Background: The mechanisms of PPARα-mediated inhibition of tumor growth and angiogenesis remain unknown.
Results: Activation of PPARα suppresses hypoxia-induced HIF-1α signaling via promoting HIF-1α degradation and diminishes hypoxia-induced VEGF secretion from cancer cells and tube formation by endothelial cells.
Conclusion: Activation of PPARα suppresses the HIF-1α signaling pathway in cancer cells.
Significance: The results support the development of PPARα agonists as anticancer agents.
Keywords: Angiogenesis, Cancer, Hypoxia-inducible Factor (HIF), Peroxisomes, Vascular Endothelial Growth Factor (VEGF), Clofibrate, PPARα
Abstract
Activation of peroxisome proliferator-activated receptor α (PPARα) has been demonstrated to inhibit tumor growth and angiogenesis, yet the mechanisms behind these actions remain to be characterized. In this study, we examined the effects of PPARα activation on the hypoxia-inducible factor-1α (HIF-1α) signaling pathway in human breast (MCF-7) and ovarian (A2780) cancer cells under hypoxia. Incubation of cancer cells under 1% oxygen for 16 h significantly induced HIF-1α expression and activity as assayed by Western blotting and reporter gene analysis. Treatment of the cells with PPARα agonists, but not a PPARγ agonist, prior to hypoxia diminished hypoxia-induced HIF-1α expression and activity, and addition of a PPARα antagonist attenuated the suppression of HIF-1α signaling. Activation of PPARα attenuated hypoxia-induced HA-tagged HIF-1α protein expression without affecting the HA-tagged HIF-1α mutant protein level, indicating that PPARα activation promotes HIF-1α degradation in these cells. This was further confirmed using proteasome inhibitors, which reversed PPARα-mediated suppression of HIF-1α expression under hypoxia. Using the co-immunoprecipitation technique, we found that activation of PPARα enhances the binding of HIF-1α to von Hippel-Lindau tumor suppressor (pVHL), a protein known to mediate HIF-1α degradation through the ubiquitin-proteasome pathway. Following PPARα-mediated suppression of HIF-1α signaling, VEGF secretion from the cancer cells was significantly reduced, and tube formation by endothelial cells was dramatically impaired. Taken together, these findings demonstrate for the first time that activation of PPARα suppresses hypoxia-induced HIF-1α signaling in cancer cells, providing novel insight into the anticancer properties of PPARα agonists.
Introduction
Hypoxia is an established characteristic of all solid tumors and is thought to be due to abnormal tumor microvasculature (1). To survive hypoxic conditions, tumor cells undergo a series of genetic and metabolic changes, such as enhanced glycolysis and survival factor overexpression (2, 3). Hypoxia-inducible factor-1α (HIF-1α),2 a transcription factor, plays a key role in hypoxia-inducible gene expression. The expression of HIF-1α is low under normoxia but highly inducible by hypoxia (3). Under hypoxic conditions, HIF-1α translocates into the nucleus, dimerizes with HIF-1β and other transcription factors, binds to the hypoxia response element (HRE), and transcriptionally activates hypoxia-inducible genes (4–6). HIF-1α is associated with tumor growth, angiogenesis, metastasis, chemo/radioresistance, and poor prognosis (7). Compelling evidence indicates that blocking HIF-1α activity or targeting HIF-1α expression slows tumor growth and increases sensitivity of tumor cells to conventional therapy (1). Thus, targeting HIF-1α is a strategy for cancer treatment.
Clofibrate and fenofibrate, two well known ligands for peroxisome proliferator-activated receptor α (PPARα), have been widely used to control plasma levels of cholesterol and triglycerides, and they increase lipoprotein lipase activity (8). Recent studies demonstrated that these two drugs have anticancer properties in various model systems (9–11), but the mechanisms behind their actions remain to be elucidated. We have reported previously that activation of PPARα mediates the anticancer action of docosahexaenoic acid, likely through down-regulation of hypoxia signaling (12, 13). We therefore hypothesized that the anticancer properties of PPARα agonists may similarly be due to suppression of HIF-1α signaling in human cancer cells. Note that activation of the HIF-1α gene has been reported to down-regulate the levels of retinoid X receptor α (RXRα), the obligate partner of PPARα, which results in decreased DNA binding activity of PPARα/RXR and reduced expression of the PPARα-activated genes (14). To date, there have been no data examining the effects of PPARα activation on HIF-1α signaling in any experimental model systems. In this study, we report the effects of clofibrate and fenofibrate on HIF-1α signaling in human breast and ovarian cancer cell model systems. We found that activation of PPARα diminished hypoxia-induced HIF-1α expression and activity, primarily through promoting HIF-1α protein degradation. As HIF-1α is known to enhance tumor growth, angiogenesis, and metastasis (15), our results support the development of PPARα agonists as therapeutic anticancer agents.
EXPERIMENTAL PROCEDURES
Materials
The pGL3-HRE-luciferase reporter construct containing the HREs of the VEGF gene promoter was kindly provided by Dr. Konstantin Salnikow (Radiation Oncology Branch, NCI, Frederick, MD). The peroxisome proliferator response element-luciferase reporter construct was obtained from Dr. Bruce Spiegelman (Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA), and the RXRα cDNA construct from Dr. Ronald C. Kahn (Joslin Diabetes Center, Harvard Medical School, Boston, MA). The pcDNA3-HA-HIF-1α and pcDNA3-HA-HIF-1α(P402A/P564A) (with two prolyl mutations) expression vectors were from Dr. William Kaelin (Dana-Farber Cancer Institute, Harvard Medical School). Anti-HIF-1α antibody was from R&D Systems (Minneapolis, MN). Anti-HA tag and anti-pVHL antibodies were from Cell Signaling Technology, Inc. (Danvers, MA). Anti-GAPDH antibody was from ProMab Biotechnologies, Inc. (Albany, CA). Anti-heme oxygenase 1 (HO-1) antibody was from Stressgen (Ann Arbor, MI). The Dual-Luciferase reporter kit was purchased from Promega (Madison, WI). The VEGF ELISA kit was purchased from R&D Systems, Inc. Anti-β-actin antibody, clofibrate, fenofibrate, troglitazone, GW6471, GW9662, and other chemical agents were analytic grade and purchased from Sigma-Aldrich.
Cell Culture
The breast cancer cell line MCF-7 and the human umbilical vein cell line EA.hy926 was purchased from American Type Culture Collection (Manassas, VA). The ovarian carcinoma cell line A2780 was a kind gift from Dr. Stephen Howell (University of California, San Diego, CA). MCF-7 and A2780 cells were cultivated in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified environment containing 5% CO2. EA.hy926 cells were cultivated in DMEM supplemented with 10% fetal bovine serum as specified by American Type Culture Collection. Cells were subcultivated twice per week in 75- or 150-mm flasks.
Transient Transfection and Luciferase Activity Assay
MCF-7 cells and A2780 cells were seeded into 100-mm cell culture dishes and reached 70–80% confluence 24 h after plating. The cells were then transfected with the pGL3-HRE-luciferase plasmid using FuGENE HD transfection reagent (Roche Applied Science) as described previously (16). The next day, cells were split into 24-well plates at a density of 2 × 105 cells/well. 48 h after transfection, cells were treated for 4 h with clofibrate, fenofibrate, troglitazone, and other reagents at the indicated concentrations and durations and then placed into a hypoxia chamber (1% O2 and 5% CO2; ProOx model C21, Biospherix, Ltd., Lacona, NY) for 16 h. Cell lysate was prepared, and luciferase activity was assayed using the Dual-Luciferase reporter kit as described (16). The firefly luciferase activity was normalized for the amount of protein used for luciferase activity assay for each sample. The data are expressed as percentages of luciferase activity detected in untreated cells.
