Background: EGFR signaling pathway activation is a crucial step in colorectal cancer tumor progression.
Results: Meprinα sheds the epidermal growth factor ligands EGF and TGFα. Phosphorylation of EGFR and ERK1/2 is increased and cell proliferation and migration is enhanced after stimulation with meprinα.
Conclusion: Meprinα transactivates the EGFR by proteolytic processing of TGFα and EGF.
Significance: Meprinα may be a therapeutic target in colorectal cancer treatment.
Keywords: Cell Proliferation, Colorectal Cancer, Epidermal Growth Factor Receptor (EGFR), ERK, Metalloprotease, Meprin, Ectodomain Shedding
Abstract
Meprinα, an astacin-type metalloprotease is overexpressed in colorectal cancer cells and is secreted in a non-polarized fashion, leading to the accumulation of meprinα in the tumor stroma. The transition from normal colonocytes to colorectal cancer correlates with increased meprinα activity at primary tumor sites. A role for meprinα in invasion and metastatic dissemination is supported by its pro-angiogenic and pro-migratory activity. In the present study, we provide evidence for a meprinα-mediated transactivation of the EGFR signaling pathway and suggest that this mechanism is involved in colorectal cancer progression. Using alkaline phosphatase-tagged EGFR ligands and an ELISA assay, we demonstrate that meprinα is capable of shedding epidermal growth factor (EGF) and transforming growth factor-α (TGFα) from the plasma membrane. Shedding was abrogated using actinonin, an inhibitor for meprinα. The physiological effects of meprinα-mediated shedding of EGF and TGFα were investigated with human colorectal adenocarcinoma cells (Caco-2). Proteolytically active meprinα leads to an increase in EGFR and ERK1/2 phosphorylation and subsequently enhances cell proliferation and migration. In conclusion, the implication of meprinα in the EGFR/MAPK signaling pathway indicates a role of meprinα in colorectal cancer progression.
Introduction
The epidermal growth factor receptor (EGFR)2 signaling pathway has critical functions in normal cellular processes such as differentiation, proliferation, migration, and the modulation of apoptosis, but it is also crucial in the pathophysiology of hyperproliferative diseases such as cancer (1). Colorectal cancer is the third most prevalent cancer and the second leading cause of cancer related deaths, worldwide (2). The analysis of tumor samples by immunohistochemistry has shown that the EGFR protein is overexpressed in 65–75% of colorectal tumors (3). The EGFR is a transmembrane receptor that is activated after binding of specific extracellular protein ligands, including epidermal growth factor (EGF) (4), heparin-binding EGF-like growth factor (HB-EGF) (5), transforming growth factor-α (TGFα) (6), betacellulin (7), amphiregulin (8), epiregulin (9), and epigen (10). The ligands are structurally and functionally related type I trans-membrane proteins that are shed after their presentation on the cell surface by an extracellular metalloprotease (11, 12). TACE/ADAM17 (a disintegrin and metalloprotease) has been identified as the main sheddase of TGFα, HB-EGF, amphiregulin, epiregulin, and epigen (13–17), and ADAM10 as the main sheddase of EGF and betacellulin (16). Furthermore, ADAM8, -9, -12, and -19, have been reported to contribute to shedding of EGFR ligands when overexpressed or deregulated, which is highly relevant in inflammation and cancer (18). Shedding of EGFR ligands by ADAMs is associated with diseases such as cancer, neurological and cardiovascular diseases, asthma, infection, and inflammation (19–21).
Recently, it has been shown that meprinα is involved in the activation of the EGFR, via release of TGFα, in human bronchial epithelial cells, 16HBE14o (22). Meprins and ADAMs both belong to the M12 family of metalloproteases (MEROPS, proteinase database) (23). Meprins are members of the astacin-family (M12A) and ADAMs of the adamalysin-family (M12B). Meprin was first discovered in proximal epithelial cells of mouse kidney in 1981 by Beynon et al. (24). In addition, two other groups discovered the same metalloprotease in the early 1980s: Sterchi et al., in 1982, described the enzyme PABA peptide hydrolase (PPH) in microvillar membranes of human small intestinal epithelial cells (25), and Kenny et al., in 1987, found the endopeptidase-2 in the microvillar membrane of proximal epithelial cells of rat kidney (26). This metalloprotease is now known as “meprin.” There are two evolutionary related isoforms: Meprinα and meprinβ. Both are synthesized as type I transmembrane proteins in the endoplasmic reticulum (27, 28). The membrane anchor of meprinα is removed intracellularly leading to the secretion of this isoform from cells, whereas meprinβ remains an integral protein of the plasma membrane (29). However, meprinα may be retained at the plasma membrane via covalent interaction with the transmembrane meprinβ (30–32). Meprinα is expressed in epithelial cells of the healthy colon mucosa where it is secreted apically into the colon lumen (33). In colorectal cancer, meprinα is released in a non-polarized fashion, leading to its accumulation in the tumor stroma (34, 35). This aberrant secretion of meprinα into the tumor stroma exposes matrix components and other stromal elements to an increased proteolytic potential (35). Once secreted, meprinα is activated in vitro and in the gut lumen by the removal of the pro-peptide through trypsin (28). An alternative activation mechanism has been suggested in colorectal cancer. In colon carcinoma cells (Caco-2), basolaterally secreted meprinα is activated by plasmin, which in turn, is activated by the fibroblast-derived urokinase-type plasminogen activator (36). Meprinα has been demonstrated to have pro-migratory and pro-angiogenic effects in colorectal cancer, and thus may be involved in the transition from benign growth (adenomas) to malignant primary tumors (37, 38).
We investigated the molecular mechanisms by which meprinα may influence tumor progression. For the first time we demonstrate that meprinα is able to shed EGF from the plasma membrane, resulting in the transactivation of EGFR signaling pathway and enhancement of Caco-2 cell proliferation and migration. We also confirm the shedding of TGFα by meprinα.
EXPERIMENTAL PROCEDURES
Antibodies and Recombinant Protein
Antibodies specific for total EGFR (monoclonal rabbit antibody) and phospho-EGFR Y1068 (monoclonal rabbit antibody) were purchased from Epitomics (Burlingame, CA); antibodies specific for total ERK1/2 (monoclonal mouse antibody) and phospho-ERK1/2 (polyclonal rabbit antibody) were from Santa Cruz Biotechnology (Heidelberg, Germany). Horseradish peroxidase-linked anti-rabbit and anti-mouse secondary antibodies were obtained from Dako Cytomation (Denmark). Recombinant active human meprinα and recombinant human pro-meprinα were generated using a baculovirus expression system in insect cells as previously described (39, 40).
Reagents
Cell culture media and all supplements were purchased from Invitrogen (Basel, Switzerland). All reagents for gel electrophoresis were obtained from Bio-Rad (Reinach, Switzerland). Complete EDTA-free protease inhibitor mixture tablets, PhosStop phosphatase inhibitor mixture tablets, and NBT/BCIP ready-to-use tablets were purchased from Roche Applied Sciences (Rotkreuz, Switzerland). MEK inhibitor U0126 was obtained from Promega (Dübendorf, Switzerland). EGF and TGFα neutralizing antibodies were purchased from R&D (Abingdon, UK). All other reagents were purchased from Sigma.
Expression Vectors for AP-tagged EGFR Ligands
Constructs of alkaline phosphatase (AP)-tagged EGFR ligands were kindly provided by Shigeki Higashiyama (EGF, TGFα, HB-EGF, amphiregulin, epiregulin, betacellulin) (16, 41) and Carl P. Blobel (epigen) (17). These vectors were constructed by inserting partial cDNAs for human TGFα, EGF, amphiregulin, epiregulin, betacellulin, and HB-EGF into the 3′-end of human placental AP cDNA in a pRc/CMV-based expression vector pAIPh (16, 41). Mouse epigen was constructed by inserting a partial cDNA for mouse epigen into the 3′-end of human placental AP in the CMV-based vector APtag-5 (17).
