Background: Diferric-tyrosyl radical [(FeIII2-Y·)(FeIII2)] cofactor-bearing subunit (β2) of ribonucleotide reductase is targeted by a Phase-II cancer drug, Triapine (3-AP).
Results: Y· loss precedes iron loss without reactive oxygen species formation.
Conclusion: Fe(II)-(3-AP) inhibits β2 catalytically resulting in iron-loaded β2 with a reduced Y·.
Significance: Susceptibility of β2 to inhibition via Y· reduction by metal complexes implicates a new avenue to develop RNR inhibitors.
Keywords: Cancer Therapy, Cell Biology, Drug Action, Enzyme Inhibitors, Reactive Oxygen Species (ROS), Ribonucleotide Reductase, Tumor Therapy, Excluding ROS, Mammalian Ribonucleotide Reductase, Triapine (3-AP) Action
Abstract
Triapine® (3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP)) is a drug in Phase II trials. One of its established cellular targets is the β2 subunit of ribonucleotide reductase that requires a diferric-tyrosyl-radical [(FeIII2-Y·)(FeIII2)] cofactor for de novo DNA biosynthesis. Several mechanisms for 3-AP inhibition of β2 have been proposed; one involves direct iron chelation from β2, whereas a second involves Y· destruction by reactive oxygen species formed in situ in the presence of O2 and reductant by Fe(II)-(3-AP). Inactivation of β2 can thus arise from cofactor destruction by loss of iron or Y·. In vitro kinetic data on the rates of 55Fe and Y· loss from [(55FeIII2-Y·)(55FeIII2)]-β2 under aerobic and anaerobic conditions reveal that Y· loss alone is sufficient for rapid β2 inactivation. OxyblotTM and mass spectrometric analyses of trypsin-digested inhibited β2, and lack of Y· loss from H2O2 and O2˙̄ treatment together preclude reactive oxygen species involvement in Y· loss. Three mammalian cell lines treated with 5 μm 3-AP reveal Y· loss and β2 inactivation within 30-min of 3-AP-exposure, analyzed by whole-cell EPR and lysate assays, respectively. Selective degradation of apo- over [(FeIII2-Y·)(FeIII2)]-β2 in lysates, similar iron-content in β2 immunoprecipitated from 3-AP-treated and untreated [55Fe]-prelabeled cells, and prolonged (12 h) stability of the inhibited β2 are most consistent with Y· loss being the predominant mode of inhibition, with β2 remaining iron-loaded and stable. A model consistent with in vitro and cell-based biochemical studies is presented in which Fe(II)-(3-AP), which can be cycled with reductant, directly reduces Y· of the [(FeIII2-Y·)(FeIII2)] cofactor of β2.
Introduction
Ribonucleotide reductases (RNRs)2 supply the monomeric precursors required for DNA replication and repair (1). Two subunits, (α2)m(β2)n (m, n = 1–3) constitute active human (h) RNR (1, 2). α2 contains the site of nucleotide reduction and binds allosteric effectors that control specificity and reduction rate (1–3). β2 houses a diferric-tyrosyl radical cofactor [(FeIII2-Y·)(FeIII2)] (Fig. 1) essential for initiating thiyl radical formation in α2, which initiates nucleotide reduction (3, 4). RNR plays a central role in nucleic acid metabolism (1, 2, 5, 6) and is the target of three cancer drugs used clinically (7), each targeting a different aspect of the RNR complex mechanism of catalysis and regulation (8–12). This paper focuses on understanding how 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (Triapine® or 3-AP, Fig. 1) specifically inactivates β2 of hRNR in vitro and in cultured mammalian cells.
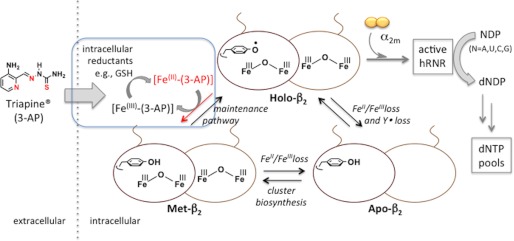
FIGURE 1.
Role of β2 in hRNR catalysis and impact of β2-specific inhibition by 3-AP. Holo-β2 upon association with (α2)m constitutes hRNR that catalyzes nucleoside diphosphate (NDP) reduction to dNDPs, one of the key upstream processes that dictates dNTP pool homeostasis. Direct inhibition of β2 can arise from either loss of Y· (Met-β2 formation) or FeIII2-Y· (apoβ2 formation). Iron-chelating small molecules such as Triapine (3-AP) may also cause indirect inhibition by depletion of intracellular labile iron pool or by Fe(II)-(3-AP)-catalyzed generation of ROS in the presence of O2. Blue inset, this study in vitro and in live mammalian cells suggests that β2-specific inhibition involves reduction of Y· elicited by the active reductant, Fe(II)-(3-AP), catalytically recyclable by intracellular reductants, e.g. GSH. This inhibition results in iron-loaded-β2 with Y· reduced, inferred in our model as Met-β2 without the need to invoke ROS.
3-AP has efficacy in a variety of cell lines and animal cancer models (13–15). It is currently in phase II clinical trials (16, 17) resulting in renewed interest in its mechanism of cytotoxicity (18–24). Studies to date suggest that multiple mechanisms of 3-AP are involved in its cytotoxicity (13–15, 18–24). 3-AP is a chelator and readily forms both Fe(II) and Fe(III) complexes (19, 25, 26). The Fe(II)-(3-AP) in the presence of O2 is able to catalyze generation of ROS (19, 27). The Fe(III)/(II)-(3-AP) reduction potential is accessible in vivo by endogenous reductants (18, 19, 25, 26).
The diverse properties of 3-AP have resulted in a number of models by which it inhibits RNR. In one model free 3-AP is proposed to chelate the Fe(III) directly from the [(FeIII2-Y·)(FeIII2)] within β2 (14, 28, 29), resulting in RNR inactivation. In a second model 3-AP is proposed to chelate iron from the intracellular iron pool(s) (18, 20) that could interfere with the essential [(FeIII2-Y·)(FeIII2)] assembly on β2 (30). A third model, which is the one currently favored in the literature, is that Fe(III)-(3-AP) is reduced to Fe(II)-(3-AP) by endogenous reductants, which in turn reacts with O2 and produces ROS that inactivate RNR (21, 27–29). A direct interaction of Y· with O2˙̄ has also been proposed to lead to β2 inhibition observed in vitro (27–29). Although depletion of the labile iron pools and ROS generation are likely involved in late-stage cytotoxic pathways such as induction of apoptosis (21, 23, 24), we will argue that neither mechanism is likely to be important in RNR-specific inhibition. Previous pharmacological studies by Sartorelli and co-workers (13) and Keppler and co-workers (22) that examined the 3-AP-induced late-stage cytotoxicity in a number of cell lines over 2–4 days noted that blockage of DNA synthesis is induced within the initial hours of 3-AP incubation. Studies by Richardson and co-workers (18) have indicated that cellular iron uptake and efflux are minimally perturbed in 3-AP-treated cultured cells in the first hours of exposure. These interesting findings prompted us to undertake a detailed investigation to understand the mechanism of 3-AP-promoted inhibition of β2 in vitro and in cell culture in the early stages of inhibitor treatment where cell viability remains high, cell cycle is not perturbed, and downstream cytotoxicity is not yet apparent.
Our studies in vitro have investigated the effect of 3-AP and its iron complexes on RNR activity, depletion of its essential Y·, and iron loss from the [(FeIII2-Y·)(FeIII2)] cluster of β2 alone and in the active holo-complex [(α2)m(β2] in a non-cycling or cycling state. The relative rates of Y· loss and iron loss have been measured and vary dependent upon the states of β2 (β2 alone, cycling- or non-cycling holo-complex). In all cases, however, Y· loss precedes iron loss, suggesting that β2-specific inhibition can be explained by the direct reduction of Y·. These data implicate Fe(II)-(3-AP) as the reductant that can rapidly reduce Y·. The observations that Y· loss occurs in the presence and, importantly, in the absence of O2 and that Y· levels are unaffected by O2˙̄ or H2O2 and the failure to detect oxidative modifications by additional studies using OxyblotTM technology and mass spectrometry on inhibited β2 rule out ROS as the basis for Y· reduction.
We have also investigated the effects of 3-AP in K562, COS-1, and hydroxyurea (HU)-resistant TA3 cells on β2 activity in cell lysates, 55Fe content of β2 immunoprecipitated from 55Fe-labeled cells, the stability of the inhibited β2, and the Y· levels in intact cells. Furthermore, OxyblotTM technology of the whole protein pool failed to reveal differences between 3-AP-treated and untreated cells. Together with in vitro data, the rapid and potent RNR inhibition in cells within 30 min of 3-AP treatment is most consistent with the formation of Fe(II)-(3-AP) from free 3-AP with intracellular Fe, which then engages in the direct reduction of the essential Y· of β2. The inhibition occurs in the period where cell viability remains high, and the activity of α2 and other abundant iron-dependent enzymes such as aconitase, a sensitive indicator of oxidative stress, are maintained. These findings further underscore the specificity of 3-AP-promoted β2-specific inhibition and downplay nonspecific indirect models discussed above. They provide initial insight into understanding how inhibition of β2 is involved in the cytotoxic effects of 3-AP.