Overexpression of Wild-type and Mutant HIF-1α in MCF-7 Cells
MCF-7 cells were seeded in 100-mm dishes and reached 70–80% confluence overnight. The cells were then transfected with the pcDNA3-HA-HIF-1α and pcDNA3-HA-HIF-1α(P402A/P564A) constructs using FuGENE HD transfection reagent as described previously (16). 72 h after transfection, overexpression of HA-tagged HIF-1α and its mutant was verified using an antibody against HA. The cells transiently overexpressing HA-HIF-1α and its mutant were subjected to the experiments testing the assumption that clofibrate may promote HIF-1α degradation in these cells.
Western Blot Analysis
Western blotting was performed as described (17, 18). In brief, cells were lysed with the lysis buffer, sonicated on ice for three strokes of 10 s each, and centrifuged at 15,000 × g for 15 min to remove insoluble material. 40 μg of protein from each sample was separated on a 10% SDS-polyacrylamide gel; transferred to a PVDF membrane; and blotted with antibodies against HIF-1α, HA, pVHL, HO-1, GAPDH, and β-actin.
Transient Knockdown of PPARα and pVHL
siRNAs for PPARα and pVHL were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Each product is a pool of three target-specific 19–25-nucleotide siRNAs designed to knock down target gene expression. Scrambled siRNAs were applied as controls. siRNAs (50 or 100 pmol) were transfected into MCF-7 cells cultured in a 6-well plate using FuGENE HD transfection reagent according to the manufacturer's protocols. 48 h after the transfection, the cells were treated with 500 mm clofibrate for 4 h and placed into a hypoxia chamber or kept under normoxic conditions for 16 h. The knockdown was confirmed by Western blot analysis. Individual siRNAs in this siRNA pool were also purchased and used to demonstrate the knockdown of PPARα and pVHL under multiple siRNA conditions in MCF-7 cells.
Co-immunoprecipitation
Co-immunoprecipitation was performed as described previously (16). In short, MCF-7 cells were treated with various agents under hypoxia. Before the cells were placed into the hypoxia chamber for 16 h, 10 mm MG132 was added to each dish (19). The cells were then washed with cold phosphate-buffered saline and harvested by adding 150 μl of immunoprecipitation buffer containing 10 mm Tris-HCl (pH 7.4), 50 mm NaCl, 0.5 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, and 1% Triton X-100. Cells were sonicated for 1 min with intervals on ice and centrifuged at 13,000 × g for 30 min to remove insoluble material. Following preclearing for 1 h at 4 °C, total cell extract (200 μg of protein) was incubated with anti-HIF-1α antibody at 4 °C with gentle rotation overnight. The antibody-protein complexes were precipitated by addition of 50 μl of protein G-agarose and rotation for 2 h at 4 °C. The supernatants were then removed by centrifugation, and the pellets were washed with immunoprecipitation buffer and subjected to Western blotting with antibodies against pVHL and HIF-1α.
RT-PCR
Total RNA was isolated from MCF-7 cells using TRIzol reagent (Invitrogen) following the manufacturer's protocol. RNA samples were reverse-transcribed with the SuperScript II kit (Invitrogen) as described previously (16). The cDNA was amplified by PCR using the following specific primers: HIF-1α, 5′-CCT CAG TCT ACA CAG CCT G-3′ (forward) and 5′-CAT ATC TGA AGA TTC AAC C-3′ (reverse); VEGF, 5′-TCG GGC CTC CGA AAC CAT G-3′ (forward) and 5′-CCT GGT GAG AGA TCT GGT TC-3′ (reverse); and β-actin, 5′-GGA AAT CGT GCG TGA CAT TA-3′ (forward) and 5′-GGA GCA ATG ATC TTG ATC TTC-3′ (reverse). The samples were initially denatured at 94 °C for 2 min prior to thermal cycling. The thermal cycle for PCR was as follows: 94 °C for 15 s, 48 °C for 30 s, and 72 °C for 1 min, for a total 30 cycles. The PCR products were separated on a 1% agarose gel containing ethidium bromide and visualized under ultraviolet light.
ELISA
Secretion of VEGF from MCF-7 cells was determined using an ELISA kit. Cells were seeded into 6-well plates at a density of 1 × 106 cells/well and treated with clofibrate or troglitazone for 4 h prior to placement into the hypoxia chamber for 16 h. The culture medium was then collected, and the level of VEGF in the medium was analyzed following the manufacturer's instructions. VEGF levels were normalized to cell numbers and are expressed as picograms/million liters of medium.
Tube Formation Analysis
The wells of a 96-well plate were coated with ice-cold BD MatrigelTM matrix gel solution (BD Biosciences) and allowed to polymerize for 1 h at 37 °C. Conditioned medium was prepared by treating MCF-7 cells with or without clofibrate under hypoxic conditions for 16 h. The medium was collected and centrifuged to remove damaged/detached cells prior to culturing endothelial cells. EA.hy926 cells, a human umbilical vein cell line with characteristics of differentiated endothelial cells, were plated into the polymerized cell matrix at a concentration of 1 × 104 cells in 150 μl of conditioned medium/well. The formation of endothelial tubes were observed microscopically and photographed after 24 h of incubation. Three experiments were performed with triplicates each time. Tube formation was quantified using the “Pattern Recognition” method specified by the manufacturer.
Statistical Analysis
All statistical analysis was done with GraphPad Prism software. One-way analysis of variance (ANOVA) with Dunnett's post-test was used to determine differences among control and experimental groups, with p < 0.05 or 0.01 as the level of statistical significance.
RESULTS
Activation of PPARα by Clofibrate and Fenofibrate in MCF-7 Cells
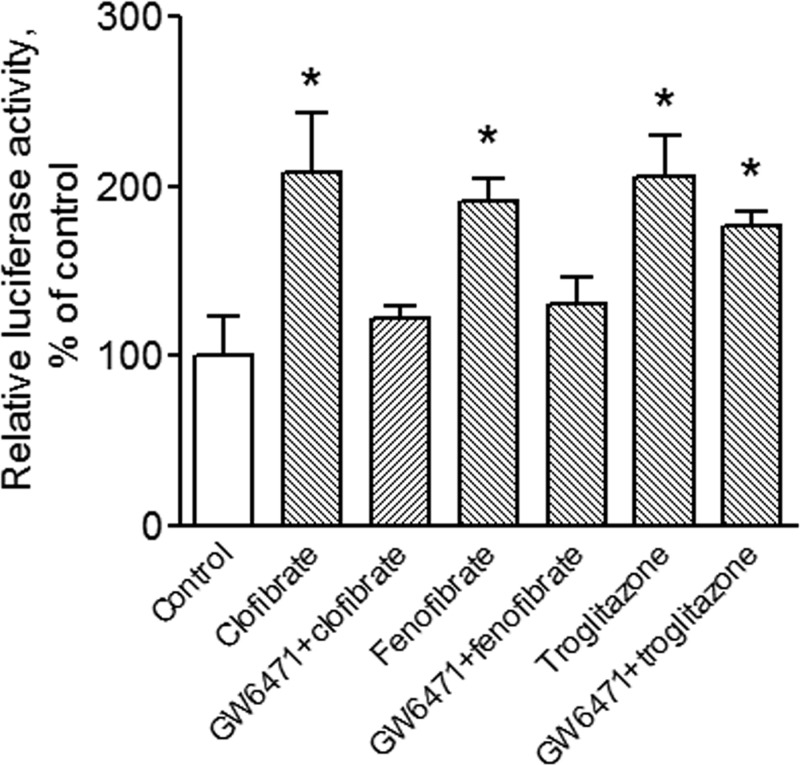
To understand whether the PPARα ligands clofibrate and fenofibrate activate PPARα signaling in MCF-7 cells, cells were transfected with 3 μg of the peroxisome proliferator response element-luciferase plasmid construct and 1 μg of RXRα cDNA (13) and treated with 500 μm clofibrate, 100 μm fenofibrate (two established PPARα agonists), or 20 μm troglitazone (a PPARγ agonist) for 4 h. The concentrations of the compounds were chosen according to previous studies (20, 21). As expected, all three compounds activated peroxisome proliferator response element-driven reporter gene activity in MCF-7 cells (Fig. 1). However, pretreatment of the cells with a well established PPARα antagonist, GW6471 (5 μm), reversed PPAR activation by clofibrate and fenofibrate but had no significant effect on troglitazone-induced reporter gene activity, indicating that clofibrate and fenofibrate activate PPARα in this model system.
