Background: Novel options should be developed for treatment of IAV infections.
Results: Obatoclax, saliphenylhalamide, and gemcitabine target host factors and inhibit IAV and several other viruses infections.
Conclusion: These compounds represent potent antiviral agents.
Significance: These compounds could be exploited in treatment of severe viral infections.
Keywords: Chemical Biology, Drug Discovery, Drug Screening, Influenza Virus, Virus Entry
Abstract
Influenza A viruses (IAVs) infect humans and cause significant morbidity and mortality. Different treatment options have been developed; however, these were insufficient during recent IAV outbreaks. Here, we conducted a targeted chemical screen in human nonmalignant cells to validate known and search for novel host-directed antivirals. The screen validated saliphenylhalamide (SaliPhe) and identified two novel anti-IAV agents, obatoclax and gemcitabine. Further experiments demonstrated that Mcl-1 (target of obatoclax) provides a novel host target for IAV treatment. Moreover, we showed that obatoclax and SaliPhe inhibited IAV uptake and gemcitabine suppressed viral RNA transcription and replication. These compounds possess broad spectrum antiviral activity, although their antiviral efficacies were virus-, cell type-, and species-specific. Altogether, our results suggest that phase II obatoclax, investigational SaliPhe, and FDA/EMEA-approved gemcitabine represent potent antiviral agents.
Introduction
Influenza A viruses (IAVs)2 are enveloped viruses with segmented negative-sense single-stranded RNA genome. The rapid accumulation of mutations in IAV genomes enables the emerging viruses to evade the immunity developed after vaccination or previous infections with IAV and cause yearly epidemics and major pandemics with high morbidity and a great number of severe and fatal cases.
Human IAVs invade the respiratory tract causing acute respiratory infections. Ocular involvement is also common. IAVs replicate in lung and retinal epithelial cells as well as in dendritic cells, type II pneumocytes, and alveolar macrophages (1–3). The replication cycle starts when the hemagglutinin (HA) proteins of the IAV virion recognize cell surface receptors. Then the IAV particle is taken up and transported via the endocytic pathway. Acidification of the endosome by cellular vacuolar ATPase (vATPase) and the virion interior by viral matrix protein 2 (M2) triggers HA-mediated fusion of viral and endosomal membranes, and the uncoating of viral ribonucleoproteins (vRNPs) from the lipid envelope and matrix protein M1 shell. The vRNPs that contain PB1, PB2, and PA polymerase subunits, nucleoprotein (NP), and genomic viral RNAs (vRNAs) are imported to the nucleus, from which the viral genes direct the production of new viral components. These assemble into new virus particles at the plasma membrane which bud and release from the cell to infect other cells.
To ensure efficient production of infectious viral particles, IAV subverts endocytosis, transcription, translation, apoptosis, and other cellular functions (4). These functions could be perturbed by small molecules to inhibit IAV infection. Here, we evaluated a set of small molecules that affect cellular functions implicated in IAV infection to find novel and validate known IAV inhibitors, to provide novel insight into the biological process of IAV-host cell interaction, and to establish a foundation for the development of host-directed antivirals. These antivirals could overcome the problem of emerging resistance to available antiinfluenza drugs that target viral neuraminidase and M2.
EXPERIMENTAL PROCEDURES
Compounds
Supplemental Table 1 summarizes compounds used in this study and their suppliers. Saliphenylhalamide (SaliPhe) was synthesized by Jef De Brabander's group as described in Ref. 5. 10 mm compounds in 100% dimethyl sulfoxide (Sigma-Aldrich) were stored at −80 °C until use.
Cells and Viruses
To obtain human macrophages, peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coat preparations of voluntary blood donors (Finnish Red Cross) using lymphocyte separation medium (PAA) and cultured for 7 days in macrophage serum-free medium (Invitrogen) supplemented with 10 ng/ml human granulocyte macrophage-colony-stimulating factor (GM-CSF; Sigma-Aldrich) (6). PBMC-derived macrophages, human telomerase reverse transcriptase (hTERT)-immortalized retinal pigment epithelial (RPE), human adenocarcinomic alveolar basal epithelial (A549), Madin-Darby canine kidney (MDCK), monkey Vero-E6 and B-Vero cells were infected with influenza A/PR8-NS116-GFP (PR8-GFP), A/WSN/33 (WSN), A/PR/8/34 (PR8), A/Sydney/5/1997 (H3N2), B/Shandong/7/97 (InfB), human echovirus 6 (Echo6, GenBank accession: JQ929657), Sindbis (SINV, Ilomantsi-2002A strain), Semliki forest (SFV), Bunyamwera (BUNV), measles (MeV, Schwarz vaccine strain), herpes simplex 1 (HSV-1, KOS strain), vaccinia virus (VACV), or mock-infected as described in Refs. 7–14. The cells and viruses were propagated at 37 °C in 5% CO2.