EXPERIMENTAL PROCEDURES
In Vitro Assays on hRNR
All in vitro studies were performed on hRNR subunits recombinantly expressed and purified from Escherichia coli (10, 11). Specific activities for CDP reduction were 700–890 nmol min−1mg−1 for α and 3000–4100 nmol min−1mg−1 for β containing 0.9–1.2 Y·/β2 and 3.6 iron/β2. The Y· in the [(FeIII2-Y·)(FeIII2)] cluster of β2 has a t½ ∼ 25 min at 37 °C in 50 mm Hepes (pH 7.6), 100 mm NaCl. All inhibition data, unless otherwise noted, have thus been adjusted for this intrinsic instability. The presence of 5 mm DTT in the storage buffer is necessary to preserve α2 activity; thus in all experiments with holo-complex, 85 μm DTT is present. All experiments were carried out at physiological concentrations of subunits (0.5–2.5 μm (monomer) in 3T6 (6, 31–33), COS-1, HeLa, and NIH-3T3 cells (12)), except the EPR experiments where sensitivity limits require 5 μm β2.
[(55FeIII2-Y·)(55FeIII2)] Cluster Assembly in β2
Labeling of the active-site diiron center with 55Fe and subsequent isolation and characterization are detailed in supplemental Materials and Methods. This procedure typically afforded 55Fe-labeled β2 with 1.0–1.2 Y·/β2 (quantitated by EPR). The ferrozine assay (34) gave 3.2–3.6 iron/β2. Non-specifically surface-bound S = 5/2 FeIII was quantitated by EPR analysis of the same samples at 77 K involving double integration of the g = 4.3 signal relative to FeIII-EDTA standard as detailed previously (35). It was less than 5% (<0.18 iron/β2) of the total iron. Specific activity of β2 reconstituted using an identical procedure to that described above with unlabeled FeCl3 was 3000–4100 nmol min−1mg−1 of β. Reconstitution and active-site labeling processes were carried out on untagged and His6-tag β2 (10) and showed that the tag does not affect activity or the amount of surface-bound iron. All the in vitro experiments thus used tagged β2. All in vitro experimental procedures are described in detail in the supplemental Materials and Methods.
Studies in Cultured Cells
COS-1, K562, and HU-resistant TA3 cells were selected as representative mammalian cell lines for the following reasons. COS-1 cells typically yield a relatively large quantity of total protein, and the endogenous β2 activity per mg of total protein is 1.5–3-fold higher than that typically obtained from HeLa and NIH-3T3. K562 was chosen, as previous studies reveal endogenous levels of Y· can be detected by whole cell EPR (36). TA3 cells further support the data from K562 due to enhanced levels of Y· and also allow study of 3-AP in HU-resistant cells. All in-cell experimental procedures are provided in the supplemental Materials and Methods.
RESULTS
Mechanism of hRNR Inhibition by 3-AP in Vitro
All in vitro studies to date on 3-AP-induced inhibition of mouse and hRNR have primarily focused on the analysis Y· loss as a measure of enzyme inactivation (27–29). Because β2 inhibition can arise from loss of iron, loss of Y·, or both, we have measured independently the effects of 3-AP and its iron complexes on the rates of Y· and active site iron loss.
Time-dependent Inhibition Assays
To establish if RNR activity is depleted in a time-dependent manner and whether this activity loss is associated with β2 and/or α2, 0.6 μm β2 (α2) was incubated with 0.3, 1, or 5 eq of 3-AP [per β(α)] for the indicated times and diluted into an assay mixture with a 7-fold excess of α2 (β2) and incubated an additional 3 min. The results shown in Fig. 2A indicate that with 1 eq or a 5-fold excess of 3-AP per β, >90% of the activity is lost within 10 and 1 min, respectively (Fig. 2A). The α2 subunit activity under the same conditions remains unchanged (Fig. 2A), validating that 3-AP is a β2 subunit-specific inhibitor. Incubation of β2 with 0.3 eq of 3-AP/β, however, resulted in 50% activity loss at 20 min (Fig. 2A), greater than expected if 3-AP itself is the inhibitor. This result suggests that 3-AP has access to iron either from the endogenous loss from β2 as previously reported in mouse (37) or from nonspecific surface-bound iron associated with the in vitro cluster assembly (10, 11). 3-AP-accelerated iron loss has previously been proposed to play a key role in β2 inhibition (14, 29). If Fe(II)/Fe(III)-(3-AP) is generated, then in the presence of DTT (85 μm) that always accompanies α2 in the assays, the metal chelate can redox-cycle (19, 25, 26), potentially causing RNR inhibition. These observations are consistent with previous proposals (27–29) that an iron-complex(es) of 3-AP is(are) the true inhibiting species. The kinetics of iron loss from β2 and whether it is accelerated by 3-AP were, therefore, examined.
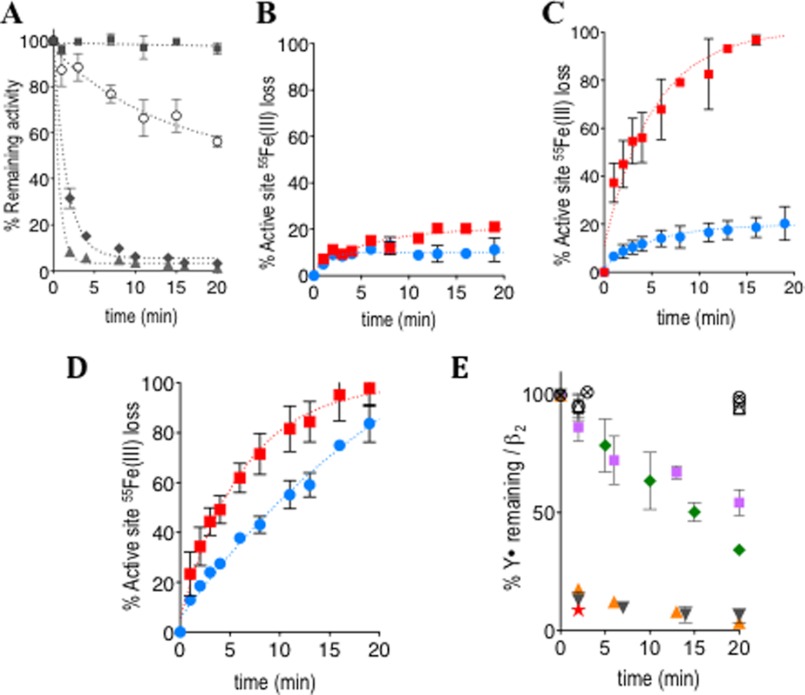
FIGURE 2.
Shown are in vitro kinetic studies on the α2 and β2 activity depletion (A), rate of active-site 55Fe loss (B–D), or Y· loss (E) in β2. All error bars are S.D. over duplicate experiments. A, 3-AP-promoted loss of α2(β2)-activity (data have been corrected for intrinsic instability) is shown. Inhibition mixture contained [r-His6-α(β)]2 = 0.6 μm, [3-AP] = 0, 0.36 (○), 1.2 (♦), or 6.0 (■ for α2; ▴, for β2) μm, and 100 μm KCl in 50 μm Hepes (pH 7.6). The assay mixture contained 0.15 μm [r-His6-α(β)]2, 1.1 μm [r-His6-β(α)]2, 3 mm ATP, 15 mm MgCl2, and 1.0 mm 5-[3H]CDP, 100 mm KCl, 100 μm Trx, 1 μm Trx reductase, 2 mm NADPH in 50 mm Hepes (pH 7.6). B–D, analysis of the rate of 55Fe loss. In all cases, the red square and blue circle, respectively, designate data with and without 3-AP. B is on β2 alone. The reaction employed 1 μm (r-His6-β)2, 10 μm 3-AP, or 5% of DMSO in double distilled H2O, 100 mm KCl, and 50 mm Hepes (pH 7.6). C is on non-cycling holo-complex. The reaction contained 1 μm (r-His6-α)2, 3 mm ATP, and 15 mm MgCl2 in addition to all the components in B. D is on the cycling holo-complex. The reaction contained 100 μm Trx, 1 μm Trx reductase, 2 mm NADPH, and 1 mm CDP in addition to all the components in C. E, shown is the rate of Y· destruction (corrected for intrinsic Y· decay) analyzed by in vitro EPR experiments: by 3-AP alone (green diamond), Fe(III)-(3-AP) (purple square), Fe(II)-(3-AP) (orange triangle), Fe(III)-(3-AP) in the presence of 5 mm GSH (black inverted triangle, orange star). The orange triangle, black inverted triangle, and orange star constitute data from strictly anaerobic experiments. (○) and (⊗) indicate the effects on β2 alone by either 0.5 μm (○) or 50 μm (⊗) H2O2 and (△) and (□) by either 50 or 1250 molar excess of O2˙̄ per β, respectively (generated using xanthine/xanthine oxidase). Representative data shown contained 5 μm (r-His6-β)2 and 50 μm 3-AP or its metal-chelate, except in orange star, where 3 μm was used. Also see supplemental Fig. S1A.