FIGURE 1.
Clofibrate and fenofibrate activate PPARα signaling in MCF-7 cells. Cells were transfected with the peroxisome proliferator response element-luciferase reporter and RXRα cDNA constructs and treated with 500 μm clofibrate, 100 μm fenofibrate, and 20 μm troglitazone in the presence or absence of 5 μm GW6471 for 4 h. Cell lysates were prepared, and luciferase activity was assayed. Data (mean ± S.E., n = 3) are expressed as percentages of the luciferase activity detected in untreated control cells. *, p < 0.05 compared with control cells using one-way ANOVA, followed by Dunnett's analysis.
Activation of PPARα Suppresses Hypoxia-induced HIF-1α Expression and Activity
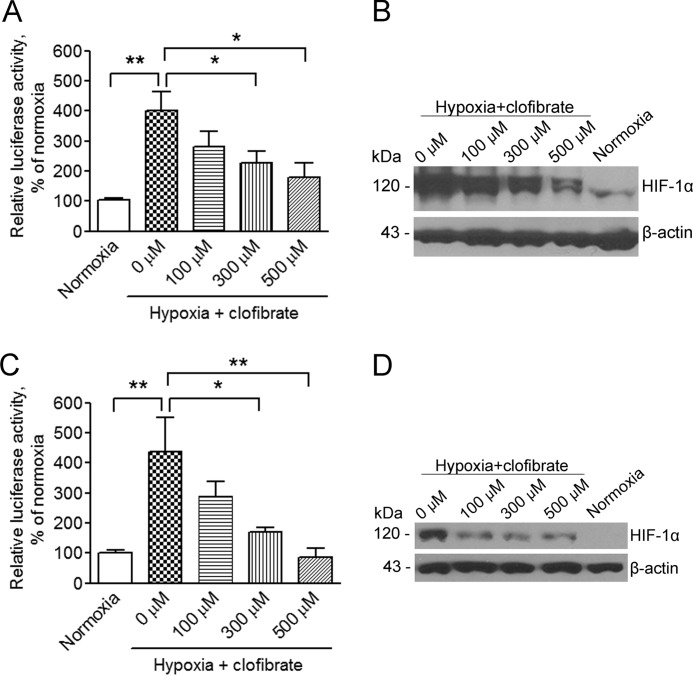
To investigate the effects of activation of PPARα on hypoxia-induced HIF-1α expression and activity, MCF-7 cells were treated with clofibrate at various concentrations for 4 h prior to placing the cells into a hypoxia chamber with a gas mixture of 94% N2, 5% CO2, and 1% O2 for 16 h. As shown in Fig. 2 (A and B), hypoxia induced a dramatic increase in HIF-1α expression as assayed by Western blotting and in HRE-driven reporter activity as analyzed using the reporter gene technique. Addition of clofibrate suppressed hypoxia-induced HIF-1α expression and activity in a concentration-dependent manner (Fig. 2, A and B). To confirm that this suppression of HIF-1α signaling is not a cell type-related event, A2780 cells were also subjected to the same experimental conditions. Hypoxia-induced HIF-1α expression and activity were also suppressed by clofibrate in A2780 cells (Fig. 2, C and D), similar to the results obtained in MCF-7 cells.
FIGURE 2.
Clofibrate suppresses hypoxia-induced HIF-1α expression and activity in a concentration-dependent manner. A, MCF-7 cells were transfected with the pGL3-HRE-luciferase reporter construct. Cells were treated with different concentrations of clofibrate for 4 h prior to placement in a hypoxia chamber for 16 h. Cell lysates were prepared, and luciferase activity was assayed. Data (mean ± S.E., n = 3) are expressed as percentages of the luciferase activity detected in untreated cells under normoxia. *, p < 0.05; **, p < 0.01 compared with untreated cells using one-way ANOVA, followed by Dunnett's analysis. B, MCF-7 cells were treated with different concentrations of clofibrate for 4 h prior to placement in a hypoxia chamber for 16 h. Cell lysates were prepared, and Western blotting was performed using antibodies against HIF-1α and β-actin. Shown are representative images of three individual experiments. C, A2780 cells were transfected with the pGL3-HRE-luciferase reporter construct. Cells were treated with different concentrations of clofibrate for 4 h prior to placement in a hypoxia chamber for 16 h. Cell lysates were prepared, and luciferase activity was assayed. Data (mean ± S.E., n = 3) are expressed as percentages of the luciferase activity detected in untreated cells under normoxia. *, p < 0.05; **, p < 0.01 compared with untreated cells using one-way ANOVA, followed by Dunnett's analysis. D, A2780 cells were treated with different concentrations of clofibrate for 4 h prior to placement in a hypoxia chamber for 16 h. Cell lysates were prepared, and Western blotting was performed using antibodies against HIF-1α and β-actin. Shown are representative images of three individual experiments.