Cell Culture and Transfection of AP-tagged EGFR Ligands
Cells were maintained at 37 °C in a humified air/CO2 (19:1) environment. Human colorectal adenocarcinoma cells (Caco-2) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% (v/v) fetal bovine serum (FBS), 2 mm glutamine, 4.5 g/liter d-glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, and non-essentials amino acids (100 μm each). Madin-Darby canine kidney cells (MDCK) were grown in minimal essential medium (MEM) supplemented with 5% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mm glutamine.
Transfection was performed using PEI (Chemie Brunschwig, Basel, Switzerland). 1.5 × 105 cells per well were seeded in a 12-well plate, 24 h before transfection. Transfection mixture (100 μl of 150 mm NaCl containing 4 μl of PEI plus 100 μl of 150 mm NaCl containing 1.5 μg DNA) was incubated for 30 min at room temperature, and then added to the cells.
Conditioned Medium and Meprinα Activity Assay
Caco-2 cells, grown 7 days over confluency, were cultured in serum-free medium for 16 h. The medium was collected (referred as conditioned medium) and accumulated meprinα was activated using trypsin (20 μg/ml) for 2 h at 37 °C. Trypsin was inhibited using soybean trypsin inhibitor (50 μg/ml). Active meprinα was inhibited using actinonin (100 nm, in excess), a meprinα inhibitor.
Meprinα activity in conditioned medium or in medium containing recombinant active meprinα was verified using the substrate N-benzoyl-l-tyrosyl-p-aminobenzoic acid (PABA peptide) as described previously (30).
Ectodomain Shedding Assay
24 h after transfection, MDCK/Caco-2 cells were stimulated with serum-free medium containing either 1 μg/ml recombinant active meprinα or 1 μg/ml recombinant meprinα inhibited with 100 nm actinonin. After stimulation, medium was collected and centrifuged for 30 min at 4 °C at maximum speed. Cells were washed twice with phosphate-buffered saline (PBS) followed by lysis on ice in 0.5 ml lysis buffer (25 mm Tris-HCl pH 8, 50 mm sodium chloride, 1% IGEPAL, 1% sodium deoxycholate, with complete EDTA-free protease inhibitor mixture tablets) for 30 min. Cells were scraped off, and cell debris was removed by centrifugation. Supernatants and lysates were kept on ice at all the times. Each data point was generated from two consecutive AP activity measurements shed from a single transfected well (n = 3 experiments).
Detection of Alkaline Phosphatase
For the spectrophotometric detection of alkaline phosphatase (AP), 100 μl of collected medium or lysate were mixed with 100 μl 4-nitro-phenyl phosphate (2 mg/ml) in AP buffer (100 mm Tris, 100 mm NaCl, 20 mm MgCl2, pH 9.5) in a 96 well plate. After incubation at 37 °C absorbance was measured at 405 nm in an ELISA reader. Absorbance was measured at different time points within a linear range (OD < 0.8) up to a maximum incubation time of 5 h. The total amount of AP measured from a single well, was used to normalize the absorbance value obtained for the supernatant of a certain condition.
For in-gel detection, AP in cell culture supernatants was concentrated using ConA beads. After elution with 50 mm Tris, pH 8.0, 0.5 m α-d-methyl-mannopyranoside, the AP-tagged EGFR ligands were loaded on a SDS-polyacrylamide gel. The SDS-gel was incubated in 2.5% Triton X-100 followed by incubation in AP buffer. AP was visualized using NBT/BCIP as substrate.
EGF-ELISA
Caco-2 cells were stimulated with medium, 1 μg/ml recombinant active meprinα, or 1 μg/ml recombinant pro-meprinα for 4 h. Supernatants were collected and released EGF was measured via the human EGF quantikine ELISA Kit (R&D, Abingdon, UK).
Phosphorylation of EGFR and ERK1/2
Caco-2 cells, seeded at a density of 5 × 105 cells per 6 cm dish, were stimulated for 0, 5, 15, 30, and 60 min with either control medium, 1 μg/ml recombinant active meprinα, 1 μg/ml recombinant pro-meprinα, or 100 ng/ml EGF (positive control). Phosphorylation induced by recombinant active meprinα, recombinant pro-meprinα, or EGF was inhibited with 2 μg/ml neutralizing EGF and TGFα antibodies, 10 μm EGFR inhibitor AG1478, or 10 μm MEK inhibitor U0126. Cells were pretreated with the inhibitors 30 min before stimulation. After stimulation, cells were washed once with PBS followed by lysis on ice for 30 min in 1 ml of cell lysis buffer (Epitomics, Burlingame, CA) supplemented with protease and phosphatase inhibitors. Cell debris was removed by centrifugation and the protein content in the lysates was determined using the bicinchoninic acid (BCA) protein assay (Pierce).
Western Blot Analysis
10 μg of protein was solubilized by boiling for 5 min in 2× Laemmli buffer. Samples were loaded on 7.5% (EGFR) or 10% (ERK1/2) SDS-polyacrylamide gels and subsequently electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Hybond P, Amersham Biosciences). Blocking was done overnight at 4 °C using T-TBS (25 mm Tris-HCl pH 7, 150 mm sodium chloride, 2.5 mm potassium chloride, 0.1% Tween-20) containing 5% dry milk. The membrane was then incubated with the first antibody (pEGFR 1:5000, pERK1/2 1:200, EGFR 1:10000, ERK1/2 1:200) in T-TBS containing 2% dry milk for 1 h at room temperature and with the appropriate horseradish peroxidase-conjugated secondary antibody (1:25,000) for 1 h at room temperature. Immune complexes were visualized using enhanced chemiluminescence (ECL Plus, Amersham Biosciences) on x-ray films. The membrane was stripped using 62.5 mm Tris-HCl pH 6.2, 2% SDS, 50 mm DTT on a shaking plate at 65 °C for 30 min followed by washing steps in T-TBS. Densitometric analysis of Western blots were performed using Image J software (Wayne Rasband, NIH).
Cell Treatment for Proliferation and Migration Experiments
For proliferation and migration experiments Caco-2 cells were stimulated with conditioned medium containing activated meprinα, inhibited meprinα, or 100 ng/ml EGF (positive control). Inhibitors were added to media containing active meprinα at the beginning of the treatment (2 μg/ml neutralizing EGF and TGFα antibodies, 10 μm EGFR inhibitor AG1478, or 10 μm MEK inhibitor U0126). Cells were pretreated with neutralizing antibodies and EGFR inhibitor for 2 h.
Alamar Blue Cell Proliferation Assay
Alamar Blue uses the natural reducing power of living cells to convert resazurin to the fluorescent molecule, resorufin (42). Caco-2 cells were seeded at a density of 1000 cells per well in a 96-well plate. After 48 h of incubation, cells were washed twice using phenol red-free medium, followed by stimulation for 24 h as described above. For the last 3 h, Alamar Blue (43) solution was added to a final concentration of 10 μg/ml. Fluorescence at 595 nm was measured directly (0 h) and 3 h after addition in a multilabel plate reader (2300 EnSpire multilabel reader; Perkin-Elmer, Turku, Finland). Values obtained at time point 0 h were subtracted from those obtained at time point 3 h (20 replicates/condition, n = 3 experiments).
