Abstract
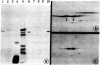
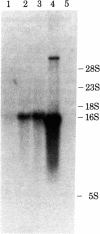
Sequences complementary to muscle poly(A)+RNA were cloned in the plasmid pBR322 and the resulting colonies were screened by colony hybridization with labeled cDNA derived from skeletal muscle and smooth muscle (gizzard). The skeletal muscle-specific clones were further screened by RNA blotting hybridization for a muscle mRNA having the size expected for a putative type M creatine kinase (M-CK) mRNA. The remaining clones with the expected hybridization properties were finally characterized by hybrid-selected translation, and a cloned sequence was shown to contain DNA hybridizing to mRNA that could be translated into M-CK. This plasmid, pMCK1, was further characterized by restriction mapping. Blot analysis of total cell RNA from differentiating myogenic cell cultures showed accumulation of M-CK mRNA in cultures older than 42 hr but not in young little-differentiated cultures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravatti M., Perriard J. C., Eppenberger H. M. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Biosynthesis of creatine kinase subunits M and B. J Biol Chem. 1979 Feb 25;254(4):1388–1394. [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- EPPENBERGER H. M., EPPENBERGER M., RICHTERICH R., AEBI H. THE ONTOGENY OF CREATINE KINASE ISOZYMES. Dev Biol. 1964 Aug;10:1–16. doi: 10.1016/0012-1606(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod A. R. Construction of bacterial plasmids containing sequences complementary to chicken alpha-tropomyosin mRNA. Nucleic Acids Res. 1981 Jun 25;9(12):2675–2689. doi: 10.1093/nar/9.12.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. S., Ricciardi R. P., Roberts B. E., Paterson B. M., Mathews M. B. Arrangement of messenger RNAs and protein coding sequences in the major late transcription unit of adenovirus 2. J Mol Biol. 1980 Oct 5;142(4):455–488. doi: 10.1016/0022-2836(80)90258-2. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perriard J. C., Caravatti M., Perriard E. R., Eppenberger H. M. Quantitation of creatine kinase isoenzyme transition in differentiating chicken embryonic breast muscle and myogenic cell cultures by immunoadsorption. Arch Biochem Biophys. 1978 Nov;191(1):90–100. doi: 10.1016/0003-9861(78)90070-x. [DOI] [PubMed] [Google Scholar]
- Perriard J. C. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Levels of mRNA for creatine kinase subunits M and B. J Biol Chem. 1979 Aug 10;254(15):7036–7041. [PubMed] [Google Scholar]
- Perriard J. C., Perriard E. R., Eppenberger H. M. Detection and relative quantitation of mRNA for creatine kinase isoenzymes in mRNA from myogenic cell cultures and embryonic chicken tissues. J Biol Chem. 1978 Sep 25;253(18):6529–6535. [PubMed] [Google Scholar]
- Robbins J., Freyer G. A., Chisholm D., Gilliam T. C. Isolation of multiple genomic sequences coding for chicken myosin heavy chain protein. J Biol Chem. 1982 Jan 10;257(1):549–556. [PubMed] [Google Scholar]
- Rosenberg U. B., Eppenberger H. M., Perriard J. C. Occurrence of heterogenous forms of the subunits of creatine kinase in various muscle and nonmuscle tissues and their behaviour during myogenesis. Eur J Biochem. 1981 May;116(1):87–92. doi: 10.1111/j.1432-1033.1981.tb05304.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. J., Rothblum K. N. Gene switching in myogenesis: differential expression of the chicken actin multigene family. Biochemistry. 1981 Jul 7;20(14):4122–4129. doi: 10.1021/bi00517a027. [DOI] [PubMed] [Google Scholar]
- Schwartz R. J., Rothblum K. Regulation of muscle differentiation: isolation and purification of chick actin messenger ribonucleic acid and quantitation with complementary deoxyribonucleic acid probes. Biochemistry. 1980 May 27;19(11):2506–2514. doi: 10.1021/bi00552a032. [DOI] [PubMed] [Google Scholar]
- Shani M., Zevin-Sonkin D., Saxel O., Carmon Y., Katcoff D., Nudel U., Yaffe D. The correlation between the synthesis of skeletal muscle actin, myosin heavy chain, and myosin light chain and the accumulation of corresponding mRNA sequences during myogenesis. Dev Biol. 1981 Sep;86(2):483–492. doi: 10.1016/0012-1606(81)90206-2. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. C., Maier V., Eppenberger H. M. Creatine kinase and aldolase isoenzyme transitions in cultures of chick skeletal muscle cells. Dev Biol. 1974 Mar;37(1):63–89. doi: 10.1016/0012-1606(74)90170-5. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Butler-Browne G. S., Schwartz K., Bouveret P., Pinset-Härstöm I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981 Aug 27;292(5826):805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]