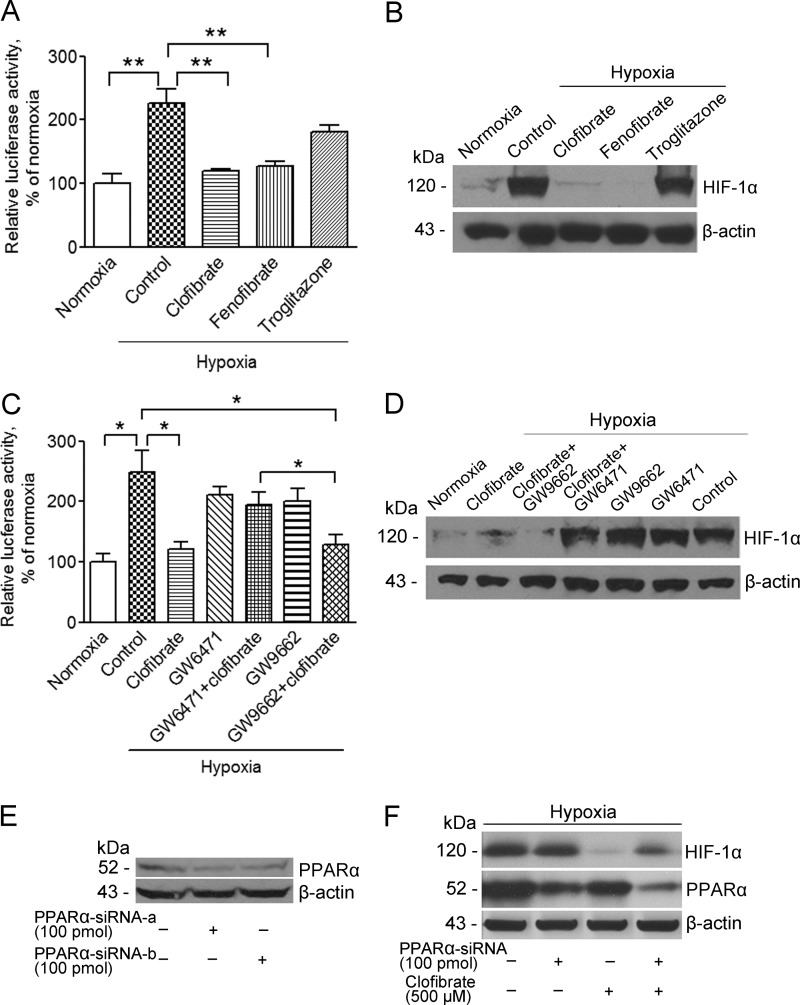
To further establish that activation of PPARα suppresses HIF-1α expression and activity, fenofibrate and troglitazone, two well established PPAR agonists, were applied to MCF-7 cells. HRE-luciferase reporter assay and Western blot analysis showed that hypoxia-induced HIF-1α expression and activity were significantly down-regulated by fenofibrate, a PPARα agonist, but not by troglitazone, a PPARγ agonist (Fig. 3, A and B). In addition, the inhibitory effects of clofibrate were reversed by GW6471 (5 μm), a potent PPARα antagonist. GW9662, a PPARγ antagonist, did not alter the inhibitory effects of clofibrate on hypoxia-induced HIF-1α expression or activity (Fig. 3, C and D). Furthermore, transient knockdown of PPARα using siRNA partially reversed clofibrate-induced suppression of HIF-1α expression in MCF-7 cells. The knockdown of PPARα was confirmed using multiple siRNA conditions (Fig. 3, E and F). Taken together, these findings indicate that it is the activation of PPARα that accounts for the suppression of hypoxia-induced HIF-1α expression and activity by clofibrate and fenofibrate.
FIGURE 3.
Clofibrate suppresses hypoxia-induced HIF-1α expression and activity through PPARα activation. A, MCF-7 cells were transfected with the pGL3-HRE-luciferase reporter construct. Cells were treated with 500 μm clofibrate, 100 μm fenofibrate, and 20 μm troglitazone for 4 h prior to placement in a hypoxia chamber for 16 h. Cell lysates were prepared, and luciferase activity was assayed. Data (mean ± S.E., n = 3) are expressed as percentages of the luciferase activity detected in untreated cells under normoxia. **, p < 0.01 compared with untreated cells using one-way ANOVA, followed by Dunnett's analysis. B, MCF-7 cells were treated with 500 μm clofibrate, 100 μm fenofibrate, and 20 μm troglitazone for 4 h prior to placement in a hypoxia chamber for 16 h. Cell lysates were prepared, and Western blotting was performed using antibodies against HIF-1α and β-actin. Shown are representative images of three individual experiments. C, MCF-7 cells were transfected with the pGL3-HRE-luciferase reporter construct. Cells were pretreated with 5 μm GW6471 or 10 μm GW9662 for 15 min prior to addition of 500 μm clofibrate for another 4 h. The cells were then placed into a hypoxia chamber for 16 h. Cell lysate was prepared, and luciferase activity was assayed. Data (mean ± S.E., n = 3–5) are expressed as percentages of the luciferase activity detected in untreated cells under normoxia. *, p < 0.05 compared with untreated cells using one-way ANOVA, followed by Dunnett's analysis. D, MCF-7 cells were pretreated with 5 μm GW6471 or 10 μm GW9662 for 15 min prior to addition of 500 μm clofibrate for another 4 h. The cells were then placed into a hypoxia chamber for 16 h. Cell lysates were prepared, and Western blotting was performed using antibodies against HIF-1α and β-actin. Shown are representative images of three individual experiments. E, MCF-7 cells were transfected with individual PPARα siRNAs (100 pmol/ml; siRNA-a or siRNA-b) for 48 h. Cell lysates were prepared, and Western blotting was performed using antibodies against PPARα and β-actin. F, MCF-7 cells were transfected with a pool of PPARα siRNAs (100 pmol/ml; siRNA-a, siRNA-b, and siRNA-c) prior to treatment with 500 μm clofibrate for 4 h. The cells were then placed into a hypoxia chamber for 16 h. Cell lysates were prepared, and Western blotting was performed using antibodies against HIF-1α, PPARα, and β-actin. Shown are representative images of three individual experiments.
Activation of PPARα Promotes HIF-1α Protein Degradation
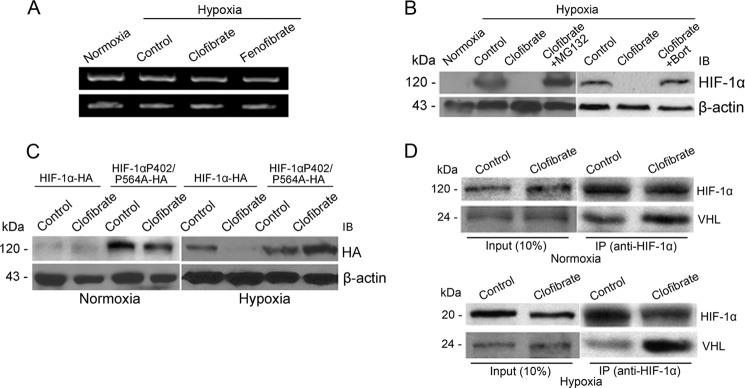
Activation of PPARα may suppress HIF-1α expression through transcriptional or post-transcriptional regulation. We therefore performed RT-PCR analysis to test whether HIF-1α mRNA expression is regulated by PPARα activation. At concentrations that suppressed hypoxia-induced HIF-1α protein expression, neither clofibrate nor fenofibrate significantly altered HIF-1α mRNA levels (Fig. 4A), suggesting that the suppression takes place at the protein level. MG132, a proteasome inhibitor that prevents degradation of ubiquitinated HIF-1α protein (19), and bortezomib, a structurally unrelated proteasome inhibitor known to enhance HIF-1α protein levels in cancer cells (22), were then used to treat MCF-7 cells prior to hypoxia. Both MG132 and bortezomib reversed the clofibrate-induced suppression of HIF-1α expression (Fig. 4B), suggesting that clofibrate suppresses hypoxia-induced HIF-1α expression by promoting HIF-1α degradation.
FIGURE 4.