Cell Titer Glo Cell Viability Assay
This assay is a method to determine the number of viable cells in culture based on quantification of the ATP present. Consequently, ATP levels represent the number of metabolically active cells (44). 1000 Caco-2 cells/well were seeded in a 96-well plate. After 48 h, cells were serum-starved overnight followed by 24 h treatment with the different stimuli. Subsequently, 100 μl of Cell Titer Glo reagent were added to each well, cells were incubated for 10 min in the dark, and luminescence was measured in a 2300 EnSpire multilabel plate reader (3 replicates/condition, n = 3 experiments).
BrdU Incorporation
Cell proliferation was also determined by bromodeoxyuridine (BrdU) incorporation analysis. We used the In situ cell proliferation kit (Roche Applied Sciences; Rotkreuz, Switzerland) according to the manufacturer's instructions. Briefly, Caco-2 cells were plated at a density of 2 × 104 cells per well of an 8 chambers culture slide (Lab-Tek). After 48 h, cells were serum-starved overnight, followed by treatment for 24 h with the different stimuli. During the last 90 min of the treatment, BrdU at a final concentration of 10 μm was added to the medium to allow BrdU incorporation. Cells were fixed in 70% ethanol for 45 min at room temperature, and incubated with anti-BrdU antibody in the presence of nuclease for DNA denaturation. Cells were counterstained with 5 μg/ml DAPI for 5 min. BrdU incorporation into cellular DNA was visualized by fluorescence microscopy. In three independent experiments a total number of 12 high-power fields (40×), and at least 800 cells per condition were analyzed. The proliferation rate was determined as a proportion of the total DAPI-positive nuclei. The value for untreated control cells was arbitrarily set to 0.
In Vitro Wound-healing Assay
2 × 105 Caco-2 cells per well were seeded in a 12-well plate and grown to confluency. The cell monolayer was wounded by scratching, using a 200 μl pipette tip. After washing with PBS the cells were incubated with the corresponding stimuli. At time points 0 h and 16 h the same positions along the scratch wound were photographed using an inverted-phase-contrast microscope (Nikon microscope TS100 fluorescence and video camera) and Adobe Photoshop was used for quantification of the scratch wound. Three measurements per scratch were performed (2 replicates/condition, n = 3 experiments).
Transwell Migration Assay
5 × 104 Caco-2 cells were seeded on top of transwell filters (polyethylene terephthalate (PET), 8 μm pores, 24-well format) from BD Biosciences. Cells were allowed to grow for 48 h followed by serum starvation for 24 h in medium containing 1% FBS. Then, medium in the lower chamber was replaced by conditioned medium containing 20% FBS and the stimuli. Medium in filter inserts was replaced by serum-free conditioned medium containing the corresponding stimuli. Cells were treated for 36 h and at the end of the treatment cells were washed twice with PBS followed by fixation for 15 min using 4% paraformaldehyde. Cells on the upper side of the transwell filters were removed with a cotton swab and cells on the lower side were stained for 5 min with 5 μg/ml DAPI. Pictures were taken (Nikon microscope TS100) and migrated cells were counted using Image J software (Wayne Rasband, NIH) (3 replicates/condition, n = 3 experiments).
Statistical Analysis
Data were analyzed using PRISM 5.0 software package (GraphPad, San Diego, CA). Results are shown as the mean ± S.E. Statistical differences between two groups were determined by unpaired Student's t test. As significant differences considered were p < 0.05.
RESULTS
EGF and TGFα Are Shed by Meprinα
To investigate the role of meprinα in ectodomain shedding of EGF and TGFα, a cell-based assay using AP-tagged EGFR ligands was used. Shedding of EGF and to a lesser extent, TGFα was significantly stimulated in MDCK cells (p < 0.001; p < 0.05; Fig. 1, A and B) as well as in Caco-2 cells (p < 0.001; p < 0.01; Fig. 1, D and E) by meprinα. Addition of the meprinα inhibitor actinonin showed a significant decrease in ligand shedding in MDCK cells (p < 0.01; p < 0.05; Fig. 1, A and B) and in Caco-2 cells (p < 0.01; p < 0.01; Fig. 1, D and E). Actinonin did not influence constitutive shedding, suggesting that this is catalyzed by a different metalloprotease (data not shown). Results obtained with the spectrophotometric assay were confirmed by in-gel detection of AP-tagged ligands, using NBT/BCIP as a substrate for the alkaline phosphatase (Fig. 1, C and F).
FIGURE 1.
EGF and TGFα are shed by meprinα. MDCK or Caco-2 cells, transiently transfected with AP-EGF or AP-TGFα, were stimulated for 1 h with serum-free medium, recombinant active meprinα, or recombinant inhibited meprinα. Alkaline phosphatase (AP) in cell culture supernatant was detected using 4-nitro-phenyl phosphate as substrate. Absorbance value obtained for EGF and TGFα shedding are shown for MDCK cells (A/B) and for Caco-2 cells (D/E). C and F show in-gel detection of AP from shed EGFR ligands after renaturation in SDS gel in MDCK cells (C) and Caco-2 cells (F). Lane 1: protein molecular weight marker. Lane 2: AP-tagged forms of EGF and TGFα released in cell culture supernatant after 1 h of stimulation with serum-free medium (control). Lane 3: EGFR ligands released after 1 h from the same well after stimulation with recombinant active meprinα. Lane 4: EGFR ligands released from a separate well after stimulation for 1 h with recombinant inhibited meprinα. Quantification of shed endogenous EGF is shown in G. Caco-2 cells were stimulated with media, recombinant active meprinα, or recombinat inhibited meprinα, and the media were analyzed using ELISA. n = 3, ± S.E.; Student's t test ***, p ≤ 0.001; **, p ≤ 0.01; *, p ≤ 0.05.
Endogenous EGF released from Caco-2 cells into the medium was quantified by EGF ELISA (Fig. 1G). Stimulation of Caco-2 cells with recombinant active meprinα resulted in a significant increase of soluble EGF compared with control values (p < 0.001) and recombinant pro-meprinα, the inactive form of meprinα (p < 0.001). Quantification of soluble TGFα upon meprinα activation by ELISA has been shown before (22). Together these data suggest that meprinα acts as a sheddase for EGF and TGFα.
Meprinα Induces EGFR and ERK1/2 Phosphorylation
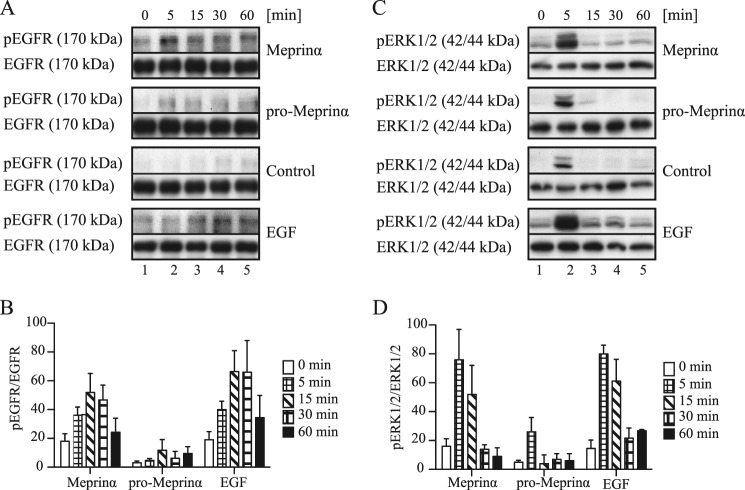
EGFR ligands, once released from the plasma membrane bind to the EGFR, which in turn is phosphorylated at several amino acid positions (Tyr-992, Tyr-1068, Tyr-1086, Tyr-1148, and Tyr-1173) (45). To investigate the effect of meprinα on the phosphorylation of EGFR via EGF or TGFα shedding, Caco-2 cells were stimulated for various periods of time with either medium alone, recombinant active meprinα, recombinant pro-meprinα, or with EGF (positive control). Fig. 2A shows representative Western blots of the phosphorylation experiment, and Fig. 2B shows the densitometric analysis of four individual experiments. In the latter, control values were subtracted and the values were normalized against total EGFR. After 5 min of treatment with meprinα (Fig. 2A, lane 2), an increase in phosphorylation was observed. A maximum activation of EGFR was achieved between 15 min and 30 min of treatment (Fig. 2A, lanes 3 and 4), followed by a decrease (Fig. 2A, lane 5). Non-active pro-meprinα showed only a very slight increase in phosphorylation of the EGFR. Minor amounts of pro-meprinα might be activated over time, for instance by plasmin or kallikreins (KLKs) (40, 46). Treatment with recombinant EGF resulted in a similar EGFR activation pattern to meprinα albeit with a stronger signal, which may have been due to the relatively high concentration of EGF used. These data indicate that the proteolytic activity of meprinα is required for EGFR phosphorylation.
