced membrane mechanical stability and that this effect was mediated primarily by phosphorylation of dematin. As there were subtle increases in phosphorylation of 4.1R and adducin following activation of PKA, we cannot completely rule out the possibility of the potential contribution of the phosphorylation of these two proteins to the observed decreases in membrane mechanical stability. However, our data strongly imply that dematin plays a key role in maintaining the functional integrity of the membrane skeleton via facilitating lateral interaction between spectrin and F-actin.
Our data also show that treatment of red cells with calyculin A alone, a phosphatase inhibitor, resulted in a modest decrease in membrane mechanical stability. Increased phosphorylation of β-spectrin and dematin following treatment with calyculin A suggests that phosphorylation of dematin alone or in combination with phosphorylation of β-spectrin by cAMP-independent kinase modulates membrane mechanical stability. Casein kinase is one of the candidate kinases because we previously demonstrated that phosphorylation of β-spectrin by casein kinase decreases membrane mechanical stability (12). Whether or not dematin phosphorylation by casein kinase affects membrane stability needs to be further explored.
The original study characterizing erythrocyte dematin established it to be an actin-binding protein and stated that dematin does not bind spectrin (14). However, a subsequent study suggested binding of dematin to spectrin (16), but this finding has largely been overlooked. In this study, we unambiguously demonstrated that dematin specifically binds to the tail region of spectrin with high affinity (on the order of 10−8 m). The apparent discrepant findings are likely due to the fact that the original study might have used purified phosphorylated dematin for binding studies (14), and we have clearly shown that dematin loses its ability to bind spectrin upon phosphorylation. Further studies are needed to precisely determine the dematin-binding site on spectrin and the spectrin-binding site on dematin.
It now appears that at least three distinct skeletal proteins are involved in reinforcing spectrin-actin interaction, i.e. 4.1R, adducin, and dematin. The fact that a number of different proteins play a redundant role emphasizes the importance of spectrin-actin interaction in erythrocyte physiology, especially in regulating membrane mechanical functions. It is interesting that the phosphorylation of these proteins by various kinases alters their ability to mediate spectrin-actin interaction. In solution, PKA phosphorylation has been shown to abolish the ability of adducin to enhance spectrin-actin binding (26). We have recently found that an adenosine receptor agonist induces an increase in intracellular cAMP levels and phosphorylation of adducin, showing that mature erythrocytes are able to respond to extracellular stimuli (27). It will be of interest to examine if stimulation by physiological agonists induces PKA phosphorylation of dematin and/or adducin and hence affects erythrocyte membrane mechanical properties. Previously, we have shown that PKC phosphorylation of 4.1R decreases membrane mechanical stability (13). In that study, we surmised that phosphorylation of 4.1R is primarily responsible for the membrane instability and did not consider the effect of dematin because dematin had not been previously implicated in regulating membrane stability, though dematin is also a substrate for PKC. Given that dematin also facilitates spectrin-actin interaction, the effect of dematin phosphorylation by PKC on membrane stability needs to be further explored.
We have previously demonstrated that the treatment of erythrocytes with lidocaine, an amphipathic local anesthetic, blocks agonist-induced increases in intracellular cAMP levels through disruption of lipid raft-mediated signal transduction (27). We also reported that lidocaine treatment of erythrocytes inhibits invasion of erythrocytes by Plasmodium falciparum, a causative protozoan parasite of the most severe form of human malaria (28). Furthermore, it has been reported that increases or decreases in cAMP levels parallel increases or decreases in P. falciparum invasion of erythrocytes, respectively (29). During the invasion process, the P. falciparum merozoite invaginates the erythrocyte membrane and is enclosed within a vacuolar membrane, which is free of membrane skeletal proteins (19). These observations raise the possibility that the membrane skeleton is locally and transiently disrupted during invasion by cAMP-dependent mechanism(s). Based on our present findings, PKA phosphorylation of dematin might be involved in disruption of spectrin-actin interaction locally to facilitate efficient parasite invasion. Our findings have uncovered a novel function for dematin in regulating red cell membrane function, and we anticipate that future studies will unravel new insights into the role of dematin in erythrocyte physiology and pathology.