Quantitative Assessment of the Rate of Active-site Iron Loss in the Presence and Absence of 3-AP
55Fe-Labeled β2 was assembled in vitro from apoβ2, resulting in specific radioactivity of 652 cpm/nmol of iron with 3.6 iron/β2 and specific activity in nucleotide reduction and Y· content identical to our previous reports (10, 11). Importantly, EPR analysis showed that the presence of non-specifically surface-bound iron is less than 5% of total iron. Thus 55Fe is predominantly associated with the active site, and our assays monitor the rate of 55Fe loss from this site. A time course for the rate of iron release at 37 °C in the absence and presence of 3-AP was examined under three different sets of conditions: β2 alone and β2 with α2 in a 1:1 molar ratio (1 μm) either in the presence of the allosteric effector ATP (non-cycling holo-complex) or in the presence of the ATP, CDP, thioredoxin (Trx)/thioredoxin reductase/NADPH reducing system (cycling holo-complex). In the absence of 3-AP, the data reveal that the [(FeIII2-Y·)(FeIII2)] exhibits very different intrinsic stabilities in the three cases (Fig. 2, B–D). When β2 is alone (Fig. 2B) or with α2 in a non-cycling state (Fig. 2C), 20% of the 55Fe is lost over 20 min. Because non-specifically bound 55Fe is <5% that of total iron, the loss detected is associated with loss from metallo-cofactor. The data additionally demonstrate that the intrinsic iron loss is not associated with DTT. DTT is absent in the β2 alone experiment but present at 85 μm in the non-cycling holo-complex (compare, Fig. 2, B and C). DTT is required to preserve α2 stability and is thus carried over from the α2 storage solution in all in vitro studies on the holo-complex. In contrast, 90% of the iron is spontaneously lost in 20 min under cycling conditions (Fig. 2D). The observed intrinsic lability in the cycling holo-complex is consistent with previous reports on 59Fe release from the cycling mouse β2 in the presence of DTT (∼60% loss in 30 min) (37). Our data reveal that the intrinsic lability of the cofactor is DTT-independent (Fig. 2, B and C) and that the cycling state of holo-hRNR provides an additional mechanism for iron loss.
Identical experiments to those described above were then carried out in the presence of 3-AP. In the case of β2 alone, a 5-eq excess of 3-AP over β (Fig. 2B) only elicits 20% iron loss in 20 min, similar to the results in its absence (Fig. 2B). This provides compelling evidence that the free ligand does not accelerate active site iron loss on β2 alone. In contrast, with the addition of α2, under conditions that result in non-cycling- or cycling-holo-complex, 3-AP greatly enhances the rate of iron release, leading to almost complete loss in 20 min with a t½ of 4.8 min in both cases (Fig. 2, C and D). The observed increase in loss of iron from the holo-complex in the presence of 3-AP likely implies that formation of the holo-complex may lead to a state(s) that allows the free 3-AP to access iron from the active site, a process not feasible in the case of β2 alone (Fig. 2B). Alternatively, the presence of reductant DTT (carried over with α2), although having no effect on the intrinsic lability of the cofactor, could redox-cycle the in situ-assembled Fe(III)-(3-AP) such that the diferric center in holo-complex is labilized by reduction. Future experiments should provide a mechanistic explanation of these results. Our focus at this time was to determine whether the varied rates and amounts of iron loss observed in the three states of β2 studied, and whether Y· loss or both are responsible for β2 inhibition.
Rate of Y· Loss from [(FeIII2-Y·)(FeIII2)]-β2
EPR studies that monitor Y· were carried out first on β2 alone under a variety of conditions: with 3-AP alone, with Fe(III)-(3-AP), with Fe(II)-(3-AP) ± O2, and with Fe(III)-(3-AP) and GSH ± O2 (Fig. 2E). The Y· in hRNR-β2 is inherently unstable; t½ of 25 min at 37 °C (11). Control experiments measuring the effect of Fe(II), Fe(III), GSH, and combinations of these on the rate of Y· loss indicated minimal changes relative to inherent instability. The data shown in Fig. 2E have been adjusted for the respective intrinsic rates of Y· loss.
In the first experiment 5 eq 3-AP/β was incubated with β2 alone, and as shown in Fig. 2E, 80% of Y· is lost within 20 min. The measured rate of Y· loss induced by the 3-AP alone is similar to that reported for mouse β2 in the absence of DTT (29). However, comparison of the rate of Y· loss with the rate of 55Fe loss for β2 alone (Fig. 2B) provides the first quantitative evidence that 3-AP effectively targets Y· during a time frame in which its impact on the diferric center remains minimal (Fig. 2, B versus E). Our in vitro data on β2 alone thus suggest that Y· quenching is the principle mechanism of enzyme inhibition and leads to iron-loaded β2.
To further investigate if Y· quenching precedes iron loss in non-cycling holo-complex in the presence of 3-AP, similar EPR analyses were performed and compared with the rates of 55Fe loss (Fig. 2, C and E). Y· loss was 80% complete in 1 min (supplemental Fig. S1B), whereas 40% of total iron was lost in the same timeframe (Fig. 2C). Whether the mechanism of iron loss is coupled to Y· loss in this short time frame remains to be established. However, if a single mechanism is at play, iron is lost at a rate 5× slower than Y· over the 20-min duration of the assay.
Rapid Y· Reduction Mediated by Fe(II)-(3-AP)
In an effort to understand the form of 3-AP responsible for inhibition of β2, loss of Y· was also examined in the presence of Fe(III)-(3-AP), Fe(II)-(3-AP) ± O2, and with Fe(III)-(3-AP) and GSH ± O2 (Fig. 2E). With 5 eq of preassembled Fe(III)-(3-AP) (per β), the rate of Y· loss was similar to the case with 5 eq of free 3-AP/β (Fig. 2E). In contrast, when preassembled Fe(II)-(3-AP) was examined, Y· destruction was complete within the first time point. The same rapid Y· loss with a t½ of <0.5 min was observed when the experiment was repeated under strictly anaerobic conditions (Fig. 2E). These data suggest that Fe(II)-(3-AP) is the active species and that ROS are unlikely to be involved.
To provide additional support for proposed role of Fe(II)-(3-AP), the EPR experiment with preassembled Fe(III)-(3-AP) complex and β2 alone was replicated in an anaerobic chamber in the presence of 5 mm GSH. GSH was chosen as a reductant due to its physiological role and abundance, and the reported range of redox potentials suggests its ability to reduce Fe(III)-(3-AP) (38). The rapid rate of Y· loss with GSH and in the absence of O2 (Fig. 2E) is consistent with Fe(II)-(3-AP) being the active inhibitor. Importantly, rapid Y· loss is also achieved with substoichiometric amounts of Fe(III)-(3-AP) (0.3 eq/β) when GSH is present (Fig. 2E). These results together suggest that reduction of Y· can be elicited by Fe(II)-(3-AP) catalytically without ROS formation. The chemical mechanism and kinetics of Y· reduction will be the focus of future studies.
EPR Analysis of Y· in β2 Alone in the Presence of H2O2 and O2˙̄
The effect of H2O2 and O2˙̄ was next evaluated. Earlier studies on E. coli β2 at 25 °C reported H2O2-dependent Y· loss (∼30% loss by 5.5 mm H2O2 in 1 h) (39) as well as irreversible enzyme inactivation by enzymatically generated O2˙̄ (∼80% loss by 3 μm steady-state O2˙̄ in 0.5 h) (40). Corresponding experiments on eukaryotic β2 have not been reported. Incubation of 5 μm hRNR β2 with 50 or 0.5 mm H2O2 at 37 °C (Fig. 2E) showed no appreciable loss of Y· with respect to controls. Similar experiments in the presence of steady-state concentrations of 0.3 and 3 μm O2˙̄ generated using xanthine/xanthine oxidase (41–43) revealed that the Y· levels remained unaltered (Fig. 2E). Thus H2O2 and O2˙̄ are both incapable of inducing Y· loss at the rate observed in the presence of Fe(II)-(3-AP).
In Vitro OxyblotTM Analysis of Inhibited β2 Alone and Cycling- and Non-cycling Holo-complexes
A hallmark of ROS action on proteins via metal-catalyzed oxidation is the formation of protein carbonyls (44). These chemotypes can be probed using OxyblotTM (Millipore) analysis in which the carbonyls are derivatized to 2,4-dinitrophenyl hydrazones, which are then detected by Western blotting (45). Thus Oxyblots were used to determine if nonspecific oxidative damage could be responsible for β2 inhibition by 3-AP. β2 alone and non-cycling- and cycling holo-complexes were incubated with and without 3-AP for 20 min. Proteins in controls and samples with 3-AP were derivatized with 2,4-dinitrophenylhydrazine and subjected to gel electrophoresis followed by Western blot analysis. Protein carbonyls were not detected within β2 treated with 3-AP under any conditions examined (supplemental Fig. S2A). A positive control was carried out with β2 treated with excess ascorbate and iron. SDS-PAGE/Western analysis revealed that β (47 kDa monomer) in these conditions underwent a gel shift to higher a molecular weight species, presumably associated with cross-linking during oxidative damage. Interestingly, Oxyblot analysis on the cycling holo-complex indicated that Trx reductase was oxidized, further indicating that the Oxyblot could readily detect oxidation; no oxidation, however, was associated with β2 (supplemental Fig. S2A). Omission of 2,4-dinitrophenylhydrazide resulted in complete absence of all the bands. Given the reported sensitivity (fM) of the Oxyblot method, the data imply that Fe(II)-(3-AP)-catalyzed protein oxidation is not involved in β2 inhibition.
Trypsin Digest LCMS Analysis of Inhibited β2
To further determine if modification accompanies inhibition of β2, LCMS analyses were performed on in-gel-digested samples of 5 μm β2 treated in vitro with no 3-AP but 50 μm concentrations of either Fe(II)-(3-AP) or H2O2 for 20 min at 37 °C. The resulting peptides were examined for side-chain oxidation (supplemental Table S1A) and hydroxylation (supplemental Table S1B). Qualitatively, there was no significant difference between these three samples. The modifications detected are likely caused by sample handling during protein isolation, gel electrophoresis, or the in-gel digestion process. The Oxyblot and mass spectrometric data suggest inhibition of β2 by 3-AP does not result in significant modification of β2 primary structure.
