Abstract
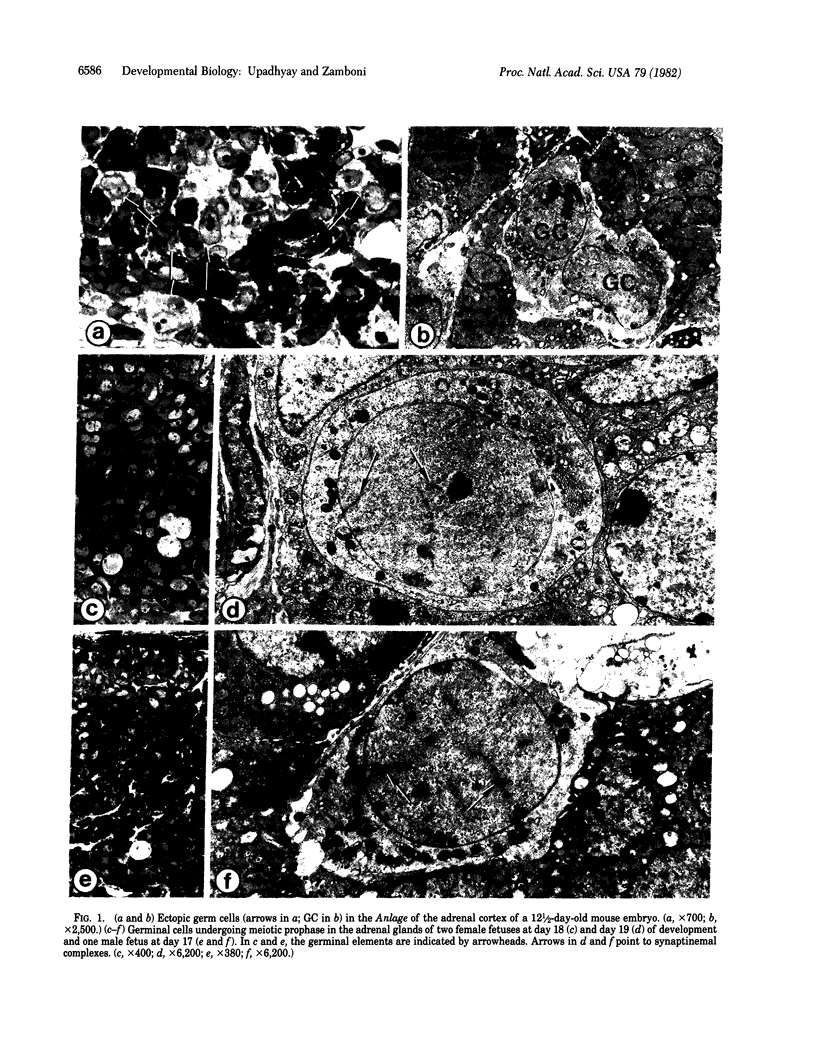
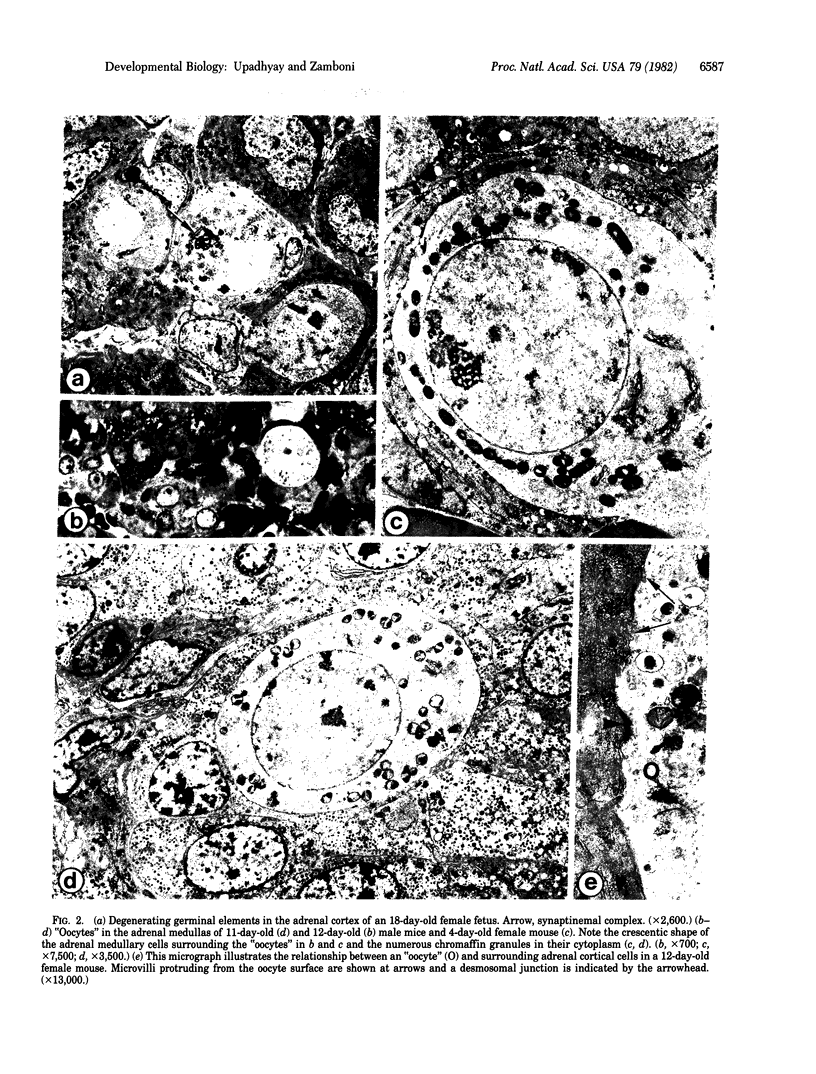
In the course of a study on the morphogenesis of the adrenal gland in random-bred Swiss albino mice, we noted the presence of ectopic germ cells in the adrenal cortexes and medullas in animals of both sexes, from day 12 1/2 of fetal development to postnatal day 12. Up to day 15 of fetal development, the cells exhibited characteristics of primordial germ cells. At day 17, and irrespective of the sex of the fetus, they all entered meiosis in synchrony with those in the ovary. Postnatally, in females as well as males, all ectopic germ cells displayed morphologic characteristics identical to those of young oocytes in unilaminar ovarian follicles. No germinal elements were seen in the adrenal glands past day 12 of life. Our study shows that mammalian germ cells are capable of undergoing sustained differentiation outside the gonads and that, in ectopic sites, they all differentiate into oocytes as they normally would in the ovary, even in males.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byskov A. G. Does the rete ovarii act as a trigger for the onset of meiosis? Nature. 1974 Nov 29;252(5482):396–397. doi: 10.1038/252396a0. [DOI] [PubMed] [Google Scholar]