This work was supported by National Institutes of Health Grants DK26263, DK32094, and HL31579. This work was also supported by the Takada Scientific Foundation and by Grant-in-aid for Scientific Research 18590408 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
- DI
- deformability index.
REFERENCES
- 1. Mohandas N., Chasis J. A. (1993) Red blood cell deformability, membrane material properties, and shape: regulation by transmembrane, skeletal, and cytosolic proteins and lipids. Semin. Hematol. 30, 171–192 [PubMed] [Google Scholar]
- 2. Mohandas N., Gallagher P. G. (2008) Red cell membrane: past, present, and future. Blood 112, 3939–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. (1979) In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature 280, 811–814 [DOI] [PubMed] [Google Scholar]
- 4. Gardner K., Bennett V. (1987) Modulation of spectrin-actin assembly by erythrocyte adducin. Nature 328, 359–362 [DOI] [PubMed] [Google Scholar]
- 5. Mische S. M., Mooseker M. S., Morrow J. S. (1987) Erythrocyte adducin: a calmodulin-regulated actin-bundling protein that stimulates spectrin-actin binding. J. Cell Biol. 105, 2837–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takakuwa Y., Tchernia G., Rossi M., Benabadji M., Mohandas N. (1986) Restoration of normal membrane stability to unstable protein 4.1-deficient erythrocyte membranes by incorporation of purified protein 4.1. J. Clin. Invest. 78, 80–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robledo R. F., Ciciotte S. L., Gwynn B., Sahr K. E., Gilligan D. M., Mohandas N., Peters L. L. (2008) Targeted deletion of α-adducin results in absent β- and γ-adducin, compensated hemolytic anemia, and lethal hydrocephalus in mice. Blood 112, 4298–4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. An X., Salomao M., Guo X., Gratzer W., Mohandas N. (2007) Tropomyosin modulates erythrocyte membrane stability. Blood 109, 1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moyer J. D., Nowak R. B., Kim N. E., Larkin S. K., Peters L. L., Hartwig J., Kuypers F. A., Fowler V. M. (2010) Tropomodulin 1-null mice have a mild spherocytic elliptocytosis with appearance of tropomodulin 3 in red blood cells and disruption of the membrane skeleton. Blood 116, 2590–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khanna R., Chang S. H., Andrabi S., Azam M., Kim A., Rivera A., Brugnara C., Low P. S., Liu S. C., Chishti A. H. (2002) Headpiece domain of dematin is required for the stability of the erythrocyte membrane. Proc. Natl. Acad. Sci. U.S.A. 99, 6637–6642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen C. M., Gascard P. (1992) Regulation and post-translational modification of erythrocyte membrane and membrane-skeletal proteins. Semin. Hematol. 29, 244–292 [PubMed] [Google Scholar]
- 12. Manno S., Takakuwa Y., Nagao K., Mohandas N. (1995) Modulation of erythrocyte membrane mechanical function by β-spectrin phosphorylation and dephosphorylation. J. Biol. Chem. 270, 5659–5665 [DOI] [PubMed] [Google Scholar]
- 13. Manno S., Takakuwa Y., Mohandas N. (2005) Modulation of erythrocyte membrane mechanical function by protein 4.1 phosphorylation. J. Biol. Chem. 280, 7581–7587 [DOI] [PubMed] [Google Scholar]
- 14. Siegel D. L., Branton D. (1985) Partial purification and characterization of an actin-bundling protein, band 4.9, from human erythrocytes. J. Cell Biol. 100, 775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Husain-Chishti A., Faquin W., Wu C. C., Branton D. (1989) Purification of erythrocyte dematin (protein 4.9) reveals an endogenous protein kinase that modulates actin-bundling activity. J. Biol. Chem. 264, 8985–8991 [PubMed] [Google Scholar]