Mechanism of 3-AP Inhibition in Mammalian Cells
Our attention turned to determine if a similar mechanism occurs in COS-1-, K562-, and HU-resistant TA3 cells exposed to 3-AP.
Loss of β Activity in Cell Lysates from COS-1 and K562 and HU-resistant TA3 Cells Treated with 3-AP
Studies from independent laboratories have reported that cultured cells (HL60 and L1210) exposed to 1–10 μm 3-AP for 0.5–4 h result in complete inhibition of DNA synthesis long before loss of cell viability (2–4 days) (13, 22). Thus our experiments with COS-1 and K562 cells to study the RNR inhibition mechanism were carried out on cells treated with 3-AP (5- 500 μm) over 0.5–3 h where the cells maintained high viability (supplemental Figs. S3 and S4). As shown in supplemental Fig. S5, 30 min of exposure of cells to 3-AP at 5 μm, similar to the in vivo concentration for β2 (∼2.5 μm monomer) (6, 31–33), resulted in no detectable β2 activity in the resulting lysates. Under the same conditions, α2-activity was unaffected. Similar experiments were also carried out with HU-resistant ΤΑ3 cells, previously shown to overexpress β2 and to have elevated concentrations of Y· by whole-cell EPR analysis (37, 46). Consistent with the higher levels of Y·, our lysate assay data in these cells showed that β2 activity is ∼4-fold higher than that in COS-1 cells (Fig. 3A versus supplemental Fig. S5A). Treatment of TA3 cells with 5 μm 3-AP for 30 min still completely abolished β2 activity (Fig. 3A). Our lysate assay data in all three cell lines thus provided direct evidence that 3-AP promotes rapid loss of RNR activity in live cells and is consistent with previous reports on the early inhibition of DNA synthesis in other cell lines studied (13, 22).
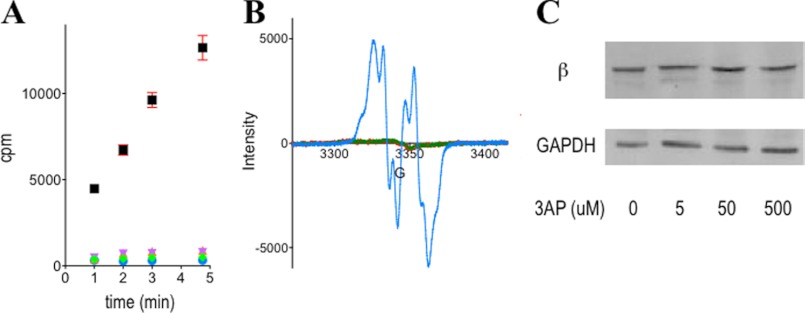
FIGURE 3.
3-AP treatment of HU-resistant TA3 cells results in complete loss of β2-specific activity (A) and Y· (B) but no change in β2 protein levels (C). A, β2 activity in resulting lysates from 30-min-3-AP-treated cells (black square, vehicle; purple inverted triangle, 500 μm; orange triangle, 50 μm; green diamond, 5 μm; blue circle, background). See supplemental Fig. S5 for the results with COS-1 and K562 cells. Error bars are S.D. over two independent experiments. B, Y· signal in treated (3 h) (green lines, 5 μm; red lines, 50 μm) and untreated (blue dots) intact TA3 cells analyzed by whole cell EPR. See supplemental Fig. S 6, C and D for the data at 30 min of treatment in TA3 and K562 cells. C, Western blot of β2 protein levels in treated and untreated TA3 cells at 30 min. See supplemental Fig. S7 for the data from COS-1 and K562 cells. GAPDH was used as the loading control.
Whole Cell EPR Analysis Links β2 Inhibition to Y· Loss
Whole cell EPR experiments were undertaken using analogous procedures to the previously published reports (36, 37, 46) to probe whether Y· loss from the [(FeIII2-Y·)(FeIII2)] cofactor of β2 could account for RNR inactivation. Y· was first shown to be detectable in untreated K562 and TA3 cells at 30 K (supplemental Fig. S6). In the TA3 cells, the higher level of Y· was reflected in a strong signal that are easily monitored even at 77 K (supplemental Fig. S6A), and the signal was identical to that from recombinant [(FeIII2-Y·)(FeIII2)]-β2 reconstituted in vitro (supplemental Fig. S6B). Treatment of either TA3 or K562 cells with 5 μm 3-AP abolished the Y· in as early as 30 min (Fig. 3B and supplemental Fig. S6, C and D). Note that the lower concentration of Y· in β2 from K562 cells is accompanied by higher background signals (supplemental Fig. S6D) as previously reported (36). Thus whole cell EPR analyses and lysate activity assays together demonstrate that 3-AP targets β2 in these cells.
Levels of β2 Are Maintained in 3-AP-treated Cells 12 h Subsequent to Activity Loss
The absence of β2 activity and loss of Y· could be accompanied by conversion of β2 to an iron-loaded or apo form (Fig. 1). To address this issue, Western blot analysis was carried out on non-synchronized TA3, K562, and COS-1 cells at 30 min (supplemental Fig. S7). These studies showed that in all three cell lines, β2 levels were similar to untreated cells (Fig. 3C and supplemental Fig. S7). In addition, a time course study showed that β2 expression was unchanged up to a 12-h period of exposure to 5 μm 3-AP (Fig. 4B). Cells also maintained their high viability over this time (supplemental Fig. S3).
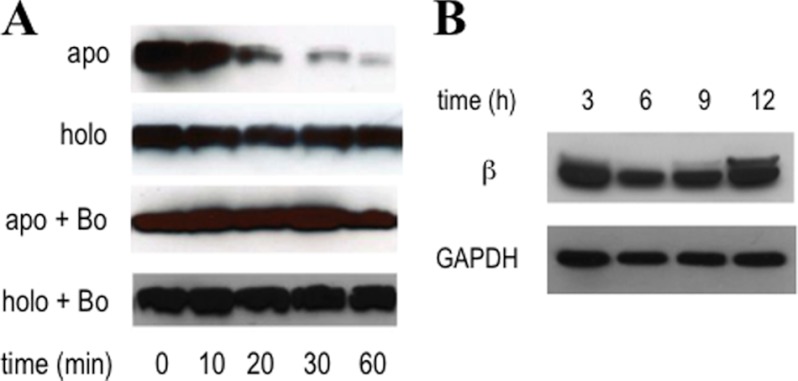
FIGURE 4.
Selective degradation of apoβ2 over holo-β2 by the proteasome in COS-1 lysates (A) and the prolonged stability of inhibited β2 in 3-AP-treated COS-1 cells (B). A, apoβ2 is selectively primed for degradation with t½ = 25 min, but holo-β2 persists. Specific proteasome inhibiting drug, bortezomib (Bo), prevents apoβ2 degradation. B, β2 protein levels were maintained over the 12-h treatment of COS-1 cells with 5 μm 3-AP. GAPDH was used as the loading control. High cell viability is maintained throughout this period (supplemental Fig. S3A). Activity depletion occurs within 30 min of exposure to 3-AP (supplemental Fig. S5A). Shown here in each diagram are representative Western blots from duplicate sets of experiments.
Analysis of Stability of Apoβ2 and Holo-β2 in Mammalian Lysates to Proteasomal Degradation
The in vivo data thus far suggest that 3-AP-dependent inhibition is associated with formation of iron-loaded-β2 but with Y· reduced or apoβ2. However, our in vitro analyses suggest that Y· loss alone is sufficient to account for β2 inhibition (Fig. 2, B–D, and supplemental Fig. S1). Two approaches were undertaken to determine the cofactor status in inhibited β2. The cell cycle-dependent periodicity of mammalian β2 is regulated by ubiquitin-mediated proteasomal degradation during the passage into mitotic (M) phase (6, 47). This knowledge suggests that β2 can be primed for degradation by the 26 S proteasome, although the cofactor status of the β2 being degraded during the M phase remains an outstanding interesting question. We thus started our study using a proteasomal degradation assay (48). COS-1 lysates were chosen to allow comparison with Western blot analysis above. Freshly prepared lysates (1 mg/ml) were supplemented with an ATP-regenerating system and incubated with either apoβ2 or [(FeIII2-Y·)(FeIII2)]-β2. Aliquots of each reaction were removed at the indicated time points from 0 to 60 min and quenched in Laemmli buffer. Western blot analysis revealed that apoβ2 had a t½ of 25 min, whereas the holo-β2 level remained unaltered at 1 h (Fig. 4A). To verify that the proteasome was involved in this degradation, bortezomib, a clinically used proteasome inhibitor (49), was added to separate lysates from the same batch as those shown to degrade apoβ2, and the experiment was repeated (Fig. 4A). No degradation was observed, confirming that apoβ2 is targeted by the proteasome.