Targeted Screening of Chemical Compounds against Reporter IAV Strain
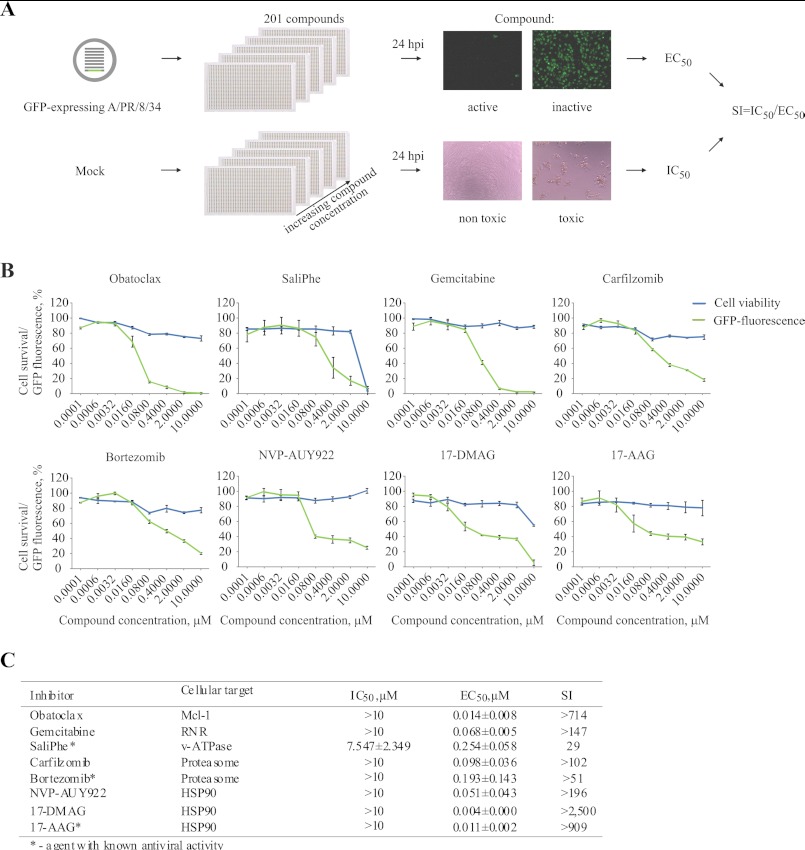
The compound efficacy testing against PR8-GFP was performed in 384-well plates in RPE cells, which mimic the natural environment for IAV infection (15, 16). Approximately 2500 RPE cells/well were seeded using Multidrop 384 (Thermo) in 25 μl of Dulbecco's modified Eagle's medium/Nutrient F-12 Ham (DMEM:F-12; Sigma-Aldrich) medium containing, 10% heat-inactivated fetal bovine serum (FBS; Invitrogen), 2 mm l-glutamine (Lonza), 50 units/ml penicillin-streptomycin mix (PenStrep; Lonza), and 0.348% NaHCO3 (Invitrogen). Cells were grown for 24 h. The growth medium was changed to the virus growth medium (VGM) containing 0.2% BSA (Sigma-Aldrich), 2 mm l-glutamine, 0.348% NaHCO3 and 1 μg/ml l-1-tosylamido-2-phenylethyl chloromethyl ketone-trypsin (TPCK)-trypsin (Sigma-Aldrich) in DMEM:F-12. The compounds were added to the cells in 10-fold serial dilutions at five different concentrations starting from 10 μm using an Echo acoustic dispenser (Labsite). 0.1% dimethyl sulfoxide was added in the control wells. The RPE cells were infected with PR8-GFP IAV at multiplicity of infection (m.o.i.) 3. At 24 h postinfection (hpi) GFP fluorescence was measured using PHERAstar FS plate reader (BMG Labtech). The effective concentrations of the compounds (EC50) were calculated with SigmaPlot 11 (Systat Software GmbH). Cytotoxicity testing was performed in parallel using Cell Titer Glo viability assay, luminescence was read with PHERAstar FS plate reader (CTG); Promega), and IC50 values were calculated. Selectivity indexes (SI = IC50/EC50) were calculated to distinguish the anti-IAV effect from toxic side effects of the compounds. The compounds with SI >10 were validated further.
Compound Validation against WT IAV Strains
Selected compounds were further validated against PR8 and WSN IAV strains in RPE cells or in human PBMC-derived macrophages. Efficacy and cytotoxicity testing were performed in 96-well plate format. Typically, 40,000 RPE cells or 600,000 PBMCs were seeded per well and grown for 24 h or 7 days, respectively, in appropriate cell growth media. RPE cells and PBMC-derived macrophages were washed twice with phosphate-buffered saline (PBS) and covered with appropriate VGM. The compounds were added to the cells in 3-fold dilutions at seven different concentrations starting from 10 μm. No compounds were added to the control wells. The cells were infected with the viruses at m.o.i. 3 or 10, or noninfected. When a virus-induced cytopathic effect was observed, cell viability was measured using a CTG assay.
For each virus-compound pair we calculated the quantitative activity and toxicity score (ATS),
where A is the maximal antiviral activity (with a virus), T is the toxic side effects (without a virus), M is the concentration level of maximal activity (over the whole dose range), and B refers to the base-line concentration level (i.e. the lowest dose). In case the maximal activity AM is >100%, we set AM = 100, and if M = B (i.e. the maximal activity is reached already at the lowest dose), we set ATS = 0. Using these constraints, ATS varies between −100 and +100, where negative values indicate excessive toxicity and highest positive values indicate most potent compounds.
Compound Efficacy Testing against Other Viruses
Compound efficacy and cytotoxicity testing against A/Sydney/5/1997(H3N2), InfB, BUNV, MeV, SINV, SFV, Echo6, HSV-1, and VACV was performed in dedicated cell lines using a CTG assay.
Virus Titration
Compound antiviral efficacies were further validated using plaque assays. Cells were treated with a compound at effective but noncytotoxic concentrations or remained nontreated and infected with dedicated virus at m.o.i. 0.1. Supernatants were collected 24–72 hpi. IAV-containing supernatants were diluted in DMEM-based VGM containing 0.2% BSA, 50 units/ml PenStrep, 2 mm l-glutamine, and 1 μg/ml TPCK-trypsin and added to MDCK cells in 6-well plates. 1 h later the cells were overlaid with Avicel medium (AM) containing 1.2% Avicel (FMC Biopolymer), 0.2% BSA, 2 mm l-glutamine, 50 units/ml PenStrep, and 1 μg/ml TPCK-trypsin in minimal essential medium (Invitrogen) and incubated for 2 days. The cells were fixed using 4% formaldehyde (Sigma-Aldrich) in PBS and stained with 0.1% crystal violet (Sigma-Aldrich) in 1% methanol (Sigma-Aldrich), 20% ethanol (Altia Oy), and 3.6% formaldehyde (Sigma-Aldrich). Plaque-forming units were calculated.