Clofibrate promotes HIF-1α proteasomal degradation. A, MCF-7 cells were treated with 500 μm clofibrate and 100 μm fenofibrate for 4 h prior to placement in a hypoxia chamber for 16 h. RT-PCR was performed to determine the mRNA levels of HIF-1α and β-actin. Shown are representative images of three individual experiments. B, MCF-7 cells were treated with 500 μm clofibrate in the presence or absence of 10 μm MG132 or 1 μm bortezomib (Bort) for 4 h prior to placement in a hypoxia chamber for 16 h. Cell lysates were prepared, and Western blotting was performed using antibodies against HIF-1α and β-actin. Shown are representative images of three individual experiments. IB, immunoblot. C, MCF-7 cells were transfected with either HA-HIF-1α expression vector or HA-HIF-1α(P402A/P564A) mutant vector. After 48 h of transfection, cells were treated with 500 μm clofibrate for 4 h prior to placement in a hypoxia chamber or kept under normoxic conditions for 16 h. Cell lysates were prepared, and Western blotting was performed using antibodies against HA and β-actin. Shown are representative images of three individual experiments. D, MCF-7 cells were treated with 500 μm clofibrate for 4 h prior to addition of 10 μm MG132. The cells were then either placed into a hypoxia chamber or kept under normoxic conditions for 16 h. Cell extracts were prepared, and equal amounts of cell extracts from each sample were immunoprecipitated (IP) with HIF-1α antibody. The immunoprecipitates were separated on a 10% SDS-polyacrylamide gel and blotted with antibodies against HIF-1α and pVHL. Shown are representative images of three individual experiments.
It is well established that degradation of HIF-1α protein is regulated by oxygen-dependent prolyl hydroxylation (23). Hydroxylation of prolines 402 and 564 in HIF-1α by prolyl hydroxylases is required for the recognition of HIF-1α protein by pVHL. The recognition of HIF-1α by pVHL allows ubiquitination and subsequent HIF-1α degradation (24–26). To investigate whether degradation of HIF-1α induced by PPARα activation is involved with hydroxylation of Pro-402 and Pro-564, we overexpressed HA-tagged HIF-1α protein and its mutant with two prolyl mutations (HIF-1α(P402A/P564A)) in MCF-7 cells. As shown in Fig. 4C, the hypoxia-induced wild-type HA-HIF-1α level was diminished by clofibrate, but HA-HIF-1α(P402A/P564A) was expressed at similar levels in control and clofibrate-treated cells (Fig. 4C). This provides strong evidence demonstrating that PPARα activation promotes HIF-1α protein degradation and that Pro-402 and Pro-564 of HIF-1α are essential for this effect.
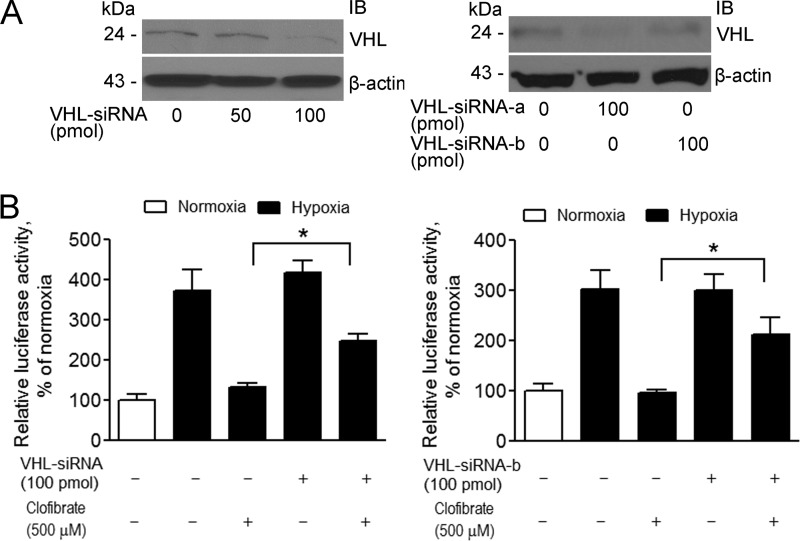
Because the interaction of HIF-1α and pVHL is critical for degradation of HIF-1α, we performed co-immunoprecipitation to test whether clofibrate affects the binding of HIF-1α to pVHL under hypoxia, using MG132 to block HIF-1α degradation (Fig. 4D). pVHL expression was detected after immunoprecipitation with anti-HIF-1α antibody, confirming an interaction of HIF-1α with pVHL in this model system. Semiquantification of the Western blots by densitometric analysis showed that this interaction was enhanced by clofibrate treatment (Fig. 4D and Table 1), an observation consistent with the conclusion that activation of PPARα promotes HIF-1α degradation, thereby suppressing HIF-1α signaling in cancer cells. To further confirm the involvement of pVHL in PPARα-mediated suppression of HIF-1α signaling, we knocked down pVHL expression in MCF-7 cells using multiple siRNA conditions (Fig. 5A). The suppression of HIF-1α signaling by clofibrate was attenuated by knocking down pVHL (Fig. 5B), supporting a critical role of pVHL in PPARα-mediated down-regulation of HIF-1α signaling.
TABLE 1.
Clofibrate enhances the interaction of HIF-1α and pVHL (densitometric analysis of the Western blot data presented in Fig. 4D)
Intensities were normalized to input levels of the same proteins and are expressed as levels relative to controls under normoxia (n = 3).
| Normoxia |
Hypoxia |
|||
|---|---|---|---|---|
| Control | Clofibrate | Control | Clofibrate | |
| pVHL | 1.0 | 1.3 ± 0.3 | 0.9 ± 0.3 | 1.6 ± 0.4a |
| HIF-1α | 1.0 | 0.8 ± 0.2 | 1.2 ± 0.3 | 0.9 ± 0.2 |
a p < 0.05 compared with the control using one-way ANOVA.
FIGURE 5.
Knockdown of pVHL attenuates clofibrate-induced suppression of HIF-1α signaling. A, left panel, MCF-7 cells were transfected with a pool of pVHL siRNAs (50–100 pmol/ml; siRNA-a, siRNA-b, and siRNA-c) for 48 h. Right panel, MCF-7 cells were transfected with individual pVHL siRNAs (100 pmol/ml; siRNA-a or siRNA-b) for 48 h. Cell lysates were prepared, and Western blotting was performed using antibodies against pVHL and β-actin. Shown are representative images of three individual experiments. IB, immunoblot. B, left panel, MCF-7 cells were cotransfected with a pool of pVHL siRNAs (100 pmol/ml) and the pGL3-HRE-luciferase reporter construct. Right panel, MCF-7 cells were cotransfected with pVHL siRNA-b (100 pmol/ml) and the pGL3-HRE-luciferase reporter construct. 48 h after transfection, cells were treated with 500 μm clofibrate for 4 h prior to placement in a hypoxia chamber for 16 h. Cell lysates were prepared, and luciferase activity was assayed. Data (mean ± S.E., n = 3) are expressed as percentages of the luciferase activity detected in untreated cells under normoxia. *, p < 0.05 analyzed by one-way ANOVA analysis.
Activation of PPARα Inhibits Expression of the Hypoxia-inducible Genes and Tube Formation by EA.hy926 Cells
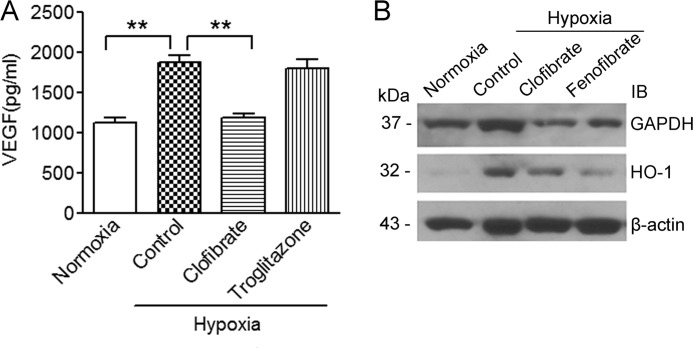
To understand the consequences of the suppression of HIF-1α signaling through activation of PPARα, we analyzed the expression of VEGF (15), HO-1 (27), and GAPDH (28), three well recognized HIF-1α target genes in MCF-7 cells. Under hypoxic conditions, the level of VEGF in the medium after culturing MCF-7 cells was significantly higher than that under normoxia. Treatment with clofibrate, but not troglitazone, reversed hypoxia-induced VEGF secretion from MCF-7 cells (Fig. 6A). The hypoxia-induced mRNA expression of the VEGF gene was also diminished by clofibrate and fenofibrate (data not shown), indicating that activation of PPARα suppresses expression of the HIF-1α target genes. This was further confirmed by the observation that protein expression of both HO-1 and GAPDH induced by hypoxia was reversed by treatment with clofibrate and fenofibrate in this model system (Fig. 6B).