- Byskov A. G., Grinsted J. Feminizing effect of mesonephros on cultured differentiating mouse gonads and ducts. Science. 1981 May 15;212(4496):817–818. doi: 10.1126/science.7221564. [DOI] [PubMed] [Google Scholar]
- Byskov A. G., Saxén L. Induction of meiosis in fetal mouse testis in vitro. Dev Biol. 1976 Sep;52(2):193–200. doi: 10.1016/0012-1606(76)90239-6. [DOI] [PubMed] [Google Scholar]
- Evans E. P., Ford C. E., Lyon M. F. Direct evidence of the capacity of the XY germ cell in the mouse to become an oocyte. Nature. 1977 Jun 2;267(5610):430–431. doi: 10.1038/267430a0. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Byskov A. G., Andreasen M. P. Induction of meiosis in fetal mouse testis in vitro by rete testis tissue from pubertal mice and bulls. J Reprod Fertil. 1979 Jul;56(2):653–656. doi: 10.1530/jrf.0.0560653. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Byskov A. G. Meiosis-inducing and meiosis-preventing substances in human male reproductive organs. Fertil Steril. 1981 Feb;35(2):199–204. [PubMed] [Google Scholar]
- O W., Baker T. G. Initiation and control of meiosis in hamster gonads in vitro. J Reprod Fertil. 1976 Nov;48(2):399–401. doi: 10.1530/jrf.0.0480399. [DOI] [PubMed] [Google Scholar]
- Upadhyay S., Zamboni L. Preliminary observations on the role of the mesonephros in the development of the adrenal cortex. Anat Rec. 1982 Jan;202(1):105–111. doi: 10.1002/ar.1092020112. [DOI] [PubMed] [Google Scholar]