For other viruses the titration procedure slightly differed from the one described above. Echo6 virus was titered on A549 cells, and both VGM and AM contained 0.4 μg/ml TPCK-trypsin. SINV, SFV, HSV-1, and VACV were titered on Vero-E6 cells, and VGM was supplemented with 5% FBS, 2 mm l-glutamine, and 50 units/ml PenStrep in DMEM, and TPCK-trypsin was omitted. BUNV was titered on Vero-E6 cells, and supernatants were diluted in PBS containing 2% newborn calf serum (Invitrogen), AM contained 0.6% Avicel, and 2% newborn calf serum in minimal essential medium, and cells were incubated with AM for 3 days. Titers of HSV-1 were determined by infecting 12-well plates of B-Vero cells with serial dilutions of supernatants in DMEM containing 7% heat-inactivated fetal calf serum (FCS; Invitrogen) and 20 μg/ml human immunoglobulin G (Baxter). Cells were fixed with methanol for 3 min and stained with 0.1% crystal violet in 2% ethanol. The degree of inhibition mediated by a compound was calculated as a ratio between virus titers in nontreated and compound-treated cells.
Immunofluorescense
Compound-treated or nontreated RPE cells were infected with WSN IAV at m.o.i. 30 on ice for 1 h. Cells were washed twice with ice-cold VGM, overlaid with the media with or without compound, and incubated at 37 °C for 1–4 h. Cells were fixed with 4% paraformaldehyde (in PBS). PBS with 1% BSA and 0.1% Triton X-100 was used for blocking and permeabilization of the fixed cells and for dilution of antibodies. NP and M1 of WSN were stained with corresponding rabbit polyclonal antibodies (1:1000; from I. J. laboratory), and the secondary antibody was Alexa Fluor 488 goat anti-rabbit IgG (H+L) (1:1000, Invitrogen Molecular Probes). Mcl-1 was stained with anti-human MCL-1 (1:100; clone 22/Mcl-1; BD Transduction Laboratories). Secondary antibody was Alexa Fluor 594 goat anti-mouse IgG (1:2000; Invitrogen). Nuclei were counterstained with DAPI. Images were captured with Nikon 90i microscope and processed with NIS Elements AR software. Note, obatoclax produces autofluorescence (absorbance peak, 490 nm; emission peak, 550 nm (17)).
Immunoblots
RPE cells were treated with 1 μm SaliPhe, 10 μm gemcitabine or 1 μm obatoclax or remained untreated and then infected with WSN IAV at m.o.i. 3. At 4, 8, or 12 hpi cells were washed twice with PBS, lysed with 2× SDS-sample buffer, and sonicated to dissolve aggregates. Proteins were resolved by electrophoresis in gradient 4–20% SDS-PAGE and transferred to polyvinylidene fluoride membrane (GE Healthcare). Membrane was blocked with 5% milk in Tris-buffered saline (TBS) and incubated with primary guinea pig anti-NS1 (1:2000), rabbit anti-M1 (1:2000), mouse anti-LRP130 (1:100, clone G-10; Santa Cruz Biotechnology), rabbit anti-NP (1:500; from I. J. laboratory), or mouse anti-β-actin (1:5000; Sigma-Aldrich) antibody for 1 h at room temperature. Membrane was washed three times for 10 min with TBS buffer containing 0.3% Tween 20 (Tween/TBS) and incubated for 1 h at room temperature with secondary antibodies conjugated to infrared dyes 680LT or 800CW (1:20,000, Li-Cor Biosciences). After three washes for 10 min with Tween/TBS buffer and one with TBS, membranes were scanned on an Odyssey scanner (Li-Cor Biosciences).
Strand-specific Viral RNA Detection
Reverse-transcription and strand-specific quantitative PCRs were performed as described in Ref. 18.
Cytokine/Chemokine Profiling
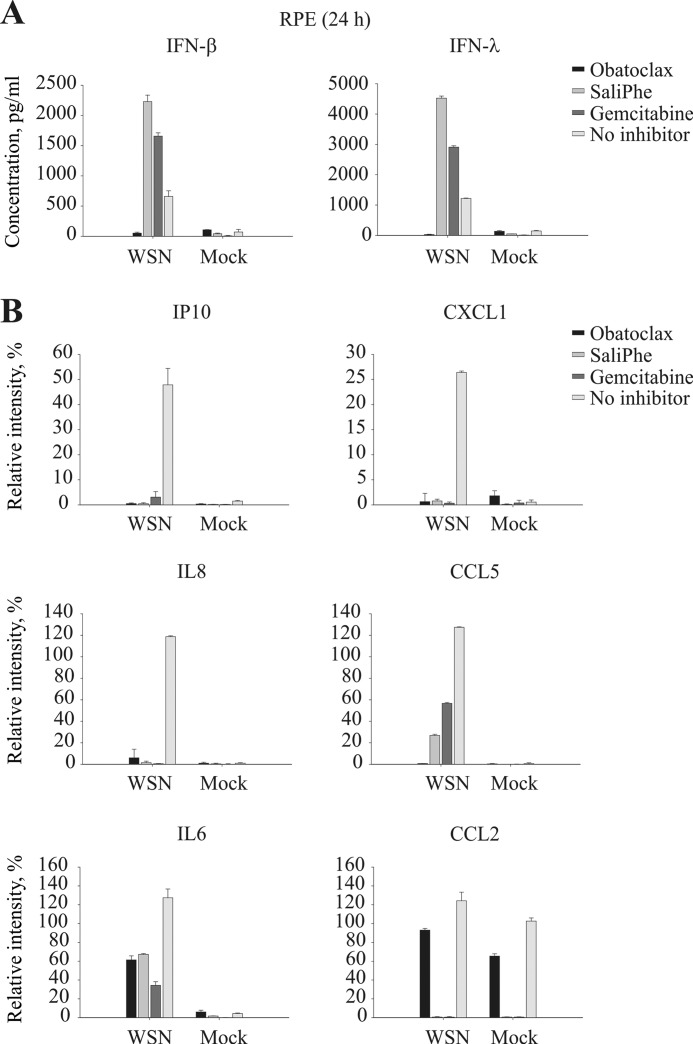
Compound-treated or nontreated RPE cells were infected with WSN at m.o.i. 3. The medium was replaced after 1 h of infection. At 24 hpi the supernatants were collected and assayed for interferon β and λ levels by ELISA (PBL Interferon source). In addition, the levels of 26 different chemokines/cytokines in the supernatants were assayed using human cytokine array panel A kit (R&D Systems).