FIGURE 6.
Clofibrate suppresses hypoxia-inducible gene expression. A, MCF-7 cells were treated with 500 μm clofibrate or 20 μm troglitazone for 4 h prior to placement in a hypoxia chamber for 16 h. The amount of VEGF in the medium was analyzed using an ELISA assay kit. Data (mean ± S.D., n = 3) are expressed as picograms/million liters of medium. **, p < 0.01 compared with untreated cells using one-way ANOVA, followed by Dunnett's analysis. B, MCF-7 cells were treated with 500 μm clofibrate or 100 μm fenofibrate for 4 h prior to placement in a hypoxia chamber for 16 h. Cell lysates were prepared, and Western blotting was performed using antibodies against GAPDH, HO-1, and β-actin. Shown are representative images of three individual experiments. IB, immunoblot.
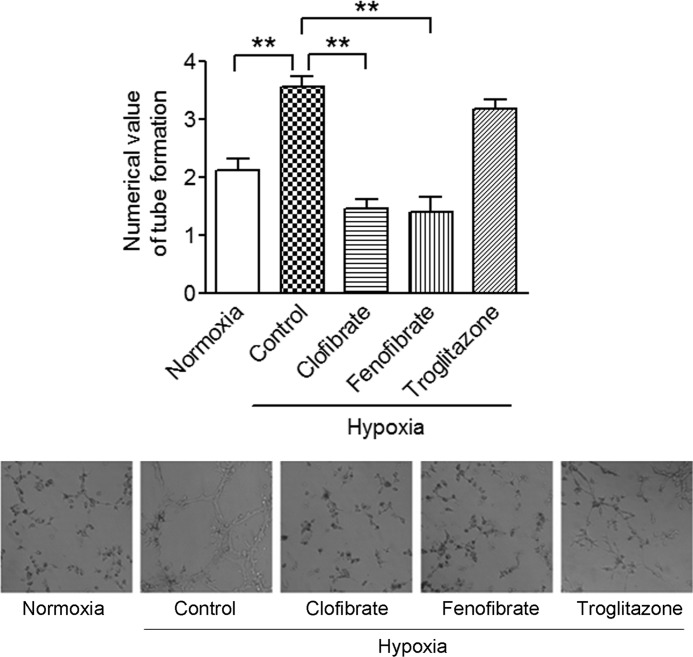
To further elucidate the consequences of PPARα-mediated suppression of HIF-1α signaling, we examined the formation of tubes by the endothelial cell line EA.hy926, utilizing a well recognized in vitro angiogenesis model system (5, 29–32). Tube formation by EA.hy926 cells was significantly increased when they were cultured in medium conditioned by the prior growth of MCF-7 cells grown under hypoxic conditions. If the conditioned medium was obtained from hypoxic MCF-7 cells treated with clofibrate or fenofibrate, tube formation was not enhanced, consistent with a decreased content of VEGF in the conditioned medium. The presence of troglitazone during the period when hypoxic MCF-7 cells conditioned the medium did not alter tube formation by the EA.hy926 cells (Fig. 7). This observation is consistent with the suppression of hypoxia-induced VEGF secretion from MCF-7 cells, suggesting that activation of PPARα inhibits tumor angiogenesis.
FIGURE 7.
Tube formation of EA.hy926 cells cultured in conditioned media. EA.hy926 cells resuspended in conditioned media were plated onto 96-well plates coated with Matrigel. The conditioned media were prepared from the media used to culture MCF-7 cells under various conditions (normoxia, hypoxia, hypoxia plus clofibrate, hypoxia plus fenofibrate, and hypoxia plus troglitazone). Tube formation of EA.hy926 cells was recorded 24 h after plating. Shown are representative images of three individual experiments (lows panels) and quantitative analysis following the manufacturer's formula (upper panel). **, p < 0.01 analyzed by one-way ANOVA, followed by Dunnett's analysis.
DISCUSSION
PPARα is a nuclear receptor that belongs to the PPAR family, the members of which function as transcription factors regulating expression of many genes (33). Activation of PPARα has been shown to play a key role in lipid metabolism, fatty acid oxidation, glucose homeostasis, and the inflammatory process (34). In recent years, PPARα-specific agonists have been reported to inhibit the growth of various cancer cells in cultured cell lines and in xenograft nude mouse models (35–37). However, little is known about the molecular and cellular mechanisms of PPARα-mediated growth inhibition of cancer cells. In this study, we demonstrated for the first time that activation of PPARα suppresses hypoxia-induced HIF-1α expression and activity and that this suppression is mediated through promoting HIF-1α protein degradation in human cancer cells. These novel observations improve our understanding of the interaction of PPARα and HIF-1α signaling in human cancer cells.
We have made several interesting findings in the context of PPARα and HIF-1α signaling in cancer cells. The first relates to the clear demonstration that activation of PPARα suppresses HIF-1α signaling in both breast and ovarian cancer cells. The interaction of PPARα and HIF-1α signaling has been previously investigated in other model systems. An early study demonstrated that HIF-1α mediates the inhibition of PPARα expression under hypoxia in epithelial cells in vitro and in vivo (38). The down-regulation of PPARα by hypoxia was recently observed in a study using human placentas (39). HIF-1α was also shown to inhibit PPARα signaling by reducing its DNA binding activity in cardiomyocytes (14). However, how PPARα might regulate HIF-1α signaling has not been described in any model systems, and the relationship between these two important nuclear receptor signaling pathways in cancer cells has been less characterized. The facts that PPARα agonists are reported to inhibit tumor growth in various cancer model systems (10, 35–37) and that HIF-1α is an established regulator of tumor growth, angiogenesis, and metastasis (15) led us to examine how activation of PPARα might affect HIF-1α expression and activity in cancer cells. We have shown that the PPARα agonists clofibrate and fenofibrate diminish hypoxia-induced HIF-1α expression and activity in our model systems. This is the first demonstration that activation of PPARα suppresses HIF-1α expression and activity, thus providing novel insight into the interaction of these two important signaling pathways in cancer.