Stability of Inhibited β2 Over Prolonged Periods
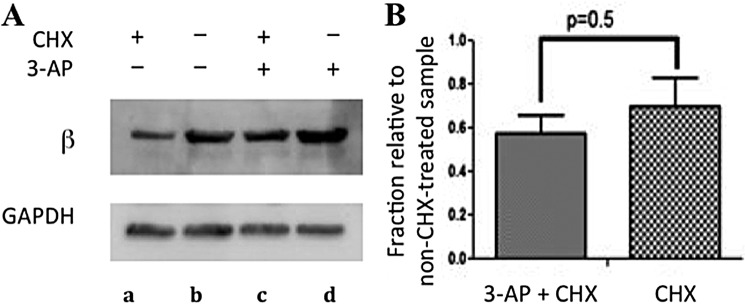
At this point it is unclear whether β2 levels (Fig. 4B) are unchanged or they appear unchanged due to up-regulation of β2 expression resulting from 3-AP treatment compensated for by increased β2 degradation (consistent with apoβ2 formation). Up-regulation in expression of target enzymes upon inhibitor treatment has been reported in mammalian cells (50 and 68) and in many cases and can occur on a 3-h time scale (51). Cycloheximide (CHX), an inhibitor of new protein synthesis (52), was used with 3-AP-treated and untreated cells to gain mechanistic insight. Because β2 has a t½ ∼ 3 h in mammalian cells (6, 46, 53), we opted to compare the effect of 3-AP on the levels of β2 expression in the presence of CHX. Non-synchronized COS-1 cells in logarithmic phase were treated with CHX (0.2 mg/ml) or DMSO for 0.5 h, at which time the media in half of the CHX-treated samples as well as half of DMSO-treated samples were replaced with media that contained 5 μm 3-AP and CHX (0.2 mg/ml) (Fig. 5A, lane c) or 5 μm 3-AP only (lane d). The data from this set were labeled (3-AP + CHX; Fig. 5B). The other half of the samples was retreated with CHX (Fig. 5A, lane a) or DMSO only (lane b). The data from this set were labeled CHX (Fig. 5B). All samples were then incubated for an additional 3 h. This time frame also limits the off-target effects from CHX-induced cytotoxicity (54). Western blot analyses showed that the addition of CHX, as expected, decreased the steady-state level of β2 by ∼60% over 3 h (Fig. 5, A, compare lanes a and b, and B, CHX), consistent with the reported t½ of β2 in TA3 and 3T6 cells (6, 46, 53). When this experiment was repeated with 5 μm 3-AP (Fig. 5, A, compare lanes c and d, and B, 3-AP + CHX), a similar ∼60% decrease in β2 levels was also observed. Thus β2 stability was unaffected by 3-AP treatment. Given that apoβ2 appears to be unstable in the lysate degradation assay, iron-loaded β2 is the most probable species remaining in the 3-AP-treated cells.
FIGURE 5.
The observed stability of inhibited β2 is not a coincidental result of reduced stability of β2 from 3-AP treatment that is offset by the new protein synthesis. A, COS-1 cells were treated with either CHX (0.2 mg/ml) (lanes a and c) or DMSO (lanes b and d) for 0.5 h, at which time media were replaced. Half of the plates were re-treated with either 5 μm 3-AP + DMSO (lane d) or 5 μm 3-AP + 0.2 mg/ml CHX (lane c) for 3-h-labeled, 3-AP+CHX in B. The other half was retreated with either 0.2 mg/ml CHX (lane a) or DMSO (lane b)-labeled CHX in B. B, shown is a histogram of data from density analysis of four independent experiments, a representative of which is shown in A. Error bars are S.D. over quadruplicate sets of experiments.
55Fe-Pulse Labeling and Immunoprecipitation of β2 Demonstrates No Change in Iron Loss ± 3-AP-treated Cells
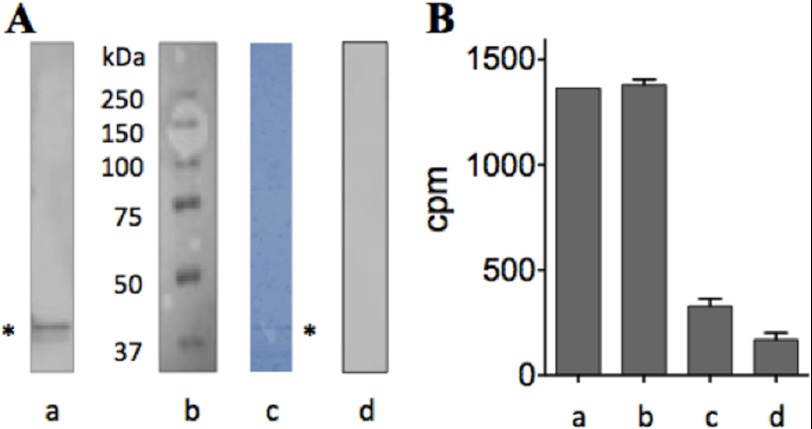
The second approach undertaken to examine the state of inhibited β2 involves immunoprecipitation from 3-AP-treated and -untreated cells that have been prelabeled with 55Fe. The labile nature of the diferric center revealed by our in vitro studies on 55Fe-labeled β2 (Fig. 2, B–D) mandates rapid isolation of β2. An optimized protocol typically affords ∼50 ng of homogeneous β from ∼80 × 106 K562 cells (Fig. 6A). Pulse-labeling was performed according to published protocols (55) using in vitro 55Fe-labeled human transferrin (Tf) (2.4 × 105 cpm/nmol of iron with 2 iron/Tf), which was added (at 25 μg/ml) to logarithmically growing K562 cells. Subsequent to 18 h of incubation, the 55Fe-labeled Tf-containing media was replaced with fresh media, and the cells were treated with 5 μm 3-AP for 0.5 h or DMSO (control). The cells were then harvested, and the relative levels of 55Fe-labeled β2 between 3-AP-treated and control cells were determined using immunoprecipitation. The results are shown in Fig. 6. Importantly, the observed values were appreciably above the non-specifically bound background values obtained in the replica pulldown experiments in which the antibody was omitted, but the resin maintained (Fig. 6, A, lane d, and B, lane c). As an additional control, the lysate from the untreated, labeled cells was treated with 10 mm HU during the antibody incubation step, and the immunoprecipitation process was repeated. HU is known to labilize the diiron center from the mouse and hRNR β2 in vitro (56)3 and in mammalian lysates (57). Consistent with these reports, only background levels of iron are associated with β2 when HU is added (Fig. 6B, lane d). These data in addition to the degradation studies suggest that the iron of β2 is largely retained during the 30 min of exposure of cells to 5 μm 3-AP, although Y· is reduced.
FIGURE 6.
Effect of 3-AP on the diferric cluster of β2 immunopurified from treated and untreated 55Fe-pulse-labeled K562 cells. A, rapid immunoprecipitation was performed with anti-β2 antibody (Abcam) conjugated to Protein G-Dyna beads (Invitrogen). a, immunoprecipitated human β2 band (*, monomer, 45 kDa) was confirmed with Western blotting using human β2-specific antibody (Ab57653). b, Mr ladders (Bio-Rad). c, PVDF membrane in a was stained with Coomassie Brilliant Blue. * indicates immunoprecipitated β monomer. d, control as in a except buffer replaced Ab57653 but Protein G-Dyna beads were kept. B, shown is the amount of radioactivity associated with isolated β2 subsequent to 30-min treatment of the prelabeled cells with 5 μm inhibitor. Shown are the results from 3-AP-untreated (a) and -treated cells (b). c, treatment was identical to a, except anti-β2 antibody was omitted but the beads were retained. d, treatment was a except 10 mm HU was added to the lysate. Error bars are S.D. over duplicate sets of experiments.
An Oxyblot of Lysates of 3-AP-treated Cells Shows the Absence of Protein Carbonyls
As shown above in vitro (supplemental Fig. S2A), further validation of the absence of oxidative carbonylation was probed in whole cell lysates resulting from 3-AP-treated cells and a comparison was made with untreated controls. No change in the extent of carbonylation was observed in any of the cell lines studied (supplemental Fig. S2B), suggesting that 3-AP treatment of the mammalian cells over this time period does not induce oxidative damage.
3-AP Is Specific for hRNR over Other Iron/Sulfur-requiring Enzymes: Lack of Inhibition of Aconitase
Given the recent report on the interconnections between iron/sulfur centers, non-heme iron and heme cofactor biogenesis, and iron homeostasis (58, 59), we examined whether 3-AP was selective for β2 or whether it might affect the activity of other iron-requiring enzymes. Aconitase that contains an essential [4Fe4S] cluster (60) was chosen as a representative enzyme, as its activity is readily assayed in lysates, the cluster is very sensitive to ROS, and one iron is readily lost from the cluster (61). The proposed chelation property of 3-AP could thus affect the availability of labile iron pools from which the iron/sulfur cluster is biosynthesized and repaired (58, 62). Thus, aconitase is a sensitive probe of several mechanisms postulated for 3-AP. However, subsequent to 3 h of treatment of COS-1 cells with 5 μm 3-AP, no changes in aconitase activity relative to the control were observed. These observations are consistent with other data in this study which indicate that 3-AP plays an active role in RNR inhibition. These results further show that the action of 3-AP is β2-specific.
DISCUSSION
Phase II clinical trials on 3-AP have sparked a resurgence of interest in the mechanisms by which 3-AP and other semicarbazones result in cell cytotoxicity (16, 17). The studies are complex, as 3-AP and similar species are typically excellent iron chelators (19, 20, 25, 26), and the reduction potential of these complexes allows redox cycling under physiological conditions (19, 25, 26), which can result in production of ROS (21, 27–29). 3-AP, in contrast to other iron chelators, does not appear to sequester iron effectively in the cell (18); thus, it is unlikely to be involved in disruption of biosynthesis and repair of many metallo-cofactor-requiring proteins (30, 62, 63). Early studies in cultured cells established that their treatment with 3-AP resulted in rapid DNA inhibition within the initial hours (13, 22) and that this effect was related to loss of activity of RNR known to require a [(FeIII2-Y·)(FeIII2)] cofactor (13, 14). This cofactor is common to all class Ia RNRs (holo-β2, Fig. 1) (3, 4, 30), although the in vitro the half-life of the Y· varies from days (E. coli) to minutes (Pseudomonas aeruginosa and human) (11, 64). In addition, the mouse β2 under conditions in which deoxynucleoside diphosphates are produced has been reported to spontaneously lose iron (∼60% loss in 30 min at 37 °C) (37). Because the [(FeIII2-Y·)(FeIII2)] cofactor is essential for RNR activity (3, 4), loss of either Y· or iron cluster results in inhibition.