RNAi
RPE cells were transfected with Hs_MCL1_12 FlexiTube siRNA (SI04949721; Qiagen) or control siRNA using Oligofectamine transfection reagent (Invitrogen) according to the manufacturer's instructions. 48 h after transfection the expression levels of Mcl-1 were analyzed in siRNA-Mcl-1- and siRNA-Ctrl-transfected cells by immunoblotting using primary rabbit anti-Mcl-1 (1:200, clone S-19; Santa Cruz Biotechnology) and secondary donkey anti-rabbit IRDye 800 (1:20,000, Li-Cor Biosciences) antibodies.
In a parallel experiment, the medium was replaced with VGM at 48 h after transfection, and the cells were infected with PR8-GFP IAV at m.o.i. 3. At 24 hpi bright field and fluorescent images were captured using a Leica microscope. GFP-fluorescense and cell survival (CTG) were measured using PHERAstar FS plate reader.
Ethics
Virus experiments were carried out under BSL-2 conditions and in compliance with regulations of the University of Helsinki (permit 21/M/09). Buffy coat fractions of voluntary blood donors were obtained under permission from the Finnish Red Cross Blood Transfusion Service and approved by the ethical review committee of the University of Helsinki Central Hospital, Finland (165/13/03/00/2011).
RESULTS
Eight Host-directed Compounds Inhibit Infection of Human Nonmalignant Cells with Reporter IAV Strain
A total of 201 host-directed chemical compounds have been preselected for this study (supplemental Table S1). These include known IAV inhibitors, such as gemfibrozil and TOFA (lipid metabolism), 3-methyladenine and wortmannin (autophagy), 17-AAG and bortezomib (protein quality control), fluorouracil and cytarabine (de novo nucleotide biosynthesis). Notably, the antiviral efficacy of these compounds has been demonstrated mainly in cancer cell lines. Our drug set also contains functional analogs of known IAV inhibitors, such as erlotinib and gefitinib (analogs of PD153035), 17-DMAG (analog of 17-AAG), carfilzomib (analog of bortezomib), leflunomide and gemcitabine (analog of fluorouracil), and a pool of potential IAV inhibitors that target the cell cycle, apoptotic pathways, and other functions that could be essential for IAV infection of a host cell.
We have tested the set of 201 compounds in nonmalignant human RPE cells at different concentrations against infection with reporter IAV strain (PR8-GFP). This strain expresses the NS11–116-GFP fusion which allows monitoring antiviral efficacy of the compounds by measuring GFP fluorescence and identification of small molecules that target stages of IAV infection ranging from IAV attachment to viral protein synthesis and quality control (9). Compound cytotoxicity testing was performed in parallel to calculate selectivity indexes (Fig. 1A). We identified five novel (obatoclax, gemcitabine, carfilzomib, NVP-AUY922, and 17-DMAG) and validated three known (SaliPhe, bortezomib, and 17-AAG (9, 19, 20)) IAV inhibitors that selectively suppressed IAV-mediated GFP expression at noncytotoxic concentrations in dose-dependent manner in RPE cells (Fig. 1, B and C).
FIGURE 1.
8 of 201 potent host-directed IAV inhibitors attenuate infection of reporter IAV strain in human nonmalignant RPE cells at noncytotoxic concentrations. A, schematic represents the efficacy and cytotoxicity testing of 201 potential and known inhibitors of IAV infection in human RPE cells. B, 8 hit compounds targeting indicated host factors were validated against reporter PR8-GFP IAV (m.o.i. 3) in RPE cells (24 hpi). Green curves show the percent GFP fluorescence of cells with the increasing concentrations of compounds. Blue curves show percent viability of noninfected cells with increasing concentrations of compounds. The error bars represent the S.D. C, cytotoxicity (IC50), antiviral efficacy (EC50), and selectivity index (SI) of the hit compounds were calculated based on the data from B.
Three Host-directed Compounds Inhibit Infection of Human Nonmalignant Cells with Wild Type IAV Strains
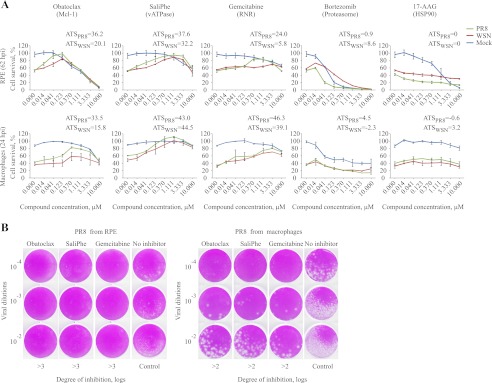
The identified compounds have been demonstrated to target cellular vATPase, proteasome, heat shock protein 90 (HSP90), ribonucleotide reductase, or induced myeloid leukemia cell differentiation protein Mcl-1 (5, 17, 21, 22). Therefore, we next selected representative inhibitors for each of the 5 targets and tested them against two wild type IAV strains (PR8 and WSN) in human RPE cells and PBMC-derived macrophages. The effect of the drugs on cell survival and virus progeny production was examined. Potent inhibitors of Mcl-1, ribonucleotide reductase, and vATPase were found to exhibit dose-dependent antiviral activity which could be expressed in positive ATS. Inhibitors of proteasomes showed a moderate effect, and HSP90 inhibitors were ineffective at the selected concentrations, which could be expressed in neutral or negative ATS, respectively (Fig. 2, A and B). These results suggest that cellular Mcl-1, ribonucleotide reductase, and vATPase could represent essential host factors for wild type IAV infection. The proteasome could also be involved, but the therapeutic window was too narrow to allow a final conclusion. By contrast, HSP90 is not required by wild type, but is exploited by the mutant IAV strain containing an altered NS1, indicating that full-length NS1 could perform HSP90 function during IAV infection.