FIGURE 2.
Meprinα induces EGFR and ERK1/2 phosphorylation. A, time course (0, 5, 15, 30, and 60 min) of EGFR and ERK1/2 phosphorylation is shown for Caco-2 cells treated with either control media, 1 μg/ml recombinant active meprinα, 1 μg/ml recombinant pro-meprinα, or 100 ng/ml EGF (positive control). A and C show representative Western blots for EGFR and ERK1/2, respectively. Immunoblots of total EGFR or ERK1/2 were used as loading control. Densitometric quantifications of phospho-EGFR relative to total EGFR are shown in B and densitometric quantifications of phospho-ERK1/2 relative to total ERK1/2 are shown in D. Control values were subtracted from the other values at the corresponding time points. n = 4 for all densitometric quantifications, ± S.E.
EGFR phosphorylation leads to the activation of intracellular pathways, such as the mitogen-activated protein kinase (MAPK) pathway. The classical MAP kinases, extracellular-signal-regulated kinases 1 and 2 (ERK1/2), are intracellular signaling molecules that are preferentially activated in response to growth factors and phorbol esters (47). To determine whether ERK1/2 are transactivated upon treatment of Caco-2 cells with meprinα, the lysates obtained from the EGFR phosphorylation experiment were also analyzed for phosphorylated ERK1/2 (Fig. 2C). Phosphorylation was calculated by densitometric measurements (Fig. 2D). Control values were subtracted and data were normalized against total ERK1/2. Similar to the treatment with EGF, stimulation with meprinα led to a peak in phosphorylation after 5 min (Fig. 2C, lane 2), which was attenuated over time. Pro-meprinα as well as the negative control showed an increase in phosphorylation at time point 5 min, although to a lesser degree (Fig. 2C, lane 2). We assume that this ERK1/2 phosphorylation after 5 min is a transient process that might be caused by the change of culture medium. Taken together, we conclude that active meprinα leads to the activation and phosphorylation of EGFR and consequently, transactivates the MAPK pathway, which culminates in ERK1/2 phosphorylation.
EGFR and ERK1/2 Phosphorylation Are Meprinα- dependent
To analyze whether the EGFR/MAPK signaling pathway is activated by meprinα via EGF and TGFα shedding, phosphorylation experiments using neutralizing EGF and TGFα antibodies were performed (Fig. 3A). Caco-2 cells were stimulated for 5 or 15 min with meprinα, pro-meprinα or EGF in the absence or presence of the neutralizing antibodies. In the absence of EGF and TGFα neutralizing antibodies, EGFR and ERK1/2 were phosphorylated when stimulated with meprinα or EGF but not with pro-meprinα. Cells treated with neutralizing EGF and TGFα antibodies showed EGFR and ERK1/2 phosphorylation reduced to control levels, after stimulation with meprinα. After stimulation with EGF, EGFR and ERK1/2 remained phosphorylated to a certain extent in the presence of neutralizing antibodies. This may be the result of ligand excess compared with the amount of antibodies used. EGFR and ERK1/2 phosphorylation remained the same after stimulation with pro-meprinα. Total EGFR and ERK1/2 were not affected by the neutralizing antibodies. We conclude, that EGFR transactivation by meprinα occurs via shedding of EGF and TGFα from the plasma membrane by meprinα.
FIGURE 3.
EGFR and ERK1/2 phosphorylation are meprinα-dependent. Caco-2 cells were stimulated for 5 or 15 min with 1 μg/ml recombinant active meprinα, 1 μg/ml recombinant pro-meprinα, or 100 ng/ml EGF in the presence or absence of neutralizing EGF and TGFα antibodies (nAB EGF/nAB TGFα) (A), EGFR inhibitor AG1478 (B), or MEK inhibitor U0126 (C). Cells were pretreated with the inhibitors for 30 min. Phosphorylation of EGFR and ERK1/2 were determined using antibodies detecting the phosphorylated form of EGFR and ERK1/2. Total EGFR and ERK1/2 were used as loading control and were not affected by neutralizing EGF and TGFα antibodies, or EGFR and ERK1/2 inhibitor n = 3.
We wondered if meprinα-induced ERK1/2 phosphorylation was entirely mediated by transactivation of EGFR. For this reason, we tested the effect of the EGFR inhibitor AG1478, (Fig. 3B). Phosphorylation of EGFR and ERK1/2 was detected after stimulation of Caco-2 cells with meprinα and EGF but not after stimulation with pro-meprinα. Inhibition of EGFR led to less EGFR phosphorylation while ERK1/2 phosphorylation was completely abrogated. Hence, meprinα activates ERK1/2 mostly via EGFR. We further confirmed that the MEK inhibitor U0126, which was later used in our functional assays, is a potent agent that completely abrogates meprinα-induced ERK1/2 phosphorylation (Fig. 3C).
Meprinα Enhances Caco-2 Cell Proliferation
Activation of the EGFR pathway plays an important role in the regulation of cellular processes such as cell proliferation and migration. The functional relevance of meprinα-dependent EGFR/ERK1/2 signaling on cell proliferation was studied in three independent assays: Alamar Blue, Cell Titer Glo cell viability assay, and BrdU incorporation. Cells were stimulated with conditioned medium containing activated meprinα, inhibited meprinα, or EGF. In addition, cell proliferation, induced by meprinα, was analyzed in the presence of neutralizing antibodies for EGF and TGFα, or inhibitors against EGFR and MEK. The cell proliferation rate for Alamar Blue and Cell Titer Glo experiments is shown in Fig. 4, A and B. A significant increase in proliferation was detected when cells were treated with active meprinα (p < 0.001; p < 0.001). Compared with that, inhibited meprinα reduced cell-proliferation to a level slightly above that of controls (p < 0.001; p < 0.01). This may be due to incomplete inhibition of meprinα by actinonin.3 EGF stimulation that was used as a positive control exhibited a comparable cell proliferation rate to meprinα. In both assays, the increase in cell proliferation monitored after stimulation with meprinα was significantly reduced in the presence of neutralizing EGF and TGFα antibodies (p < 0.05; p < 0.01), EGFR inhibitor (p < 0.01; p < 0.01) or MEK inhibitor (p < 0.01; p < 0.01). DNA synthesis was quantified by BrdU uptake in Caco-2 cells after treatment with the different stimuli. Representative photographs are shown in Fig. 4D and quantification of the results of three independent experiments are shown in Fig. 4C. Nuclear BrdU labeling was significantly increased after stimulation with active meprinα (p < 0.001) and significantly decreased when treated with inhibited meprinα (p < 0.001). BrdU uptake was significantly reduced in the presence of neutralizing EGF and TGFα antibodies (p < 0.01), EGFR inhibitor (p < 0.01), or MEK inhibitor (p < 0.01). Altogether, these data demonstrate that the proliferative effect of meprinα in Caco-2 cells is regulated through the EGFR/MAPK pathway.