- 16. Rana A. P., Ruff P., Maalouf G. J., Speicher D. W., Chishti A. H. (1993) Cloning of human erythroid dematin reveals another member of the villin family. Proc. Natl. Acad. Sci. U.S.A. 90, 6651–6655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Azim A. C., Knoll J. H., Beggs A. H., Chishti A. H. (1995) Isoform cloning, actin binding, and chromosomal localization of human erythroid dematin, a member of the villin superfamily. J. Biol. Chem. 270, 17407–17413 [DOI] [PubMed] [Google Scholar]
- 18. Husain-Chishti A., Levin A., Branton D. (1988) Abolition of actin bundling by phosphorylation of human erythrocyte protein 4.9. Nature 334, 718–721 [DOI] [PubMed] [Google Scholar]
- 19. Lauer S., VanWye J., Harrison T., McManus H., Samuel B. U., Hiller N. L., Mohandas N., Haldar K. (2000) Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 19, 3556–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen H., Khan A. A., Liu F., Gilligan D. M., Peters L. L., Messick J., Haschek-Hock W. M., Li X., Ostafin A. E., Chishti A. H. (2007) Combined deletion of mouse dematin headpiece and β-adducin exerts a novel effect on the spectrin-actin junctions leading to erythrocyte fragility and hemolytic anemia. J. Biol. Chem. 282, 4124–4135 [DOI] [PubMed] [Google Scholar]
- 21. Khan A. A., Hanada T., Mohseni M., Jeong J. J., Zeng L., Gaetani M., Li D., Reed B. C., Speicher D. W., Chishti A. H. (2008) Dematin and adducin provide a novel link between the spectrin cytoskeleton and human erythrocyte membrane by directly interacting with glucose transporter-1. J. Biol. Chem. 283, 14600–14609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gallagher P. G., Forget B. G. (2010) Williams Hematology, 8th Ed., McGraw-Hill Medical Publishing Division, New York [Google Scholar]
- 23. Mohandas N., Clark M. R., Health B. P., Rossi M., Wolfe L. C., Lux S. E., Shohet S. B. (1982) A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood 59, 768–774 [PubMed] [Google Scholar]
- 24. Correas I., Leto T. L., Speicher D. W., Marchesi V. T. (1986) Identification of the functional site of erythrocyte protein 4.1 involved in spectrin-actin associations. J. Biol. Chem. 261, 3310–3315 [PubMed] [Google Scholar]
- 25. Vardar D., Chishti A. H., Frank B. S., Luna E. J., Noegel A. A., Oh S. W., Schleicher M., McKnight C. J. (2002) Villin-type headpiece domains show a wide range of F-actin-binding affinities. Cell Motil. Cytoskeleton 52, 9–21 [DOI] [PubMed] [Google Scholar]
- 26. Matsuoka Y., Hughes C. A., Bennett V. (1996) Adducin regulation. Definition of the calmodulin-binding domain and sites of phosphorylation by protein kinases A and C. J. Biol. Chem. 271, 25157–25166 [DOI] [PubMed] [Google Scholar]
- 27. Kamata K., Manno S., Ozaki M., Takakuwa Y. (2008) Functional evidence for presence of lipid rafts in erythrocyte membranes: Gαs in rafts is essential for signal transduction. Am. J. Hematol. 83, 371–375 [DOI] [PubMed] [Google Scholar]
- 28. Koshino I., Takakuwa Y. (2009) Disruption of lipid rafts by lidocaine inhibits erythrocyte invasion by Plasmodium falciparum. Exp. Parasitol. 123, 381–383 [DOI] [PubMed] [Google Scholar]
- 29. Harrison T., Samuel B. U., Akompong T., Hamm H., Mohandas N., Lomasney J. W., Haldar K. (2003) Erythrocyte G protein-coupled receptor signaling in malarial infection. Science 301, 1734–1736 [DOI] [PubMed] [Google Scholar]