FIGURE 2.
Obatoclax, gemcitabine, and SaliPhe efficiently attenuate replication of wild type IAV strains in human RPE cells and PBMC-derived macrophages at noncytotoxic concentrations. A, the antiviral efficacy and cytotoxicity of five representative inhibitors of Bcl2-like proteins, ribonucleotide reductase, vATPase, proteasome, or HSP90 were examined in noninfected (blue curves) and PR8- or WSN-infected (green and red curves) RPE cells and macrophages using a CTG assay. The error bars represent the S.D. The ATS are shown. B, PR8 IAVs produced in nontreated or obatoclax- (0.4 μm), gemcitabine- (1 μm), or SaliPhe- (3 μm) treated human macrophages and RPE cells were titered on MDCK cells. Representative plaque assays and degrees of inhibition of PR8 virus production are shown.
Virus-, Cell Type-, and Species-specific Antiviral Activity of Three Compounds
We next tested whether obatoclax, gemcitabine, and SaliPhe inhibit seasonal H3N2 IAV and InfB infections. Supplemental Fig. S1 shows that obatoclax and SaliPhe, by contrast to gemcitabine, rescued RPE cells from infections with these viruses. This result indicates that obatoclax and SaliPhe could possess anti-IAV and anti-InfB activity, whereas gemcitabine activity could be limited to certain influenza strains.
We next tested whether these compounds are able to rescue cancer cells from IAV infection. We found that obatoclax and SaliPhe protected human adenocarcinomic alveolar basal epithelial (A549) cells from the challenge with WSN virus and that the antiviral effect of this compound was achieved at lower concentrations than those needed to mediate cancer cell death (supplemental Fig. S1). Interestingly, gemcitabine was unable to rescue IAV-infected A549 cells, suggesting that gemcitabine activity could be cell type-specific. We also tested whether obatoclax, gemcitabine, and SaliPhe possess species-specific antiviral activity and found that obatoclax, by contrast to gemcitabine and SaliPhe, was less effective in MDCK cells than in RPE or A549 (Fig. 2A and supplemental Fig. S1).
We next explored whether these compounds display antiviral activity against several other viruses of different families. Compound efficacy and cytotoxicity testing was performed against BUNV, MeV, SINV, SFV, Echo6, HSV-1, and VACV in dedicated cell lines (supplemental Fig. S2). Further, we titered the viruses produced by cells nontreated or treated with effective but noncytotoxic concentration of the compound. Table 1 shows the degrees of inhibition of virus production mediated by the compounds. We found that obatoclax and SaliPhe efficiently (>2 logs reduction in virus titers) attenuated infections of BUNV and SINV, whereas gemcitabine suppressed SINV and HSV-1.
TABLE 1.
Viruses tested for their susceptibility to obatoclax, gemcitabine, and SaliPhe
| Virus | Group | Family | Cell line | Degree of inhibition (compound concentration) |
||
|---|---|---|---|---|---|---|
| Obatoclax | SaliPhe | Gemcitabine | ||||
| BUNV | (−) ssRNA | Bunyaviridae | Vero | >6 logs (1 μm) | >4 logs (3 μm) | NDb |
| MeV | (−) ssRNA | Paramyxoviridae | Vero | ND | ND | ND |
| SINV | (+) ssRNA | Togaviridae | Vero | >2 logs (0.3 μm) | >3 logs (0.3 μm) | >2 logs (3 μm) |
| SFV | (+) ssRNA | Togaviridae | Vero | (0.3 μm)a | (0.3 μm)a | >1 log (3 μm) |
| Echo6 | (+) ssRNA | Picornaviridae | A549 | >1 log (1 μm) | ND | >1 log (3 μm) |
| HSV-1 | dsDNA | Herpesviridae | RPE | ND | ND | >4 logs (3 μm) |
| VACV | dsDNA | Poxviridae | RPE | ND | ND | ND |
a , the compound did not affect virus production and survival of infected cells at indicated concentrations.
b ND, not determined because the compound did not protect cells from IAV-mediated death (see Fig. 7).
Altogether, these results suggest that obatoclax, SaliPhe, and gemcitabine possess virus-, cell type-, and species-specific antiviral activities, indicating that different viruses could possess differential preferences for cellular targets of obatoclax, SaliPhe, and gemcitabine upon infection.
Obatoclax, SaliPhe, and Gemcitabine Inhibit Virus Entry
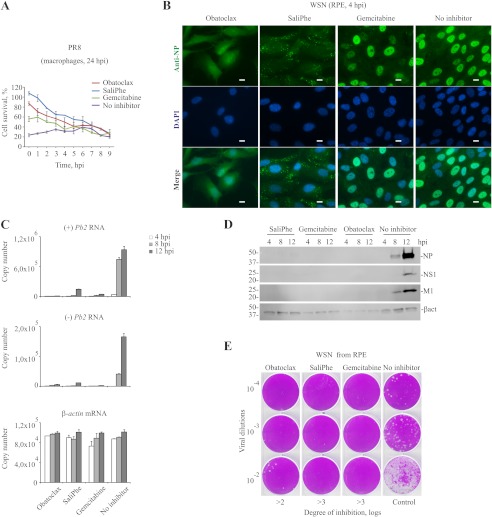
A time-of-compound-addition experiment in macrophages demonstrated that obatoclax, gemcitabine, and SaliPhe inhibited early steps of IAV infection (Fig. 3A). An immunofluorescence experiment monitoring the viral NP at 4 hpi revealed that obatoclax and SaliPhe treatment prevented nuclear accumulation of vRNPs which was observed in nontreated and gemcitabine-treated RPE cells (Fig. 3B). Obatoclax, SaliPhe, and gemcitabine treatment affected subsequent vRNA transcription and replication, viral protein synthesis, and infectious viral particle production (Fig. 3, C–E). Thus, these three compounds inhibit entry of IAV into the cells.