Studies in cultured cells and xenograft models treated with 3-AP on the other hand have in general focused on assessment of 3-AP-promoted late-stage cytotoxicity (13–15, 18–24, 28, 29). These data suggest that multiple pathways are operative. Postulated mechanisms include deficiency in DNA lesion repair (15), depletion of intracellular iron storage pools (18, 20), ROS-mediated oxidative damage (21, 24, 27–29), and initiation of apoptotic signals (21, 23, 24). Our data now provide a window into the initial period of 3-AP treatment of cells before loss of regulation at multiple levels impact cell viability. A unified model results from comparison of in vitro and cell culture studies despite many remaining questions that need to be resolved.
In vitro
The currently favored model for the mechanism of RNR inhibition by 3-AP in vitro is that Fe(III)-(3-AP) can be reduced to Fe(II)-(3-AP) by DTT in assay buffers (27–29) or β2 itself (29) and generate ROS that reduce the Y·. Yen and co-workers (27) studying hRNR β2 alone by EPR analysis showed complete loss of Y· subsequent to a 30-min incubation with a catalytic amount of Fe(II)-(3-AP) (preassembled in the presence of excess DTT). Under the same conditions, Fe(III)-(3-AP) without DTT resulted in ∼60% Y· loss, whereas 3-AP alone resulted in ∼40% Y· loss (see Fig. 4 in Yen and co-workers (27)). These results are similar to our EPR data shown in Fig. 2E. Yen and co-workers (27) further showed using a 5,5-dimethyl-1-pyrroline-N-oxide as a radical trap that incubation of Fe(II)-(3-AP) with O2 generates ROS and that superoxide dismutase and catalase partially prevented Y· loss. From all their data they concluded that O2 is important in Y· destruction. Their complex experimental protocol, however, involved preincubation of Fe(II)-(3-AP) for 30 min with catalase alone or in combination with superoxide dismutase before addition to β2 followed by a further 30-min incubation. The experimental design (27) and the observation that DTT, Fe(II), and O2 can rapidly regenerate Y· (65) suggest that an alternative interpretation of their results is possible; that is, that Y· is regenerated.
Gräslund and co-workers (29) has also reported time-dependent Y· loss on mouse β2 alone. Their EPR data in the presence of DTT (see Fig. 6 in Gräslund and co-workers (29)) support Fe(II)-(3-AP) as the active species in Y· reduction, consistent with Yen and co-workers (27, 28) and our own data. However, as in Yen and co-workers (27, 28), Gräslund and co-workers (29) favor the importance of ROS in Y· reduction. This proposal was based on the observation that they observed 100% loss in Y· in 5 min at 37 °C with substoichiometric amounts of Fe(III)-(3-AP) relative to β2 but only 20% loss under anaerobic conditions (see Fig. 6a in Gräslund and co-workers (29)). The source of reducing equivalents essential for reduction of Fe(III)-(3-AP) to Fe(II)-(3-AP) was suggested to be mouse β2 itself. We note that with Fe(III)-(3-AP) and O2, neither our laboratory (Fig. 2E) nor Yen and co-workers (27) see 100% Y· loss, although the possibility remains that hRNR-β2 is distinct from the mouse enzyme. The basis for the differences in the results remains unresolved. Finally, although both the Yen (27, 28) and Gräslund laboratories (29) have suggested that 3-AP can bind to β2, in the former case based on binding experiments with 3-[3H]AP and the latter case based on molecular docking and computational analysis, in neither case is the proposal compelling.
We propose an alternative model, consistent with our biochemical studies, that accommodates most of the previously reported results. Our kinetic data on Y· and 55Fe loss from β2 suggest that rapid inhibition of hRNR-β2 results from reduction of Y· by Fe(II)-(3-AP) by outer sphere electron transfer (Fig. 2, B–E). This mechanism also can account for catalytic cycling of this reductant but does not involve O2 (Fig. 2E). Our studies further suggest that met-β2 is likely generated (Fig. 2B) and that this state persists for 20 min, the duration of the assay. Given recent studies on the P. aeruginosa β (64), however, we cannot rule out the possibility that FeII2 is formed and remains tightly bound. Our additional experiments reveal that presence of α2 appears to allow 3-AP to remove the iron from the holo-complex, although the rate of iron loss is slower than Y· loss (Fig. 2, C and D, supplemental Fig. S1B). Finally, efforts to detect evidence for damage to the inhibited β2 using oxyblot methods (supplemental Fig. S2A) and mass spectroscopy analysis of peptides of in-gel-trypsinized β2 (supplemental Table S1) failed to reveal protein damage that could account for its inhibition. Thus our data in vitro show that the inhibition of β2 occurs without the need to invoke ROS formation. We also observe a distinction between the effects of 3-AP on Y· reduction and chelation of active-site iron (Fig. 2, B--E). A detailed mechanism of Fe(II)-(3-AP) reduction of the Y· remains to be established in vitro.
In Vivo
Our studies in cell culture further support our in vitro mechanism for 3-AP-mediated β2 inhibition that occurs in the initial hours of drug treatment (0.5–4 h) (13, 22). Evidence is provided from whole cell EPR methods to monitor Y· (Fig. 3B, supplemental Fig. S6, C and D), 55Fe-labeled Tf labeling, and immunoprecipitation of the inhibited β2 to assess iron content (Fig. 6) and oxyblot experiments of the 3-AP treated cells (supplemental Fig. S2B). Our results demonstrate that Y· loss occurs in multiple cell lines after only a 30-min exposure to 3-AP (Fig. 3B, supplemental Fig. S6, C and D). Assays of accompanying cell lysates revealed no detectable RNR activity (Fig. 3A, supplemental Fig. S5) or iron loss in the same time frame (Fig. 6B). Finally, no enhanced oxidation due to 3-AP treatment of the live cells was detected (supplemental Fig. S6B).
In the cell the actual state(s) of β2 (holo-complex versus β2 alone) has not been reported. Our studies indicate that the final state of β2 is iron-loaded with its Y· reduced (Figs. 4A and 6). The inhibited state of β2 is surprisingly stable even under conditions where new protein synthesis is inhibited (Fig. 5). Our findings suggest that apoβ2 is not the end product in 3-AP-treated cells. Finally, α2 is also present in cells, conditions where 3-AP can remove iron from β2 in a holo-complex based on our in vitro findings (Fig. 2, C and D). Thus, although a portion of β2 may undergo iron loss, it may be reloaded, giving rise to the observed protein stability in 3-AP-treated cells (Figs. 4B and 5). Preservation of the diiron center in β2 is consistent with previous studies with SK-N-MC cells treated with 50 μm 3-AP for 3 h which revealed that iron uptake from 59Fe-labeled Tf (∼20%) and iron release from 59Fe pulse-labeled cells (25%) were both minimal relative to the other iron chelators studied (18). Consistent with these results, whole cell EPR analyses of 3-AP-treated K562 cells (5 or 500 μm) after 0.5, 3, and 12 h showed no change in the size of the intracellular g = 4.3 signals between the treated and untreated cells (data not shown). These observations contrast to the phenotype typically observed when chelation and depletion of intracellular iron pools are in operation (36).
Our studies thus raise interesting questions about the mechanisms by which the diiron cluster of β2 persists and whether the [(FeIII2-Y·)(FeIII2)]-β2 can be regenerated by a maintenance pathway in vivo, with the Y· continually being re-reduced by cycling Fe(II)-(3-AP). The identification of a small molecule that can negatively intercept the regeneration of active cofactor in living mammalian cells (Fig. 1) may ultimately shed light on the [(FeIII2-Y·)(FeIII2)] cluster biosynthetic and maintenance pathways (30).
Our model is that Fe(II)-(3-AP) is the active species involved in β2 inhibition (Fig. 1). This proposal is in accord with the reported detection of EPR signals (g = 2) in peripheral blood mononuclear cells collected from patients treated with 3-AP (66) within 2 h of treatment, proposed to be iron- and copper-bound 3-AP. Given the intracellular prevalence of iron relative to Cu (63) and the stability constants for 3-AP (19, 24, 25, 29), Fe(II)-(3-AP) is the most likely active species. Our in vitro studies with 55Fe-labeled β2 suggest the protein itself is capable of providing at least catalytic amounts of iron in all states of β2 (Fig. 2, B–D); in vivo, however, multiple sources of iron are present. In the continued presence of free ligand in cultured cells, RNR inhibition can persist by the redox cycling capacity of Fe(II)/(III) complex (19, 25, 26), facilitated by intracellular reductants such as GSH (38). Finally, although our model (Fig. 1) primarily focuses on targeting β2 alone, our data in vitro and in non-synchronized cells suggest that the holo-complex is also susceptible to 3-AP-promoted inhibition by direct Y· reduction (Fig. 2, C and D and supplemental Fig. S1B).
Our findings demonstrate that Y· within hRNR β2 in any state (alone or non-cycling or cycling holo-complex) is highly susceptible to reduction while being stable to a range of reactive small-molecule oxidants. This observation is significant because a pool of β2 unbound to (α2)m almost certainly exists in cells, and our in vitro data show that this form of β2 is relatively inert to iron chelation, at least by 3-AP. Thus to design more effective semicarbazone anticancer agents, the efficiency of the reduction of hRNR Y· by the semicarbazone Fe(III)/(II) complexes should be considered alongside other pharmacokinetic properties. Furthermore, because Y· is highly susceptible to reduction, alternative reductants/radical quenchers should be investigated as potential β2 inhibitors (19). Finally, our studies provide a simple series of experiments that can be used to distinguish between ROS effects and Y· reduction both in vitro and in cells, allowing a more thorough evaluation/optimization of other semicarbazones as potential therapeutics.