FIGURE 4.
Meprinα enhances Caco-2 cell proliferation. Caco-2 cells were treated with conditioned medium containing activated meprinα, inhibited meprinα, or 100 ng/ml EGF (positive control). Further, the effect of inhibitors (neutralizing EGF and TGFα antibodies (nAB EGF/nAB TGFα), EGFR inhibitor AG1478, or MEK inhibitor U0126) on meprinα- induced proliferation was analyzed. Proliferation was measured by three independent assays: Alamar Blue (A), Cell Titer Glo cell viability (B), and BrdU incorporation (C/D). D shows representative pictures for each condition and in C, quantification of three separate experiments is shown. For all experiments, Caco-2 cell proliferation in response to the corresponding stimuli is shown as percentage increase. Control values were set as 0. n = 3; ± S.E., Student's t test ***, p ≤ 0.001; **, p ≤ 0.01; *, p ≤ 0.05.
Migration of Caco-2 Cells Is Enhanced by Meprinα Activity
The effect of meprinα on the migration behavior of Caco-2 cells was assessed using an in vitro wound-healing assay. A scratch was induced to confluent Caco-2 cells using a 200-μl pipette tip. We compared migration of Caco-2 cells treated with conditioned medium containing activated meprinα, inhibited meprinα, or EGF (positive control). Furthermore, the effect of inhibitors (neutralizing EGF and TGFα antibodies, EGFR inhibitor, or MEK inhibitor) on meprinα-induced migration was analyzed. Representative photographs, taken at time point 0 h and 16 h of the identical location, are shown in Fig. 5A. Quantification of the results of six separate experiments is shown in Fig. 5B. Under all conditions a closing of the wound was observed. A significant enhancement in wound closure was detected in cells exposed to active meprinα compared with inhibited meprinα and control values (p < 0.001, p < 0.01). EGF and TGFα inhibition through neutralizing antibodies as well as EGFR inhibition revealed a significant reduction in meprinα-induced wound closure (p < 0.05, p < 0.05), as did ERK1/2 inhibition (p < 0.001).
FIGURE 5.
Migration of Caco-2 cells is increased through meprinα activity. Cells were treated with conditioned media containing activated meprinα (in the presence or absence of neutralizing EGF and TGFα antibodies (nAB EGF/nAB TGFα), EGFR inhibitor AG1478, or MEK inhibitor U0126), inhibited meprinα, or EGF. A/B, in vitro wound-healing assay. One representative of three independent experiments is shown in A, and in B quantification of the results of three separate assays is shown. C, transwell migration assay of Caco-2 cells. Cells fixed and stained with DAPI were counted. In C, the percentage increase compared with control values is shown and the control values were set as 0. n = 3; ±S.E., Student's t test ***, p ≤ 0.001; **, p ≤ 0.01; *, p ≤ 0.05.
The in vitro wound-healing assay represents a combination of cell migration and cell proliferation. To avoid the proliferative effect, we also performed a transwell migration assay. Caco-2 cells were cultivated on 8 μm pore size filters in a 24-well culture plate with the same conditions as used for the in vitro wound-healing assay. Migrated cells were found under all conditions, but a significant increase in migration was monitored after stimulation with meprinα compared with control values (p < 0.001). Inhibited meprinα showed a significant decrease (p < 0.01; Fig. 5C) compared with active meprinα, and stimulation with EGF led to a slightly higher increase in migration compared with meprinα. The increase in migrated cells monitored after stimulation with meprinα was significantly reduced in the presence of neutralizing EGF and TGFα antibodies (p < 0.01), and EGFR inhibitor (p < 0.01), Inhibition of ERK1/2 led to less migration than the controls resulting in a negative value in the diagram (Fig. 5C). ERK1/2 is a key enzyme of many signaling cascades. Therefore, inhibition of ERK1/2 interferes with meprinα-induced migration and most likely with supplemental pathways triggering cell migration. In conclusion, we show that meprinα activity enhances migration of Caco-2 cells and this effect is dependent on the transactivation of EGFR/MAPK signaling.
DISCUSSION
In this study, we set out to identify EGFR ligands as substrates for meprinα. Our data demonstrate that human meprinα is effectively capable of shedding EGF. Additionally, we could confirm the shedding of TGFα by meprinα, which was shown previously in lung epithelial cells (22). We also demonstrate that active meprinα transactivates the EGFR and ERK1/2 and subsequently increases Caco-2 cell proliferation and migration.
Shedding of EGF and TGFα was enhanced in cells treated with active recombinant meprinα, and inversely, was reduced after inhibition of meprinα by actinonin, indicating that the proteolytic activity is required for shedding of EGF and TGFα. Two other groups have demonstrated TGFα shedding by meprin in two individual assays (22, 48). Back in 1991, Choudry et al., have identified the growth factor TGFα as an in vitro substrate for endopeptidase-2 (now known as meprin) (48). Using recombinant human TGFα and purified endopeptidase-2 from rat kidney, they showed that TGFα was processed in a time-dependent manner. In the presence of actinonin no hydrolysis was observed. Recently, Bergin et al. have analyzed shedding of TGFα by meprinα in human bronchial epithelial cells (16HBE14o-cells) (22). Using an ELISA assay, they found elevated levels of TGFα in the medium of cells after treatment with recombinant meprinα. This effect was also inhibited by the addition of actinonin. The authors suggested that meprinα is activated by neutrophil elastase and, via TGFα precursor processing, induces Il-8 expression (22).
With our experimental setup using AP-tagged constructs of EGFR ligands we confirm TGFα shedding by meprinα in MDCK and Caco-2 cells. Furthermore, we show that EGF, another EGFR ligand, is also shed by meprinα. Accordingly, we found increased levels of soluble EGF in the media (ELISA). Compared with TGFα, EGF was shed by meprinα to a higher extent, and cleavage of both ligands was abrogated when meprinα was inhibited by actinonin. Other EGFR ligands were also analyzed as potential substrates for meprinα. Epigen and betacellulin were not shed by meprinα, HB-EGF, amphiregulin, and epiregulin were shed but shedding was not inhibited by actinonin, indicating that another protease was involved (data not shown).
Differentiated Caco-2 cells express meprinα endogenously, which makes them a preferred cell culture system for the analysis of meprinα function (35). Therefore, we used Caco-2 cells in our studies to investigate the consequences of meprinα expression on cell behavior in the context of colorectal cancer. We carried out shedding experiments using MDCK cells to confirm our results acquired with recombinant meprinα in a second cell line. MDCK cells do not express endogenous meprinα. Nevertheless, the WT form in combination with recombinant meprin and stably transfected cell lines are widely used and established in meprin research (29, 33).
Meprin consists of two homologous isoforms, meprinα and meprinβ. We have previously shown that meprinβ cleaves and releases E-cadherin, which is considered to act as a tumor suppressor (49). Thus, we also considered meprinβ as a potential sheddase for EGFR ligands. In contrast to meprinα, meprinβ is not implicated in the shedding of EGFR ligands (data not shown). Although, both isoforms have related cleavage sites, different substrate specificities have been described (50, 51) (Jefferson et al., 62), which most likely is the reason for their different behavior toward EGFR ligands. Additionally, meprinα and β exhibit remarkable differences in their activation (46) and regulation by inhibitors (52) (Jefferson et al. 62). This certainly contributes to the different functions in cell proliferation and migration as observed previously (40). In the large intestine, only minor amounts of meprinβ are expressed. Hence, most meprinα is released into the gut lumen in vivo and may be rapidly diluted. However in colorectal cancer, meprinα accumulates in the tumor stroma and persists close to the cell plasma membrane (34, 35).