FIGURE 3.
Obatoclax, SaliPhe, and gemcitabine inhibit IAV entry. A, PR8-infected (m.o.i. 3) PBMC-derived macrophages were treated with obatoclax (0.4 μm), SaliPhe (3 μm), or gemcitabine (1 μm) at the indicated time points. Control cells remained nontreated. Cell viability was measured at 24 hpi using a CTG assay. The error bars represent the S.D. B, RPE cells were treated with obatoclax (0.4 μm), SaliPhe (3 μm), gemcitabine (1 μm), or nontreated, infected with WSN IAV at m.o.i. 30, fixed at 4 hpi, and imaged for the viral NP. DAPI stains the nucleus. Scale bars, 10 μm. C, RPE cells were treated with obatoclax (0.4 μm), SaliPhe (3 μm), gemcitabine (1 μm), or nontreated followed by infection with WSN IAV at m.o.i. 3. Total RNA was extracted at the indicated time points and subjected to quantitative PCRs detecting viral-positive (+) and -negative (−) strands of Pb2 and cellular β-actin RNA. The error bars represent the S.D. D, cells were treated and infected as in A. Total cell lysates were obtained at the indicated time points and analyzed by immunoblotting. E, WSN IAVs produced in nontreated or obatoclax- (0.4 μm), gemcitabine- (1 μm), or SaliPhe- (3 μm) treated RPE cells were titered on MDCK cells. Representative plaque assays and degrees of inhibition of PR8 virus production are shown.
Mcl-1 Is a Novel Antiviral Target
Obatoclax is a novel anti-IAV agent, whereas SaliPhe and analogs of gemcitabine have been shown to possess anti-IAV activity (9, 23). Therefore, we investigated the possible mechanism of action of obatoclax.
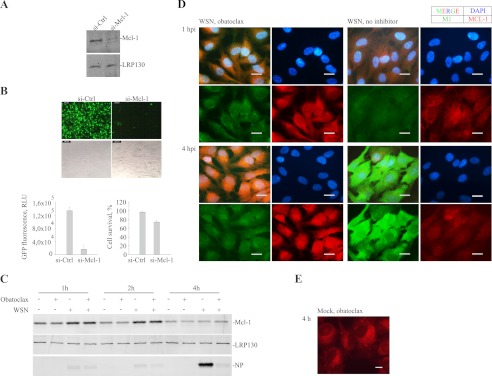
It was demonstrated that obatoclax preferentially binds Mcl-1 (17); however, the Mcl-1 involvement in IAV infection has not been shown. To prove that Mcl-1 is essential for IAV entry we partially silenced Mcl-1 using Mcl-1-specific siRNA (Fig. 4A), infected these cells with PR8-GFP virus and analyzed virus-mediated GFP expression. Silencing of Mcl-1 substantially reduced IAV-mediated GFP expression and slightly affected the viability of infected cells (Fig. 4B), suggesting that Mcl-1 is involved in IAV infection and apoptosis. Notably, Mcl-1 is up-regulated during the first hours of IAV infection (Fig. 4C) as with other viral infections (24, 25). This result indicates that Mcl-1 could also be involved in host antiviral responses. An immunofluorescence experiment showed that Mcl-1 has a localization pattern similar to that of viral M1 (Fig. 4D), indicating that Mcl-1 could be involved in the recognition of IAVs.
FIGURE 4.
Mcl-1 is a target for obatoclax and an essential host factor for IAV infection. A, RPE cells were transfected with Mcl-1-specific or control siRNA, and Mcl-1 levels were analyzed 48 h later by immunoblotting. B, si-Mcl-1 and si-Ctrl RPE cells were prepared as in A and infected with PR8-GFP virus. 24 hpi representative fluorescent and bright-field images were taken, and GFP expression and cell viability were quantified. The error bars represent the S.D. C, obatoclax-treated or nontreated RPE cells were WSN- (m.o.i. 3) or MOCK-infected. Cells were harvested at indicated times, and Mcl-1, NP, and LRP130 (loading control) levels were analyzed by immunoblotting. D, RPE cells were treated with obatoclax (0.4 μm) or remained nontreated and infected with WSN IAV at m.o.i. 30, fixed at 1 and 4 hpi, and imaged for the viral M1 and Mcl-1. DAPI stains the nucleus. Scale bars, 20 μm. E, RPE cells were treated with obatoclax (0.4 μm), stained, and imaged 4 h later. Autofluorescent obatoclax localizes to the perinuclear region of the cells. Scale bars, 10 μm.
Effect of Obatoclax, SaliPhe, and Gemcitabine on IAV-mediated Cellular Antiviral and Proinflammatory Responses
We analyzed the effect of obatoclax, SaliPhe, and gemcitabine on cytokine/chemokine production by IAV-infected and mock-infected cells. We found that all three compounds suppressed WSN-mediated production of proinflammatory IP10, CXCL1, IL6, IL8, CCL2, and CCL5 in RPE cells (supplemental Fig. S3 and Fig. 5). Surprisingly, obatoclax inhibited, whereas SaliPhe and gemcitabine stimulated, production of antiviral IFN-β and IFN-λ (Fig. 5). These results indicate that obatoclax, in contrast to SaliPhe and gemcitabine, could prevent activation of cellular antiviral responses, most probably, via suppression of Mcl-1-type I IFN signaling axis.
FIGURE 5.
Obatoclax, by contrast to SaliPhe and gemcitabine, suppressed production of IFNs by IAV-infected cells. RPE cells were infected with WSN at m.o.i. 3. At 24 hpi the supernatants were collected and assayed for chemokine/cytokine levels.