Supplementary Material
Acknowledgments
We thank Professor Lars Thelander of Umeå University, Sweden, for the generous provision of hydroxyurea-resistant TA3 cell lines and Dr. Ioannis Papayannopoulos, Principal Scientist and Director of Proteomics Facility, Koch Institute for Integrative Cancer Research at the Massachusetts Institute of Technology, for discussion and help with the analysis of mass spectrometry data.
This work was supported, in whole or in part, by National Institutes of Health Grant GM29595 (to J. S.). This work was also supported by Damon Runyon Cancer Research Fellowship DRG2015-09 (to Y. A.) and a Howard Hughes Medical Institute international student fellowship (to M. J. C. L.).

This article contains supplemental Materials and Methods, references, Figs. S1–S7, and Table S1.
Y. Aye and J. Stubbe, unpublished data.
- RNR
- ribonucleotide reductase
- hRNR
- human RNR
- 3-AP
- Triapine®
- CHX
- cycloheximide
- HU
- hydroxyurea
- ROS
- reactive oxygen species
- Trx
- thioredoxin
- Tf
- transferrin.
REFERENCES
- 1. Nordlund P., Reichard P. (2006) Ribonucleotide reductases. Annu. Rev. Biochem. 75, 681–706 [DOI] [PubMed] [Google Scholar]
- 2. Hofer A., Crona M., Logan D. T., Sjöberg B. M. (2012) DNA building blocks. Keeping control of manufacture. Crit. Rev. Biochem. Mol. Biol. 47, 50–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stubbe J., van Der Donk W. A. (1998) Protein radicals in enzyme catalysis. Chem. Rev. 98, 705–762 [DOI] [PubMed] [Google Scholar]
- 4. Stubbe J., Nocera D. G., Yee C. S., Chang M. C. (2003) Radical initiation in the class I ribonucleotide reductase. Long-range proton-coupled electron transfer. Chem. Rev. 103, 2167–2201 [DOI] [PubMed] [Google Scholar]
- 5. Zhou B. B., Elledge S. J. (2000) The DNA damage response. Putting checkpoints in perspective. Nature 408, 433–439 [DOI] [PubMed] [Google Scholar]
- 6. Thelander L. (2007) Ribonucleotide reductase and mitochondrial DNA synthesis. Nat. Genet. 39, 703–704 [DOI] [PubMed] [Google Scholar]
- 7. Shao J., Zhou B., Chu B., Yen Y. (2006) Ribonucleotide reductase inhibitors and future drug design. Curr. Cancer Drug Targets 6, 409–431 [DOI] [PubMed] [Google Scholar]
- 8. Krakoff I. H. (1975) in Antineoplastic and Immunosuppressive Agents, Part II (Sartorelli A. C., Johns D. G., eds.) pp. 789–792, Springer-Verlag New York Inc., New York [Google Scholar]
- 9. Gandhi V., Plunkett W. (2006) in Cancer Drug Discovery and Development: Deoxynucleoside Analogs in Cancer Therapy (Peters G. J., ed.) pp. 153–171, Humana Press, Totowa, NJ [Google Scholar]
- 10. Wang J., Lohman G. J., Stubbe J. (2007) Enhanced subunit interactions with gemcitabine 5′-diphosphate inhibit ribonucleotide reductases. Proc. Natl. Acad. Sci. U.S.A. 104, 14324–14329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aye Y., Stubbe J. (2011) Clofarabine 5′-di and -triphosphates inhibit human ribonucleotide reductase by altering the quaternary structure of its large subunit. Proc. Natl. Acad. Sci. U.S.A. 108, 9815–9820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aye Y., Brignole E. J., Long M. J., Chittuluru J., Drennan C. L., Asturias F. J., Stubbe J. (2012) Clofarabine targets the large subunit (α) of human ribonucleotide reductase in live cells by assembly into persistent hexamers. Chem. Biol. 19, 799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cory J. G., Cory A. H., Rappa G., Lorico A., Liu M. C., Lin T. S., Sartorelli A. C. (1994) Inhibitors of ribonucleotide reductase. Comparative effects of amino- and hydroxyl-substituted pyridine-2-carboxaldehyde thiosemicarbazones. Biochem. Pharm. 48, 335–344 [DOI] [PubMed] [Google Scholar]
- 14. Finch R. A., Liu M. C., Cory A. H., Cory J. G., Sartorelli A. C. (1999) Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP)). An inhibitor of ribonucleotide reductase with antineoplastic activity. Adv. Enzyme Regul. 39, 3–12 [DOI] [PubMed] [Google Scholar]
- 15. Finch R. A., Liu M., Grill S. P., Rose W. C., Loomis R., Vasquez K. M., Cheng Y., Sartorelli A. C. (2000) Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone). A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem. Pharm. 59, 983–991 [DOI] [PubMed] [Google Scholar]
- 16. Nutting C. M., van Herpen C. M., Miah A. B., Bhide S. A., Machiels J. P., Buter J., Kelly C., de Raucourt D., Harrington K. J. (2009) Phase II study of 3-AP triapine in patients with recurrent or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 20, 1275–1279 [DOI] [PubMed] [Google Scholar]
- 17. Traynor A. M., Lee J. W., Bayer G. K., Tate J. M., Thomas S. P., Mazurczak M., Graham D. L., Kolesar J. M., Schiller J. H. (2010) A phase II trial of Triapine (NSC# 663249) and gemcitabine as second line treatment of advanced non-small cell lung cancer. Eastern cooperative oncology group study 1503. Invest. New Drugs 28, 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaston T. B., Lovejoy D. B., Watts R. N., Richardson D. R. (2003) Examination of the antiproliferative activity of iron chelators. Multiple cellular targets and the different mechanism of action of triapine compared with desferrioxamine and the potent pyridoxal isonicotinoyl hydrazine analogue 311′. Clin. Cancer Res. 9, 402–414 [PubMed] [Google Scholar]
- 19. Richardson D. R., Kalinowski D. S., Richardson V., Sharpe P. C., Lovejoy D. B., Islam M., Bernhardt P. V. (2009) 2-Acetylpyridine thiosemicarbazones are potent iron chelators and antiproliferative agents. Redox activity, iron complexation, and characterization of their antitumor activity. J. Med. Chem. 52, 1459–1470 [DOI] [PubMed] [Google Scholar]
- 20. Yu Y., Wong J., Lovejoy D. B., Kalinowski D. S., Richardson D. R. (2006) Chelators at the cancer coalface. Desferrioxamine to triapine and beyond. Clin. Cancer Res. 12, 6876–6883 [DOI] [PubMed] [Google Scholar]
- 21. Merlot A. M., Kalinowski D. S., Richardson D. R. (2012) Novel chelators for cancer treatment. Where are they now? Antioxid. Redox. Signal. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22. Kowol C. R., Trondl R., Heffeter P., Arion V. B., Jakupec M. A., Roller A., Galanski M., Berger W., Keppler B. K. (2009) Impact of metal coordination on cytotoxicity of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (triapine) and novel insights into terminal dimethylation. J. Med. Chem. 52, 5032–5043 [DOI] [PubMed] [Google Scholar]
- 23. Alvero A. B., Chen W., Sartorelli A. C., Schwartz P., Rutherford T., Mor G. (2006) Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) induces apoptosis in ovarian cancer cells. J. Soc. Gynecol. Investig. 13, 145–152 [DOI] [PubMed] [Google Scholar]
- 24. Yu Y., Gutierrez E., Kovacevic Z., Saletta F., Obeidy P., Suryo Rahmanto Y., Richardson D. R. (2012) Iron chelators for the treatment of cancer. Curr. Med. Chem. 19, 2689–2702 [DOI] [PubMed] [Google Scholar]
- 25. Enyedy E. A., Nagy N. V., Zsigó, Kowol C. R., Arion V. B., Keppler B. K., Kiss T. (2010) Comparative solution equilibrium study of the interactions of copper (II), iron (II) and zinc (II) with triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) and related ligands. Eur. J. Inorg. Chem. 2010, 1717–1728 [Google Scholar]
- 26. Enyedy É. A., Primik M. F., Kowol C. R., Arion V. B., Kiss T., Keppler B. K. (2011) Interaction of triapine and related thiosemicarbazones with iron(III)/(II) and gallium(III). A comparative solution equilibrium study. Dalton Trans. 40, 5895–5905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shao J., Zhou B., Di Bilio A. J., Zhu L., Wang T., Qi C., Shih J., Yen Y. (2006) A ferrous-triapine complex mediates formation of reactive oxygen species that inactivate human ribonucleotide reductase. Mol. Cancer Ther. 5, 586–592 [DOI] [PubMed] [Google Scholar]
- 28. Zhu L., Zhou B., Chen X., Jiang H., Shao J., Yen Y. (2009) Inhibitory mechanisms of heterocyclic carboxaldehyde thiosemicarbazones for two forms of human ribonucleotide reductase. Biochem. Pharm. 78, 1178–1185 [DOI] [PubMed] [Google Scholar]