Two membrane-anchored metalloproteases, ADAM10 and ADAM17, were found to have critical roles in the release of EGFR ligands (14–17, 53). Most likely the basal amounts of shed EGF and TGFα that we found in the ectodomain shedding assay are due to these enzymes. We inhibited ADAMs to exclude that these ADAMs interfere with the shedding of TGFα and EGF (data not shown). The inhibition of ADAMs led to a minimization of the constitutive shedding without affecting the shedding by meprinα. In addition, meprinα generates a slightly smaller TGFα fragment (Fig. 1, C and F) to that obtained with ADAM17, indicating that the cleavage site of meprinα differs from that of ADAM17.
Mice lacking ADAM17 expression die perinatally and have a similar phenotype to TGFα−/− mice (54). This points to ADAM17 as the main sheddase for TGFα. In mouse cells ADAM10 is responsible for EGF shedding. Mice lacking ADAM10 die very early during embryogenesis and hence, determination of the physiological contribution to EGF signaling in animals remains to be determined (55). In cell-based assays, ADAM10 has been identified as a sheddase that can release TGFα almost as efficiently as its primary sheddase, in ADAM17−/− cells (16, 18). This implies that the function of ADAMs may be replaced by other metalloproteases in tissues where ADAMs are not expressed or are not stimulated. We therefore propose a physiological role for meprinα in the local transactivation of the EGFR pathway.
Aberrant expression and/or activities of EGF family members and their receptors have been reported in solid tumors including colorectal cancer (56–59). We have previously analyzed meprinα mRNA levels, protein expression, and proteolytic activity in colonic adenomas, primary tumors and liver metastases from colorectal cancer patients. Varied levels of meprinα mRNA were detected in all specimens. While expression of meprinα protein was very weak in adenomas, it was detected in primary tumor tissue as well as in liver metastases. In advanced primary tumors (UICC stages III and IV), subpopulations of cells were detected with a strong expression of meprinα protein. The activity of meprinα correlated mostly with protein expression except for liver metastases where activity was as low as in adenomas. This implies that the spreading of cancer cells correlates with increased meprinα protein as well as meprinα activity (37).
To further investigate the mechanism that leads to the spreading of colorectal cancer in response to meprinα, we analyzed the ability of meprinα to transactivate the EGFR. Stimulation of Caco-2 cells with active meprinα showed a significant increase in EGFR phosphorylation compared with pro-meprinα (Fig. 2, A and B). Ligand binding to the EGFR activates two main intracellular pathways known to play a role in colorectal cancer: the mitogen-activated protein kinase (MAPK) pathway and the phosphatidylinositol-3-kinase- (PI3K-) protein kinase (AKT) pathway (60). In mammals five distinct groups of MAPKs have been characterized, with the most prominent being ERK1/2. Our data using neutralizing EGF and TGFα antibodies, as well as EGFR and ERK1/2 inhibitors (Fig. 3), imply that EGFR phosphorylation occurs after shedding of EGF or TGFα by meprinα and that this transactivation of EGFR leads to the activation of the MAPK signaling cascade and consequently to the phosphorylation of ERK1/2 (Figs. 2C and 3). ERK1/2 are preferentially activated in response to growth factors and have been implicated in cell migration, invasion, proliferation, angiogenesis, cell differentiation, and cell survival (60).
Proliferation and migration experiments were performed using conditioned media from Caco-2 cells, which endogenously express meprinα. Meprinα is secreted as a zymogen into culture media due to constitutive proteolytic removal of the C-terminal transmembrane and cytosolic domain (34). Trypsin activation on cells is difficult to achieve as meprinα is removed with each washing step. Therefore culture medium was collected and accumulated meprinα was activated (see “Experimental Procedures”).
Cell proliferation is a cellular response known to be enhanced in colorectal cancer upon EGFR activation (61). In three independent assays we demonstrate that Caco-2 cell proliferation is significantly increased in response to meprinα. Further we demonstrate that inhibition of EGF and TGFα, EGFR or ERK1/2 leads to a significant reduction in meprinα-induced proliferation. Therefore, we conclude that the increase in cell proliferation occurs via transactivation of the EGFR/MAPK signaling pathway by a meprinα-dependent mechanism.
We have previously demonstrated a pro-migratory effect induced by meprinα in MDCK cells using videomicroscopy (37). In that study plasmin-activated meprinα was used and migration was induced by hepatocyte growth factor (HGF). In the present study, we demonstrate increased migration of colorectal cancer cells in response to meprinα in an in vitro scratch assay and in a transwell migration assay (Fig. 5). Caco-2 cells endogenously express meprinα, and represent a more natural environment for meprinα function. Inhibition of EGF and TGFα, EGFR, or ERK1/2 showed a significant reduction in meprinα-induced migration in both assays. Altogether, both approaches highlight that meprinα enhances cell migration via EGFR/MAPK signaling pathway.
The identification of EGFR ligands as substrates for meprinα and the known pro-migratory, pro-proliferative, and pro-angiogenic effects of meprinα in vitro, lay the foundation for further analysis on the role of meprinα in colorectal cancer. Experiments, including the use of a transgenic meprinα KO mouse model, will be necessary to address the biological relevance of meprinα in shedding of endogenous forms of EGFR ligands in vivo. Further, in vitro experiments focusing on the role of meprinα in metastasis of colorectal cancer cells would be of great interest. The transactivation of the EGFR may be critical for the transition from benign growth to malignant primary tumors in colorectal cancer and thus meprinα may be an interesting target for the design of drugs that modulate its activity.
Acknowledgments
We thank Ursula Luginbühl for excellent technical support, and Shigeki Higashiyama and Carl P. Blobel for providing AP-tagged EGFR ligand constructs.
This work was supported by Swiss National Science Foundation Grant 31003A 125212/1 and by the Deutsche Forschungsgemeinschaft (DFG) Grant BE 4086/1-2 and SFB877 (project A9), and the Cluster of Excellence “Inflammation at Interfaces” (to C. B. P.).
E. E. Sterchi, unpublished data.
- EGFR
- epidermal growth factor receptor
- EGF
- epidermal growth factor
- HB-EGF
- heparin-binding EGF-like growth factor
- TGFα
- transforming growth factor-α
- ERK
- extracellular-signal-regulated
- AP
- alkaline phosphatase
- MDCK
- Madine-Darby canine kidney
- PABA peptide
- N-benzoyl-l-tyrosyl-p-aminobenzoic acid.