DISCUSSION
Here, we developed a systematic approach to search for potential antiviral compounds and to provide a foundation for their further development. Using this approach we evaluated a set of carefully preselected potential and known host-directed antiinfluenza agents. We found that obatoclax, SaliPhe, and gemcitabine inhibited IAV and several other virus infections at noncytotoxic concentrations. Using these small molecules we provided novel insight into IAV-host cell interactions. In particular, the link was established between compound targets and stages of virus infection.
Obatoclax is a phase II anticancer and a novel anti-IAV agent that binds cellular Mcl-1. Silencing of Mcl-1 using Mcl-1-directed siRNA confirmed the importance of the Mcl-1-mediated pathways for efficient IAV infection.
Mcl-1 was up-regulated during IAV infection. This indicates that Mcl-1 could be implicated in host antiviral responses. Indeed, inhibition of Mcl-1 with obatoclax suppressed the type I IFN responses in IAV-infected cells. This could be achieved via Mcl-1-mediated regulation of STAT3 signaling pathway which controls type I IFN responses (26–28). Thus, Mcl-1 could be involved in cellular antiviral and apoptosis responses and IAV uptake.
Gemcitabine is an FDA/EMEA-approved anticancer and a novel anti-IAV agent that alters viral RNA transcription and replication. This observation is in line with previously reported findings showing that the analogs of this drug possess anti-IAV activity by targeting ribonucleotide reductase which is essential for de novo pyrimidine biosynthesis (23).
SaliPhe is an investigational anticancer and anti-IAV compound (5, 9, 29). It inhibits vATPase activity, and similarly to ammonium chloride, it prevents the acidification of endosomes and arrests IAV in the endocytic compartments.
In conclusion, we identified obatoclax, gemcitabine, and SaliPhe as potent inhibitors of virus-host interactions, and these results could provide a foundation for clinical development of these host-directed antivirals. These antivirals could overcome the problem of emerging resistance to available virus-directed anti-influenza drugs.
Supplementary Material
Acknowledgments
We thank Alun Parsons for critical reading of the manuscript; Markus Wolschek for the gift of PR8-GFP IAV strain; Stephen L. White from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, NCI, National Institutes of Health, for compound sets; Ulrich Lauer, from the University Hospital Tuebingen, for the gift of MeV; and Jani Saarela, Caroline Heckman, Maria Anastasina, and Minttu Kaloinen for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants CA90349 and CA95471. This work was also supported by the Jane and Aatos Erkko Foundation, Centre for International Mobility (CIMO), the Academy of Finland Grants 138644 and 255852, and Robert A. Welch Foundation Grant I-1422.

This article contains supplemental Table 1, Figs. S1 and S2, and additional references.
- IAV
- influenza A virus
- AM
- Avicel medium
- ATS
- activity and toxicity score
- CTG
- Cell Titer Glo viability assay
- hpi
- hours postinfection
- Mcl
- myeloid leukemia cell
- MDCK
- Madin-Darby canine kidney
- m.o.i.
- multiplicity of infection
- NP
- nucleoprotein
- PBMC
- peripheral blood mononuclear cell
- RNP
- ribonucleoprotein
- RPE
- retinal pigment epithelial
- SaliPhe
- saliphenylhalamide
- TPCK
- l-1-tosylamido-2-phenylethyl chloromethyl ketone
- v
- vacuolar
- VGM
- virus growth medium.
REFERENCES
- 1. Gu J., Xie Z., Gao Z., Liu J., Korteweg C., Ye J., Lau L. T., Lu J., Gao Z., Zhang B., McNutt M. A., Lu M., Anderson V. M., Gong E., Yu A. C., Lipkin W. I. (2007) H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet 370, 1137–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurtz J., Manvell R. J., Banks J. (1996) Avian influenza virus isolated from a woman with conjunctivitis. Lancet 348, 901–902 [DOI] [PubMed] [Google Scholar]
- 3. Weinheimer V. K., Becher A., Tönnies M., Holland G., Knepper J., Bauer T. T., Schneider P., Neudecker J., Ruckert J. C., Szymanski K., Temmesfeld-Wollbrueck B., Gruber A. D., Bannert N., Suttorp N., Hippenstiel S., Wolff T., Hocke A. C. (2012) J. Infect. Dis., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Müller K. H., Kakkola L., Nagaraj A. S., Cheltsov A. V., Anastasina M., Kainov D. E. (2012) Emerging cellular targets for influenza antiviral agents. Trends Pharm. Sci. 33, 89–99 [DOI] [PubMed] [Google Scholar]
- 5. Lebreton S., Jaunbergs J., Roth M. G., Ferguson D. A., De Brabander J. K. (2008) Evaluating the potential of vacuolar ATPase inhibitors as anticancer agents and multigram synthesis of the potent salicylihalamide analog saliphenylhalamide. Bioorg. Med. Chem. Lett. 18, 5879–5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lietzén N., Ohman T., Rintahaka J., Julkunen I., Aittokallio T., Matikainen S., Nyman T. A. (2011) Quantitative subcellular proteome and secretome profiling of influenza A virus-infected human primary macrophages. PLoS Pathogens 7, e1001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guse K., Sloniecka M., Diaconu I., Ottolino-Perry K., Tang N., Ng C., Le Boeuf F., Bell J. C., McCart J. A., Ristimäki A., Pesonen S., Cerullo V., Hemminki A. (2010) Antiangiogenic arming of an oncolytic vaccinia virus enhances antitumor efficacy in renal cell cancer models. J. Virol. 84, 856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heikkilä O., Susi P., Stanway G., Hyypiä T. (2009) Integrin αVβ6 is a high-affinity receptor for coxsackievirus A9. J. Gen. Virol. 90, 197–204 [DOI] [PubMed] [Google Scholar]