Our second new finding relates to the observation that clofibrate promotes the degradation of HIF-1α protein in cancer cells. It is well known that HIF-1α protein degradation is regulated by oxygen (27). Under normoxia, HIF-1α is hydroxylated by prolyl hydroxylases at Pro-402 and Pro-564, present in the oxygen-dependent degradation domain of the protein. These modifications allow HIF-1α to be recognized by pVHL, which is the recognition component of an E3 ubiquitin ligase, leading to HIF-1α ubiquitination and subsequent proteasomal degradation (40). Several lines of evidence from our study support the conclusion that activation of PPARα enhances HIF-1α degradation in cancer cells. First, we have shown that HIF-1α mRNA expression was unchanged after addition of clofibrate under hypoxic conditions, indicating that transcription of the HIF-1α gene is not affected by activation of PPARα. Second, when MG132 and bortezomib, two well established proteasome inhibitors (22, 41, 42), were applied to the cells under hypoxia, clofibrate-induced suppression of HIF-1α expression was reversed, indicating that HIF-1α protein degradation is targeted by clofibrate. Third, we demonstrated that when HIF-1α was overexpressed in MCF-7 cells, clofibrate treatment suppressed wild-type HIF-1α expression but had no effect on mutant HIF-1α (with mutation of Pro-402 and Pro-564) expression, strongly indicating that activation of PPARα promotes HIF-1α protein degradation. Finally, our co-immunoprecipitation experiments showed that clofibrate enhanced the interaction of HIF-1α with pVHL under hypoxic conditions, suggesting that more HIF-1α proteins are processed into the ubiquitin-proteasome degradation pathway upon activation of PPARα in our model system. This was further confirmed by the observation that knockdown of pVHL significantly attenuated clofibrate-induced suppression of HIF-1α signaling. Given the importance of both PPARα and HIF-1α signaling in cancer as well as in other diseases, the detailed mechanisms of how PPARα activation leads to an enhanced interaction of pVHL with HIF-1α protein, resulting in rapid degradation of HIF-1α under hypoxia, merit further investigation.
Another interesting finding of this study relates to the consequences of PPARα-mediated suppression of hypoxia-induced HIF-1α expression and activity. This includes primarily the demonstration that clofibrate treatment down-regulates hypoxia-induced expression of VEGF in and secretion of VEGF from MCF-7 cells and suppresses hypoxia-induced tube formation (angiogenesis) by endothelial cells. The VEGF gene is a well established target of HIF-1α signaling (15). Suppression of VEGF expression by PPARα agonists has been described in other cancer model systems (43–46). Our findings complement previous reports and provide a mechanistic explanation of how PPARα agonists suppress VEGF expression and angiogenesis, thus supporting the development of PPARα agonists as effective anti-angiogenic agents for cancer therapy.
In conclusion, we have demonstrated that activation of PPARα suppresses hypoxia-induced HIF-1α signaling via promotion of HIF-1α degradation in human cancer cells. These findings provide new insight into our understanding of the two important signaling pathways in cancer cells and support the use of PPARα agonists as therapeutic anticancer agents.
Acknowledgment
We thank Dr. Minghui Zou (Department of Medicine, University of Oklahoma Health Sciences Center) for use of the hypoxia chamber.
This work was supported, in whole or in part, by National Institutes of Health Oklahoma IDeA Network of Biomedical Research Excellence (OK-INBRE) Program Grant 3P20RR016478-09S2. This work was also supported by American Cancer Society Grant CNE-117557, Susan G. Komen for the Cure Foundation Grant KG081083, and Oklahoma Center for the Advancement of Science and Technology Grant HR09-025.
- HIF-1α
- hypoxia-inducible factor-1α
- HRE
- hypoxia response element
- PPARα
- peroxisome proliferator-activated receptor α
- RXRα
- retinoid X receptor α
- HO-1
- heme oxygenase 1
- ANOVA
- analysis of variance.
REFERENCES
- 1. Poon E., Harris A. L., Ashcroft M. (2009) Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev. Mol. Med. 11, e26. [DOI] [PubMed] [Google Scholar]
- 2. Kunz M., Ibrahim S. M. (2003) Molecular responses to hypoxia in tumor cells. Mol. Cancer 2, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris A. L. (2002) Hypoxia–a key regulatory factor in tumor growth. Nat. Rev. Cancer 2, 38–47 [DOI] [PubMed] [Google Scholar]
- 4. Kaelin W. G., Jr., Ratcliffe P. J. (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 [DOI] [PubMed] [Google Scholar]
- 5. Manalo D. J., Rowan A., Lavoie T., Natarajan L., Kelly B. D., Ye S. Q., Garcia J. G., Semenza G. L. (2005) Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105, 659–669 [DOI] [PubMed] [Google Scholar]
- 6. Elvidge G. P., Glenny L., Appelhoff R. J., Ratcliffe P. J., Ragoussis J., Gleadle J. M. (2006) Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1α, HIF-2α, and other pathways. J. Biol. Chem. 281, 15215–15226 [DOI] [PubMed] [Google Scholar]
- 7. Brown J. M., Wilson W. R. (2004) Exploiting tumor hypoxia in cancer treatment. Nat. Rev. Cancer 4, 437–447 [DOI] [PubMed] [Google Scholar]
- 8. Urbanska K., Pannizzo P., Grabacka M., Croul S., Del Valle L., Khalili K., Reiss K. (2008) Activation of PPARα inhibits IGF-I-mediated growth and survival responses in medulloblastoma cell lines. Int. J. Cancer 123, 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zak Z., Gelebart P., Lai R. (2010) Fenofibrate induces effective apoptosis in mantle cell lymphoma by inhibiting the TNFα/NF-κB signaling axis. Leukemia 24, 1476–1486 [DOI] [PubMed] [Google Scholar]
- 10. Yokoyama Y., Xin B., Shigeto T., Umemoto M., Kasai-Sakamoto A., Futagami M., Tsuchida S., Al-Mulla F., Mizunuma H. (2007) Clofibric acid, a peroxisome proliferator-activated receptor α ligand, inhibits growth of human ovarian cancer. Mol. Cancer Ther. 6, 1379–1386 [DOI] [PubMed] [Google Scholar]
- 11. Grabacka M., Placha W., Plonka P. M., Pajak S., Urbanska K., Laidler P., Slominski A. (2004) Inhibition of melanoma metastases by fenofibrate. Arch. Dermatol. Res. 296, 54–58 [DOI] [PubMed] [Google Scholar]
- 12. Tuller E. R., Beavers C. T., Lou J. R., Ihnat M. A., Benbrook D. M., Ding W. Q. (2009) Docosahexaenoic acid inhibits superoxide dismutase 1 gene transcription in human cancer cells: the involvement of peroxisome proliferator-activated receptor α and hypoxia-inducible factor-2α signaling. Mol. Pharmacol. 76, 588–595 [DOI] [PubMed] [Google Scholar]
- 13. Tuller E. R., Brock A. L., Yu H., Lou J. R., Benbrook D. M., Ding W. Q. (2009) PPARα signaling mediates the synergistic cytotoxicity of clioquinol and docosahexaenoic acid in human cancer cells. Biochem. Pharmacol. 77, 1480–1486 [DOI] [PubMed] [Google Scholar]
- 14. Belanger A. J., Luo Z., Vincent K. A., Akita G. Y., Cheng S. H., Gregory R. J., Jiang C. (2007) Hypoxia-inducible factor-1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor α/retinoid X receptor. Biochem. Biophys. Res. Commun. 364, 567–572 [DOI] [PubMed] [Google Scholar]