REFERENCES
- 1. Fischer O. M., Hart S., Gschwind A., Ullrich A. (2003) EGFR signal transactivation in cancer cells. Biochem. Soc. Trans. 31, 1203–1208 [DOI] [PubMed] [Google Scholar]
- 2. Parkin D. M., Bray F., Ferlay J., Pisani P. (2005) Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 [DOI] [PubMed] [Google Scholar]
- 3. Yarom N., Jonker D. J. (2011) The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov. Med. 11, 95–105 [PubMed] [Google Scholar]
- 4. Cohen S. (1965) The stimulation of epidermal proliferation by a specific protein (EGF). Dev. Biol. 12, 394–407 [DOI] [PubMed] [Google Scholar]
- 5. Higashiyama S., Abraham J. A., Miller J., Fiddes J. C., Klagsbrun M. (1991) A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 251, 936–939 [DOI] [PubMed] [Google Scholar]
- 6. Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. (1984) Human transforming growth factor-α: precursor structure and expression in E. coli. Cell 38, 287–297 [DOI] [PubMed] [Google Scholar]
- 7. Shing Y., Christofori G., Hanahan D., Ono Y., Sasada R., Igarashi K., Folkman J. (1993) Betacellulin: a mitogen from pancreatic beta cell tumors. Science 259, 1604–1607 [DOI] [PubMed] [Google Scholar]
- 8. Shoyab M., Plowman G. D., McDonald V. L., Bradley J. G., Todaro G. J. (1989) Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science 243, 1074–1076 [DOI] [PubMed] [Google Scholar]
- 9. Toyoda H., Komurasaki T., Uchida D., Takayama Y., Isobe T., Okuyama T., Hanada K. (1995) Epiregulin. A novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J. Biol. Chem. 270, 7495–7500 [DOI] [PubMed] [Google Scholar]
- 10. Strachan L., Murison J. G., Prestidge R. L., Sleeman M. A., Watson J. D., Kumble K. D. (2001) Cloning and biological activity of epigen, a novel member of the epidermal growth factor superfamily. J. Biol. Chem. 276, 18265–18271 [DOI] [PubMed] [Google Scholar]
- 11. Harris R. C., Chung E., Coffey R. J. (2003) EGF receptor ligands. Exp. Cell Res. 284, 2–13 [DOI] [PubMed] [Google Scholar]
- 12. Prenzel N., Zwick E., Daub H., Leserer M., Abraham R., Wallasch C., Ullrich A. (1999) EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402, 884–888 [DOI] [PubMed] [Google Scholar]
- 13. Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., Boyce R. W., Nelson N., Kozlosky C. J., Wolfson M. F., Rauch C. T., Cerretti D. P., Paxton R. J., March C. J., Black R. A. (1998) An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284 [DOI] [PubMed] [Google Scholar]
- 14. Merlos-Suárez A., Ruiz-Paz S., Baselga J., Arribas J. (2001) Metalloprotease-dependent protransforming growth factor-α ectodomain shedding in the absence of tumor necrosis factor-α-converting enzyme. J. Biol. Chem. 276, 48510–48517 [DOI] [PubMed] [Google Scholar]
- 15. Sunnarborg S. W., Hinkle C. L., Stevenson M., Russell W. E., Raska C. S., Peschon J. J., Castner B. J., Gerhart M. J., Paxton R. J., Black R. A., Lee D. C. (2002) Tumor necrosis factor-α converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J. Biol. Chem. 277, 12838–12845 [DOI] [PubMed] [Google Scholar]
- 16. Sahin U., Weskamp G., Kelly K., Zhou H. M., Higashiyama S., Peschon J., Hartmann D., Saftig P., Blobel C. P. (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 164, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sahin U., Blobel C. P. (2007) Ectodomain shedding of the EGF-receptor ligand epigen is mediated by ADAM17. FEBS Lett. 581, 41–44 [DOI] [PubMed] [Google Scholar]
- 18. Horiuchi K., Le Gall S., Schulte M., Yamaguchi T., Reiss K., Murphy G., Toyama Y., Hartmann D., Saftig P., Blobel C. P. (2007) Substrate selectivity of epidermal growth factor-receptor ligand sheddases and their regulation by phorbol esters and calcium influx. Mol. Biol. Cell 18, 176–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edwards D. R., Handsley M. M., Pennington C. J. (2008) The ADAM metalloproteinases. Mol. Aspects Med. 29, 258–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mochizuki S., Okada Y. (2007) ADAMs in cancer cell proliferation and progression. Cancer Sci. 98, 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tousseyn T., Jorissen E., Reiss K., Hartmann D. (2006) (Make) stick and cut loose–disintegrin metalloproteases in development and disease. Birth Defects Res. C. Embryo Today 78, 24–46 [DOI] [PubMed] [Google Scholar]
- 22. Bergin D. A., Greene C. M., Sterchi E. E., Kenna C., Geraghty P., Belaaouaj A., Taggart C. C., O'Neill S. J., McElvaney N. G. (2008) Activation of the epidermal growth factor receptor (EGFR) by a novel metalloprotease pathway. J. Biol. Chem. 283, 31736–31744 [DOI] [PubMed] [Google Scholar]
- 23. Barrett A. J., Rawlings N. D., O'Brien E. A. (2001) The MEROPS database as a protease information system. J. Struct. Biol. 134, 95–102 [DOI] [PubMed] [Google Scholar]
- 24. Beynon R. J., Shannon J. D., Bond J. S. (1981) Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem. J. 199, 591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterchi E. E., Green J. R., Lentze M. J. (1982) Non-pancreatic hydrolysis of N-benzoyl-l-tyrosyl-p-aminobenzoic acid (PABA-peptide) in the human small intestine. Clin. Sci. 62, 557–560 [DOI] [PubMed] [Google Scholar]
- 26. Kenny A. J., Ingram J. (1987) Proteins of the kidney microvillar membrane. Purification and properties of the phosphoramidon-insensitive endopeptidase ('endopeptidase-2') from rat kidney. Biochem. J. 245, 515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bode W., Gomis-Rüth F. X., Huber R., Zwilling R., Stöcker W. (1992) Structure of astacin and implications for activation of astacins and zinc-ligation of collagenases. Nature 358, 164–167 [DOI] [PubMed] [Google Scholar]
- 28. Grünberg J., Dumermuth E., Eldering J. A., Sterchi E. E. (1993) Expression of the alpha subunit of PABA peptide hydrolase (EC 3.4.24.18) in MDCK cells. Synthesis and secretion of an enzymatically inactive homodimer. FEBS Lett. 335, 376–379 [DOI] [PubMed] [Google Scholar]
- 29. Dumermuth E., Eldering J. A., Grünberg J., Jiang W., Sterchi E. E. (1993) Cloning of the PABA peptide hydrolase α subunit (PPH α) from human small intestine and its expression in COS-1 cells. FEBS Lett. 335, 367–375 [DOI] [PubMed] [Google Scholar]
- 30. Sterchi E. E., Naim H. Y., Lentze M. J., Hauri H. P., Fransen J. A. (1988) N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase: a metalloendopeptidase of the human intestinal microvillus membrane which degrades biologically active peptides. Arch. Biochem. Biophys. 265, 105–118 [DOI] [PubMed] [Google Scholar]
- 31. Marchand P., Tang J., Bond J. S. (1994) Membrane association and oligomeric organization of the α and β subunits of mouse meprin A. J. Biol. Chem. 269, 15388–15393 [PubMed] [Google Scholar]
- 32. Ishmael F. T., Norcum M. T., Benkovic S. J., Bond J. S. (2001) Multimeric structure of the secreted meprin A metalloproteinase and characterization of the functional protomer. J. Biol. Chem. 276, 23207–23211 [DOI] [PubMed] [Google Scholar]
- 33. Eldering J. A., Grünberg J., Hahn D., Croes H. J., Fransen J. A., Sterchi E. E. (1997) Polarized expression of human intestinal N-benzoyl-l-tyrosyl-p-aminobenzoic acid hydrolase (human meprin) α and β subunits in Madin-Darby canine kidney cells. Eur. J. Biochem. 247, 920–932 [DOI] [PubMed] [Google Scholar]