- 9. Müller K. H., Kainov D. E., El Bakkouri K., Saelens X., De Brabander J. K., Kittel C., Samm E., Muller C. P. (2011) The proton translocation domain of cellular vacuolar ATPase provides a target for the treatment of influenza A virus infections. Br. J. Pharmacol. 164, 344–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sane J., Kurkela S., Desdouits M., Kalimo H., Mazalrey S., Lokki M. L., Vaheri A., Helve T., Törnwall J., Huerre M., Butler-Browne G., Ceccaldi P. E., Gessain A., Vapalahti O. (2012) Prolonged myalgia in sindbis virus infection: case description and in vitro infection of myotubes and myoblasts. J. Infect. Dis. 206, 407–414 [DOI] [PubMed] [Google Scholar]
- 11. Schneider J., Dauber B., Melén K., Julkunen I., Wolff T. (2009) Analysis of influenza B virus NS1 protein trafficking reveals a novel interaction with nuclear speckle domains. J. Virol. 83, 701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwarz A. J. (1962) Preliminary tests of a highly attenuated measles vaccine. Am. J. Dis. Child 103, 386–389 [DOI] [PubMed] [Google Scholar]
- 13. Shi X., Elliott R. M. (2009) Generation and analysis of recombinant Bunyamwera orthobunyaviruses expressing V5 epitope-tagged L proteins. J. Gen. Virol. 90, 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spuul P., Balistreri G., Hellström K., Golubtsov A. V., Jokitalo E., Ahola T. (2011) Assembly of α virus replication complexes from RNA and protein components in a novel trans-replication system in mammalian cells. J. Virol. 85, 4739–4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang X. R., Jimenez G., Chang E., Frolkis M., Kusler B., Sage M., Beeche M., Bodnar A. G., Wahl G. M., Tlsty T. D., Chiu C. P. (1999) Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat. Genet. 21, 111–114 [DOI] [PubMed] [Google Scholar]
- 16. Michaelis M., Geiler J., Klassert D., Doerr H. W., Cinatl J., Jr. (2009) Infection of human retinal pigment epithelial cells with influenza A viruses. Invest. Ophthalmol. Vis. Sci. 50, 5419–5425 [DOI] [PubMed] [Google Scholar]
- 17. Nguyen M., Marcellus R. C., Roulston A., Watson M., Serfass L., Murthy Madiraju S. R., Goulet D., Viallet J., Bélec L., Billot X., Acoca S., Purisima E., Wiegmans A., Cluse L., Johnstone R. W., Beauparlant P., Shore G. C. (2007) Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc. Natl. Acad. Sci. U.S.A. 104, 19512–19517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng L., Lintula S., Ho T. H., Anastasina M., Paju A., Haglund C., Stenman U. H., Hotakainen K., Orpana A., Kainov D., Stenman J. (2012) Technique for strand-specific gene-expression analysis and monitoring of primer-independent cDNA synthesis in reverse transcription. BioTechniques 52, 263–270 [DOI] [PubMed] [Google Scholar]
- 19. Chase G., Deng T., Fodor E., Leung B. W., Mayer D., Schwemmle M., Brownlee G. (2008) Hsp90 inhibitors reduce influenza virus replication in cell culture. Virology 377, 431–439 [DOI] [PubMed] [Google Scholar]
- 20. Dudek S. E., Luig C., Pauli E. K., Schubert U., Ludwig S. (2010) The clinically approved proteasome inhibitor PS-341 efficiently blocks influenza A virus and vesicular stomatitis virus propagation by establishing an antiviral state. J. Virol. 84, 9439–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bajwa N., Liao C., Nikolovska-Coleska Z. (2012) Inhibitors of the anti-apoptotic Bcl-2 proteins: a patent review. Expert Opin. Ther. Pat. 22, 37–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gesto D. S., Cerqueira N. M., Fernandes P. A., Ramos M. J. (2012) Gemcitabine: a critical nucleoside for cancer therapy. Curr. Med. Chem. 19, 1076–1087 [DOI] [PubMed] [Google Scholar]
- 23. Meneghesso S., Vanderlinden E., Stevaert A., McGuigan C., Balzarini J., Naesens L. (2012) Synthesis and biological evaluation of pyrimidine nucleoside monophosphate prodrugs targeted against influenza virus. Antiviral Res. 94, 35–43 [DOI] [PubMed] [Google Scholar]
- 24. Rosebeck S., Sudini K., Chen T., Leaman D. W. (2011) Involvement of Noxa in mediating cellular ER stress responses to lytic virus infection. Virology 417, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Venticinque L., Meruelo D. (2010) Sindbis viral vector induced apoptosis requires translational inhibition and signaling through Mcl-1 and Bak. Mol. Cancer 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen K. F., Su J. C., Liu C. Y., Huang J. W., Chen K. C., Chen W. L., Tai W. T., Shiau C. W. (2012) A novel obatoclax derivative, SC-2001, induces apoptosis in hepatocellular carcinoma cells through SHP-1-dependent STAT3 inactivation. Cancer Lett. 321, 27–35 [DOI] [PubMed] [Google Scholar]
- 27. Koskela H. L., Eldfors S., Ellonen P., van Adrichem A. J., Kuusanmäki H., Andersson E. I., Lagström S., Clemente M. J., Olson T., Jalkanen S. E., Majumder M. M., Almusa H., Edgren H., Lepistö M., Mattila P., Guinta K., Koistinen P., Kuittinen T., Penttinen K., Parsons A., Knowles J., Saarela J., Wennerberg K., Kallioniemi O., Porkka K., Loughran T. P., Jr., Heckman C. A., Maciejewski J. P., Mustjoki S. (2012) Somatic STAT3 mutations in large granular lymphocytic leukemia. New Engl. J. Med. 366, 1905–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang W. B., Levy D. E., Lee C. K. (2011) STAT3 negatively regulates type I IFN-mediated antiviral response. J. Immunol. 187, 2578–2585 [DOI] [PubMed] [Google Scholar]
- 29. Qin A., Cheng T. S., Lin Z., Cao L., Chim S. M., Pavlos N. J., Xu J., Zheng M. H., Dai K. R. (2012) Prevention of wear particle-induced osteolysis by a novel V-ATPase inhibitor saliphenylhalamide through inhibition of osteoclast bone resorption. PloS One 7, e34132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.