- 15. Rankin E. B., Giaccia A. J. (2008) The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 15, 678–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X., Yu H., Lou J. R., Zheng J., Zhu H., Popescu N. I., Lupu F., Lind S. E., Ding W. Q. (2011) MicroRNA-19 (miR-19) regulates tissue factor expression in breast cancer cells. J. Biol. Chem. 286, 1429–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding W. Q., Liu B., Vaught J. L., Palmiter R. D., Lind S. E. (2006) Clioquinol and docosahexaenoic acid act synergistically to kill tumor cells. Mol. Cancer Ther. 5, 1864–1872 [DOI] [PubMed] [Google Scholar]
- 18. Ding W. Q., Liu B., Vaught J. L., Yamauchi H., Lind S. E. (2005) Anticancer activity of the antibiotic clioquinol. Cancer Res. 65, 3389–3395 [DOI] [PubMed] [Google Scholar]
- 19. Nardinocchi L., Pantisano V., Puca R., Porru M., Aiello A., Grasselli A., Leonetti C., Safran M., Rechavi G., Givol D., Farsetti A., D'Orazi G. (2010) Zinc down-regulates HIF-1α and inhibits its activity in tumor cells in vitro and in vivo. PLoS ONE 5, e15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Göttlicher M., Widmark E., Li Q., Gustafsson J. A. (1992) Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc. Natl. Acad. Sci. U.S.A. 89, 4653–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adams M., Montague C. T., Prins J. B., Holder J. C., Smith S. A., Sanders L., Digby J. E., Sewter C. P., Lazar M. A., Chatterjee V. K., O'Rahilly S. (1997) Activators of peroxisome proliferator-activated receptor γ have depot-specific effects on human preadipocyte differentiation. J. Clin. Invest. 100, 3149–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birle D. C., Hedley D. W. (2007) Suppression of the hypoxia-inducible factor-1 response in cervical carcinoma xenografts by proteasome inhibitors. Cancer Res. 67, 1735–1743 [DOI] [PubMed] [Google Scholar]
- 23. Brahimi-Horn C., Pouysségur J. (2006) The role of the hypoxia-inducible factor in tumor metabolism growth and invasion. Bull. Cancer 93, E73–E80 [PubMed] [Google Scholar]
- 24. Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr. (2001) HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 [DOI] [PubMed] [Google Scholar]
- 25. Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 26. Hoffman M. A., Ohh M., Yang H., Klco J. M., Ivan M., Kaelin W. G., Jr. (2001) von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to down-regulate HIF. Hum. Mol. Genet. 10, 1019–1027 [DOI] [PubMed] [Google Scholar]
- 27. Semenza G. L. (2003) Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 [DOI] [PubMed] [Google Scholar]
- 28. Iyer N. V., Kotch L. E., Agani F., Leung S. W., Laughner E., Wenger R. H., Gassmann M., Gearhart J. D., Lawler A. M., Yu A. Y., Semenza G. L. (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor-1α. Genes Dev. 12, 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bauer J., Margolis M., Schreiner C., Edgell C. J., Azizkhan J., Lazarowski E., Juliano R. L. (1992) In vitro model of angiogenesis using a human endothelium-derived permanent cell line: contributions of induced gene expression, G-proteins, and integrins. J. Cell. Physiol. 153, 437–449 [DOI] [PubMed] [Google Scholar]
- 30. Benndorf R., Böger R. H., Ergün S., Steenpass A., Wieland T. (2003) Angiotensin II type 2 receptor inhibits vascular endothelial growth factor-induced migration and in vitro tube formation of human endothelial cells. Circ. Res. 93, 438–447 [DOI] [PubMed] [Google Scholar]
- 31. Shim J. S., Matsui Y., Bhat S., Nacev B. A., Xu J., Bhang H. E., Dhara S., Han K. C., Chong C. R., Pomper M. G., So A., Liu J. O. (2010) Effect of nitroxoline on angiogenesis and growth of human bladder cancer. J. Natl. Cancer Inst. 102, 1855–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cui Y., Han Z., Hu Y., Song G., Hao C., Xia H., Ma X. (2012) MicroRNA-181b and microRNA-9 mediate arsenic-induced angiogenesis via NRP1. J. Cell. Physiol. 227, 772–783 [DOI] [PubMed] [Google Scholar]
- 33. Schoonjans K., Staels B., Auwerx J. (1996) The peroxisome proliferator-activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim. Biophys. Acta 1302, 93–109 [DOI] [PubMed] [Google Scholar]
- 34. Desvergne B., Wahli W. (1999) Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 20, 649–688 [DOI] [PubMed] [Google Scholar]
- 35. Shigeto T., Yokoyama Y., Xin B., Mizunuma H. (2007) Peroxisome proliferator-activated receptor α and γ ligands inhibit the growth of human ovarian cancer. Oncol. Rep. 18, 833–840 [PubMed] [Google Scholar]
- 36. Drukala J., Urbanska K., Wilk A., Grabacka M., Wybieralska E., Del Valle L., Madeja Z., Reiss K. (2010) ROS accumulation and IGF-IR inhibition contribute to fenofibrate/PPARα-mediated inhibition of glioma cell motility in vitro. Mol. Cancer 9, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grabacka M., Plonka P. M., Urbanska K., Reiss K. (2006) Peroxisome proliferator-activated receptor α activation decreases metastatic potential of melanoma cells in vitro via down-regulation of Akt. Clin. Cancer Res. 12, 3028–3036 [DOI] [PubMed] [Google Scholar]
- 38. Narravula S., Colgan S. P. (2001) Hypoxia-inducible factor-1-mediated inhibition of peroxisome proliferator-activated receptor α expression during hypoxia. J. Immunol. 166, 7543–7548 [DOI] [PubMed] [Google Scholar]
- 39. Chang T. T., Shyu M. K., Huang M. C., Hsu C. C., Yeh S. Y., Chen M. R., Lin C. J. (2011) Hypoxia-mediated down-regulation of OCTN2 and PPARα expression in human placentas and in BeWo cells. Mol. Pharmaceutics 8, 117–125 [DOI] [PubMed] [Google Scholar]
- 40. Pouysségur J., Dayan F., Mazure N. M. (2006) Hypoxia signaling in cancer and approaches to enforce tumor regression. Nature 441, 437–443 [DOI] [PubMed] [Google Scholar]
- 41. Meriin A. B., Gabai V. L., Yaglom J., Shifrin V. I., Sherman M. Y. (1998) Proteasome inhibitors activate stress kinases and induce Hsp72. Diverse effects on apoptosis. J. Biol. Chem. 273, 6373–6379 [DOI] [PubMed] [Google Scholar]
- 42. Pan J. A., Ullman E., Dou Z., Zong W. X. (2011) Inhibition of protein degradation induces apoptosis through a microtubule-associated protein 1 light chain 3-mediated activation of caspase-8 at intracellular membranes. Mol. Cell. Biol. 31, 3158–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meissner M., Stein M., Urbich C., Reisinger K., Suske G., Staels B., Kaufmann R., Gille J. (2004) PPARα activators inhibit vascular endothelial growth factor receptor 2 expression by repressing Sp1-dependent DNA binding and transactivation. Circ. Res. 94, 324–332 [DOI] [PubMed] [Google Scholar]
- 44. Panigrahy D., Kaipainen A., Huang S., Butterfield C. E., Barnés C. M., Fannon M., Laforme A. M., Chaponis D. M., Folkman J., Kieran M. W. (2008) PPARα agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc. Natl. Acad. Sci. U.S.A. 105, 985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grau R., Punzón C., Fresno M., Iñiguez M. A. (2006) Peroxisome proliferator-activated receptor α agonists inhibit cyclooxygenase 2 and vascular endothelial growth factor transcriptional activation in human colorectal carcinoma cells via inhibition of activator protein 1. Biochem. J. 395, 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grau R., Díaz-Muñoz M. D., Cacheiro-Llaguno C., Fresno M., Iñiguez M. A. (2008) Role of peroxisome proliferator-activated receptor α in the control of cyclooxygenase 2 and vascular endothelial growth factor: involvement in tumor growth. PPAR Res. 2008, 352437. [DOI] [PMC free article] [PubMed] [Google Scholar]