- 34. Lottaz D., Hahn D., Müller S., Müller C., Sterchi E. E. (1999) Secretion of human meprin from intestinal epithelial cells depends on differential expression of the α and β subunits. Eur. J. Biochem. 259, 496–504 [DOI] [PubMed] [Google Scholar]
- 35. Lottaz D., Maurer C. A., Hahn D., Büchler M. W., Sterchi E. E. (1999) Nonpolarized secretion of human meprin α in colorectal cancer generates an increased proteolytic potential in the stroma. Cancer Res. 59, 1127–1133 [PubMed] [Google Scholar]
- 36. Rösmann S., Hahn D., Lottaz D., Kruse M. N., Stöcker W., Sterchi E. E. (2002) Activation of human meprin-α in a cell culture model of colorectal cancer is triggered by the plasminogen-activating system. J. Biol. Chem. 277, 40650–40658 [DOI] [PubMed] [Google Scholar]
- 37. Lottaz D., Maurer C. A., Noel A., Blacher S., Huguenin M., Nievergelt A., Niggli V., Kern A., Muller S., Seibold F., Friess H., Becker-Pauly C., Stocker W., Sterchi E. E. (2011) Enhanced activity of meprin-alpha, a pro-migratory and pro-angiogenic protease, in colorectal cancer. PLoS ONE 6, e26450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schutte A., Hedrich J., Stocker W., Becker-Pauly C. (2010) Let it flow: Morpholino knockdown in zebrafish embryos reveals a pro-angiogenic effect of the metalloprotease meprin alpha2. PLoS ONE 5, e8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Becker C., Kruse M. N., Slotty K. A., Köhler D., Harris J. R., Rösmann S., Sterchi E. E., Stöcker W. (2003) Differences in the activation mechanism between the α and β subunits of human meprin. Biol. Chem. 384, 825–831 [DOI] [PubMed] [Google Scholar]
- 40. Becker-Pauly C., Höwel M., Walker T., Vlad A., Aufenvenne K., Oji V., Lottaz D., Sterchi E. E., Debela M., Magdolen V., Traupe H., Stöcker W. (2007) The α and β subunits of the metalloprotease meprin are expressed in separate layers of human epidermis, revealing different functions in keratinocyte proliferation and differentiation. J. Invest Dermatol. 127, 1115–1125 [DOI] [PubMed] [Google Scholar]
- 41. Tokumaru S., Higashiyama S., Endo T., Nakagawa T., Miyagawa J. I., Yamamori K., Hanakawa Y., Ohmoto H., Yoshino K., Shirakata Y., Matsuzawa Y., Hashimoto K., Taniguchi N. (2000) Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J. Cell Biol. 151, 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahmed S. A., Gogal R. M., Jr., Walsh J. E. (1994) A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170, 211–224 [DOI] [PubMed] [Google Scholar]
- 43. Anoopkumar-Dukie S., Carey J. B., Conere T., O'Sullivan E., van Pelt F. N., Allshire A. (2005) Resazurin assay of radiation response in cultured cells. Br. J. Radiol 78, 945–947 [DOI] [PubMed] [Google Scholar]
- 44. Crouch S. P., Kozlowski R., Slater K. J., Fletcher J. (1993) The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 160, 81–88 [DOI] [PubMed] [Google Scholar]
- 45. Wells A. (1999) EGF receptor. Int. J. Biochem. Cell Biol. 31, 637–643 [DOI] [PubMed] [Google Scholar]
- 46. Ohler A., Debela M., Wagner S., Magdolen V., Becker-Pauly C. (2010) Analyzing the protease web in skin: meprin metalloproteases are activated specifically by KLK4, 5 and 8 vice versa leading to processing of proKLK7 thereby triggering its activation. Biol. Chem. 391, 455–460 [DOI] [PubMed] [Google Scholar]
- 47. Roux P. P., Blenis J. (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol. Biol. Rev. 68, 320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choudry Y., Kenny A. J. (1991) Hydrolysis of transforming growth factor-α by cell-surface peptidases in vitro. Biochem. J. 280, 57–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huguenin M., Müller E. J., Trachsel-Rösmann S., Oneda B., Ambort D., Sterchi E. E., Lottaz D. (2008) The metalloprotease meprinbeta processes E-cadherin and weakens intercellular adhesion. PLoS ONE 3, e2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bertenshaw G. P., Turk B. E., Hubbard S. J., Matters G. L., Bylander J. E., Crisman J. M., Cantley L. C., Bond J. S. (2001) Marked differences between metalloproteases meprin A and B in substrate and peptide bond specificity. J. Biol. Chem. 276, 13248–13255 [DOI] [PubMed] [Google Scholar]
- 51. Becker-Pauly C., Barre O., Schilling O., Auf dem Keller U., Ohler A., Broder C., Schutte A., Kappelhoff R., Stocker W., Overall C. M. (2011) Proteomic analyses reveal an acidic prime side specificity for the astacin metalloprotease family reflected by physiological substrates. Mol. Cell Proteomics 10, M111 009233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hedrich J., Lottaz D., Meyer K., Yiallouros I., Jahnen-Dechent W., Stöcker W., Becker-Pauly C. (2010) Fetuin-A and cystatin C are endogenous inhibitors of human meprin metalloproteases. Biochemistry 49, 8599–8607 [DOI] [PubMed] [Google Scholar]
- 53. Jackson L. F., Qiu T. H., Sunnarborg S. W., Chang A., Zhang C., Patterson C., Lee D. C. (2003) Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 22, 2704–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mann G. B., Fowler K. J., Gabriel A., Nice E. C., Williams R. L., Dunn A. R. (1993) Mice with a null mutation of the TGF α gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell 73, 249–261 [DOI] [PubMed] [Google Scholar]
- 55. Hartmann D., de Strooper B., Serneels L., Craessaerts K., Herreman A., Annaert W., Umans L., Lübke T., Lena Illert A., von Figura K., Saftig P. (2002) The disintegrin/metalloprotease ADAM 10 is essential for Notch signaling but not for α-secretase activity in fibroblasts. Hum. Mol. Genet. 11, 2615–2624 [DOI] [PubMed] [Google Scholar]
- 56. Half E., Broaddus R., Danenberg K. D., Danenberg P. V., Ayers G. D., Sinicrope F. A. (2004) HER-2 receptor expression, localization, and activation in colorectal cancer cell lines and human tumors. Int. J. Cancer 108, 540–548 [DOI] [PubMed] [Google Scholar]
- 57. Yasui W., Sumiyoshi H., Hata J., Kameda T., Ochiai A., Ito H., Tahara E. (1988) Expression of epidermal growth factor receptor in human gastric and colonic carcinomas. Cancer Res. 48, 137–141 [PubMed] [Google Scholar]
- 58. Ciardiello F., Kim N., Saeki T., Dono R., Persico M. G., Plowman G. D., Garrigues J., Radke S., Todaro G. J., Salomon D. S. (1991) Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc. Natl. Acad. Sci. U.S.A. 88, 7792–7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tampellini M., Longo M., Cappia S., Bacillo E., Alabiso I., Volante M., Dogliotti L., Papotti M. (2007) Co-expression of EGF receptor, TGFα and S6 kinase is significantly associated with colorectal carcinomas with distant metastases at diagnosis. Virchows Arch. 450, 321–328 [DOI] [PubMed] [Google Scholar]
- 60. Citri A., Yarden Y. (2006) EGF-ERBB signaling: towards the systems level. Nat. Rev. Mol. Cell Biol. 7, 505–516 [DOI] [PubMed] [Google Scholar]
- 61. Yarden Y., Sliwkowski M. X. (2001) Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 [DOI] [PubMed] [Google Scholar]
- 62. Jefferson T., Auf dem Keller U., Bellac C., Metz V. V., Broder C., Hedrich J., Ohler A., Maier W., Magdolen V., Sterchi E., Bond J. S., Jayakumar A., Traupe H., Chalaris A., Rose-John S., Pietrzik C. U., Postina R., Overall C. M., Becker-Pauly C. (2012) The substrate degradome of meprin metalloproteases reveals an unexpected proteolytic link between meprin beta and ADAM10. Cell. Mol. Life Sciences, in press [DOI] [PMC free article] [PubMed] [Google Scholar]