- 29. Popovi-Bijeli A., Kowol C. R., Lind M. E., Luo J., Himo F., Enyedy E. A., Arion V. B., Gräslund A. (2011) Rinoculeotide reductase inhibition by metal complexes of triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone). A combined experimental and theoretical study. J. Inorg. Biochem. 105, 1422–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cotruvo J. A., Stubbe J. (2011) Class I ribonucleotide reductase. Metallocofactor assembly and repair in vitro and in vivo. Annu. Rev. Biochem. 80, 733–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akerblom L., Ehrenberg A., Gräslund A., Lankinen H., Reichard P., Thelander L. (1981) Overproduction of the free radical of ribonucleotide reductase in hydroxyurea-resistant mouse fibroblast 3T6 cells. Proc. Natl. Acad. Sci. U.S.A. 78, 2159–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Engström Y., Eriksson S., Jildevik I., Skog S., Thelander L., Tribukait B. (1985) Cell cycle-dependent expression of mammalian ribonucleotide reductase. J. Biol. Chem. 260, 9114–9116 [PubMed] [Google Scholar]
- 33. Håkansson P., Hofer A., Thelander L. (2006) Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J. Biol. Chem. 281, 7834–7841 [DOI] [PubMed] [Google Scholar]
- 34. Fish W. W. (1988) Rapid colorimetric micromethod for the quantitation of complexed iron in biological samples. Methods Enzymol. 158, 357–364 [DOI] [PubMed] [Google Scholar]
- 35. Bou-Abdallah F., Chasteen N. D. (2008) Spin concentration measurements of high-spin (g′ = 4.3) rhombic iron(III) ions in biological samples. Theory and application. J. Biol. Inorg. Chem. 13, 15–24 [DOI] [PubMed] [Google Scholar]
- 36. Cooper C. E., Lynagh G. R., Hoyes K. P., Hider R. C., Cammack R., Porter J. B. (1996) The relationship of intracellular iron chelation to the inhibition and regulation of human ribonuleotide reductase. J. Biol. Chem. 271, 20291–20299 [DOI] [PubMed] [Google Scholar]
- 37. Nyholm S., Mann G. J., Johansson A. G., Bergeron R. J., Gräslund A., Thelander L. (1993) Role of ribonucleotide reductase in inhibition of mammalian cell growth by potent iron chelators. J. Biol. Chem. 268, 26200–26205 [PubMed] [Google Scholar]
- 38. Barycki J. J. (2008) in Redox Biochemistry (Banerjee R., Becker D., eds) pp. 11–21, John Wiley & Sons, Inc., Hoboken, NJ [Google Scholar]
- 39. Sahlin M., Sjöberg B. M., Backes G., Loehr T., Sanders-Loehr J. (1990) Activation of the iron-containing B2 protein of ribonucleotide reductase by hydrogen peroxide. Biochem. Biophys. Res. Commun. 167, 813–818 [DOI] [PubMed] [Google Scholar]
- 40. Gaudu P., Nivière V., Pétillot Y., Kauppi B., Fontecave M. (1996) The irreversible inactivation of ribocucleotide reductase from Escherichia coli by superoxide radicals. FEBS Lett. 387, 137–140 [DOI] [PubMed] [Google Scholar]
- 41. McCord J. M., Fridovich I. (1969) Superoxide dismutase. An enzymatic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055 [PubMed] [Google Scholar]
- 42. Del Maestro R. F., Björk J., Arfors K. E. (1981) Increase in microvascular permeability induced by enzymatically generated free radicals. Microvasc. Res. 22, 239–254 [DOI] [PubMed] [Google Scholar]
- 43. Imlay J. A., Fridovich I. (1991) Assay of metabolic superoxide production in Escherichia coli. J. Biol. Chem. 266, 6957–6965 [PubMed] [Google Scholar]
- 44. Stadtman E. R. (1993) Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Ann. Rev. Biochem. 62, 797–821 [DOI] [PubMed] [Google Scholar]
- 45. Hernebring M., Brolén G., Aguilaniu H., Semb H., Nyström T. (2006) Elimination of damaged proteins during differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 103, 7700–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eriksson S., Gräslund A., Skog S., Thelander L., Tribukait B. (1984) Cell cycle-dependent regulation of mammalian ribonucleotide reductase. The S-phase correlated increase in subunit M2 is regulated by de novo protein synthesis. J. Biol. Chem. 259, 11695–11700 [PubMed] [Google Scholar]
- 47. Chabes A. L., Pfleger C. M., Kirschner M. W., Thelander L. (2003) Mouse ribonucleotide reductase R2 protein. A new target for anaphase-promoting complex-Cdh1-mediated proteolysis. Proc. Natl. Acad. Sci. U.S.A. 100, 3925–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hershko A., Ciechanover A. (1998) The ubiquitin system. Ann. Rev. Biochem. 67, 425–479 [DOI] [PubMed] [Google Scholar]
- 49. Adams J., Kauffman M. (2004) Development of the proteasome inhibitor Velcade® (Bortezomib). Cancer Invest. 22, 304–311 [DOI] [PubMed] [Google Scholar]
- 50. Stargell L. A., Heruth D. P., Gaertig J., Gorovsky M. A. (1992) Drugs affecting microtubule dynamics increase α-tubulin mRNA accumulation via transcription in Tetrahymena thermophila. Mol. Cell. Biol. 12, 1443–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hsieh Y. C., Skacel N. E., Bansal N., Scotto K. W., Banerjee D., Bertino J. R., Abali E. E. (2009) Species-specific differences in translational regulation of dihydrofolate reductase. Mol. Pharmacol. 76, 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Obrig T. G., Culp W. J., McKeehan W. L., Hardesty B. (1971) The mechanism by which cycoheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J. Biol. Chem. 246, 174–181 [PubMed] [Google Scholar]
- 53. Chabes A., Thelander L. (2000) Controlled protein degradation regulates ribonucleotide reductase activity in proliferating mammalian cells during the normal cell cycle and in response to DNA damage and replication blocks. J. Biol. Chem. 275, 17747–17753 [DOI] [PubMed] [Google Scholar]
- 54. Christner C., Wyrwa R., Marsch S., Küllertz G., Thiericke R., Grabley S., Schumann D., Fischer G. (1999) Synthesis and cytotoxic evaluation of cycloheximide derivatives as potential inhibitors of FKBP12 with neuroregenerative properties. J. Med. Chem. 42, 3615–3622 [DOI] [PubMed] [Google Scholar]
- 55. Fuller S. D., Simons K. (1986) Transferrin receptor polarity and recycling accuracy in “tight” and “leaky” strains of Madin-Darby canine kidney cells. J. Cell Biol. 103, 1767–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nyholm S., Thelander L., Gräslund A. (1993) Reduction and loss of the iron center in the reaction of the small subunit of mouse ribonucleotide reductase with hydroxyurea. Biochemistry 32, 11569–11574 [DOI] [PubMed] [Google Scholar]
- 57. McClarty G. A., Chan A. K., Choy B. K., Wright J. A. (1990) Increased ferritin gene expression is associated with increased ribonucleotide reductase gene expression and the establishment of hydroxyurea resistance in mammalian cells. J. Biol. Chem. 265, 7539–7547 [PubMed] [Google Scholar]
- 58. Mühlenhoff U., Molik S., Godoy J. R., Uzarska M. A., Richter N., Seubert A., Zhang Y., Stubbe J., Pierrel F., Herrero E., Lillig C. H., Lill R. (2010) Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 12, 373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Y., Liu L., Wu X., An X., Stubbe J., Huang M. (2011) Investigation of in vivo diferric tyrosyl radical formation in Saccharomyces cerevisiae Rnr2 protein. Requirement of Rnr4 and contribution of Grx3/4 and Dre2 proteins. J. Biol. Chem. 286, 41499–41509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rose I. A., O'Connell E. L. (1967) Mechanism of aconitase action. J. Biol. Chem. 242, 1870–1879 [PubMed] [Google Scholar]
- 61. Gardner P. R., Nguyen D. D., White C. W. (1994) Aconitase is a sensitive and critical target of oxygen poisoning in cultured cells and in rat lungs. Proc. Natl. Acad. Sci. U.S.A. 91, 12248–12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lill R. (2009) Function and biogenesis of iron-sulfur proteins. Nature 460, 831–838 [DOI] [PubMed] [Google Scholar]
- 63. Hentze M. W., Muckenthaler M. U., Andrews N. C. (2004) Balancing acts. Molecular control of mammalian iron metabolism. Cell 117, 285–297 [DOI] [PubMed] [Google Scholar]
- 64. Torrents E., Westman M., Sahlin M., Sjöberg B. M. (2006) Ribonucleotide reductase modularity. Atypical duplication of the ATP-cone domain in Pseudomonas aeruginosa. J. Biol. Chem. 281, 25287–25296 [DOI] [PubMed] [Google Scholar]
- 65. Thelander L., Gräslund A. (1983) Mechanism of inhibition of mammalian ribonucleotide reductase by the iron chelate of 1-formylisoquinoline thiosemicarbazone. J. Biol. Chem. 258, 4063–4066 [PubMed] [Google Scholar]
- 66. Kolesar J. M., Schelman W. R., Geiger P. G., Holen K. D., Traynor A. M., Alberti D. B., Thomas J. P., Chitambar C. R., Wilding G., Antholine W. E. (2008) Electron paramagnetic resonance study of peripheral blood mononuclear cells from patients with refractory solid tumors treated with Triapine®. J. Inorg. Biochem. 102, 693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deleted in proof
- 68. Berg R. W., Ferguso P. J., DeMoor J. M., Vincen M. D., Koropatnick J. (2002) The means to an end of tumor cell resistance to chemotherapeutic drugs targeting thymidylate synthase. Shoot the messenger. Curr. Drug Targets 3, 297–309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.